Abstract
Background
A high prevalence (92.3%) of hepatitis C virus (HCV) co-infection among HIV patients identified during a large HIV outbreak associated with injection of oxymorphone in Indiana prompted genetic analysis of HCV strains.
Methods
Molecular epidemiological analysis of HCV-positive samples included genotyping, sampling intra-host HVR1 variants by next-generation sequencing (NGS) and constructing transmission networks using Global Hepatitis Outbreak and Surveillance Technology (GHOST).
Findings
Results from the 492 samples indicate predominance of HCV genotypes 1a (72.2%) and 3a (20.4%), and existence of 2 major endemic NS5B clusters involving 49.8% of the sequenced strains. Among 76 HIV co-infected patients, 60.5% segregated into 2 endemic clusters. NGS analyses of 281 cases identified 826,917 unique HVR1 sequences and 51 cases of mixed subtype/genotype infections. GHOST mapped 23 transmission clusters. One large cluster (n = 130) included 50 cases infected with ≥2 subtypes/genotypes and 43 cases co-infected with HIV. Rapid strain replacement and superinfection with different strains were found among 7 of 12 cases who were followed up.
Interpretation
GHOST enabled mapping of HCV transmission networks among persons who inject drugs (PWID). Findings of numerous transmission clusters, mixed-genotype infections and rapid succession of infections with different HCV strains indicate a high rate of HCV spread. Co-localization of HIV co-infected patients in the major HCV clusters suggests that HIV dissemination was enabled by existing HCV transmission networks that likely perpetuated HCV in the community for years. Identification of transmission networks is an important step to guiding efficient public health interventions for preventing and interrupting HCV and HIV transmission among PWID.
Fund
US Centers for Disease Control and Prevention, and US state and local public health departments.
Research in context.
Evidence before this study
Acute Hepatitis C Virus (HCV) infections have increased 167% in recent years in the USA; majority of these infections are believed to be attributable to injection drug use. The Centers for Disease Control and Prevention (CDC) recently reported injection-drug use of extended-release oxymorphone within a network of Persons Who Inject Drugs (PWID) in Indiana that led to introduction and rapid transmission of HIV. Global Hepatitis Outbreak and Surveillance Technology (GHOST), a novel disease detection tool developed by CDC, was employed to evaluate the HCV strains circulating in this community and map the landscape of HCV transmission.
Added value of this study
GHOST-assisted molecular testing revealed a large network of HCV transmission that facilitated the HIV outbreak in Indiana. This analysis shows transmissions of HCV among PWID predated the HIV outbreak, with multiple introductions of the virus into the community over several years. Widespread HCV transmission was revealed when the HIV outbreak was detected, and findings explain a large proportion of HIV-positive cases being co-infected with HCV.
Evidence of rising number of HCV cases in the region, which likely reflected a rise in the number of people abusing injection drugs, was an early signal that the community was at risk of additional public health issues, including an HIV outbreak. Similar at-risk communities across the nation might forestall massive HIV outbreaks, by keeping close tabs on acute HCV infections.
Implications of all the available evidence
This was the first real time field application of GHOST, the cyber-based HCV molecular surveillance technology, to a disease outbreak of such magnitude. GHOST integrates bioinformatics and web-based technologies with Next Generation Sequencing, and enables users, regardless of their computational expertise, to conduct independent, cost-effective, and accurate hepatitis molecular surveillance. Genetic testing coupled with new computational technologies enables rapid identification of complex transmission networks operating among PWID. Timely detection of these networks is important for disease surveillance and development of targeted public health interventions to contain infection transmission.
Alt-text: Unlabelled Box
1. Introduction
Hepatitis C virus (HCV) infection is a global public health problem, with 62.5–79.4 million persons chronically infected worldwide [1]. HCV is the most common blood-borne infection in the United States, resulting in an estimated 3.5 million individuals infected with the virus [2,3]. From 2003 to 2013, national HCV-related mortality surpassed that from human immunodeficiency virus (HIV) infection [4], combined with deaths associated with the 59 other nationally notifiable infectious conditionS [4]. HCV is efficiently transmitted via percutaneous exposure to blood and blood products. A large proportion of new HCV infections are associated with injection drug use (IDU) [[5], [6], [7], [8]]. Elevated risk of HCV infection among young persons who inject drugs (PWID) has been reported from many countries, including the United States, for more than a decade [[9], [10], [11]].
Over the last two decades, the opioid epidemic has led to an increase in IDU and associated morbidity and mortality in the United States [12,13]. Increase in acute HCV infections was recently reported, with the largest increase in Central Appalachia [14]. Surveillance data from Kentucky, Tennessee, Virginia, and West Virginia showed a 364% increase in the number of acute HCV infections from 2006 to 2012 among PWID aged ≤30 years [15]. Increase in new HCV infections was highly correlated with the epidemic of prescription opioid abuse in this region [16]. During 2010–2015, HCV incidence rates increased by 167% with the highest rates among young PWID [14]. Prevalence of HIV infection is historically low in the Mid-West [17]. However, in January 2015, the Indiana State Department of Health recorded a cluster of 11 newly diagnosed HIV infections among residents of a small rural community in Scott County, which led to detection of 181 cases of HIV infection diagnosed from November 2014 to November 2015 linked to injection use of oxymorphone [18]. This study showed that the rapid transmission of HIV was initiated by introduction of a single HIV-1 strain. Additionally, serological and molecular analyses showed that 92.3% of the HIV patients were co-infected with HCV [18], suggesting a difference in the epidemic history of HCV infection in this community.
Here, we report results of complex genetic analyses that reveal long-standing and continued HCV transmission within the affected community in Indiana. A dense and dynamic network of HCV transmission among PWID presaged the explosive HIV outbreak.
2. Methods
2.1. Specimens and associated data
The outbreak response investigation was approved by the Centers for Disease Control and Prevention (CDC) as epidemic disease control activity in response to a public health emergency; review by an Institutional Review Board was not required. Informed consent was not obtained because the activity was determined not to be research. Routine consents for HIV and HCV testing (oral or written, depending on the setting) and for contact tracing (oral) were obtained. Patient summary is shown in Table 1. CDC received all archived and new de-identified HCV-positive serum/plasma specimens collected between April and December 2015 in the course of HIV outbreak investigation [18]. Additional specimens collected in 2010 from an earlier HCV investigation in Indiana (n = 10) were tested for reference to identify HCV strains circulating in the community prior to this outbreak in 2015. The specimens were pre-screened and defined as laboratory-confirmed HCV infection by HCV antibody testing (VITROS Anti-HCV assay, Ortho Clinical Diagnostics, Raritan, NJ) and/or pooled nucleic acid amplification testing (Aptima HCV TMA, San Diego, CA). A person was considered to have acute HCV infection if HCV RNA was detectable in serum/plasma, but anti-HCV was negative. These specimens were also tested for HIV infection, and demographic and other patient associated data were collected as described in detail earlier [18].
Table 1.
emographic information, risk factors and testing information of Indiana cases with HCV and HIV infection.
| Variable demographics | Total patients (N = 492)a |
|---|---|
| Reason for testing | Number |
| Injection drug user | 274 |
| Recent exposure | 153 |
| Past exposure | 7 |
| Correctional screening | 23 |
| Routine screening | 8 |
| Confirmatory | 3 |
| Not available/unknown | 24 |
| Race | |
| White | 474 |
| Other or missing data | 18 |
| Sex | |
| Male | 313 |
| Female | 179 |
| County | |
| Scott | 295 |
| Clark | 64 |
| Jackson | 65 |
| Other | 68 |
| Age (years) | |
| <25 | 92 |
| 25–34 | 212 |
| 35–44 | 113 |
| ≥45 | 75 |
| HCV molecular data | |
| HCV RNA positive | 359 |
| HIV Co-infected HCV RNA positive | 91 |
| NS5B sanger sequencing | 299 |
| HVR1 NG sequencing | 281 |
| Sanger and/or NG sequencing | 329 |
| HCV RNA positive, no sequencing results | 20 |
| HCV Ab positive, PCR negative | 38 |
| Acute HCV cases | 12 |
| Reference HCV cases from 2010 | 10 |
Samples include anti-HCV and/or anti-HIV, anti-HCV and/or HCV RNA positive.
2.1.1. Nucleic acid extraction and PCR amplification
Samples included were anti-HCV and/or anti-HIV, anti-HCV and/or HCV RNA positive (n = 492). All HCV-positive samples were used for in-depth HCV molecular analyses. Nucleic acid was extracted from serum/plasma using the Roche MagNA Pure LC instrument and the MagNA Pure LC Total Nucleic Acid Isolation Kit (Roche Diagnostics, Mannheim, Germany). RNA was precipitated and reverse-transcribed using random hexamer primers. HCV genotyping and subtyping was performed using a 300-nucleotide (nt) NS5B coding region of the HCV genome amplified by PCR using previously described primers and conditions [19]. For determining genetic relatedness of the HCV infections of the patients, the E1/E2 junction of the HCV genome (309 nt), which contains the hypervariable region 1 (HVR1), was amplified using our nested PCR protocol as previously described [20]. The amplicons generated during first-round PCR were used as templates for nested PCR using hybrid primers composed of primer adapters, multiple identifiers, and specific sequences complementary to the HCV genome. This strategy allowed for multiplexing and downstream pyrosequencing. Resulting amplicons were quantified using the Picogreen kit (Invitrogen, Carlsbad, CA). Integrity of each fragment was evaluated using a Bioanalyzer 2100 (Agilent, Santa Clara, CA). To control for cross-contamination, a set of negative controls was introduced at each stage of the procedure starting from the extraction to the final round of PCR amplification to disqualify DNA libraries with detectable PCR products in negative controls. No cross-contamination was detected in experiments described here.
2.1.2. Next-generation sequencing (NGS)
PCR products were pooled and subjected to pyrosequencing using the GS FLX System and the GS FLX Titanium Sequencing Kit (454 Life Sciences, Roche, Branford, CT). Low-quality reads were removed using the GS Run Processor v2.3 (Roche). Initial reads were processed by matching to the corresponding identifier. The 454 files were processed using the error correction algorithms KEC and ET, which have been validated as highly accurate in finding true haplotypes, removing false haplotypes, and estimating the frequency of true haplotypes [21]. The error-corrected files were aligned using MUSCLE, and clean HVR1 sequences were obtained. Using this procedure, 2942 unique HVR1 sequences have been recovered on average from each cases.
2.1.3. Sequence analysis
For each sample of HCV sequences, a multiple sequence alignment (MSA) was created using MAFFT 7.221 [22]. The primer sequences were removed and the final sequences were 264 nucleotides in length. The amount of genetic heterogeneity of each sample was estimated by the nucleotide diversity (π) parameter, using MATLAB R2014a [23]. The sequences of every pair of samples were aligned and used to calculate genetic distances with MATLAB [24]. Phylogenetic analysis was conducted either using consensus NS5B sequences generated by Sanger sequencing as described [19], or intra-host HCV HVR1 variants sampled by NGS as described above. A maximum likelihood tree using the Tamura-Nei (TN93) substitution model was constructed using MEGA 6 [25]. The NS5B sequences were used to classify HCV strains into genotypes and subtypes by phylogenetic analysis with curated HCV NS5B reference sequences from North America archived in CDC's sequence database. Patristic distances were calculated over the phylogenetic tree using MATLAB.
2.1.4. HCV transmission network
A transmission network that represents the genetic relatedness among HCV in patients was built using the CDC in-house developed web-based (https://webappx.cdc.gov/GHOST/) Global Hepatitis Outbreak and Surveillance Technology (GHOST), hosting the sequence cleaning algorithm for the HVR1 454 NGS data [21], and the threshold method for detection of HCV transmission [26,27] implemented in MATLAB, and drawn with GEPHI.
2.1.5. k-step network
A k-step network was built as previously described [28]. The k-step network contains all possible minimum spanning trees and allows to efficiently visualize the genetic relatedness among all haplotypes present in a sample. The networks were drawn with GEPHI [29].
2.2. Role of the funding source
The study was supported by intramural CDC funding. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
3.1. Patient demographic and clinical characteristics
All samples (n = 492) collected between April and December 2015 (n = 482) and reference samples (n = 10) collected in 2010 were used for PCR and sequencing. Among the 492 tested, 12 (2.4%) were identified as acute patients, underscoring high existing prevalence of HCV in the community. A total of 339 (68.9%) samples were positive by the NS5B PCR and used for consensus sequencing of the NS5B region (n = 299), and/or by the HVR1 PCR and used for NGS of intra-host HVR1 variants (n = 281). The median age of the patients was 27 years, 63.6% were men, 96.3% were white, 60.0% resided in Indiana, and 55.7% were PWID (Table 1).
3.2. HCV inter-host diversity
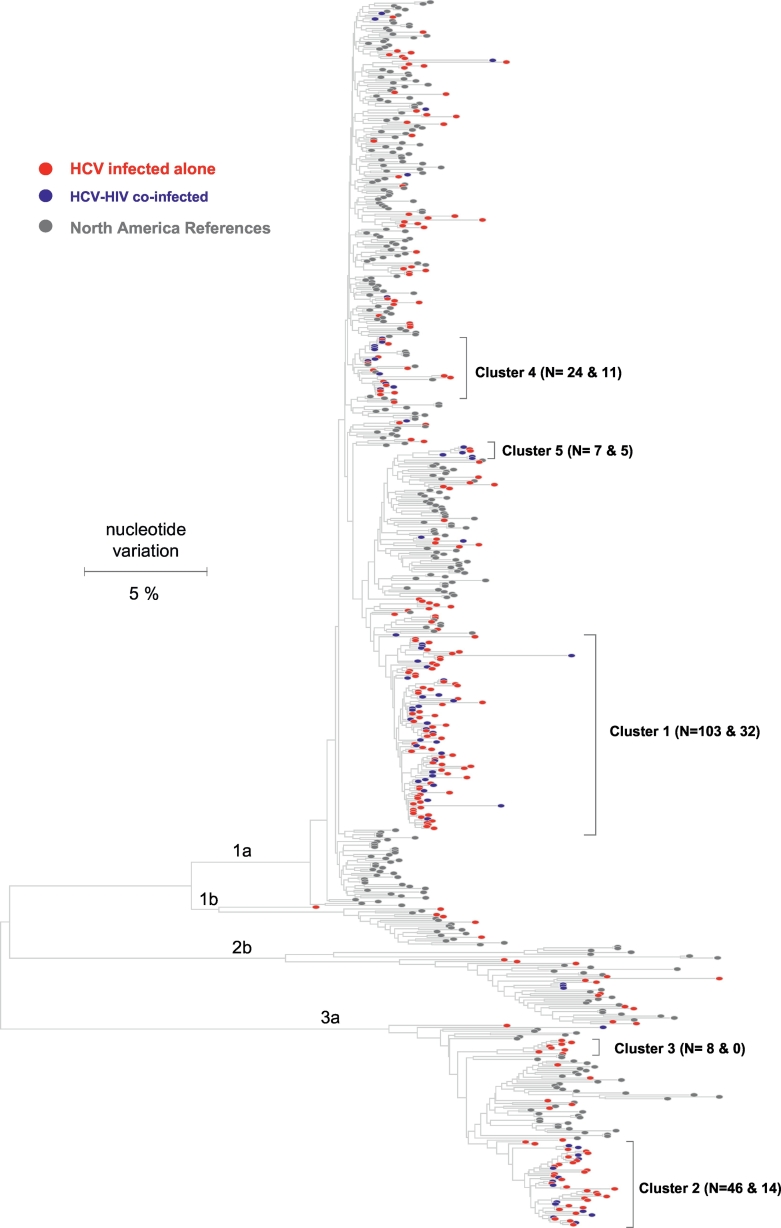
HCV strains were genetically characterized using NS5B consensus sequences obtained from 299 HCV-infected PWID. Phylogenetic analysis of the sequences showed a predominance of HCV genotype 1a (n = 216, 72.2%) and 3a (n = 61, 20.4%) followed by 2b (n = 16, 5.4%) and 1b (n = 6, 2.0%) (Fig. 1). All HCV sequences were unique and, when analyzed in the background of sequences obtained from other HCV strains circulating in the US, were found to be distributed across the entire tree, indicating numerous independent introductions of HCV strains into this region of Indiana. However, 49.8% of all HCV sequences were located in two major ‘endemic clusters’ that are composed exclusively of Indiana strains identified in our study. Cluster 1 (n = 103) included 47.7% of the genotype 1a sequences obtained from Indiana, while cluster 2 (n = 46) comprised 75.4% of the genotype 3a sequences. Together with another minor cluster 3 (n = 8), 88.5% of the genotype 3a sequences form endemic clusters (Fig. 1).
Fig. 1.
Phylogenetic analysis of HCV NS5b sequences. Colored nodes identify HCV strains sampled from Indiana in this study. Red nodes - HCV infected Indiana cases. Blue nodes - HIV-co-infected Indiana cases. Gray nodes - reference HCV strains circulating in North America. N shows the number of HCV infected (red and blue nodes) and HIV co-infected (blue nodes) cases in clusters. Endemic clusters 1, 2, 3 and 5 are composed exclusively of the Indiana HCV variants (no reference sequences - gray nodes).
3.3. HCV/HIV co-infection
The NS5B sequences were obtained from 76 patients with HCV and HIV co-infection. HCV sequences from the 76 HIV-co-infected patients were not evenly distributed across the phylogenetic tree; 62 of these NS5B sequences (81.6%) formed four co-infected clusters (blue nodes in Fig. 1). Approximately 60% of HCV strains from HIV-co-infected persons were found in endemic clusters 1 (n = 32) and 2 (n = 14), and represented ~30% of all HCV strains in each cluster.
3.4. Mixed-strain infections
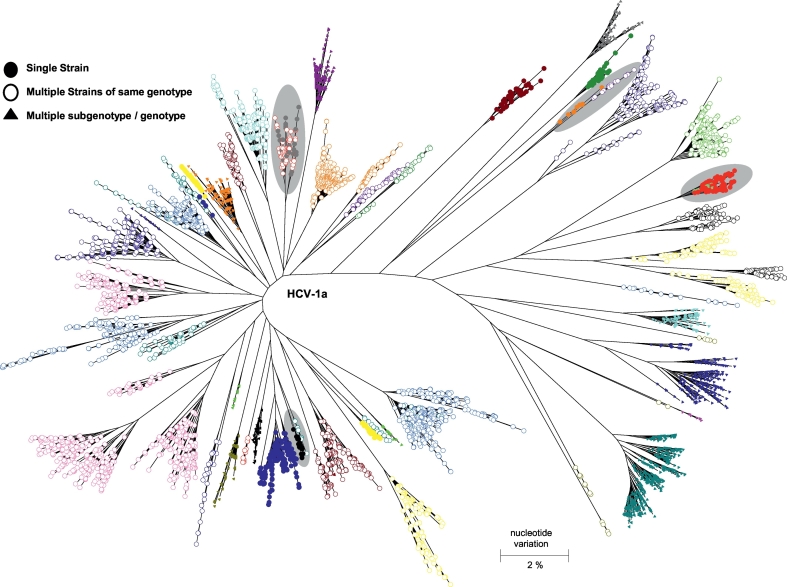
NGS analyses of the intra-host HVR1 variants in 281 patients identified a total of 826,917 unique sequences. Phylogenetic analysis of the intra-host HVR1 variants showed that, while 230 patients (81.9%) were infected with HCV strains of a single subtype, HCV strains identified in 51 patients (18.1%) belonged to more than one subtype and/or genotype (Fig. 2). One person was found to be infected with HCV strains that belonged to genotypes 1a, 1b, 3a, and 6.
Fig. 2.
Genetic complexity of intra-host HCV HVR1 variants. Phylogenetic tree of HVR1 variants from 40 cases. Each case is shown in different colour. HCV HVR1 variants sampled from cases linked by transmission are highlighted in gray shaded circles. Triangles identify HVR1 variants obtained from cases with mixed subtype/genotype infection. Open circles identify HVR1 variants obtained from cases infected with >1 HCV strain of the same subtype.
3.5. HCV transmission clusters
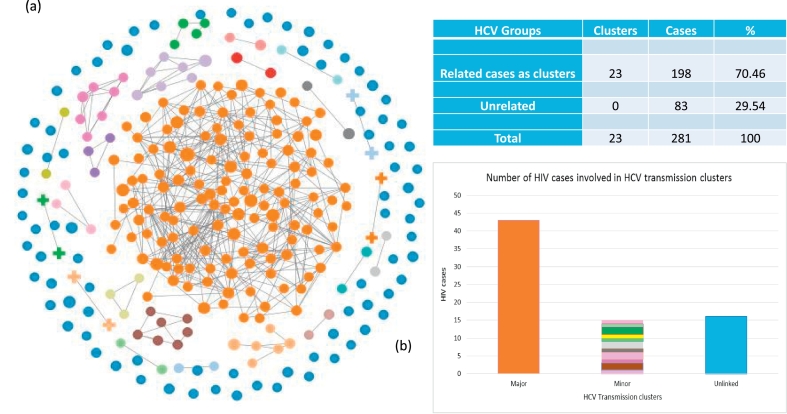
Using the intra-host HVR1 variants from all 281 cases tested by NGS, GHOST identified 23 HCV transmission clusters involving 198 persons (70.5%). The size of clusters ranged from 2 to 130 patients. Fig. 3 shows the transmission network generated by GHOST. Each node in the network represents a single infected person and a link between two persons is drawn if they share HCV strains [26]. The network shown is a set of 23 linked components representing transmission clusters, and a set of unlinked cases that do not share HCV strains with other tested members of the community. The largest cluster involves 130 persons (orange nodes), with 50 (38.5%) of them having mixed-genotype infections or 94.3% of all patients with mixed-genotype infection detected in this study. In this major cluster, 51 cases were infected with HCV strains that belonged to NS5B phylogenetic cluster 1, and 18 and 6 belonged to the NS5B clusters 2 and 3, respectively, indicating that endemic phylogenetic clusters identified using consensus NS5B sequences do not represent transmission clusters. Among cases involved in the major transmission cluster (n = 130), 4 were sampled in 2010, indicating that at least some HCV strains identified during this investigation were circulating in this community for at least 5 years. This finding underscores and supports long-term persistence of HCV infection among Indiana residents, prior to the more recent introduction of HIV.
Fig. 3.
GHOST-identified HCV transmission clusters. Each node is an HCV sample, a link is drawn if the minimal hamming distance between sequences from two samples is smaller than the validated genetic relatedness threshold [26]. A total of 23 HCV transmission clusters were identified. Clusters are colour coded (Panel a). Blue nodes represent unrelated cases. Orange nodes identify the largest transmission cluster (n = 130). Panel b represents the number of HIV cases identified in the HCV related and unrelated cases.
3.6. HIV co-infection in transmission clusters
Among sequences from 281 patients analyzed by GHOST, 74 (26.3%) patients were co-infected with HIV, of which 43 (58.1% of HIV cases; p < 0.05) belonged to the largest HCV transmission cluster of 130 cases (Fig. 4). Only 20.3% and 21.6% of the HIV co-infected persons were from all other transmission clusters and unlinked cases, respectively. This finding suggests that the major transmission cluster includes persons who occupy central positions in the syringe sharing network, and who may serve as sentinels for the early detection of introductions of other blood-borne infections that may be circulating in the same contact network.
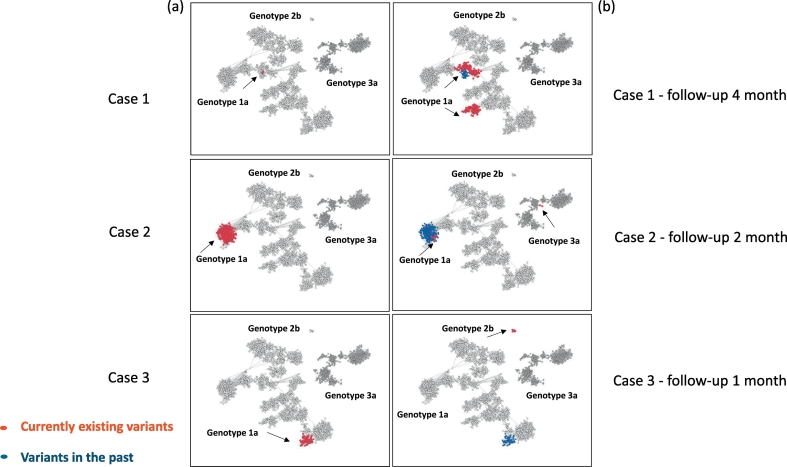
Fig. 4.
k-step network of the HCV variants found in three cases sampled at different time points of infection. Examples of evolving HCV diversity over time in 3 cases. Each node is a distinct HCV variant found in 12 infected cases who were followed up. Panel (a) shows in red HVR1 variants sampled in a case at the first time-point. Panel (b) shows in red HVR1 variants sampled from a case at the second time-point, while the variants seen at the previous time-point are shown in blue. Case 1 is infected with a second strain of the same subtype 1a; Case 2 is infected with HCV strains of different genotypes (1a to 1a/3a); Case 3 experienced a complete switch from one HCV genotype to another (genotype 1a to 2b).
3.7. Dynamics of HCV transmission
Among 281 persons from whom intra-host HCV HVR1 variants were identified by NGS, 12 were followed for up to 4 months. Fig. 4 shows examples of intra-host HCV populations sampled from three patients at two time-points (a and b). Six cases were infected with a second HCV strain after 1–4 months of follow-up, with two being infected with a second strain of the same subtype (e.g. Patient 1) and four with HCV strains of different genotypes (e.g. Patient 2), and for one case (Patient 3) a complete switch from one strain to another was observed. Combined, these results indicate a high rate of transmission of many HCV strains in this PWID community.
4. Discussion
To the best of our knowledge, this is the first comprehensive genetic evaluation of an HCV transmission network among PWID during an HIV outbreak in the United States. In the current HIV-driven field investigation, application of GHOST revealed a long-lasting local HCV epidemic. Analysis conducted using NS5B sequences indicates that each patient was infected with a unique HCV variant that belonged to one of four subtypes from three genotypes, suggesting multiple introductions of HCV into this rural PWID community. Such diversity can be achieved only by exposure of the entire community to HCV from several outside sources for many years. A long history of HCV infection among PWID in Indiana, prior to the recent introduction of a single HIV strain that caused the large HIV outbreak in 2015–2016, is supported by the detection of four HCV-positive patients, specimens from whom were collected in 2010. All four are involved in the major HCV transmission cluster of 130 cases, indicating that some HCV strains identified during the HIV outbreak were already circulating in this community for at least five years. Additionally, a long-term persistence of HCV infection among Indiana residents is strongly suggested by the observation of large endemic clusters of closely related HCV strains identified by NS5B sequence analyses. Extensive sampling from these clusters suggests a certain temporal pattern of HCV introduction when ancestral strains for each cluster have established local infections before all other detected HCV strains, so that the diverse progeny of these ancestors became dominant and more readily sampled from the affected PWID community. The earlier HCV strains, having infected this community before any other strain detected here, belonged to subtypes 1a and 3a. However, more recently introduced HCV strains belonged to subtype 1a predominantly. These findings imply a substantial increase in the introduction of new HCV strains over time, indicating a recent influx of HCV 1a infections and emphasizing possible wide-ranging contacts of the community with outside HCV sources.
The epidemiological investigation of the Indiana outbreak showed rapid spread of HIV by IDU, and occurred among PWID who shared syringes to inject the extended-release formulation of the prescription oral opioid oxymorphone [18]. Thus, our hypothesis of a recent increase in the introduction of HCV strains into this community is supported by recent reports of epidemics of prescription opioid abuse in the US [12,13], and increased numbers of patients with acute HCV infection in Central Appalachia [14]. A significant finding of our study is the detection of 51 cases (18%) with HCV mixed-genotype infections among the 281 persons tested using NGS. Spontaneous clearance of viruses from one subtype and persistence of another subtype after mixed infection was observed here in the persons who had a follow-up visit. Considering that HCV superinfections usually result in a rapid (<30 days) strain switch in affected persons [30], most probably owing to replicative exclusion [31], such a high rate of infections with multiple HCV strains indicates an extremely high rate of transmission of HCV in this rural PWID community. Co-transmission of more than a single HCV strain can also occur by the persistence of many mixed-genotype infections among PWID. However, in this case, the rate of HCV transmission in this community should have been expected to be even greater than suggested by superinfections alone, since a long-term co-existence of multiple HCV strains in a single person has been reported to be rare [31]. Detection of rapid succession of infections with different HCV strains in the patients with longitudinal follow-up specimens strongly supports the extraordinarily high rate of HCV transmission in this community of PWID. The complex and dynamic intra-host viral population is indicative of the evolving HCV epidemic, and underscores the need for a comprehensive and consistent assessment of the community in the following years. Combined, these data highlight significant epidemics of HCV among PWID in Indiana who previously have had limited access to HCV-related public health services, and how these gaps paved way for infiltration of HIV.
This is the first report where GHOST was field tested for an investigation of this scale, and our results offered high-resolution details about the HCV transmission dynamics. GHOST analysis showed that ~70% of the NGS-tested persons were organized into 23 transmission clusters, with the largest cluster including ~46% of all patients. This finding indicates the existence of a vast and highly modular HCV transmission network, reflecting the underlying network of contacts relevant to dissemination of blood-borne infections, that likely facilitated the explosive spread of HIV during the outbreak in the Indiana PWID community. Our analysis shows that these co-circulating HCV lineages preceded HIV in this community, with both viruses exploring the same transmission network among PWID, because 92.3% of all HIV-infected persons were found co-infected with HCV [18], but only 26.8% of HCV-infected cases tested here were co-infected with HIV. Segregation of 81.6% of HCV variants from HIV-co-infected patients into NS5B clusters (Fig. 1) indicates similarity in patterns of transmission between these two viruses. The major transmission cluster identified by GHOST includes 58.1% of HCV/HIV co-infected persons tested by NGS, while the cluster itself constitutes 46.3% of all HCV cases in the network. These results suggest that HIV dissemination was facilitated by the existing transmission network, which perpetuated HCV in the community for many years. The distribution of HIV co-infections predominantly among HCV patients organized into tight clusters suggests that both viral infections have similar routes of dissemination and explore the same contact network, with HCV having established infections earlier than HIV. Thus, the pattern of HCV dissemination reflects the pattern of HIV introduction to PWID in Indiana.
With the growing opioid crisis, the dual epidemics of HCV and HIV demand an integrated response. Our finding of similar transmission patterns across the PWID network suggests that targeted surveillance in PWID networks may permit a more efficient detection of new HIV infections in PWID communities infected with HCV. The high burden of HCV and HIV/HCV co-infection, coupled with lack of awareness and low-access to HCV services, highlights the need for sustained molecular surveillance of high-risk groups to understand better the landscape of HCV and HIV infections in the United States. HIV testing of persons, specifically from HCV clusters in HIV-naïve PWID populations, may facilitate early detection of HIV introduction into the community and allow early intervention to prevent future outbreaks of this magnitude. Early detection and prevention of HIV transmission would produce enormous public health and economic savings. Outcomes from the current investigation suggest that GHOST could facilitate prioritization of integrated intervention services for people at risk for both HIV and HCV infection. Implementing GHOST testing in rural high-risk communities, with PWID affected by the opioid epidemic where large HCV transmission networks with multiple genotypes are likely to exist, could help reduce existing and new HCV infections, and potentially prevent HIV introduction and transmission, if accompanied by network-targeted public health interventions such as Syringe Services Program (SSP), Medication Assisted Treatment (MAT), and HCV Direct Acting Antiviral therapy.
The limitation of this study is that GHOST testing was not conducted locally, which reduced identification of cases linked to the transmission network. Indeed, the identification of 29.5% of all cases and 21.6% of HIV-co-infected cases outside of the detected transmission clusters despite the expected high rate of HCV transmission suggests incomplete sampling from the local transmission network. At the population level, the infection network can be wider than the cases studied here, with unknown and unknowable transmissions occurring. GHOST integrates bioinformatics and web-based technologies with NGS, and enables users, regardless of their computational expertise, to conduct independent, cost-effective, and accurate hepatitis molecular surveillance. Participation of local public health institutions in GHOST-based surveillance is crucial for the most efficient detection of HCV outbreaks and transmission networks and for guiding targeted public health interventions.
In conclusion, while a single HIV strain caused the recent large HIV outbreak, numerous HCV strains have been introduced into the Indiana PWID community over many years prior to the HIV outbreak, with the rate of introductions likely increasing over time. Our investigation highlights the intersection of three major epidemics - opioid abuse, HCV, and HIV. GHOST offered detailed analysis of the genetic data and identified a vast HCV transmission network, frequent mixed-genotype infections and superinfections, indicating a complex, high-rate of HCV transmission in this community. The existing dense contact network that perpetuated HCV infections for years, likely enabled an increase in HIV infections in this population. As the heroin and opioid epidemic continues to worsen in the United States, it is imperative that susceptible populations are quickly identified to prevent new HIV outbreaks. Because the prevalence of HIV infection is generally low in the Mid-West, initiatives to identify high-risk HCV transmission networks may guide early detection of HIV outbreaks and interventions for interrupting HCV and HIV transmission among PWID in this, and other, geographic regions.
Contributors
SR and YK lead the study. SR, HT, FJ, YL, LP, LGR, GV and HR performed specimen management and laboratory testing. SR, GX, ZD, EC, WS, PS and AS analyzed data. DC, IR, SS provided computational support. SR and YK coordinated the investigation in partnership with PP, RG, PPo, JG, SB, JL, WS, ET and JW. SR and YK wrote the manuscript, and all authors contributed to the critical revision of the manuscript and approved the final version.
Acknowledgments
Acknowledgements
This study was funded by the US Centers for Disease Control and Prevention, and other state and local public health departments. The authors would like to thank the study participants and their partners, members of the Indiana HIV Outbreak Investigation Team including - Scott and Clark County Health Department, Scottsburg, IN; Saleem Kamili, Mike Purdy, Longmire Atkinson, Jon Zibbell, Philip Spradling from CDC DVH.
Disclosure
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention (CDC). The use of trade names and commercial sources is for identification only and does not imply endorsement by the CDC.
Declaration of interests
No competing interests have been declared. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Presented in part
Ramachandran S et al. Networks of HCV transmissions among persons who inject drugs: Indiana, 2015. Conference on Retroviruses and Opportunistic Infections (CROI), Boston, Abstract 149, 2016.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.10.007.
Appendix A. Supplementary data
Supplementary material
References
- 1.Polaris Observatory HCV Collaborators Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modelling study. Lancet Gastroenterol Hepatol. 2017;2(3):161–176. doi: 10.1016/S2468-1253(16)30181-9. [DOI] [PubMed] [Google Scholar]
- 2.Denniston M.M., Jiles R.B., Drobeniuc J., Klevens R.M., Ward J.W., McQuillan G.M. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293–300. doi: 10.7326/M13-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edlin B.R., Eckhardt B.J., Shu M.A., Holmberg S.D., Swan T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology. 2015;62(5):1353–1363. doi: 10.1002/hep.27978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ly K.N., Hughes E.M., Jiles R.B., Holmberg S.D. Rising Mortality Associated with Hepatitis C Virus in the United States, 2003-2013. Clin Infect Dis. 2016;62(10):1287–1288. doi: 10.1093/cid/ciw111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Havens J.R., Lofwall M.R., Frost S.D., Oser C.B., Leukefeld C.G., Crosby R.A. Individual and network factors associated with prevalent hepatitis C infection among rural Appalachian injection drug users. Am J Public Health. 2013;103(1):e44–e52. doi: 10.2105/AJPH.2012.300874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease C, Prevention Hepatitis C virus infection among adolescents and young adults: Massachusetts, 2002-2009. MMWR Morb Mortal Wkly Rep. 2011;60(17):537–541. [PubMed] [Google Scholar]
- 7.Centers for Disease C, Prevention Notes from the field: Hepatitis C virus infections among young adults--rural Wisconsin, 2010. MMWR Morb Mortal Wkly Rep. 2012;61(19):358. [PubMed] [Google Scholar]
- 8.Zibbell J.E., Hart-Malloy R., Barry J., Fan L., Flanigan C. Risk factors for HCV infection among young adults in rural New York who inject prescription opioid analgesics. Am J Public Health. 2014;104(11):2226–2232. doi: 10.2105/AJPH.2014.302142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Des Jarlais D.C., Diaz T., Perlis T. Variability in the incidence of human immunodeficiency virus, hepatitis B virus, and hepatitis C virus infection among young injecting drug users in New York City. Am J Epidemiol. 2003;157(5):467–471. doi: 10.1093/aje/kwf222. [DOI] [PubMed] [Google Scholar]
- 10.Page K., Morris M.D., Hahn J.A., Maher L., Prins M. Injection drug use and hepatitis C virus infection in young adult injectors: Using evidence to inform comprehensive prevention. Clin Infect Dis. 2013;57(Suppl. 2):S32–S38. doi: 10.1093/cid/cit300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roy E., Boudreau J.F., Boivin J.F. Hepatitis C virus incidence among young street-involved IDUs in relation to injection experience. Drug Alcohol Depend. 2009;102(1–3):158–161. doi: 10.1016/j.drugalcdep.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Mateu-Gelabert P., Guarino H., Jessell L., Teper A. Injection and sexual HIV/HCV risk behaviors associated with nonmedical use of prescription opioids among young adults in New York City. J Subst Abuse Treat. 2015;48(1):13–20. doi: 10.1016/j.jsat.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lankenau S.E., Teti M., Silva K., Jackson Bloom J., Harocopos A., Treese M. Initiation into prescription opioid misuse amongst young injection drug users. Int J Drug Policy. 2012;23(1):37–44. doi: 10.1016/j.drugpo.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suryaprasad A.G., White J.Z., Xu F. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006-2012. Clin Infect Dis. 2014;59(10):1411–1419. doi: 10.1093/cid/ciu643. [DOI] [PubMed] [Google Scholar]
- 15.Zibbell J.E., Iqbal K., Patel R.C. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years - Kentucky, Tennessee, Virginia, and West Virginia, 2006-2012. MMWR Morb Mortal Wkly Rep. 2015;64(17):453–458. [PMC free article] [PubMed] [Google Scholar]
- 16.Valdiserri R., Khalsa J., Dan C. Confronting the emerging epidemic of HCV infection among young injection drug users. Am J Public Health. 2014;104(5):816–821. doi: 10.2105/AJPH.2013.301812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reif S., Pence B.W., Hall I., Hu X., Whetten K., Wilson E. HIV diagnoses, prevalence and outcomes in nine southern states. J Community Health. 2015;40(4):642–651. doi: 10.1007/s10900-014-9979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters P.J., Pontones P., Hoover K.W. HIV infection linked to injection use of oxymorphone in Indiana, 2014-2015. N Engl J Med. 2016;375(3):229–239. doi: 10.1056/NEJMoa1515195. [DOI] [PubMed] [Google Scholar]
- 19.Forbi J.C., Purdy M.A., Campo D.S., Vaughan G., Dimitrova Z.E., Ganova-Raeva L.M. Epidemic history of hepatitis C virus infection in two remote communities in Nigeria, West Africa. J Gen Virol. 2012;93(Pt 7):1410–1421. doi: 10.1099/vir.0.042184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramachandran S., Xia G.L., Ganova-Raeva L.M., Nainan O.V., Khudyakov Y. End-point limiting-dilution real-time PCR assay for evaluation of hepatitis C virus quasispecies in serum: Performance under optimal and suboptimal conditions. J Virol Methods. 2008;151(2):217–224. doi: 10.1016/j.jviromet.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Skums P., Dimitrova Z., Campo D.S. Efficient error correction for next-generation sequencing of viral amplicons. BMC Bioinform. 2012;(13 Suppl 10):S6. doi: 10.1186/1471-2105-13-S10-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tajima F. Evolutionary relationship of DNA sequences in finite populations. Genetics. 1983;105(2):437–460. doi: 10.1093/genetics/105.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathworks. Matlab. Natick, MA. 2010. [Google Scholar]
- 25.Kumar S., Tamura K., Nei M. MEGA3: Integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform. 2004;5(2):150–163. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
- 26.Campo D.S., Xia G.L., Dimitrova Z. Accurate genetic detection of hepatitis C virus transmissions in outbreak settings. J Infect Dis. 2016;213(6):957–965. doi: 10.1093/infdis/jiv542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Longmire A.G., Sims S., Rytsareva I. GHOST: Global hepatitis outbreak and surveillance technology. BMC Genomics. 2017;18(Suppl. 10):916. doi: 10.1186/s12864-017-4268-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campo D.S., Dimitrova Z., Yamasaki L. Next-generation sequencing reveals large connected networks of intra-host HCV variants. BMC Genomics. 2014;(15 Suppl 5):S4. doi: 10.1186/1471-2164-15-S5-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bastian M., Heymann S., Jacomy M. International AAAI conference on weblogs and social media. Vol. 2009. San Jose; CA, USA: 2009. Gephi: An open source software for exploring and manipulating networks. May 17–20. [Google Scholar]
- 30.Blackard J.T. HCV superinfection and reinfection. Antivir Ther. 2012;17(7 Pt B) doi: 10.3851/IMP2460. (1443–8) [DOI] [PubMed] [Google Scholar]
- 31.Laskus T., Wang L.F., Radkowski M. Exposure of hepatitis C virus (HCV) RNA-positive recipients to HCV RNA-positive blood donors results in rapid predominance of a single donor strain and exclusion and/or suppression of the recipient strain. J Virol. 2001;75(5):2059–2066. doi: 10.1128/JVI.75.5.2059-2066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material