Abstract
Trauma experienced during surgery can contribute to the development of a systemic inflammatory response that can cause multi-organ dysfunction or even failure. Post-surgical neuroinflammation is a documented phenomenon that results in synaptic impairment, neuronal dysfunction and death, and impaired neurogenesis. Various pro-inflammatory cytokines, such as TNFα, maintain a state of chronic neuroinflammation, manifesting as post-operative cognitive dysfunction and post-operative delirium. Furthermore, elderly patients with post-operative cognitive dysfunction or delirium are three times more likely to experience permanent cognitive impairment or dementia. We conducted a narrative review, considering evidence extracted from various databases including Pubmed, MEDLINE and EMBASE, as well as journals and book reference lists. We found that further pre-clinical and well-powered clinical studies are required to delineate the precise pathogenesis of post-operative delirium and cognitive dysfunction. Despite the burden of post-operative neurological sequelae, clinical studies investigating therapeutic agents, such as dexmedetomidine, ibuprofen and statins, have yielded conflicting results. In addition, evidence supporting novel therapeutic avenues, such as nicotinic and HMGB-1 targeting and remote ischaemic pre-conditioning, is limited and necessitates further investigation.
Keywords: Inflammation, Neuroinflammation, Surgery, Systemic inflammatory response syndrome, Multiple organ dysfunction syndrome, Therapeutic targets
Highlights
-
•
Recent studies have demonstrated that surgery can cause neuroinflammation.
-
•
Post-surgical neuroinflammation can cause reversible and irreversible neurological sequelae, such as delirium and dementia.
-
•
Neuroinflammatory mechanisms include microglial activation, blood-brain barrier dysfunction, and subsequent neuronal damage.
-
•
Currently licensed medications have demonstrated the ability to attenuate neuroinflammation, but results remain conflicting.
-
•
More studies are needed to develop therapeutic approaches to tackle neuroinflammation and its associated neurological effects.
Research in context.
Evidence before this study
Prior to undertaking this narrative review, we considered evidence extracted from databases including Pubmed, MEDLINE and EMBASE, as well as researching within high-impact journals including, but not limited to: Nature, Cell (Trend in Neuroscience), Lancet, Critical Care and Annals of Surgery. Furthermore, book reference lists were reviewed, for example: Perioperative Care of the Elderly Patient (Barnett & Neves, 2018), Anaesthesia and Neurotoxicity (Morimoto, 2017) and Perioperative Care of the Elderly (2017).
As this research is a narrative review, the only inclusion criteria were for clinical and pre-clinical papers to have been written in the last 5 years (1st January 2013 – 1st September 2018). Search terms included post-operative delirium, post-operative cognitive decline/dysfunction, systemic inflammatory response syndrome and post-surgical neuroinflammation.
Added value of this study
This study summarises our current understanding of the mechanisms underlying post-operative delirium and cognitive decline, in addition to current novel therapeutic approaches to manage these conditions and promising future pathways that require further investigation.
Implications of all the available evidence
With over 300 million surgical procedures performed globally and an aging population, summarising and increasing the awareness of deleterious post-surgical neurological sequalae is vital. Alarmingly, evidence suggests that post-surgical cognitive complications are not simply limited to the immediate post-surgical period but, in fact, can result in chronic cognitive decline, such as dementia. This paper has numerous implications for practice, policy and future research:
Future research: encourage further investigation into current novel medication associated with lower rates of post-operative delirium and cognitive decline. In addition, by highlighting promising pathways with the potential to be targeted, we aim to facilitate new pre-clinical basic scientific studies by highlighting unexplained clinical outcomes
Policy and practice: increasing awareness of both the short-term consequences of post-operative delirium and cognitive decline (incl. Increased length of hospitalisation and healthcare costs), as well as the long-term effects (poorer quality of life, increased morbidity and mortality). By doing so, we aim to increase funding and international awareness of the clinical, financial and socioeconomic implications of these conditions.
Alt-text: Unlabelled Box
1. Introduction
Global estimates suggest that approximately 312 million major surgical procedures were performed in 2012, representing an increase of 38% over the previous 8 years [1]. Furthermore, accepted annual global estimates of the frequency of surgery in high-income countries suggest that over 11,000 surgical procedures are performed per 100,000 individuals [1,2]. With increasing lifespan and growing populations, the number of surgical procedures performed annually is likely to continue to increase. Bearing this in mind, it is critical that the peri-operative management of surgical patients is optimised to avoid deleterious sequelae, such as post-operative neurological complications. The first large prospective study in 1998 investigating post-operative cognitive decline following non-cardiac surgery estimated that 12% of patients develop symptoms of cognitive dysfunction in the post-operative period [3]. Whilst post-operative cognitive dysfunction is often self-limiting, recent concerning findings indicate that elderly patients that develop post-operative delirium or cognitive dysfunction are three times more likely to suffer from permanent cognitive impairment or dementia [4]. Despite global improvements in post-operative outcomes, our understanding of deleterious neurological sequelae in the post-operative period remains limited.
2. Surgical trauma and inflammation
2.1. Development of systemic inflammation
An uninhibited early inflammatory response results in a well-documented disorder known as Systemic Inflammatory Response Syndrome (SIRS). Studies have shown that higher surgical trauma and stress correlates with the development of SIRS and with longer hospital stay [5,6]. The proposed mechanisms underlying SIRS are summarised in Table 1. The severity of postoperative SIRS may be predicted by the increased levels of cytokines observed [[7], [8], [9]]. SIRS can potentially lead to multi-organ injury (Fig. 1). In this review we will focus on neuroinflammation and functional changes within the brain after surgery. In addition, it is important to note that major surgery is always accompanied with anaesthesia, which possesses its own associated pathological and functional effects within the brain which is still under investigation clinically; this is out of the scope of this review.
Table 1.
Pathogenesis of the systemic inflammatory response following surgical trauma and other disease conditions per se.
| Stage | Response | Result |
|---|---|---|
| I | Initial localised inflammation to limit further injury and promote site healing following surgical trauma, infection, burns and various other conditions. | There is initiation of a local inflammatory reaction via activation of the innate immune system and recruitment of immune cells, such as macrophages and neutrophils, to the site of injury. |
| II | Activation of the early compensatory anti-inflammatory response (CARS) aims to restore immunologic balance. | CARS results in various physiological alterations including the reduction of lymphocytes via apoptosis, a dampened monocytic response to cytokine stimulation and cutaneous anergy. In addition, CARS causes a decrease in human leukocyte antigen (HLA) antigen-presenting receptors on monocytes, as well as an increased production of specific cytokines, such as IL-10, that act to suppress TNF expression. Ultimately, there are two potential outcomes for patients in stage II: a. Restoration of immunologic balance and dampening of the pro-inflammatory response b. An overactive systemic inflammatory response prevails over CARS. |
| III | The overactive pro-inflammatory SIRS reaction predominates over the anti-inflammatory response. | SIRS causes endothelial dysfunction, increased microvascular permeability, profound vasodilation and activation of the coagulation system. The uninhibited action of NF-κβ results in the release of pro-inflammatory cytokines, including TNFα and IL-1. Other cytokines, particularly IL-6, stimulate the release of acute-phase reactants such as C-reactive protein (CRP), whilst activation of the complement cascade, particularly via C3a and C5a, promotes vasodilation and increased vascular permeability. |
| IV | The anti-inflammatory response is upregulated in order to compensate for the vigour of the systemic pro-inflammatory state. | The CARS eventually becomes excessive, resulting in profound immunosuppression or immune paralysis. This predisposes the individual to nosocomial or secondary infections, re-initiating the vicious cycle of systemic inflammation. |
| V | Prolonged dysregulation of the SIRS and CARS response. | This stage is termed immunologic dissonance. The prolonged action of pro-inflammatory cytokines, such as TNFα and IL-1, directly alters endothelial surfaces, resulting in the increased expression of tissue factor (TF). TF initiates the production of thrombin, promoting coagulation and also acting as a pro-inflammatory mediator itself. TNFα and IL-1 promote the production of plasminogen activator inhibitor-1, inhibiting the process of fibrinolysis. The pro-coagulant state is further upregulated via the activation of the complement cascade, which disrupts the action of anti-thrombin and activated protein-C. Persistence of the pro-coagulant state results in various complications of microvascular thrombosis, ultimately resulting in multiple organ dysfunction or failure and, in the most severe cases, death. |
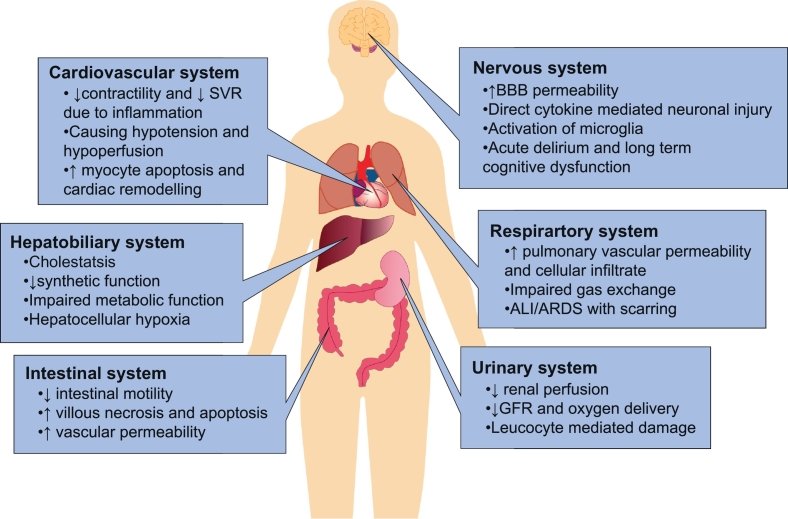
Fig. 1.
End organ effects of postoperative systemic inflammatory response syndrome (SIRS).
Surgical dissection and its associated trauma cause cell death which, in turn, results in the release of intracellular components into the extracellular space. These include immunogenic compounds such as RNA and DAMPs (HMGB-1, ATP and Histone) which bind to and activate specific toll-like receptors (TLRs), driving the NFκB mediated transcription of pro-inflammatory cytokines. Furthermore, activated T cells release pro-inflammatory mediators and can cause direct cytotoxicity. These processes result in tissue injury, oedema and inflammation and ultimately damage organs. ALI, acute lung injury. ARDS, acute respiratory distress syndrome. BBB, blood brain barrier. GFR, glomerular filtration rate. SVR, systemic vascular resistance.
2.2. Severe SIRS and pathological changes in the brain
2.2.1. Disruption of BBB – The hallmark of neuroinflammation
Following surgical trauma, the innate immune system is activated in a nuclear factor-κβ (NF-κβ)-dependent manner. This results in the release of a variety of pro-inflammatory mediators, such as TNFα, which ultimately causes blood-brain barrier (BBB) compromise and promotes monocyte-derived migration of macrophages into brain parenchyma [10]. In turn, intensification of CD11b immunoreactivity occurs, which has been identified in the surgical phenotype [11]. In addition, a murine study demonstrated that surgery and anaesthesia both play a role in reducing the levels of tight junction proteins including cloudin, occludin and ZO-1 [12]. This reduction of tight junction proteins may be caused by an increase of IL-6, which is essential in the metabolism of β-catenin resulting in a reduction of tight junction proteins [12]. The mechanisms underlying the development of BBB dysfunction and neuroinflammation are highlighted in Fig. 2
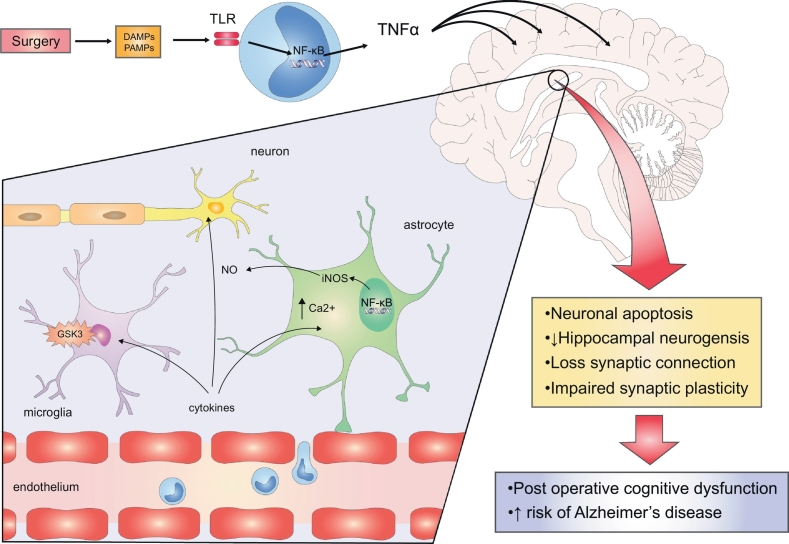
Fig. 2.
Mechanism of post-operative neuroinflammation.
DAMPs and PAMPs activate the downstream pathway involved in inducing the production of TNFα, as well as other proinflammatory mediators via NF-κB. This causes the loss of blood-brain barrier (BBB) integrity, due to endothelial dysfunction occurs and increased permeability of the BBB. As a result, there is a recruitment of circulating lymphocytes into the neuronal tissue, and microglia and astrocytes are activated. Cytokines induce the synthesis and release of NO via inducible nitric oxide synthase from the activated microglia and astrocytes. Also, cytokines cause an increase in intracellular Ca2+. In addition, GSK-3 dysfunction occurs in neuroinflammation, which potentiates microglial activation and migration and stimulates cells to produce NO and TNFα via NF-κB activation. Subsequently, neuroinflammation leads to neuronal apoptosis, reduced hippocampal neurogenesis, impaired synaptic plasticity and a loss of synaptic connections. All of this, in turn, leads to neurodegenerative conditions such as post-operative cognitive dysfunction and increased risk of Alzheimer's disease. BBB, blood brain barrier. DAMP, danger associated molecular pattern. GSK-3, Glycogen synthase kinase-3. iNOS, inducible nitric oxide synthase. NO, nitric oxide. TLR, toll like receptor.
Surprisingly, mast cells can also be found in the brain and appear to contribute to the disruption of the BBB following surgery. An increased number of mast cells is seen in the hippocampus in mice following tibial fracture surgery, which is associated with increased permeability of the BBB [13]. Although it is not yet fully understood, mast cells are shown to play a role in reducing the levels of tight junction proteins, including occludin and claudin-5 [13]. Mast cells may also indirectly disrupt the BBB by upregulating MMP-2 and 9, which subsequently break down the basal lamina and tight junction proteins of the BBB upon the release of serine protease tryptase and chymase.
2.2.2. The role of microglia and astrocytes
Both microglia and astrocytes have shown to play a key role in neuroinflammation. The release of pro-inflammatory cytokines during surgery-induced neuroinflammation results in the transformation of inactivated microglia to an activated, phagocytic state. Studies have demonstrated that the inhibition of microglia can lead to a reduction of proinflammatory cytokines including TNFα and IL-1β, as well as chemokines such as MCP-1, which are all important factors in causing neuroinflammation [14]. Evidence has shown that astrocytes and microglia can communicate with each other upon surgical trauma, resulting in a pro-inflammatory state. It appears that CCL2, a chemokine also known as MCP-1, is released by astrocytes following surgery, which binds to its receptor CCR2 in microglia causing activation of microglia and transformation into M1 phenotype [15]. Ultimately, there is an increased production of pro-inflammatory cytokines such as TNF-α and IL-1β, causing neuroinflammation and neuronal apoptosis [15]. Astrocytes may also contribute to neuroinflammation and neuronal damage by producing amyloid proteins following surgery. IL-17A is a cytokine that is upregulated in multiple inflammatory diseases, including neuroinflammation following surgery [16,17]. It has been demonstrated that IL-17A causes an increased production of Aβ1–42 via the TGFβ/Smad signalling pathway inside the astrocyte, a mechanism that is also observed in Alzheimer's' disease [18]. It is also important to note that the production of reactive oxygen species (ROS) in microglia is increased following surgery, contributing to neuroinflammation [19].
2.2.3. The role of DAMPs
Following surgical trauma, danger-associated molecular patterns (DAMPs) are released, which adhere to pattern recognition receptors (PRRs). PRRs are present within the BBB endothelium, and macrophages, whilst interaction between DAMPs and PRRs results in further activation of pro-inflammatory pathways. S100A8 is a cytosolic protein that acts as a DAMP and is upregulated following surgical trauma, associated with microgliosis and macrophage migration into the brain via the TLR4/MyD88 signalling pathway [20]. Interestingly, S100A8 expression is reduced in TLR4 knock-out (TLR4−/−) mice, suggesting a loop relationship between activation of the TLR4/MyD88 pathway and the ligand S100A8.
Other DAMPs, including the high-mobility group box 1 protein (HMGB-1), are also increased after surgery and cause neuroinflammation, particularly within the perivascular space and brain parenchyma [21]. It has been shown that HMGB1 can lead to microglial activation, which is a crucial step in neuroinflammation, ultimately resulting in cognitive dysfunction [22]. Furthermore, circulating HMGB-1 from a distant site can also be found in the brain [23]. These findings suggest that HMGB-1 may enter the disrupted BBB following surgery and cause neuroinflammation.
2.2.4. The role of BDNF
Brain-derived neurotrophic factor (BDNF) is a neurotrophin involved in neurogenesis, synaptogenesis and regulating neural plasticity, as well as learning and memory. Reduction of BDNF is seen in many neurodegenerative diseases including Alzheimer's disease and Parkinson's disease [24,25]. Interestingly, a decreased level of BDNF is also observed in parallel with neuroinflammation following surgery [26,27]. It is likely that surgery causes neuroinflammation and further downregulates both BDNF and its receptor TrkB, leading to a reduction of p-TrkB and inhibition of its downstream signalling pathway, which is important for learning and memory [28,29]. BNDF reduction may be mediated by phosphorylation of the glucocorticoid receptor GR [26].
2.3. Iron accumulation
Iron is necessary to ensure a normally-functioning CNS, as it is involved in DNA and RNA synthesis, myelin formation, and serves as a co-factor for multiple reactions [30]. As the brain ages there is also an accumulation of iron, and this has been associated with neurodegenerative diseases, such as Parkinson's disease and Alzheimer's disease [31,32]. Iron accumulation may also be a result of surgical trauma, in which oxidative stress may contribute to POCD in rodents [34]. Peripheral surgical trauma has been shown to cause changes in hippocampal iron homeostasis, resulting in post-surgical neuroinflammation and cognitive decline, which is attenuated with the administration of deferoxamine [35]. Deferoxamine is thought to reduce the effects of post-surgical hippocampal iron accumulation by ameliorating oxidative stress, microglial activation and neuronal apoptosis, thus potentially reducing the incidence of POCD [36]. Clinical studies are required to determine if there is a significant relationship between iron accumulation and post-operative neurological disease in humans.
2.4. Surgical stress response and the cholinergic anti-inflammatory pathway
As discussed, direct traumatic injury results in the activation of local inflammatory processes, whilst unregulated stress responses may lead to SIRS and multi-organ failure. It is important to note that the stress response to surgical trauma is comprised of a sequence of physiological processes involving biochemical alterations and changes to the metabolic, cardiovascular, endocrine and immune systems.
Local tissue injury acts as an afferent stimulus which causes activation of autonomic and somatic responses. This results in stimulation of the sympathetic nervous system and activation of the hypothalamic-pituitary-adrenal (HPA) axis. Following activation of the hypothalamus, there is an increase in adrenocorticotropic hormone (ACTH) release from the anterior pituitary and a subsequent increase in cortisol release from the adrenal medulla. Cortisol, in humans, and corticosterone, in rodents, are the primary glucocorticoid hormones responsible for the stress response. Overall, the release of cortisol causes an increase in blood glucose levels due to significant changes in protein, fat and carbohydrate metabolism. Furthermore, activation of the HPA axis also causes an increased synthesis of adrenaline and noradrenaline within the adrenal medulla, resulting in hypertension and tachycardia. Other hormonal changes include an increase in glucagon, anti-diuretic hormone and growth hormone, as well as activation of the renin-angiotensin-aldosterone system.
Activation of the cholinergic anti-inflammatory pathway is a vital response in dampening the effects of the sympathetic nervous system following surgical trauma. Cholinergic inhibition is mediated via the vagus reflex, whereby afferent vagus nerve fibres sense peripheral inflammatory mediators and convey these signals to the dorsal motor nucleus, subsequently inducing an efferent signal and acetylcholine release which acts on nicotinic α-7 receptors of macrophages and pro-inflammatory cells. Vagal activation therefore suppresses local and systemic inflammation by inhibiting the release of pro-inflammatory cytokines, including IL-1β, IL-18 and TNF-α [37]. Following surgical trauma, the body needs to maintain an intricate balance between an inflammatory state, which is required for physiological processes such as wound healing, and the cholinergic anti-inflammatory response, which is important in preventing SIRS and its associated complications [38].
3. Consequences of neuroinflammation
Post-surgical neuroinflammation causes a variety of deleterious structural and functional alterations within the brain, ultimately resulting in neurological impairment.
3.1. Synaptic dysfunction
Whilst synaptic impairment has classically been considered a characteristic feature of late stage neurodegeneration, it has more recently been heralded as an early indicator of dementia progression [39]. Surgical trauma results in the pathological activation of astrocytes, thus causing the release of pro-inflammatory cytokines and subsequent synaptic dysfunction [40]. The release of pro-inflammatory mediators triggers a vicious cycle whereby cytokines cause a reduction in glutamate-induced Ca2+ increase in astrocytes, thus resulting in a detrimental effect on astrocyte reactivity, and subsequent synaptic dysfunction [41]. Synaptic dysfunction has been shown to precede the development of irreversible neurodegenerative pathology, such as tauopathies [42].
3.2. Neuronal death
The process of post-surgical neuroinflammation involves several pro-apoptotic pathways. Activation of the TNF receptor-1 (TNFR1) signalling pathway results in the promotion of neuronal apoptosis, whilst increased levels of TNFα correlate with increased hippocampal neuron apoptosis [43]. In addition to cytokines, the release of NO from activated microglia and astrocytes further promotes the inflammatory response, thus resulting in neuronal apoptosis.
Zhang et al. showed that activated mast cells following surgery can induce microglial activation via MAPK signalling pathway, resulting in neuronal apoptosis [44]. In addition, activated mast cells are also directly involved in neuronal apoptosis, as indicated by increased levels of proapoptotic factors Bax/Bcl-2 and caspase-3 following surgery [44].
Recent studies have identified that peripheral surgery causes a reduction in cerebral acetylcholine, thus triggering a complex neuroinflammatory response [45], as well as subsequent degeneration of cholinergic neurons. Plaschke et al. have demonstrated that cholinergic signalling mediates the secretion of pro-inflammatory markers, such as IL-1β, following surgical stress [45], whilst decreases in cerebral acetylcholine results in an increased secretion of numerous pro-inflammatory cytokines [46]. Furthermore, increasing acetylcholine levels by inhibiting acetylcholinesterase causes a sustained anti-inflammatory effect, which may be a therapeutic option in reducing post-surgical neuroinflammation [47].
3.3. Neurogenesis impairment
Neurogenesis describes the maturation and differentiation of neural progenitor cells (NPCs) to neurons. The process of neurogenesis is confined to subventricular zone on the walls of the lateral ventricles, and the subgranular zone of the dentate gyrus within the hippocampus. Pro-inflammatory cytokines can inhibit neurogenesis by causing the death of NPCs and limiting neuronal differentiation. It has been proposed that an age-related decline in hippocampal neurogenesis may contribute, at least partially, to the cognitive deficits observed in neurodegenerative diseases. This may potentially indicate a relationship between neuroinflammation and the cognitive deficits observed in conditions such as dementia [48].
3.4. GSK3 dysregulation
Glycogen synthase kinase-3 (GSK3) is a serine/threonine protein kinase, which possesses significant pro-inflammatory effects within the brain [49]. GSK3 potentiates the activation and migration of microglia, and stimulates cells to produce pro-inflammatory mediators, including nitric oxide and TNFα, via the activation of NFκβ-mediated pathways. In addition, a positive correlation between GSK3 concentration and BBB permeability has also been demonstrated [50], whilst GSK3 also facilitates the migration of leukocytes across the BBB. Inhibition of GSK3 is associated with an upregulation of IL-10 mediated anti-inflammatory pathways, as well as a decrease in pro-inflammatory markers [49]. As GSK3 has demonstrated a role in the pathophysiology of neuroinflammation, it has been reasoned that it may possess a role in the development of neurodegenerative pathology, whilst its inhibition may be a novel therapeutic option to potentially dampen the neuroinflammatory response associated with surgical trauma.
4. Post-surgical neuroinflammation related neuro-disorders
4.1. Post-operative delirium (POD)
Delirium is an acute confusional state that is characterised by its fluctuating nature and reversibility, associated dysfunctional mental state and inattention, and typical time course. A diagnosis is usually made when the patient conveys differentiating perceptions. POD has an incidence of 37–46%, which differs depending on the type of surgical-procedure carried out, with reports as high as 51% [51]. Adverse events are also common; these include increased length of hospital stay and associated healthcare costs, and increased mortality. Whilst anaesthesia has classically been considered the major contributor to post-operative delirium, animal studies indicate that surgery-induced inflammation may be a significant cause of POD-associated neurological impairment [45,47].
The peripheral inflammatory response affects the brain, underlying the pathogenesis of POD. Cytokines, especially IL-1β, TNF-α and IL-6, are believed to interact with the brain. The mechanism by which peripheral cytokines make their way to the brain is either via vagal afferents, via the blood-brain barrier, or through the circumventricular region [52]. These peripheral cytokines cause microglial-induced cytokine synthesis and result in a cycle of neuroinflammation. A recent meta-analysis of human observational studies demonstrated that an increase in the concentration of peripheral and cerebrospinal fluid (CSF) inflammatory markers, such as CRP and IL-6, has demonstrated an association with both POD and POCD [53]. Furthermore, an increase in central and peripheral levels of S-100β and IL-8 levels has been shown to be significantly associated with POD, rather than POCD.
Further clinical evidence is required to understand these underlying mechanisms in humans.
4.2. Post-operative cognitive dysfunction (POCD)
Due to the surgery-induced systemic inflammatory response, patients may suffer ‘post-operative cognitive dysfunction’ (POCD). POCD is characteristically observed in the immediate post-operative period and manifests as a transient disturbance in cognition. This disturbance presents as a spectrum, from lethargy to social withdrawal and reduced concentration, to severe cognitive dysfunction. POCD may also result in fluctuating consciousness and sleep-wake-cycle irregularities.
Elderly patients have the highest incidence of POCD. Amongst hospitalized post-operative patients aged 60 years and older, rates as high as 40% have been observed [55]. Whilst a transient fluctuation in cognition within the post-operative period is often not considered to be particularly concerning, it is important to note that POCD has been shown to predict the onset of dementia in the future and may contribute to chronic neurodegeneration, particularly with repeated surgical procedures [4,56].
An increase in pro-inflammatory cytokines intra-operatively potentiates short-term cognitive impairment [57]. In addition, reports from animal studies have suggested that TNFα-mediated dysfunction of the BBB, and the subsequent migration of leukocytes into the hippocampus, has the ability to cause memory impairment [58].
POCD is more common following major surgery and in the presence of postoperative complications. Although POCD is traditionally considered a complication that occurs following cardiac surgery, epidemiological evidence suggests that the condition is also prevalent following various other surgical procedures, including abdominal surgery [59]. Both surgical trauma and neurodegenerative disorders are associated with increased cytokine release, resulting in numerous preliminary studies that have suggested that patients with Alzheimer's disease may be prone to the development of post-operative cognitive decline [60,61]. Furthermore, this relationship is thought to be due to a peripheral inflammatory stress response that promotes a neuroinflammatory process that results in cognitive dysfunction [62,63]. Increased levels of CRP and IL-6 have been noted in both POCD and POD, whilst increased levels of neuron-specific enolase (NSE) centrally and peripherally in patients with POCD specifically has been reported [53].
4.3. Other neurodegenerative conditions
Alzheimer's disease (AD) is a chronic neurodegenerative condition and is the most frequently diagnosed dementia. Both POCD and AD share the common feature of increased microglial activity, which ultimately results in profound neuroinflammation, cholinergic dysfunction and synaptic impairment. It is important to consider the fact that these inflammatory processes are not resolved as efficiently in elderly patients, in comparison to the younger population. It is hypothesized that this is due to a process known as microglial priming, whereby the accumulation of abnormally folded proteins and neurodegenerative changes result in abnormal microglial activation and multiplication [64]. Furthermore, this may explain why retrospective studies have suggested that the rates of POCD and post-operative dementia are high in older patients. For instance, Vanderweyde et al. (2010) states that 1/3 of patients aged 65 and over develop POCD, with 70% of these patients developing dementia 3 to 5 years later [65].
As discussed previously, GSK3 dysregulation has been hypothesized to play a role in post-surgical neuroinflammation due to its ability to control cellular proliferation and migration, inflammation, apoptosis and immune modulation. However, GSK-3 dysregulation has also been described as a hallmark of Alzheimer's disease [66,67], thus suggesting a common pathogenesis between stress-induced neuroinflammation and neurodegenerative disorders. Despite this, there have been limited studies investigating the direct impact of surgical trauma on the development of dementia. Evidence is predominantly limited to animal studies [68], whilst a retrospective cohort study demonstrated an increased 5-year risk of developing AD after coronary artery bypass grafting compared to patients who underwent transluminal coronary angioplasty [69]. This is corroborated in other surgeries, including dermatological, musculoskeletal, genitourinary, ophthalmological, and ENT, where there is almost a reported two-fold increase in dementia within 3–7 years [70].
5. Therapeutic options for post-operative neurological complications
A summary of the potential drugs that may be effective in treating POCD, as well as their proposed mechanisms, are summarised in Table 2.
Table 2.
Potential drugs that may be effective in treating POCD and their proposed effects.
| Drugs | Proposed effects | References |
|---|---|---|
| Dexamethasone | 71, 72 | |
| Parocoxib | Reduction in IL-1β, TNF-α, prostaglandin E | 73, 74, 75 |
| Paracetamol | Reduction in IL-1β, TNF-α, IL-6 | 76 |
| Ibuprofen | COX-2 inhibition and a reduction in peripheral cytokines, cortisol, and catecholamines | 77, 78, 79 |
| Dexmedetomidine | Reduction in IL-1β, TNF-α, IL-6, TLR-4 | 80–85 |
| Statins | Upregulation of α-secretase non-amyloidogenic pathway of APP processing | 92–97 |
| Ulinastatin | Reduction of inflammatory mediators and blood brain barrier permeability | 102, 103 |
| Nicotinic stimulation | Reduction of TNF-α | 36, 44, 46, 104 |
| HMGB-1 | Reduction of inflammatory mediators | 22, 105, 106 |
| Hydrogen sulphide donor | 107, 108, 109 | |
| Nt-p65-TMD | Inhibition of NF-κB p65 | 110 |
5.1. Novel use of existing licenced medications
5.1.1. Anti-inflammatory drugs
Due to the close association between neuroinflammation and post-operative neurological complications, several studies have investigated the effect of both steroids and non-steroid anti-inflammatory drugs.
The therapeutic value of peri-operative corticosteroids is conflicted in the current literature. Several randomised control trials of peri-operative methylprednisolone administration failed to show any improvement in post-operative cognitive function [71,72]. However, more recent studies using high dose intra-operative Dexamethasone (8 mg to 1 mg/kg) have reported a significantly lower rate of POD and POCD [73,74].
Parecoxib is a cyclooxygenase (COX)-2 selective NSAID thought to have good CNS distribution. Animal models of post-operative SIRS have reported that parecoxib administration results in significantly lower IL-1β and TNF-α expression, as well as lower Prostaglandin E levels in the hippocampus [75]. This is associated with better-preserved cognitive performance. In clinical trials, both single-dose parecoxib during anaesthesia and regular parecoxib in the post-operative period are associated with significantly lower rates of POD and POCD [76,77].
Paracetamol is a centrally acting antipyretic and analgesic thought to act through inhibition of the COX enzyme in the central nervous system. A recent animal study by Zhao et al. reported significantly reduced hippocampal IL-1 β, IL-6, TNF-α, as well as better cognitive performance associated with paracetamol administration [78]. However, this has not yet been confirmed in human studies.
Ibuprofen works by inhibiting both COX-1 and COX-2. Its inhibition of COX-1 may be responsible for its unwanted gastrointestinal effects [79]. Conversely, the inhibition of COX-2 provides ibuprofen with its analgesic, antipyretic, and anti-inflammatory properties. Research on mice by Huang et al. [80], has demonstrated improved cognitive performance, reduced systemic inflammation and glial activation upon the utilisation of ibuprofen perioperatively. Pre-operative administration of IV ibuprofen has been shown to improve post-operative cognitive function in the clinical setting, where the level of cytokines, cortisol, and catecholamines were reduced [81].
5.1.2. Dexmedetomidine
Dexmedetomidine is a α2 adrenoceptor agonist first licenced for clinical use in 1999 by FDA, with an initial use for sedation. In animal models of SIRS and surgical insult, dexmedetomidine has been shown to reduce the severity of neuroinflammation and neuroapoptosis [[82], [83], [84], [85], [86]]. Dexmedetomidine administration is associated with reduced hippocampal expression of IL-1β, IL-6, TNF-α and TLR-4, as well as reduced astrocyte and microglial activities [83,87].
Dexmedetomidine has demonstrated promising therapeutic benefits in clinical trials. A randomised control trial by Su et al. reported a 60% reduced rate of post-operative delirium after an infusion of dexmedetomidine [88]. Furthermore, a recent randomised controlled trial discovered improved cognitive function and quality of life in 3-year survivors, as well as increasing survival up to 2-years after a low-dose dexmedetomidine infusion in non-cardiac surgery [89]. In a meta-analysis of 13 studies with over 1300 patients, Zhou et al. estimated an effect size of 40% reduction in the risk of POCD [90]. In conjunction with these findings, a meta-analysis of 18 human studies has also demonstrated a significantly reduced POD risk after an administration of dexmedetomidine [91].
Whilst dexmedetomidine has demonstrated promising results, due to concerns over its potential side effects on cardiopulmonary system [92,93], its use needs to be further optimised in various clinical settings.
5.1.3. Statins
Statins, as shown through experimental models, have demonstrated a reduction in the production of Aβ plaques. This is achieved by downregulating neuroinflammation secondary to an interrupted secretase enzyme function. The α-secretase non-amyloidogenic pathway of amyloid precursor protein (APP) processing is upregulated by statins, in addition to β-secretase dimerization's inhibition by statins [[94], [95], [96], [97]]. As such, statins in epidemiological studies have demonstrated a reduction in AD incidence and POCD development [98,99]. Despite this, it is important to note that the association between statins and AD in humans varies across different properties of statins, patient gender and ethnicity [100]. Furthermore, evidence from both observational and randomised trials have produced conflicting results, with no well-powered high-quality data indicating a significant reduction in dementia with statin use, including the Medical Research Council (MRC) and British Heart Foundation (BHF) Heart Protection Study [101], pravastatin in elderly individuals at risk of vascular disease trial (PROSPER) [102]and Cochrane review and analysis [103]. Whilst these studies are dated, they provide the most robust and well-powered evidence regarding the relationship between statins and cognitive impairment.
5.2. Novel therapeutic targets
Ulinastatin is associated with reduced circulating inflammatory mediators and reduced blood brain barrier permeability in animal models of SIRS [106]. A recent meta-analysis of 5 studies and over 450 patients has reported a 60% reduction in the rate of POCD [107].
Nicotinic stimulation, in the form of a selective α7 nicotinic acetylcholine receptor agonist, has demonstrated improved hippocampal-associated memory deficits and neuroinflammation. The proposed mechanism involves the modulation of nuclear factor-kappa B (NF-κB) activity in monocytes, as well as the attenuation of oxidative stress responses by downregulating nicotinamide adenine dinucleotide phosphate's (NADPH) signalling [108], and the eventual inhibition of TNF- α [37]. Furthermore, it has been suggested that utilising procholinergic drugs, such as physostigmine, may also be therapeutic options in downregulating SIRS and neuroinflammation [45,47].
High mobility group box 1 (HMGB-1) is a DAMP that interacts with TLR-4 to induce the expression of NFκB and other inflammatory mediators, as well as being involved in the recruitment of bone marrow derived macrophages. In animal studies, administration of HMGB-1 has been shown to lead to memory impairment [109], whilst inhibition of HMGB-1 production and administration of anti-HMGB-1 antibodies are both associated with reduced neuroinflammation and better cognitive function [22,110].
Although hydrogen sulphide donors are toxic at high concentrations, research in recent years suggests that at smaller doses they may have possesscytoprotective effects [111,112]. Several animal studies have demonstrated that pre-treatment with sodium hydrosulphide is associated with significantly reduced SIRS-induced neuroinflammation and cognitive impairment [113].
Inhibition of NF-κB p65 activation using the cell-penetrating fusion protein, nt-p65-TMD, regulates and attenuates post-surgical systemic inflammation [114]. These preliminary results indicate that NF-κB p65 inhibition may be a potential future therapeutic option in the prevention of POCD.
5.3. Other novel approaches
Remote ischaemic preconditioning (rIPC), a technique whereby blood flow to a limb is repeatedly impaired, has shown promising neuroprotective results. Rat studies have demonstrated that the rIPC is neuroprotective against cerebral ischaemia by altering peripheral immune responses [115]. Clinical studies indicate that implementing rIPC in colon and cardiac surgery results in improved post-operative cognitive function [116,117]. Improved cerebral blood flow is believed to play a part in this neuroprotection, as mediated by circulating nitrite and its associated nitrosylation of complex I [118]. In addition, clinical studies also suggest that rIPC prevents the deterioration of short-term post-operative cognitive function in cardiac surgery patients [117], as well as reduces post-operative ischaemic tissue damage in brain tumour surgery [119]. However, the role of rIPC in neuroprotection has produced conflicting results, with findings from the multicentre, randomised RIPHeart (Remote Ischaemic Preconditioning for Heart Surgery) study demonstrating no effect on neurocognitive function or long-term outcomes in cardiac surgery patients [120].
The term human ‘microbiota’ refers to the microbes, including bacteria, fungi, arachaea and viruses, found within a specific environment, whilst the microbiome refers to their respective genomes. It has been suggested that microbiota is critically involved in normal brain development [121,122], whilst changes in gastrointestinal microbiota may contribute to memory decline and cognitive dysfunction in elderly patients [ [121,123]. Furthermore, significant differences in microbiota have been found between healthy, cognitively in-tact individuals and patients with depression, autism and neurodegenerative disorders [121,[123], [124], [125], [126]]. In fact, administration of a combination of antimicrobials, including amipicillin, meropenum, vancomycin, neomycin and bacitracin, causes a reduction of gut microbiota and subsequently impairs novel object recognition memory [127]. In addition, the systemic use of cefazolin, an antibiotic commonly used in the peri-operative period, causes changes in gut microbiota resulting in increased inflammation in the brain and gut, as well as learning impairment and cognitive dysfunction [128]. Studies exploring specific microbiomic patterns that may predispose to post-operative cognitive delirium and dysfunction may allow the therapeutic use of microbiome interference to reduce unwanted post-operative neurological sequelae. Furthermore, avoiding specific combinations of anti-microbial agents may also provide benefit in reducing post-operative cognitive dysfunction. However, further pre-clinical and clinical studies are warranted.
6. Concluding remarks and future perspectives
Studies have shown that surgery results in a systemic inflammatory response, which may potentiate neuroinflammation and, in turn, trigger deleterious neurological sequelae in some patients. Evidence suggests that post-surgical neuroinflammation may result in delirium and cognitive dysfunction, as well as long-term, irreversible disorders such as dementia per se.
Various therapeutic agents have been investigated for their neuroprotective effects, including dexmedetomidine and statins, as well as other novel agents targeting nicotinic and HMGB-1 pathways. Unfortunately, clinical studies investigating the utility of these agents are currently limited by number and quality, with dexmedetomidine demonstrating significant promise in recent studies. In addition, further investigation of other therapeutic avenues, including remote ischaemic pre-conditioning and microbiome interference, is required.
Overall, further pre-clinical and well-powered clinical studies are required to further investigate the precise pathogenesis underlying the relationship between surgery, neuroinflammation and the development of neurological disorders, as well as the efficacy and safety profile of therapeutic agents in ameliorating these post-surgical complications.
Funding sources
The Work has been supported by the fellowship and BOC Chair grant to Prof Daqing Ma from British Journal of Anaesthesia and Royal College of Anaesthetists, London, UK. The funding sources had no role in study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Declarations of interests
All authors declare no conflicts of interest.
Author's contribution
All authors contributed equally.
References
- 1.Weiser T.G., Haynes A.B., Molina G., Lipsitz S.R., Esquivel M.M., Uribe-Leitz T. Estimate of the global volume of surgery in 2012: an assessment supporting improved health outcomes. Lancet. 2015 Apr 27;385(Suppl. 2) doi: 10.1016/S0140-6736(15)60806-6. S11–6736(15)60806–6. [Epub 2015 Apr 26] [DOI] [PubMed] [Google Scholar]
- 2.Weiser T., Haynes A., Molina G., Lipsitz S., Esquivel M., Uribe-Leitz T. Size and distribution of the global volume of surgery in 2012. 2012. http://www.who.int/bulletin/volumes/94/3/15-159293.pdf Available at: [DOI] [PMC free article] [PubMed]
- 3.Moller J.T., Cluitmans P., Rasmussen L.S., Houx P., Rasmussen H., Canet J. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International study of post-operative cognitive dysfunction. Lancet. 1998 Mar 21;351(9106):857–861. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 4.Sprung J., Roberts R.O., Weingarten T.N., Nunes Cavalcante A., Knopman D.S., Petersen R.C. Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br. J. Anaesth. 2017;119(2):316–323. doi: 10.1093/bja/aex130. [DOI] [PubMed] [Google Scholar]
- 5.Pittet D., Rangel-Frausto S., Li N., Tarara D., Costigan M., Rempe L. Systemic inflammatory response syndrome, sepsis, severe sepsis and septic shock: incidence, morbidities and outcomes in surgical ICU patients. Intensive Care Med. 1995 Apr;21(4):302–309. doi: 10.1007/BF01705408. [DOI] [PubMed] [Google Scholar]
- 6.Takenaka K., Ogawa E., Wada H., Hirata T. Systemic inflammatory response syndrome and surgical stress in thoracic surgery. J. Crit. Care. 2006 Mar;21(1):48–53. doi: 10.1016/j.jcrc.2005.07.001. [discussion 53–5] [DOI] [PubMed] [Google Scholar]
- 7.Mokart D., Merlin M., Sannini A., Brun J.P., Delpero J.R., Houvenaeghel G. Procalcitonin, interleukin 6 and systemic inflammatory response syndrome (SIRS): early markers of postoperative sepsis after major surgery. Br. J. Anaesth. 2005 Jun;94(6):767–773. doi: 10.1093/bja/aei143. [DOI] [PubMed] [Google Scholar]
- 8.Aosasa S., Ono S., Mochizuki H., Tsujimoto H., Osada S., Takayama E. Activation of monocytes and endothelial cells depends on the severity of surgical stress. World J. Surg. 2000 Jan;24(1):10–16. doi: 10.1007/s002689910003. [DOI] [PubMed] [Google Scholar]
- 9.Abe T., Oka M., Tangoku A., Hayashi H., Yamamoto K., Yahara N. Interleukin-6 production in lung tissue after transthoracic esophagectomy. J. Am. Coll. Surg. 2001 Mar;192(3):322–329. doi: 10.1016/s1072-7515(00)00805-x. [DOI] [PubMed] [Google Scholar]
- 10.Lv S., Song H.L., Zhou Y., Li L.X., Cui W., Wang W. Tumour necrosis factor-alpha affects blood-brain barrier permeability and tight junction-associated occludin in acute liver failure. Liver Int. 2010 Sep;30(8):1198–1210. doi: 10.1111/j.1478-3231.2010.02211.x. [DOI] [PubMed] [Google Scholar]
- 11.Weaver L.C., Bao F., Dekaban G.A., Hryciw T., Shultz S.R., Cain D.P. CD11d integrin blockade reduces the systemic inflammatory response syndrome after traumatic brain injury in rats. Exp. Neurol. 2015 Sep;271:409–422. doi: 10.1016/j.expneurol.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang S., Gu C., Mandeville E.T., Dong Y., Esposito E., Zhang Y. Anesthesia and surgery impair Blood-brain barrier and cognitive function in mice. Front. Immunol. 2017;8 doi: 10.3389/fimmu.2017.00902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang S., Dong H., Zhang X., Li N., Sun J., Qian Y. Cerebral mast cells contribute to postoperative cognitive dysfunction by promoting blood brain barrier disruption. Behav. Brain Res. 2016 Feb 1;298(Pt B):158–166. doi: 10.1016/j.bbr.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Feng X, Valdearcos M, Uchida Y, Lutrin D, Maze M, Koliwad SK. Microglia mediate postoperative hippocampal inflammation and cognitive decline in mice. JCI Insight;2(7). [DOI] [PMC free article] [PubMed]
- 15.Xu J., Dong H., Qian Q., Zhang X., Wang Y., Jin W. Astrocyte-derived CCL2 participates in surgery-induced cognitive dysfunction and neuroinflammation via evoking microglia activation. Behav. Brain Res. 2017 Aug 14;332:145–153. doi: 10.1016/j.bbr.2017.05.066. [DOI] [PubMed] [Google Scholar]
- 16.Tian A., Ma H., Zhang R., Tan W., Wang X., Wu B. Interleukin17A promotes postoperative cognitive dysfunction by triggering Î2-amyloid accumulation via the transforming growth factor-Î2 (TGFÎ2)/smad signaling pathway. PLoS One. 2015;10(10) doi: 10.1371/journal.pone.0141596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Onishi R.M., Gaffen S.L. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology. 2010 Mar;129(3):311–321. doi: 10.1111/j.1365-2567.2009.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Docagne F., Gabriel C., Lebeurrier N., Lesne S., Hommet Y., Plawinski L. Sp1 and Smad transcription factors co-operate to mediate TGF-beta-dependent activation of amyloid-beta precursor protein gene transcription. Biochem. J. 2004 Oct 15;383(Pt 2):393–399. doi: 10.1042/BJ20040682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiu L.L., Ji M.H., Zhang H., Yang J.J., Sun X.R., Tang H. NADPH oxidase 2-derived reactive oxygen species in the hippocampus might contribute to microglial activation in postoperative cognitive dysfunction in aged mice. Brain Behav. Immun. 2016 Jan;51:109–118. doi: 10.1016/j.bbi.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Lu S.M., Yu C.J., Liu Y.H., Dong H.Q., Zhang X., Zhang S.S. S100A8 contributes to postoperative cognitive dysfunction in mice undergoing tibial fracture surgery by activating the TLR4/MyD88 pathway. Brain Behav. Immun. 2015 Feb;44:221–234. doi: 10.1016/j.bbi.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Lin G.X., Wang T., Chen M.H., Hu Z.H., Ouyang W. Serum high-mobility group box 1 protein correlates with cognitive decline after gastrointestinal surgery. Acta Anaesthesiol. Scand. 2014 Jul;58(6):668–674. doi: 10.1111/aas.12320. [DOI] [PubMed] [Google Scholar]
- 22.Terrando N., Yang T., Wang X., Fang J., Cao M., Andersson U. Systemic HMGB1 neutralization prevents postoperative neurocognitive dysfunction in aged rats. Front. Immunol. 2016;7 doi: 10.3389/fimmu.2016.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He H.J., Wang Y., Le Y., Duan K.M., Yan X.B., Liao Q. Surgery upregulates high mobility group box-1 and disrupts the blood-brain barrier causing cognitive dysfunction in aged rats. CNS Neurosci. Ther. 2012 Dec;18(12):994–1002. doi: 10.1111/cns.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howells D.W., Porritt M.J., Wong J.Y., Batchelor P.E., Kalnins R., Hughes A.J. Reduced BDNF mRNA expression in the Parkinson's disease substantia nigra. Exp. Neurol. 2000 Nov;166(1):127–135. doi: 10.1006/exnr.2000.7483. [DOI] [PubMed] [Google Scholar]
- 25.Lee J.G., Shin B.S., You Y.S., Kim J.E., Yoon S.W., Jeon D.W. Decreased serum brain-derived neurotrophic factor levels in elderly korean with dementia. Psychiatry Investig. 2009 Dec;6(4):299–305. doi: 10.4306/pi.2009.6.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian X.S., Tong Y.W., Li Z.Q., Li L.X., Zhang T., Ren T.Y. Surgical stress induces brain-derived neurotrophic factor reduction and postoperative cognitive dysfunction via glucocorticoid receptor phosphorylation in aged mice. CNS Neurosci. Ther. 2015 May;21(5):398–409. doi: 10.1111/cns.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hovens I.B., Schoemaker R.G., van der Zee E.A., Absalom A.R., Heineman E., van Leeuwen B.L. Postoperative cognitive dysfunction: involvement of neuroinflammation and neuronal functioning. Brain Behav. Immun. 2014 May;38:202–210. doi: 10.1016/j.bbi.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Wei P., Zheng Q., Liu H., Wan T., Zhou J., Li D. Nicotine-induced neuroprotection against cognitive dysfunction after partial hepatectomy involves activation of BDNF/TrkB signaling pathway and inhibition of NF-kappaB signaling pathway in aged rats. Nicotine Tob. Res. 2017 Jul 27;20(4):515–522. doi: 10.1093/ntr/ntx157. [DOI] [PubMed] [Google Scholar]
- 29.Fan D., Li J., Zheng B., Hua L., Zuo Z. Enriched environment attenuates surgery-induced impairment of learning, memory, and neurogenesis possibly by preserving BDNF expression. Mol. Neurobiol. 2016 Jan;53(1):344–354. doi: 10.1007/s12035-014-9013-1. [DOI] [PubMed] [Google Scholar]
- 30.Singh N., Haldar S., Tripathi A.K., Horback K., Wong J., Sharma D. Brain iron homeostasis: from molecular mechanisms to clinical significance and therapeutic opportunities. Antioxid. Redox Signal. 2014 Mar 10;20(8):1324–1363. doi: 10.1089/ars.2012.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rouault T.A., Cooperman S. Brain iron metabolism. Semin. Pediatr. Neurol. 2006 Sep;13(3):142–148. doi: 10.1016/j.spen.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Smith M.A., Zhu X., Tabaton M., Liu G., McKeel D.W., Jr., Cohen M.L. Increased iron and free radical generation in preclinical Alzheimer disease and mild cognitive impairment. J. Alzheimers Dis. 2010;19(1):363–372. doi: 10.3233/JAD-2010-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.An L.N., Yue Y., Guo W.Z., Miao Y.L., Mi W.D., Zhang H. Surgical trauma induces iron accumulation and oxidative stress in a rodent model of postoperative cognitive dysfunction. Biol. Trace Elem. Res. 2013 Feb;151(2):277–283. doi: 10.1007/s12011-012-9564-9. [DOI] [PubMed] [Google Scholar]
- 35.Li Y., Pan K., Chen L., Ning J.L., Li X., Yang T. Deferoxamine regulates neuroinflammation and iron homeostasis in a mouse model of postoperative cognitive dysfunction. J. Neuroinflammation. 2016 Oct 12;13(1):268. doi: 10.1186/s12974-016-0740-2. [-016-0740-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan K., Li X., Chen Y., Zhu D., Li Y., Tao G. Deferoxamine pre-treatment protects against postoperative cognitive dysfunction of aged rats by depressing microglial activation via ameliorating iron accumulation in hippocampus. Neuropharmacology. 2016 Dec;111:180–194. doi: 10.1016/j.neuropharm.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Bonaz B., Sinniger V., Pellissier S. Anti-inflammatory properties of the vagus nerve: potential therapeutic implications of vagus nerve stimulation. J. Physiol. 2016 Oct 15;594(20):5781–5790. doi: 10.1113/JP271539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujii T., Mashimo M., Moriwaki Y., Misawa H., Ono S., Horiguchi K. Physiological functions of the cholinergic system in immune cells. J. Pharmacol. Sci. 2017 May;134(1):1–21. doi: 10.1016/j.jphs.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Rivera A., Vanzuli I., Arellano J.J., Butt A. Decreased regenerative capacity of oligodendrocyte progenitor cells (NG2-Glia) in the ageing brain: a vicious cycle of synaptic dysfunction, myelin loss and neuronal disruption? Curr. Alzheimer Res. 2016;13(4):413–418. doi: 10.2174/1567205013666151116125518. [DOI] [PubMed] [Google Scholar]
- 40.Newman L. Aneurysmal bone cyst--a lesion in the mandibular ramus. Br. J. Oral Maxillofac. Surg. 1987 Feb;25(1):74–78. doi: 10.1016/0266-4356(87)90160-4. [DOI] [PubMed] [Google Scholar]
- 41.Rama Rao K.V., Kielian T. Neuron-astrocyte interactions in neurodegenerative diseases: role of neuroinflammation. Clin. Exp. Neuroimmunol. 2015 Aug;6(3):245–263. doi: 10.1111/cen3.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuste J.E., Tarragon E., Campuzano C.M., Ros-Bernal F. Implications of glial nitric oxide in neurodegenerative diseases. Front. Cell Neurosci. 2015;9 doi: 10.3389/fncel.2015.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olmos G., Llado J. Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediat. Inflamm. 2014;2014:861231. doi: 10.1155/2014/861231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X., Dong H., Li N., Zhang S., Sun J., Zhang S. Activated brain mast cells contribute to postoperative cognitive dysfunction by evoking microglia activation and neuronal apoptosis. J. Neuroinflammation. 2016 May 31;13(1):127. doi: 10.1186/s12974-016-0592-9. [-016-0592-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plaschke K., Muller A.K., Kopitz J. Surgery-induced changes in rat IL-1beta and acetylcholine metabolism: role of physostigmine. Clin. Exp. Pharmacol. Physiol. 2014 Sep;41(9):663–670. doi: 10.1111/1440-1681.12267. [DOI] [PubMed] [Google Scholar]
- 46.Plaschke K., Schulz S., Rullof R., Weigand M.A., Kopitz J. In-depth characterization of the neuroinflammatory reaction induced by peripheral surgery in an animal model. J. Neural Transm. (Vienna) 2018 Oct;125(10):1487–1494. doi: 10.1007/s00702-018-1909-x. [DOI] [PubMed] [Google Scholar]
- 47.Plaschke K., Weigand M.A., Fricke F., Kopitz J. Neuroinflammation: effect of surgical stress compared to anaesthesia and effect of physostigmine. Neurol. Res. 2016 May;38(5):397–405. doi: 10.1080/01616412.2016.1173889. [DOI] [PubMed] [Google Scholar]
- 48.Ekdahl C.T., Claasen J.H., Bonde S., Kokaia Z., Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc. Natl. Acad. Sci. U. S. A. 2003 Nov 11;100(23):13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin M., Rehani K., Jope R.S., Michalek S.M. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat. Immunol. 2005 Aug;6(8):777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramirez S.H., Fan S., Dykstra H., Rom S., Mercer A., Reichenbach N.L. Inhibition of glycogen synthase kinase 3beta promotes tight junction stability in brain endothelial cells by half-life extension of occludin and claudin-5. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0055972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saczynski J.S., Inouye S.K., Kosar C., Tommet D., Marcantonio E.R., Fong T. Cognitive and brain reserve and the risk of postoperative delirium in older patients. Lancet Psychiatry. 2014 Nov;1(6):437–443. doi: 10.1016/S2215-0366(14)00009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Gool W.A., van de Beek D., Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet. 2010 Feb 27;375(9716):773–775. doi: 10.1016/S0140-6736(09)61158-2. [DOI] [PubMed] [Google Scholar]
- 53.Liu X., Yu Y., Zhu S. Inflammatory markers in postoperative delirium (POD) and cognitive dysfunction (POCD): a meta-analysis of observational studies. PLoS One. 2018 Apr 11;13(4) doi: 10.1371/journal.pone.0195659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rundshagen I. Postoperative cognitive dysfunction. Dtsch. Arztebl. Int. 2014 Feb;111(8):119–125. doi: 10.3238/arztebl.2014.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Needham M.J., Webb C.E., Bryden D.C. Postoperative cognitive dysfunction and dementia: what we need to know and do. Br. J. Anaesth. 2017 Dec 1;119(suppl_1):i115–i125. doi: 10.1093/bja/aex354. [DOI] [PubMed] [Google Scholar]
- 57.Demura S., Takahashi K., Kawahara N., Watanabe Y., Tomita K. Serum interleukin-6 response after spinal surgery: estimation of surgical magnitude. J. Orthop. Sci. 2006 May;11(3):241–247. doi: 10.1007/s00776-006-1002-4. [DOI] [PubMed] [Google Scholar]
- 58.Terrando N., Eriksson L.I., Ryu J.K., Yang T., Monaco C., Feldmann M. Resolving postoperative neuroinflammation and cognitive decline. Ann. Neurol. 2011 Dec;70(6):986–995. doi: 10.1002/ana.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Benson R.A., Ozdemir B.A., Matthews D., Loftus I.M. A systematic review of postoperative cognitive decline following open and endovascular aortic aneurysm surgery. Ann. R. Coll. Surg. Engl. 2017 Feb;99(2):97–100. doi: 10.1308/rcsann.2016.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buvanendran A., Kroin J.S., Berger R.A., Hallab N.J., Saha C., Negrescu C. Upregulation of prostaglandin E2 and interleukins in the central nervous system and peripheral tissue during and after surgery in humans. Anesthesiology. 2006 Mar;104(3):403–410. doi: 10.1097/00000542-200603000-00005. [DOI] [PubMed] [Google Scholar]
- 61.Matsumoto E.D., Margulis V., Tunc L., Taylor G.D., Duchene D., Johnson D.B. Cytokine response to surgical stress: comparison of pure laparoscopic, hand-assisted laparoscopic, and open nephrectomy. J. Endourol. 2005 Nov;19(9):1140–1145. doi: 10.1089/end.2005.19.1140. [DOI] [PubMed] [Google Scholar]
- 62.Dantzer R. Cytokine-induced sickness behavior: where do we stand? Brain Behav. Immun. 2001 Mar;15(1):7–24. doi: 10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- 63.Kapila A.K., Watts H.R., Wang T., Ma D. The impact of surgery and anesthesia on post-operative cognitive decline and Alzheimer's disease development: biomarkers and preventive strategies. J. Alzheimers Dis. 2014;41(1):1–13. doi: 10.3233/JAD-132258. [DOI] [PubMed] [Google Scholar]
- 64.Lyman M., Lloyd D.G., Ji X., Vizcaychipi M.P., Ma D. Neuroinflammation: the role and consequences. Neurosci. Res. 2014 Feb;79:1–12. doi: 10.1016/j.neures.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 65.Vanderweyde T., Bednar M.M., Forman S.A., Wolozin B. Iatrogenic risk factors for Alzheimer's disease: surgery and anesthesia. J. Alzheimers Dis. 2010;22(Suppl. 3):91–104. doi: 10.3233/JAD-2010-100843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Orellana A.M., Vasconcelos A.R., Leite J.A., de Sa Lima L., Andreotti D.Z., Munhoz C.D. Age-related neuroinflammation and changes in AKT-GSK-3beta and WNT/ beta-CATENIN signaling in rat hippocampus. Aging (Albany NY) 2015 Dec;7(12):1094–1111. doi: 10.18632/aging.100853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Plaschke K., Kopitz J. In vitro streptozotocin model for modeling Alzheimer-like changes: effect on amyloid precursor protein secretases and glycogen synthase kinase-3. J. Neural Transm. (Vienna) 2015 Apr;122(4):551–557. doi: 10.1007/s00702-014-1319-7. [DOI] [PubMed] [Google Scholar]
- 68.Xu Z., Dong Y., Wang H., Culley D.J., Marcantonio E.R., Crosby G. Age-dependent postoperative cognitive impairment and Alzheimer-related neuropathology in mice. Sci. Rep. 2014;4 doi: 10.1038/srep03766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee T.A., Wolozin B., Weiss K.B., Bednar M.M. Assessment of the emergence of Alzheimer's disease following coronary artery bypass graft surgery or percutaneous transluminal coronary angioplasty. J. Alzheimers Dis. 2005 Aug;7(4):319–324. doi: 10.3233/jad-2005-7408. [DOI] [PubMed] [Google Scholar]
- 70.Chen P.L., Yang C.W., Tseng Y.K., Sun W.Z., Wang J.L., Wang S.J. Risk of dementia after anaesthesia and surgery. Br. J. Psychiatry. 2014 Mar;204(3):188–193. doi: 10.1192/bjp.bp.112.119610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Royse C.F., Saager L., Whitlock R., Ou-Young J., Royse A., Vincent J. Impact of methylprednisolone on postoperative quality of recovery and delirium in the steroids in cardiac surgery trial: a randomized, double-blind, placebo-controlled substudy. Anesthesiology. 2017 Feb;126(2):223–233. doi: 10.1097/ALN.0000000000001433. [DOI] [PubMed] [Google Scholar]
- 72.Whitlock R.P., Devereaux P.J., Teoh K.H., Lamy A., Vincent J., Pogue J. Methylprednisolone in patients undergoing cardiopulmonary bypass (SIRS): a randomised, double-blind, placebo-controlled trial. Lancet. 2015 Sep 26;386(10000):1243–1253. doi: 10.1016/S0140-6736(15)00273-1. [DOI] [PubMed] [Google Scholar]
- 73.Dieleman J.M., Nierich A.P., Rosseel P.M., van der Maaten J.M., Hofland J., Diephuis J.C. Intraoperative high-dose dexamethasone for cardiac surgery: a randomized controlled trial. JAMA. 2012 Nov 7;308(17):1761–1767. doi: 10.1001/jama.2012.14144. [DOI] [PubMed] [Google Scholar]
- 74.Valentin L.S., Pereira V.F., Pietrobon R.S., Schmidt A.P., Oses J.P., Portela L.V. Effects of single low dose of dexamethasone before noncardiac and nonneurologic surgery and general anesthesia on postoperative cognitive dysfunction-a phase iii double blind, randomized clinical trial. PLoS One. 2016 May 6;11(5) doi: 10.1371/journal.pone.0152308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peng M., Wang Y.L., Wang F.F., Chen C., Wang C.Y. The cyclooxygenase-2 inhibitor parecoxib inhibits surgery-induced proinflammatory cytokine expression in the hippocampus in aged rats. J. Surg. Res. 2012 Nov;178(1):e1–e8. doi: 10.1016/j.jss.2012.08.030. [DOI] [PubMed] [Google Scholar]
- 76.Zhu Y.Z., Yao R., Zhang Z., Xu H., Wang L.W. Parecoxib prevents early postoperative cognitive dysfunction in elderly patients undergoing total knee arthroplasty: a double-blind, randomized clinical consort study. Medicine (Baltimore) 2016 Jul;95(28) doi: 10.1097/MD.0000000000004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mu D.L., Zhang D.Z., Wang D.X., Wang G., Li C.J., Meng Z.T. Parecoxib supplementation to morphine analgesia decreases incidence of delirium in elderly patients after hip or knee replacement surgery: a randomized controlled trial. Anesth. Analg. 2017 Jun;124(6):1992–2000. doi: 10.1213/ANE.0000000000002095. [DOI] [PubMed] [Google Scholar]
- 78.Zhao W.X., Zhang J.H., Cao J.B., Wang W., Wang D.X., Zhang X.Y. Acetaminophen attenuates lipopolysaccharide-induced cognitive impairment through antioxidant activity. J. Neuroinflammation. 2017 Jan 21;14(1):17. doi: 10.1186/s12974-016-0781-6. [-016-0781-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rao P., Knaus E.E. Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyond. J. Pharm. Pharm. Sci. 2008 Sep 20;11(2):81s–110s. doi: 10.18433/j3t886. [DOI] [PubMed] [Google Scholar]
- 80.Huang C., Irwin M.G., Wong G.T.C., Chang R.C.C. Evidence of the impact of systemic inflammation on neuroinflammation from a non-bacterial endotoxin animal model. J. Neuroinflammation. 2018 May 17;15(1) doi: 10.1186/s12974-018-1163-z. 147-018-1163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Le V., Kurnutala L., SchianodiCola J., Ahmed K., Yarmush J., Daniel Eloy J. Premedication with intravenous ibuprofen improves recovery characteristics and stress response in adults undergoing laparoscopic cholecystectomy: a randomized controlled trial. Pain Med. 2016 Feb;18 doi: 10.1093/pm/pnv113. [DOI] [PubMed] [Google Scholar]
- 82.Yamanaka D., Kawano T., Nishigaki A., Aoyama B., Tateiwa H., Shigematsu-Locatelli M. Preventive effects of dexmedetomidine on the development of cognitive dysfunction following systemic inflammation in aged rats. J. Anesth. 2017 Feb;31(1):25–35. doi: 10.1007/s00540-016-2264-4. [DOI] [PubMed] [Google Scholar]
- 83.Paeschke N., von Haefen C., Endesfelder S., Sifringer M., Spies C.D. Dexmedetomidine Prevents Lipopolysaccharide-Induced MicroRNA Expression in the Adult Rat Brain. Int. J. Mol. Sci. 2017 Aug 23;18(9) doi: 10.3390/ijms18091830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang Z., Liu G., Zeng Q., Gao R., Zhang S., Wang L. MiR-29b expression is associated with a dexmedetomidine-mediated protective effect against oxygen-glucose deprivation-induced injury to SK-N-SH cells in vitro. Cell Biol. Int. 2017 Oct;31 doi: 10.1002/cbin.10906. [DOI] [PubMed] [Google Scholar]
- 85.Chen Y., Zhang X., Zhang B., He G., Zhou L., Xie Y. Dexmedetomidine reduces the neuronal apoptosis related to cardiopulmonary bypass by inhibiting activation of the JAK2-STAT3 pathway. Drug Des. Dev. Ther. 2017 Sep 26;11:2787–2799. doi: 10.2147/DDDT.S140644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ning Q., Liu Z., Wang X., Zhang R., Zhang J., Yang M. Neurodegenerative changes and neuroapoptosis induced by systemic lipopolysaccharide administration are reversed by dexmedetomidine treatment in mice. Neurol. Res. 2017 Apr;39(4):357–366. doi: 10.1080/01616412.2017.1281197. [DOI] [PubMed] [Google Scholar]
- 87.Zhu Y.J., Peng K., Meng X.W., Ji F.H. Attenuation of neuroinflammation by dexmedetomidine is associated with activation of a cholinergic anti-inflammatory pathway in a rat tibial fracture model. Brain Res. 2016 Aug 1;1644:1–8. doi: 10.1016/j.brainres.2016.04.074. [DOI] [PubMed] [Google Scholar]
- 88.Su X., Meng Z.T., Wu X.H., Cui F., Li H.L., Wang D.X. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet. 2016 Oct 15;388(10054):1893–1902. doi: 10.1016/S0140-6736(16)30580-3. [DOI] [PubMed] [Google Scholar]
- 89.Zhang D.F., Su X., Meng Z.T., Li H.L., Wang D.X., Li X.Y. Impact of dexmedetomidine on long-term outcomes after noncardiac surgery in elderly: 3-year follow-up of a randomized controlled trial. Ann. Surg. 2018 May;8 doi: 10.1097/SLA.0000000000002801. [DOI] [PubMed] [Google Scholar]
- 90.Zhou C., Zhu Y., Liu Z., Ruan L. Effect of dexmedetomidine on postoperative cognitive dysfunction in elderly patients after general anaesthesia: a meta-analysis. J. Int. Med. Res. 2016 Dec;44(6):1182–1190. doi: 10.1177/0300060516671623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Duan X., Coburn M., Rossaint R., Sanders R.D., Waesberghe J.V., Kowark A. Efficacy of perioperative dexmedetomidine on postoperative delirium: systematic review and meta-analysis with trial sequential analysis of randomised controlled trials. Br. J. Anaesth. 2018 Aug;121(2):384–397. doi: 10.1016/j.bja.2018.04.046. [DOI] [PubMed] [Google Scholar]
- 92.Lodenius A., Ebberyd A., Hardemark Cedborg A., Hagel E., Mkrtchian S., Christensson E. Sedation with dexmedetomidine or propofol impairs hypoxic control of breathing in healthy male volunteers: a nonblinded, randomized crossover study. Anesthesiology. 2016 Oct;125(4):700–715. doi: 10.1097/ALN.0000000000001236. [DOI] [PubMed] [Google Scholar]
- 93.Gerlach A.T., Blais D.M., Jones G.M., Burcham P.K., Stawicki S.P., Cook C.H. Predictors of dexmedetomidine-associated hypotension in critically ill patients. Int. J. Crit. Illn. Inj. Sci. 2016 Jul-Sep;6(3):109–114. doi: 10.4103/2229-5151.190656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Grimm M.O.W., Mett J., Grimm H.S., Hartmann T. APP function and lipids: a bidirectional link. Front. Mol. Neurosci. 2017;10 doi: 10.3389/fnmol.2017.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hoglund K., Wallin A., Blennow K. Effect of statins on beta-amyloid metabolism in humans: potential importance for the development of senile plaques in Alzheimer's disease. Acta Neurol. Scand. Suppl. 2006;185:87–92. doi: 10.1111/j.1600-0404.2006.00691.x. [DOI] [PubMed] [Google Scholar]
- 96.Parsons R.B., Price G.C., Farrant J.K., Subramaniam D., Adeagbo-Sheikh J., Austen B.M. Statins inhibit the dimerization of beta-secretase via both isoprenoid- and cholesterol-mediated mechanisms. Biochem. J. 2006 Oct 15;399(2):205–214. doi: 10.1042/BJ20060655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Burns M.P., Igbavboa U., Wang L., Wood W.G., Duff K. Cholesterol distribution, not total levels, correlate with altered amyloid precursor protein processing in statin-treated mice. NeuroMolecular Med. 2006;8(3):319–328. doi: 10.1385/nmm:8:3:319. [DOI] [PubMed] [Google Scholar]
- 98.Shepardson N.E., Shankar G.M., Selkoe D.J. Cholesterol and statins in Alzheimer’s disease: II. Review of human trials and recommendations. Arch. Neurol. 2011 Nov;68(11):1385–1392. doi: 10.1001/archneurol.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vizcaychipi M.P., Watts H.R., O'Dea K.P., Lloyd D.G., Penn J.W., Wan Y. The therapeutic potential of atorvastatin in a mouse model of postoperative cognitive decline. Ann. Surg. 2014 Jun;259(6):1235–1244. doi: 10.1097/SLA.0000000000000257. [DOI] [PubMed] [Google Scholar]
- 100.Chu C.S., Tseng P.T., Stubbs B., Chen T.Y., Tang C.H., Li D.J. Use of statins and the risk of dementia and mild cognitive impairment: a systematic review and meta-analysis. Sci. Rep. 2018 Apr 11;8(1):5804. doi: 10.1038/s41598-018-24248-8. [-018-24248-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002 Jul 6;360(9326):7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 102.Shepherd J., Blauw G.J., Murphy M.B., Bollen E.L., Buckley B.M., Cobbe S.M. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002 Nov 23;360(9346):1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 103.McGuinness B., Craig D., Bullock R., Passmore P. Statins for the prevention of dementia. Cochrane Database Syst. Rev. 2009 Apr 15;(2):CD003160. doi: 10.1002/14651858.CD003160.pub2. doi(2):CD003160. [DOI] [PubMed] [Google Scholar]
- 106.Li Y., Zhao L., Fu H., Wu Y., Wang T. Ulinastatin suppresses lipopolysaccharide induced neuro-inflammation through the downregulation of nuclear factor-kappaB in SD rat hippocampal astrocyte. Biochem. Biophys. Res. Commun. 2015 Mar 20;458(4):763–770. doi: 10.1016/j.bbrc.2015.01.155. [DOI] [PubMed] [Google Scholar]
- 107.Lv Z.T., Huang J.M., Zhang J.M., Zhang J.M., Guo J.F., Chen A.M. Effect of ulinastatin in the treatment of postperative cognitive dysfunction: review of current literature. Biomed. Res. Int. 2016;2016:2571080. doi: 10.1155/2016/2571080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Terrando N., Yang T., Ryu J.K., Newton P.T., Monaco C., Feldmann M. Stimulation of the alpha7 nicotinic acetylcholine receptor protects against neuroinflammation after tibia fracture and endotoxemia in mice. Mol. Med. 2015 Mar 17;20:667–675. doi: 10.2119/molmed.2014.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vacas S., Degos V., Tracey K.J., Maze M. High-mobility group box 1 protein initiates postoperative cognitive decline by engaging bone marrow-derived macrophages. Anesthesiology. 2014 May;120(5):1160–1167. doi: 10.1097/ALN.0000000000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kong Z.H., Chen X., Hua H.P., Liang L., Liu L.J. The oral pretreatment of glycyrrhizin prevents surgery-induced cognitive impairment in aged mice by reducing neuroinflammation and alzheimer's-related pathology via HMGB1 inhibition. J. Mol. Neurosci. 2017 Dec;63(3–4):385–395. doi: 10.1007/s12031-017-0989-7. [DOI] [PubMed] [Google Scholar]
- 111.Dugbartey G.J., Bouma H.R., Lobb I., Sener A. Hydrogen sulfide: a novel nephroprotectant against cisplatin-induced renal toxicity. Nitric Oxide. 2016 Jul 1;57:15–20. doi: 10.1016/j.niox.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 112.Wallace J.L., Wang R. Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat. Rev. Drug Discov. 2015 May;14(5):329–345. doi: 10.1038/nrd4433. [DOI] [PubMed] [Google Scholar]
- 113.Tu F.P., Li J.X., Li Q., Wang J. Effects of hydrogen sulfide on cognitive dysfunction and NR2B in rats. J. Surg. Res. 2016 Oct;205(2):426–431. doi: 10.1016/j.jss.2016.06.071. [DOI] [PubMed] [Google Scholar]
- 114.Cheon S.Y., Kim J.M., Kam E.H., Ho C.C., Kim E.J., Chung S. Cell-penetrating interactomic inhibition of nuclear factor-kappa B in a mouse model of postoperative cognitive dysfunction. Sci. Rep. 2017 Oct 18;7(1):13482. doi: 10.1038/s41598-017-14027-2. [-017-14027-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu Z.J., Chen C., Li X.R., Ran Y.Y., Xu T., Zhang Y. Remote ischemic preconditioning-mediated neuroprotection against stroke is associated with significant alterations in peripheral immune responses. CNS Neurosci. Ther. 2016 Jan;22(1):43–52. doi: 10.1111/cns.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.He Z., Xu N., Qi S. Remote ischemic preconditioning improves the cognitive function of elderly patients following colon surgery: a randomized clinical trial. Medicine (Baltimore) 2017 Apr;96(17) doi: 10.1097/MD.0000000000006719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hudetz J.A., Patterson K.M., Iqbal Z., Gandhi S.D., Pagel P.S. Remote ischemic preconditioning prevents deterioration of short-term postoperative cognitive function after cardiac surgery using cardiopulmonary bypass: results of a pilot investigation. J. Cardiothorac. Vasc. Anesth. 2015 Apr;29(2):382–388. doi: 10.1053/j.jvca.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 118.Hess D.C., Hoda M.N., Khan M.B. Humoral mediators of remote ischemic conditioning: important role of eNOS/NO/Nitrite. Acta Neurochir. Suppl. 2016;121:45–48. doi: 10.1007/978-3-319-18497-5_8. [DOI] [PubMed] [Google Scholar]
- 119.Sales A.H.A., Barz M., Bette S., Wiestler B., Ryang Y.M., Meyer B. Impact of ischemic preconditioning on surgical treatment of brain tumors: a single-center, randomized, double-blind, controlled trial. BMC Med. 2017 Jul 25;15(1):137. doi: 10.1186/s12916-017-0898-1. [-017-0898-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Meybohm P., Kohlhaas M., Stoppe C., Gruenewald M., Renner J., Bein B. RIPHeart (Remote Ischemic Preconditioning for Heart Surgery) study: myocardial dysfunction, postoperative neurocognitive dysfunction, and 1 year follow-up. J. Am. Heart Assoc. 2018 Mar 26;7(7) doi: 10.1161/JAHA.117.008077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rogers G.B., Keating D.J., Young R.L., Wong M.L., Licinio J., Wesselingh S. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol. Psychiatry. 2016 Jun;21(6):738–748. doi: 10.1038/mp.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim S., Kim H., Yim Y.S., Ha S., Atarashi K., Tan T.G. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature. 2017 Sep 28;549(7673):528–532. doi: 10.1038/nature23910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sherwin E., Dinan T.G., Cryan J.F. Recent developments in understanding the role of the gut microbiota in brain health and disease. Ann. N. Y. Acad. Sci. 2018 May;1420(1):5–25. doi: 10.1111/nyas.13416. [DOI] [PubMed] [Google Scholar]
- 124.Vogt N.M., Kerby R.L., Dill-McFarland K.A., Harding S.J., Merluzzi A.P., Johnson S.C. Gut microbiome alterations in Alzheimer's disease. Sci. Rep. 2017 Oct 19;7(1) doi: 10.1038/s41598-017-13601-y. 13537-017-13601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vuong H.E., Yano J.M., Fung T.C., Hsiao E.Y. The microbiome and host behavior. Annu. Rev. Neurosci. 2017 Jul 25;40:21–49. doi: 10.1146/annurev-neuro-072116-031347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tse J.K.Y. Gut microbiota, nitric oxide, and microglia as prerequisites for neurodegenerative disorders. ACS Chem. Neurosci. 2017 Jul 19;8(7):1438–1447. doi: 10.1021/acschemneuro.7b00176. [DOI] [PubMed] [Google Scholar]
- 127.Frohlich E.E., Farzi A., Mayerhofer R., Reichmann F., Jacan A., Wagner B. Cognitive impairment by antibiotic-induced gut dysbiosis: analysis of gut microbiota-brain communication. Brain Behav. Immun. 2016 Aug;56:140–155. doi: 10.1016/j.bbi.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liang P., Weiran S., Zhiyi S. Perioperative use of cefazolin ameliorates postoperative cognitive dysfunction but induces gut inflammation in mice. J. Neuroinflammation. 2018;15(235) doi: 10.1186/s12974-018-1274-6. [DOI] [PMC free article] [PubMed] [Google Scholar]