Abstract
Background
Streptococcus pneumoniae is the leading cause of bacterial pneumonia worldwide. Previous reports showed that IL-20 cytokines (IL-19, IL-20 and IL-24) are induced and have an immuno-regulatory function during cutaneous infection. In the current study, our aim was to demonstrate the implication of IL-20 cytokines and their receptors and their role during experimental pneumococcal infection.
Methods
C57BL/6 mice were infected with S. pneumoniae by intranasal route. The bacterial burden, the immune response and the cytokine production were evaluated after treatment with an anti-IL-20 receptor-b (IL-20Rb) neutralizing antibody (anti-IL-20Rb).
Findings
Of interest, expression of IL-20 cytokines mRNA and protein were transiently increased in the lung tissue during infection. Blocking of the IL-20Rb decreased the bacterial burden both in the bronchoalveolar lavage and the lung whereas there was no significant drop in the blood. This treatment also reduced the pulmonary damages (as shown by the alveolar wall thickening), the recruitment of neutrophils and dendritic cells, and the levels of the pro-inflammatory cytokines IL-1β and IL-6 in the lung. Administration of the anti-IL-20Rb antibody enhanced the synthesis of the antibacterial peptide LCN2. However, this effect is transient and did not affect the survival of the infected mice.
Interpretation
Collectively, this study highlights the implication of IL-20 related cytokines during lung infection by S. pneumoniae and might have therapeutic applications in bacterial pneumonia.
Fundings
This work was supported by CNRS, INSERM, INSERM-transfert, the University of Lille and the Fondation du Souffle (Paris, France).
Keywords: Pneumonia, Cytokines, Cytokine receptor, Immunomodulation, Antimicrobial peptides
Research in context.
Evidence before the study
Exposure to microorganisms might be the cause of respiratory infections which still are a major cause of death and an important health problem. Among bacteria, Streptococcus pneumoniae was recognized as a leading cause of pneumonia and hospital-acquired opportunistic infections. The severity of the pneumonia is tightly related to the efficiency of the defense mechanism. Both innate and adaptive immune responses participate in the clearance of the pneumococci. Among the factors orchestrating the defense mechanism, IL-20 cytokines play a major role in the clearance of the bacteria and the lung homeostasis by limiting injury and maintaining tissue integrity. Whereas the role of interleukin(IL)-22 is widely demonstrated, the implication of the other IL-20 cytokines (IL-19, IL-20 and IL-24) acting through different pathways is unknown.
Added value of this study
We demonstrated that expression of IL-19, IL-20 and IL-24 were transiently increased in the lung tissue with a peak at 24 h post-infection. Blocking the binding of IL-20 cytokines to their receptor improve the bacterial clearance and decrease the inflammatory reaction and the tissue lesions during the first days of the infection. Altogether, this study identified a new mechanism inhibiting the lung defense mechanism against bacterial infection at the opposite of IL-22.
Implications of all the available evidence
These data suggest that imbalance between IL-22 and the other IL-20 cytokines might increase the susceptibility to respiratory infection often observed during chronic inflammatory diseases. Thus, targeting IL-20 cytokines might be valuable to improve the control of respiratory bacterial infection.
Alt-text: Unlabelled Box
1. Introduction
Pneumococcal lung infections are a real public health problem and are responsible for approximately 2 million deaths and cost hundred billions of dollars per year [1]. Streptococcus pneumoniae (Sp) is a facultative anaerobic Gram-positive diplococcus for which >90 serotypes have been identified [2]. It is the most common cause of bacterial pneumonia and it is also implicated in sinusitis and bacterial meningitis [3,4]. Sp colonizes the nasopharynx and can spread to the lower respiratory tract via the airways [1].
The severity of the pneumonia is tightly related to the efficiency of the anti-bacterial host response. Both innate and adaptive immune responses participate in the clearance of the pneumococci [5]. Among the factors orchestrating the anti-bacterial response, Th17-type cytokines, including interleukin (IL)-17 and IL-22, play a major role in the clearance of Sp [[6], [7], [8]] and the lung homeostasis by limiting injury and maintaining tissue integrity, by modulating remodeling and the secretion of antimicrobial peptides [6]. We also previously demonstrated that the increased susceptibility to Sp in mice chronically exposed to cigarette smoke was due to a defective IL-22 response [9]. In this situation, alteration of alveolar macrophages and dendritic cell (DC) function leads to a lower secretion of these cytokines by conventional and non-conventional T cells. IL-22 with IL-19, IL-20, IL-24 and IL-26 (IL-26 only present in humans), belong to the IL-20 cytokine subfamily, a subset of the IL-10 family [[10], [11], [12]]. IL-19, IL-20 and IL-24 all bind the type I IL-20 receptor (IL-20R), a heterodimeric receptor composed of the IL-20RA and B chains (IL-20Ra and IL- 20Rb). Moreover, IL-20 and IL-24 bind the type II IL-20R a heterodimeric receptor composed of the IL-22 receptor α1 subunit and IL-20Rb [13]. Thus, IL-20Rb subunit is the common chain to both receptor types recognizing IL-20 cytokines and blocking antibodies against this receptor can efficiently neutralize this pathway [14].
These IL-20Rb-containing receptor complexes are mainly expressed on epithelial cells, mononuclear phagocytes and some lymphocytes. Recent data also revealed that activated neutrophils expressed this receptor [15]. Moreover, the role of IL-20 related cytokines is controversial and exhibited some “anti-inflammatory” effects involved in the cutaneous tissue homeostasis [16]. However, Myles et al. have shown that IL-20 related cytokines (IL-19, IL-20 and IL-24) promote cutaneous Staphylococcus aureus infection in mice by downregulating IL-17 and IL-22 production [14]. However, their implication in lung bacterial infection has not yet been evaluated.
In this study, our aim was to demonstrate the implication during lung infection by SP and their role in this context. We hypothesized that blocking the IL-20 pathway using an anti-IL-20Rb neutralizing antibody could help to control such bacterial lung infection. For this, we first analyzed the production of IL-20-related cytokines and their receptors in lung tissues from mice infected by Sp. The impact of the treatment with a blocking anti-IL-20Rb antibody was evaluated on the bacterial clearance and lung inflammation as well as on the survival in mice infected by Sp. This study identified IL-20 related cytokines and their receptor as modulators of the host response during Sp infection. Thus, targeting IL-20Rb subunit might be valuable to improve the control of Sp infection.
2. Materials and methods
2.1. Animals
Six- to eight-week-old male wild-type C57BL/6 (H—2Db) mice were purchased from Janvier (SOPF animal facility, Le Genest-St.-Isle, France). All animal work conformed to the guidelines of Animal Care and Use Committee from Nord Pas-de-Calais (agreement no. AF 16/20,090). Mice were maintained in a temperature-controlled (23 °C) facility with a strict 12 h light/dark cycle with food and water provided ad libitum.
2.2. Infection with streptococcus pneumoniae and bacterial counts
A clinical isolate S. pneumoniae serotype 1 (clinical isolate E1586) was obtained from the National Reference Laboratory, Ministry of Health, Uruguay and was grown as described previously [17]. For infection, frozen working stocks were diluted in phosphate- buffered saline (PBS). The quantity of bacteria in the frozen stock was systematically verified in each experiment and the quantity of bacteria was approximately the same before and after frozen. Anesthetized mice (intraperitoneal injection of ketamine (75 mg/Kg)-xylazine (5 mg/Kg) (50% of each compound used in proportion) (Rompun 2% xylazine, Bayer and Imalgene 1000, Merial) were intranasally infected with 2 × 106 bacteria (in 50 μl). For the evaluation of the lung response to Sp, mice were sacrificed by cervical dislocation at 12, 24 or 48 h post-infection. Bacterial burden was measured by plating serial dilutions of lung extracts (from the middle lobe grinded in 1 ml of PBS), bronchoalveolar lavage (BAL) (pure, 1/10 to 1/1000) and blood (1/10 to 1/10000) samples onto chocolate agar plates (Chocolate agar + PolyViteX™, Biomérieux). Colony-Forming Units (CFU) were enumerated 24 h later and expressed as CFU/ml. Survival experiments were also performed by measuring the weight loss and the survival in infected mice during 6 days. In these experiments, mice were sacrificed if we observed a weight loss higher than 20% of the initial weight associated with behavior parameters showing lethargy.
2.3. Treatment of mice with anti-IL-20Rb neutralizing antibody and Isotype control
Treated animals received the endotoxin-free anti-IL-20Rb neutralizing antibody (clone 20RNTC, 50 μg/100 μl; eBioscience) by intraperitoneal route, 24 h before and at day 1 and 3 after Sp infection. The 13R4 IgG2a- antibody was used as the isotype control (Evitria).
2.4. Broncho-alveolar lavage and tissue preparation
BAL was performed by washing the lungs five times with 0.5 ml of phosphate-buffered saline solution (PBS) plus 2% fetal bovine serum (FBS) (Gibco). After centrifugation at 400g for 6 min at 4 °C, the supernatant (cell-free BAL fluid) was stored at −20 °C for cytokine analysis, and the pellet was used for flow cytometry analysis.
The left lobe of the lung were mashed with a sterile blade then digested with collagenase (Collagenase Type VI 17104–019 Gibco by Life technologies) at 37 °C. After 15 min of digestion, lungs were homogenized with an 18G needle and digested for 15 min. After centrifugation at 400g 6 min at 4 °C, the pellets were resuspended in a 30% Percoll solution (Percoll TM GE Healthcare 17–0891-01) and centrifuged at 500g for 15 min. The pellets were resuspended in red blood cells (RBC) lysis buffer during 5 min at 20 °C, to remove erythrocytes. The reaction of RBC lysis was stopped with PBS 2% FBS (Gibco). After centrifugation at 400g for 6 min at 4 °C, pulmonary cells were resuspended in PBS 2% FBS, then enumerated and used for flow cytometry.
2.5. Flow cytometer reagents
The cell phenotype on BAL and pulmonary cell suspension were analyzed by flow cytometry using the following antibodies:
Monoclonal antibodies (mAbs) against mouse CD5 (ref130–102-574, FITC-conjugated), Tetramer CD1d (NIH facility, PE-conjugated), NK1.1 (ref 130–103-963, PerCp-Cy5.5–conjugated), CD4 (ref 130–102-411, PE-Cy7- conjugated), CD25 (ref 130–102-550, APC-conjugated), CD69 (ref 561,238, Alexa700-conjugated), TCRγδ (ref 130–104-016, APC- Vio770 conjugated), TCR-β (ref 130–104-815, V450-conjugated), CD8 (ref 130–109-252, V500-conjugated), CD45 (ref BLE103140, BV605-conjugated) (BD Biosciences, Biolegend and Myltenyi Biotech), I-Ab (ref 130–102-168, FITC- conjugated), F4/80 (ref 130–102-422, PE conjugated), CD103 (ref 563,637, PerCP-Cy5.5-conjugated), CD11c (ref 558,079, PE Cy7-conjugated), CD86 (ref 560,581, Alexa-700 conjugated), Ly6G (ref 560,600, APC-H7 conjugated), CD11b (ref 560,455, V45O conjugated), CD45 (ref 130–402-512, V500 conjugated), Ly6C (ref BLE128036, BV605-conjugated) (BD Biosciences, Biolegend and Myltenyi Biotech) and CCR2 (ref FAB 5538A, R&D systems, APC conjugated). Data were acquired on a LSR Fortessa (BD Biosciences) and analyzed with FlowJo™ software v7.6.5 (Stanford, CA, USA). Gating strategies are described in supplementary Fig. 1 (See supplementary information). cDC1 were identified among DC, as CD103+ CD11b− cells whereas cDC2 were CD11b+ CD103−.
2.6. Lung immunohistochemistry
For histopathology, the posterior lobe of lungs were fixed by inflation and immersion in paraformaldehyde (PFA; 4%) and embedded in paraffin. To evaluate airway inflammation, lung sections (4-μm thick) were stained by hematoxylin & eosin. On these slides, we have measured both lung remodeling and inflammation through the use of lung injury scoring as defined in the supplementary table 1 (See supplementary information).
For immunohistochemistry, paraffin-embedded lungs sections were deparaffinized into two successive baths of xylene (Acros Organics) during 10 min and rehydrated with successive baths of ethanol (successively, two baths of 100%, one bath of 90%, 80% and 50% during 5 min for all and one bath of water during 5 min). The unmasking of epitope was carried out in pH 6.0 citrate buffer during 15 min at 90 °C. The kit ab80436 – Expose Mouse and Rabbit Specific HRP/DAB Detection IHC Kit (Abcam) was used according to the manufacturer's recommendation for detection of IL-19, IL-20 and IL-24. For IL-20 receptors, Fast Red (SIGMAFAST™ Fast Red TR/Naphthol AS-MX Tablets) is used for revelation. Counterstaining was performed with Hematoxylin (Interchim).
We used as primary antibodies, an anti-IL-19 antibody [EPNCIR168] ab154187 (Abcam); an anti-IL-20 antibody orb13501 (Biorbyt), anti-IL-24 antibody orb228807 (Biorbyt), anti-IL-20Ra antibody bs-2619R (Bioss), anti-IL-20Rb clone 20RNTC (eBiosciences) and an anti-IL-22Ra1 clone 496,514 (R&D systems), all at 0.01 mg/ml.
In order to quantify the remodeling within the alveoli, we measured the alveolar wall thickening in the peribronchial area by using the software Image J (NIH). We analyzed at least 10 alveolar walls in the lungs of 4 different mice for each group.
2.7. Cytokine measurement by individual ELISA system kit
IL-1β, IL-6, IL-19, and IFN-γ (eBiosciences), TNF-α (R&D (Abingdon, UK), IL-20 (Boster Biological Technology) and IL-24 (Elabscience) were determined in BAL and lung homogenates by enzyme-linked immunosorbent assay (ELISA), using commercial kits according to the manufacturer's recommendations.
2.8. RNA isolation and quantitative RT-PCR
Total RNA was isolated from homogenized mouse lung (the cranial lobe) using TRIzolR Reagent (Ambion) according to manufacturer's instructions and quantified by NanoVue Plus (Healthcare Bio-sciences AB). Reverse transcription (RT) was performed with High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) according to manufacturer's instructions. cDNA were subjected to quantitative PCR (QuantStudio 12 K Flex Applied Biosystems) using primers for mouse reported in supplementary Table 2 (See supplementary information) (Eurofins Genomics). Relative transcript expression of a gene is given as −2∆∆C using GAPDH as a housekeeping gene.
2.9. Statistical analyses
The experiments evaluating the response to Sp infection at 12, 24 and 72 h were repeated 3 times with 4–5 mice per group. In addition, two experiments were performed with 10 mice per group for the survival analysis. Results are expressed as the mean ± SEM. Mann-Whitney U analysis and non-parametric Kruskal-Wallis followed by Dunns post tests were performed to determine significant differences between groups using GraphPad Prism software version 5.00. Data are expressed as means ± SEM. Statistically significant differences were defined as *P < .05, **P < .01 and ***P < .001.
3. Results
3.1. Infection with Sp initiates a pulmonary inflammatory reaction and triggers septicemia
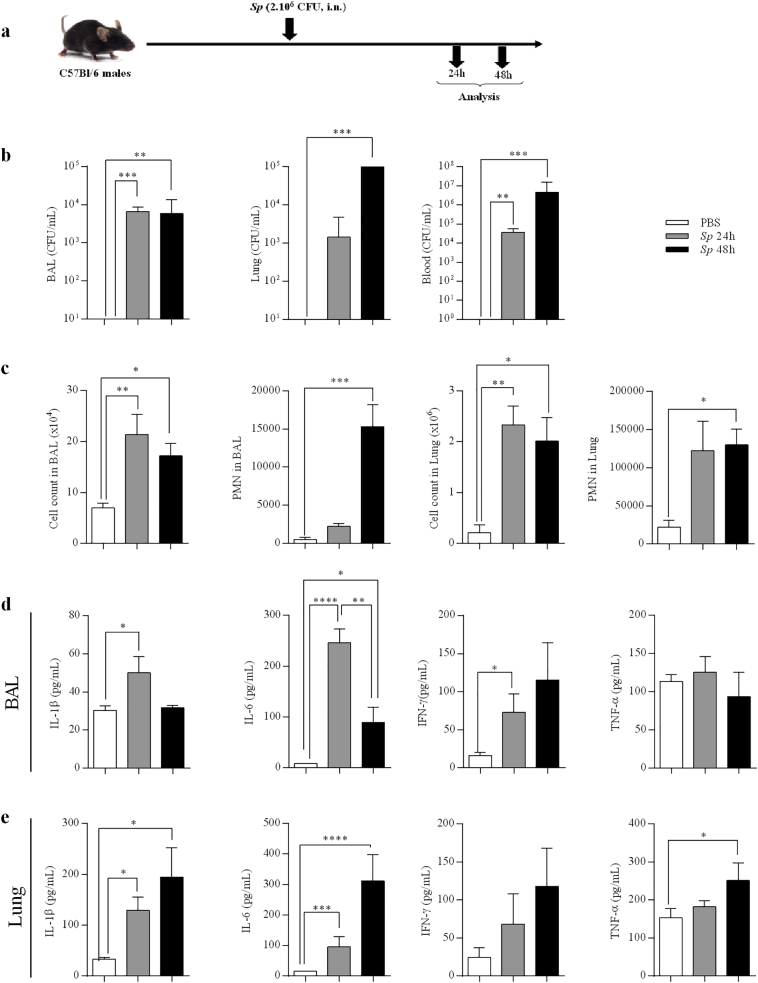
Mice were infected by intranasal administration of Sp (Fig. 1a). Whereas the bacterial counts were unchanged in the BAL at 24 and 48 h post- infection (p.i), these numbers were strongly increased at 48 h in the lung tissue and the blood as compared to 24 h (Fig. 1b). Moreover, total cell numbers were significantly increased in BAL and lungs of infected mice at both time points in comparison to the baseline which was control mice (PBS), associated with neutrophil recruitment (Fig. 1c). The concentrations of IL-1β, IL-6 and IFN-γ were significantly increased in the BAL of infected mice particularly at 24 h (Fig. 1d). In thelung, the production of these cytokines as well as TNF-α continued to increase until 48 h p.i (Fig. 1e). No significant change for these parameters was observed at 12 h p.i. (data not shown).
Fig. 1.
Infection with Streptococcus pneumoniae induces a pro-inflammatory response and bacterial dissemination in the blood. All data were represented at 24 and 48h post-infection with S. pneumoniae(Sp, 2.106 CFU). (a) Protocol of infection by Sp. (b) Bacterial load in BAL, lung tissues and blood as CFU counts per mL. (c) Total cell count in BAL and lung tissues (obtained from the middle right lobe), and neutrophils (PMN) number in lung tissue evaluated by flow cytometry. (d-e) IL-1β, IL-6, IFN-γ, and TNF-α levels in BAL (d) and lung lysates (the cranial lobe) (e), evaluated by ELISA (pg/mL). Results were expressed as mean ± SEM and statistical analyses were performed by Mann-Whitney U analysis and non-parametric Kruskal-Wallis followed by Dunns posttest in comparison with PBS control. *P<.05, **P<.01 and ***P<.001. Three independent experiments have been performed with 4-5 mice in each group.
3.2. Infection with Sp triggers the lung expression of IL-20 cytokines and their receptors
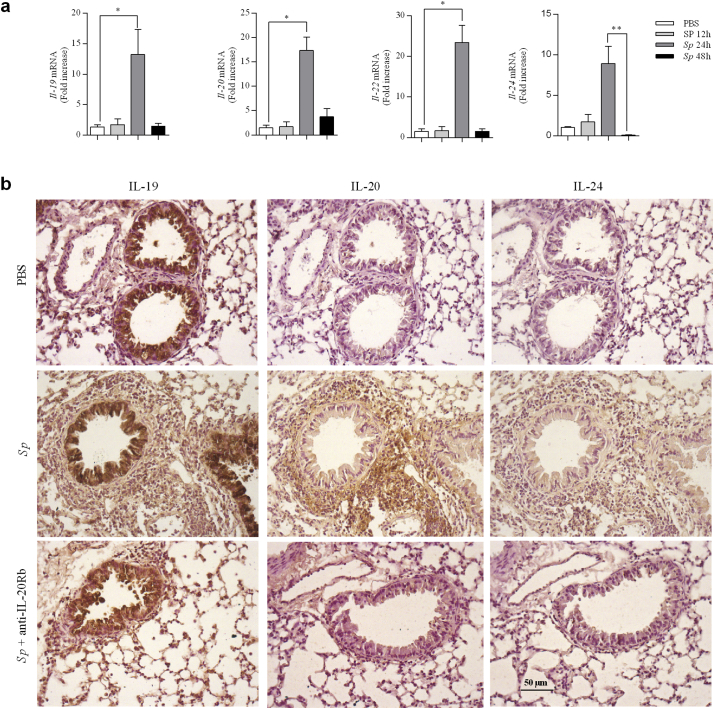
We next investigated the pulmonary amount of IL-20 related cytokines during the course of Sp infection. Quantitative RT-PCR analysis indicated enhanced expression of transcripts for Il-19, Il-20 and Il-24 as well as for Il-22 at 24 h p.i, that decreased at 48 h p.i (Fig. 2a). No changes were detected at 12 h p.i.
Fig. 2.
Streptococcus pneumoniae infection triggers IL-20 cytokine expression in the lung. Lungs from PBS group and from mice infected with S. pneumoniae(Sp, 2.106 CFU) were collected at 12, 24 and 48h p.i.. (a) Il-19, Il-20, Il-22 and Il-24 mRNA levels were evaluated by RT-qPCR in lung tissue (the cranial lobe) of Spinfected mice and controls. Results were expressed as fold increase compared to the mice exposed to PBS and using expression of Gapdh as a housekeeping gene. (b) Expression of IL-19, IL-20 and IL-24 was evaluated on lung sections by immunohistochemistry (magnification x200) at 24h p.i. Lung sections were prepared from not infected mice (PBS), mice infected with Sp and infected with Sp and treated aby anti-IL-20Rb antibody (Sp + anti-IL-20). Results were expressed as mean ± SEM and statistical analyses were performed by Mann-Whitney U analysis and non-parametric Kruskal-Wallis followed by Dunns posttest in comparison with PBS controls, or with Sp at 48h p.i. *P<.05 and **P<.01. Three independent experiments have been performed with 4-5 mice in each group.
Immunohistochemistry staining revealed that IL-20 and IL-24 were nearly undetectable at baseline (PBS condition), whereas airway epithelial cells were strongly positive for IL-19. Infection with Sp markedly increased bronchial and peribronchial staining of these cytokines at 24 h post-infection (see Table 1). In addition to bronchial epithelial cells, pneumocytes as well as inflammatory cells were positive for these cytokines (Fig. 2b). No staining was reported with the isotype controls as shown in the supplementary fig. 2 (See supplementary information).
Table 1.
Number of cells expressing IL-20 related cytokines and their receptors in peribronchial area from lung sections.
| IL-19 | IL-20 | IL-24 | IL-20Ra | IL-20Rb | IL-22Ra | |
|---|---|---|---|---|---|---|
| PBS | 24.8 ± 2.3 | 9.6 ± 1.6 | 13.9 ± 1.5 | 70.6 ± 5.2 | 33.2 ± 11.9 | 31.4 ± 4.8 |
| Sp | 84.4 ± 1.0 | 55.4 ± 5.9 | 64.5 ± 28.2 | 85.3 ± 2.3 | 51.9 ± 3.2 | 85.3 ± 2.3 |
|
Sp + anti-IL20Rb |
62.9 ± 5.3 | 32.9 ± 5.5 | 56.5 ± 3.2 | 42.8 ± 9.8 | 25.3 ± 7.9 | 15.6 ± 2.1 |
Immunohistological staining of IL-20 related cytokines (IL-19, IL-20, and IL-24) and their receptors (IL-20Ra, IL-20Rb, IL-22Ra) were performed on lung tissues of PBS -, Sp – infected mice and Sp - infected mice treated with the anti-IL-20Rb neutralizing antibody. Bronchial epithelial cells were not included in the count since most of them are positive particularly for IL-19 and IL-20Ra. The results are expressed as the mean number per mm2 of positive cells in 3 to 5 fields with standard deviation.
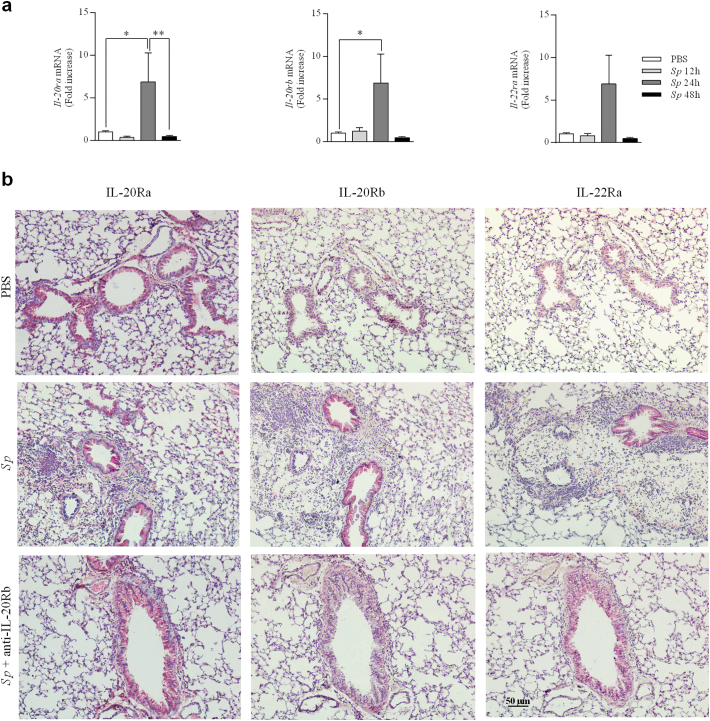
Since IL-20 related cytokines were induced after Sp infection, we then evaluated the expression of their receptors in the lung tissues of infected mice. In contrast with Il-22ra mRNA, expression of Il-20ra and Il-20rb mRNA were significantly increased in lungs of infected mice at 24 h p.i., in comparison to PBS mice. Transcript levels were similar to baseline at 12 h and 48 h p.i (Fig. 3a). Immunohistochemical analysis revealed the IL-20Ra was strongly expressed by airway epithelial cells at baseline (PBS mice) whereas a light staining was detected for IL-20Rb and IL-22Ra. Sp markedly increased bronchial and peribronchial staining of IL-20Rb and with a lower intensity, of IL-22Ra at 24 h p.i (Fig. 3b and Table 1). Some binding was also detected within the inflammatory cells in Sp mice. In contrast, the expression of IL-20Ra was unchanged in the airway epithelial cells. Taken together, our data reveals a basal expression for IL-19 and an enhanced expression of IL-20 cytokines in the airways and in the inflammatory peribronchial area early after infection with Sp. This is associated with an enhanced expression of IL-20Rb and IL-22Ra mainly in airway epithelial cells whereas IL-20Ra was strongly expressed at baseline and not modulated by the infection.
Fig. 3.
Expression of IL-20 receptors is amplified in lungs from mice infected with Streptococcus pneumoniae. Lungs from PBS group and from mice infected with S. pneumoniae (Sp, 2.106 CFU) were collected at 12, 24 and 48 h p.i.. (a) Il-20ra, Il-20rb and Il-22ra mRNA levels in lung tissue of Sp infected mice were evaluated by RT-qPCR in lung tissue of Sp infected mice and controls. Results were expressed as fold increase compared to the mice exposed to PBS and using expression of Gapdh as a housekeeping gene. (b) Expression of the IL-20Ra, IL-20Rb and IL-22Ra was evaluated by immunohistochemistry (magnification x200) on lung sections collected at 24 h p.i. Lung sections were prepared from not infected mice (PBS), mice infected with Sp (Sp) and infected with Sp and treated aby anti-IL-20Rb antibody (Sp + anti-IL-20). Results were expressed as mean ± SEM and statistical analyses were performed by Mann-Whitney U analysis and non-parametric Kruskal-Wallis followed by Dunns posttest in comparison with PBS controls. *P < .05, and **P < .01. Three independent experiments have been performed with 4–5 mice in each group.
3.3. Treatment with anti-IL-20Rb antibody decreases the bacterial load and the inflammatory reaction
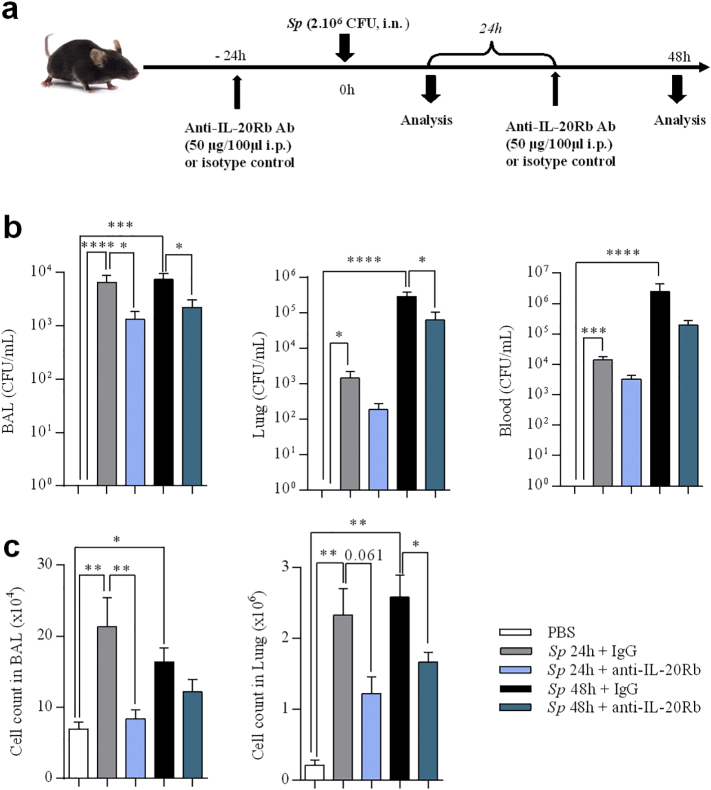
We next analyzed whether the neutralization of the IL-20Rb sub-unit could affect the outcome of the pulmonary infection by Sp (Fig. 4a).
Fig. 4.
Anti-IL-20Rb neutralizing antibody increases bacterial clearance in Streptococcus pneumoniae -infected mice and limits the inflammatory reaction. (a) Protocol of infection by Sp and treatment with anti-IL-20Rb neutralizing antibody or the isotype control. (b) CFU counts per mL in BAL, lung tissues (middle lobe) and blood 24 h p.i. (c) Total cell count in BAL and lung tissues (left lobe). Results were expressed as mean ± SEM and statistical analyses were performed by Mann-Whitney U analysis and non-parametric Kruskal-Wallis followed by Dunns posttest in comparison with PBS controls or with Sp + IgG. *P < .05, **P < .01 and ***P < .001. Three independent experiments have been performed with 4–5 mice in each group.
Blocking the IL-20Rb receptor significantly decreased the bacterial burden in the BAL at 24 h p.i, and in the BAL and the lung 48 h p.i (23% and 38% of inhibition respectively) (Fig. 4b). Treatment with the anti-IL-20Rb antibody also decreased the number of total cells in the BAL at 24 h, and in the lung tissues at 48 h (Fig. 4c), while there was a trend to decrease the total cell number at the other time point. This was confirmed by the analysis of lung sections. Indeed, histopathological scores exhibited a reduction of lung inflammation and lesions 24 h p.i (anti-IL-20Rb treated Sp mice: 8.67 ± 1.58 vs IgG-treated Sp mice: 13.3 ± 2.04) and 48 h p.i (anti-IL-20Rb treated Sp mice: 9.5 ± 1.41 vs IgG-treated Sp mice: 13.83 ± 1.58) compared with uninfected control mice (3.4 ± 0.68). We have also measured the alveolar wall thickness in the three groups and our results showed that Sp infection induced a significant thickening in alveolar walls as reported in the supplementary fig. 3 (See supplementary information). Moreover, treatment with the anti-IL-20Rb antibody completely prevent the effect of the infection.
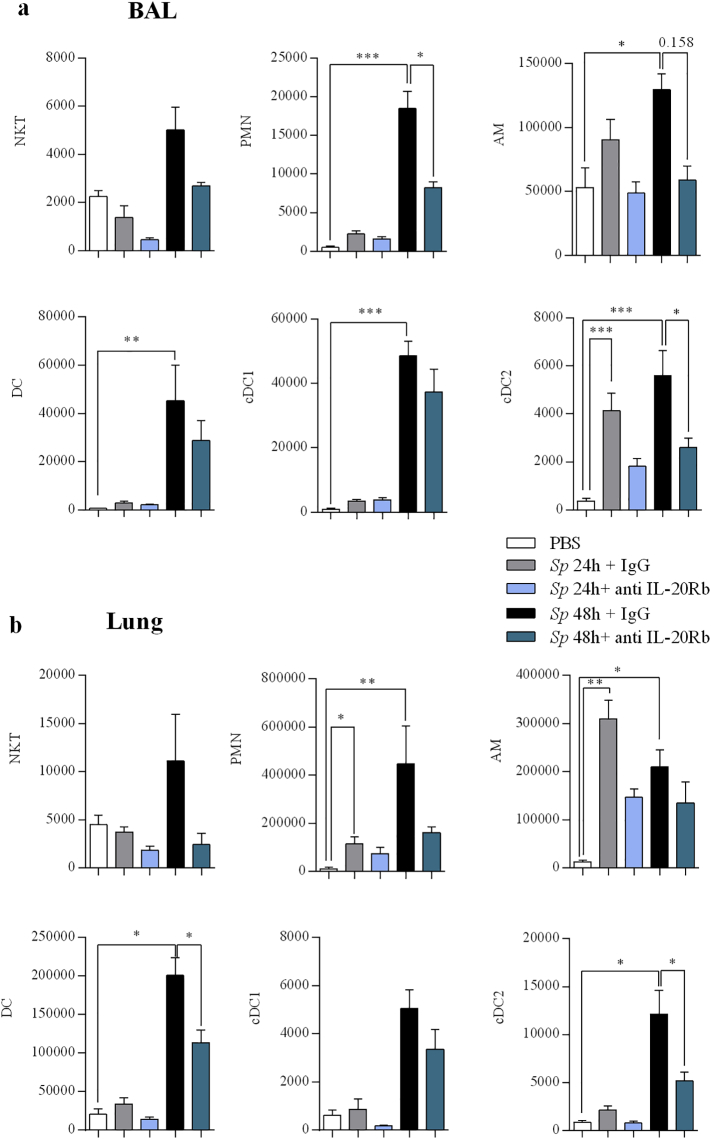
To determine which cell populations were impacted by the treatment, we numbered the major subpopulations of inflammatory cells by flow cytometry. Interestingly, our data revealed that the numbers of neutrophils, and of cDC2 in the BAL were lower in mice treated with anti-IL-20Rb than in infected controls while there was a trend to decrease the number of alveolar macrophages (Fig. 5a). In the lung tissues, treatment with the blocking anti-IL-20Rb antibodies decreased the number of neutrophils and of cDC2 (as well as the total number of DC) at 48 h p.i (Fig. 5b).
Fig. 5.
Anti IL-20Rb neutralizing antibody limits cell infiltration in BAL and lung of treated mice. Natural Killer T (NKT) cells, polymorphonuclear neutrophils (PMN), alveolar macrophages (AM), dendritic cells (DC), and resident DC (cDC1) and inflammatory DC (cDC2) were numbered by flow cytometry in the BAL (a) and the lung tissues (b) at 24 h p.i. Results were expressed as mean ± SEM of absolute numbers calculated using the corresponding total cell counts. Results were expressed as mean ± SEM and statistical analyses were performed by Mann-Whitney U analysis and non-parametric Kruskal-Wallis followed by Dunns posttest in comparison with PBS controls, or with Sp + IgG. *P < .05, **P < .01 and ***P < .001. Three independent experiments have been performed with 4–5 mice in each group.
Taken together, blocking the IL-20Rb pathway decreased the bacterial load and the inflammatory cell recruitment (namely of neutrophils and DC) after respiratory infection by Sp.
3.4. Anti-IL-20Rb antibody modulates the production of inflammatory cytokines and anti-microbial peptides
Since the blockade of the IL-20 pathway altered the inflammatory cell recruitment after infection with Sp, the cytokine production was analyzed in the BAL and the lung tissues (Fig. 6).
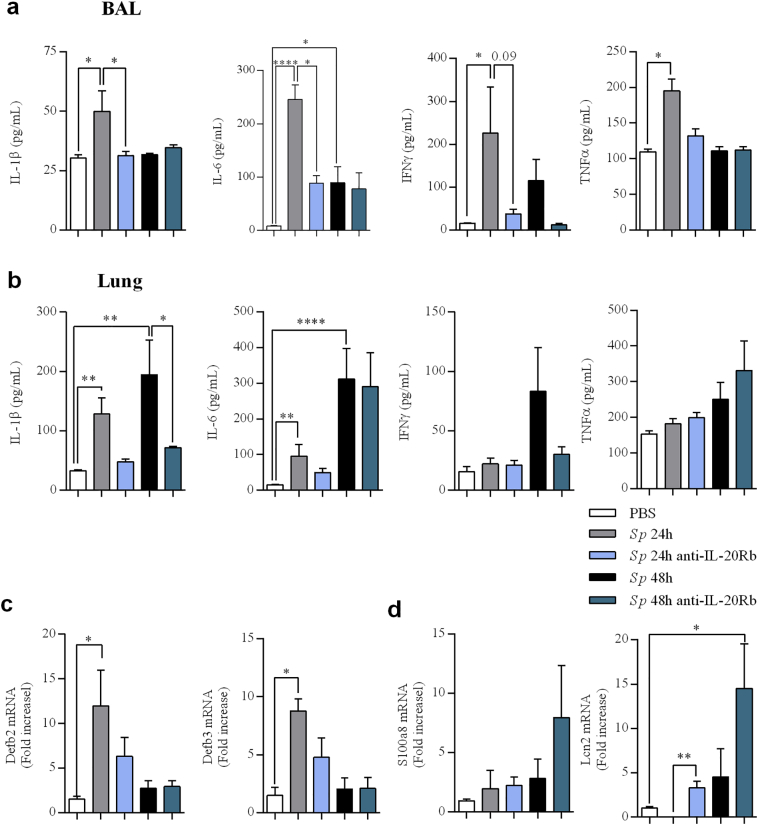
Fig. 6.
Anti-IL-20Rb neutralizing antibody modulates the production of pro- inflammatory cytokines and anti-microbial peptides. (a-b) IL-1β, IL-6, IFN-γ, and TNF-α levels evaluated by ELISA (pg/mL) in BAL (a) and lung lysates (b) 24 h p.i. (c-d) mRNA level of the antimicrobial peptides β-defensins (Defb2 and Defb3) (c) and S100a8 and lipocalin-2 (lcn2) (d) were evaluated by RT-qPCR in lung tissues (cranial lobe) at 24 h p.i. Results were expressed as fold increase compared to the mice exposed to PBS and using expression of GAPDH as a housekeeping gene. Results were expressed as mean ± SEM. Results were expressed as mean ± SEM and statistical analyses were performed by Mann-Whitney U analysis and non-parametric Kruskal-Wallis followed by Dunns posttest in comparison with PBS controls, or with Sp + IgG. *P < .05, **P < .01 and ***P < .001. Three independent experiments have been performed with 4–5 mice in each group.
Treatment using anti-IL-20Rb antibody significantly decreased the concentrations of IL-1β and IL-6 in the BAL collected at 24 h p.i. in infected mice, in association with a trend for lower levels of IFN-γ (Fig. 6a). In the lung tissues, the treatment with the anti-IL-20Rb antibody only reduced IL-1β (Fig. 6b). We also measured the levels of Th17 cytokines in these samples and we did not observe any modulation of the levels for IL-17 protein and IL-22 mRNA after blocking the IL-20 pathway (supplementary Fig. 4 (See supplementary information)).
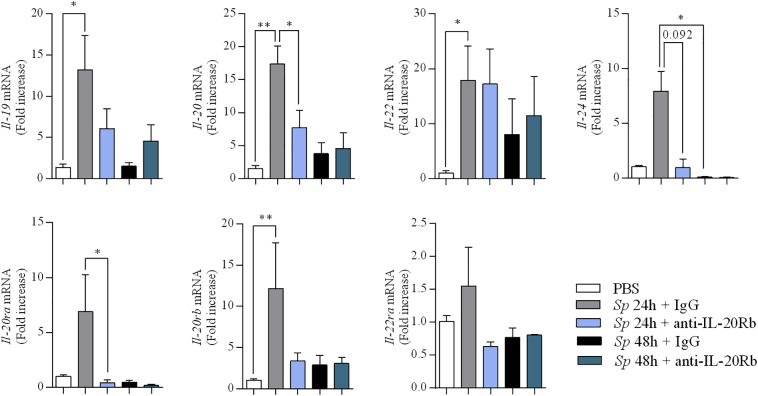
We also checked the impact of the treatment with the anti-IL-20Rb antibody on the expression of IL-20 cytokines themselves and on their receptors. Compared to IgG-treated mice, administration of anti-IL-20Rb antibody reduced the mRNA expression of IL-20 and IL-24 without impacting IL-22 mRNA expression at 24 h p.i. (Fig. 7). This treatment also affects the expression of IL-20Ra. This was confirmed and extended by the immunohistochemistry analysis. The administration of the anti-IL-20Rb antibody decreased the peribronchial and bronchial expression of IL-19, IL-20 and IL-24 (Fig. 2b), and. IL- 20Ra, IL-20Rb, and IL-22Ra (Fig. 3b). It also diminished the expression of IL-20Rb in airway epithelial cells.
Fig. 7.
Anti IL-20Rb neutralizing antibody reduces the expression of IL-20 cytokines and their receptors in the lung of Streptococcus pneumoniae -infected mice. mRNA levels of the IL-20 related cytokines (IL-19, IL-20, IL-22, IL-24) and their receptors (IL-20Ra, IL-20Rb and IL-22Ra) in lung tissue of mice infected with Sp evaluated by RT-qPCR. Results were expressed as fold increase compared to the mice exposed to PBS and using expression of GAPDH as a housekeeping gene. Results were expressed as mean ± SEM and statistical analyses were performed by Mann-Whitney U analysis and non-parametric Kruskal-Wallis followed by Dunns posttest in comparison with PBS controls, or with Sp + IgG. *P < .05, **P < .01 and ***P < .001. Three independent experiments have been performed with 4–5 mice in each group.
We also analyzed the expression of anti-microbial peptides involved in the anti-infectious defense in the lung by RT-PCR. Sp infection amplified the expression of Defb2 and Defb3 mRNA at 24 h p.i. (Fig. 6c) but not the expression of S100a8 and Lcn2 mRNA (Fig. 6d) as well as S100a9 mRNA (supplementary Fig. 5 (See supplementary information)). Administration of anti-IL-20Rb neutralizing antibody enhanced the Lcn2 mRNA expression while there was a trend for S100A8 and S100a9, mainly 48 h p.i. This suggests that IL-20Rb blockade amplifies the expression some antimicrobial peptides which in turn, can facilitate the bacterial clearance.
3.5. Anti-IL-20Rb antibody did not affect the weight loss and the survival in mice infected by Sp
In order to determine the impact of the treatment with anti-IL-20Rb antibody on the evolution of the infection in mice, we analyzed the weight loss and the survival during 6 days. Control mice lose >20% of their weight at 4–5 days p.i. and the treatment with anti-IL-20Rb antibody did not affect this drop (supplementary Fig. 6 (See supplementary information)). In parallel, some mice died at the same period and once again, the treatment did not significantly modify their survival.
4. Discussion
It has been described previously that IL-20 related cytokines and their receptors play a role in promoting S.aureus skin infections [14]. Moreover, it is known that IL-20 family cytokines are expressed in lung epithelial cells [18], suggesting their implication in the lung. we have demonstrated that infection by Sp promotes the production of mostly IL-19 and IL-20, as well as the expression of IL-20Rb subunit of the receptor. We also showed that the peak of IL-20 cytokine expression was concomitant with an effect of these cytokines on the bacterial clearance and the inflammatory reaction, particularly by decreasing neutrophil and dendritic cell influx. Altogether, we have uncovered a critical role for IL-20 receptors.
Sp colonizes the lower respiratory tract via its spreading through the airways. Recognition of bacterial cell wall components of Sp (lipoteichoic acids; LTA and peptidoglycan; PG) by Toll-like receptor 2 (TLR2) [19,20] induces the activation of the canonical nuclear factor (NF)-ƙB pathway in antigen presenting cells [21]. The activation of this pathway leads to the release of pro-inflammatory cytokines and chemokines, such as IL-1β, IL-6 and TNF-α. Our data concerning the evaluation of the inflammation and the cytokines in the BAL and lung tissues of mice validated our experimental model with a production of pro-inflammatory cytokines between 24 and 48 h p.i. We have shown that IL-20 related cytokines (IL-19, IL-20, and IL-24 as well as IL-22) are induced during this cytokine burst, at 24 h p.i both at the protein and the mRNA levels. However, the mRNA expression of Il-19, Il-20, Il-22, Il-24, Il-20ra, Il-20rb and Il-22ra mRNA nearly returns to baseline levels at 48 h p.i. whereas these levels were not significantly different to the PBS controls 12 h p.i As previously described for the majority of NF-ƙb dependent cytokines in the lung, the protein expression for these cytokines rapidly declines [8]. Recent data underlines that the production of IL-20 cytokines is dependent on NF-ƙB [16] and could be induced by TLR2 and TLR4 stimulation, both receptors being mobilized by Sp [19,20]. At the mRNA level, the production of IL-19 and IL-20 is markedly increased, despite no significant effect for IL-24. Interestingly some recent data showed that this cytokine is induced by respiratory viruses suggesting that it could be more associated with viral infection [22]. Interestingly, we detected a strong expression of IL-19 in the bronchial epithelium of control mice whereas we did not detect a difference between the mRNA expression of Il-19, Il-20 and Il-24 in the lung tissue as well as in bronchial epithelial cell cultures at baseline (data not shown). This suggests that IL-19 is tightly post-transcriptionally regulated and that the protein might be stored within the cytoplasm of these cells. After Sp challenge, we detected an increased expression in airway epithelial cells (within the bronchi and the alveoli) as well as in the inflammatory infiltrate. DC and macrophages are able to produce these cytokines and we observed that monocyte-derived DC secretes these cytokines after activation by Sp (manuscript in preparation). Of note, using multiple Sp inocula in our experiments instead of a lethal dose of Sp could have also been informative to better decipher the role of IL-20 cytokines.
It has been shown in a model of cutaneous infection, that IL-20 family cytokines are initially secreted by infiltrating innate immune cells and lymphocytes 24 h after challenge [14]. Most research has been focused on IL-22, but we know that during inflammation, IL-19, IL-20 and IL-24, binding to their receptors, induce cell recruitment (neutrophils, dendritic cells and T cells), antimicrobial peptides expression (β-defensin or S100A peptides) as well as chemokines and cytokines production [16]. Among the cytokines produced, the binding of IL-20 related cytokines to their receptors allow a positive autocrine loop expression of IL-19, IL-20 and IL-24, which amplify their effect during inflammation [16,23]. Our data showing that neutralization of IL-20Rb subunit decreases the expression of IL-20 family cytokines (IL-19, IL-20 and IL-24) and their receptors could potentially be explained by an autocrine loop. Interestingly, the inflammatory cell recruitment (specifically, neutrophils and DC as well as AM in the BAL) due to Sp infection is reduced after blocking the IL-20 pathway suggesting that this process might also participate in the lower level IL-20 cytokine expression. Moreover, this decreased recruitment was associated with a lower production of pro-inflammatory cytokines like IL-1β, IL- 6 and IFN-γ. Indeed, inflammatory cells are potential sources for these cytokines. Myles et al. reported that IL-20 related cytokines promote cutaneous Staphylococcus aureus infection in mice by downregulating the Th17 cytokines-dependent pathway [14]. Since we are unable to detect an increased production of IL-17/IL-22 and pro-Th17 cytokines (such as IL-1β and IL-6) in lung tissues of Sp mice treated with anti-IL-20Rb neutralizing antibody, we speculate that IL-20 family cytokines boost the anti-bacterial activity of these effector cells as shown in vitro in neutrophils [15]. Indeed, blocking IL-20Rb pathway promotes bacterial clearance in BAL and lung of treated mice, although it decreases the influx of neutrophils and macrophages. Additional experiments are required to analyze the effector function within these cells in infected mice treated with the anti-IL-20Rb blocking antibody. The treatment with the anti-IL-20Rb antibody also amplifies the mRNA expression of selected antimicrobial peptides including Lcn2 while there was a trend for S100a8 mRNA. Administration of the anti-IL-20Rb antibody did not modulate the β-defensin mRNA expression (Defb2, Defb3) which are usually produced by pulmonary epithelial cells and neutrophils during Sp infection [24]. Interestingly, it has been shown that S100 anti-microbial peptides (expressed by neutrophils, monocytes and activated endothelial and epithelial cells) control bacterial clearance by inducing lung recruitment of neutrophils and macrophages during Sp infection [25,26]. Lcn2-deficient animals also exhibit higher susceptibility to infection compared to control animals [27].
The understanding of the mechanisms underlying the beneficial effects of the treatment using anti-IL-20Rb antibody is ongoing and will be helpful to limit pulmonary bacterial infections. Interestingly, this effect is associated with a lower histopathological score measuring both the inflammation and the airway remodeling as illustrated in the Figs. 2b and 3b, a result confirmed at the level of neutrophils, dendritic cells and macrophages by flow cytometry analysis. However, this effect was not associated with a significant impact on mice survival and loss weight which is prominent after day 3 p.i. This is probably related to the drop in the production of IL-20 cytokines observed at day 2 p.i. in our acute model of infection. These data suggest that it is necessary to associate this treatment with another therapeutic approach such as antibiotic, in order to improve the efficiency of the anti-IL-20Rb blocking antibody.
In conclusion, we show that IL-20 related cytokines and their receptors are induced during Sp lung infection and that the treatment using an anti-IL-20Rb neutralizing antibody limits inflammation due to Sp. Moreover, combined treatment of lung infection associating a molecule blocking the IL-20 pathway with antibiotics might be helpful to accelerate bacterial clearance and to prevent the tissue lesions associated with uncontrolled inflammation.
Acknowledgments
Acknowledgements
We thank Fiordiligie Casilag for proofreading. We also thank the Bio Imaging Center platform (Bicel) for the microscopy and flow cytometry part, especially Sophie Salome-Desnoulez and Hélène Bauderlique. We would also like to thank the animal Biosafety Level-2 facility, in strict accordance with Lille Pasteur Institute guidelines on animal care. We also thank Oncovet Clinical Research for the histological analysis of lung sections.
Funding sources
This work was supported by the Centre National de la Recherche Scientifique (CNRS), the Institut National de la Santé et de la Recherche Médicale (INSERM), INSERM-transfert (Paris, France), the University of Lille (Lille, France) and Fondation de Recherche en Santé Respiratoire (Paris, France). Funders had no role in study design, data collection, data analysis, interpretation, and writing of the report.
Declaration of interests
All the authors have no conflict of interests.
Author contributions
F.M. & O·B worked together for all experiments. G.K. performed colony-forming units (CFU) experiments. P.G & M.P. participated to the experiments and supervised the project with FT. The manuscript was written by F.M. and O·B. under the supervision of P.G. and M.P.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.10.031.
Appendix A. Supplementary data
Supplementary material
References
- 1.Dockrell D.H., Whyte M.K.B., Mitchell T.J. Pneumococcal pneumonia: mechanisms of infection and resolution. Chest. 2012;142:482–491. doi: 10.1378/chest.12-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Lieshout M.H.P., de Vos A.F., Dessing M.C., de Porto A., de Boer O.J., de Beer R. ASC and NLRP3 impair host defense during lethal pneumonia caused by serotype 3 Streptococcus pneumoniae in mice. Eur J Immunol. 2018;48:66–79. doi: 10.1002/eji.201646554. [DOI] [PubMed] [Google Scholar]
- 3.Sethi S., Murphy T.F. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355–2365. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 4.Xu F., Droemann D., Rupp J., Shen H., Wu X., Goldmann T. Modulation of the inflammatory response to Streptococcus pneumoniae in a model of acute lung tissue infection. Am J Respir Cell Mol Biol. 2008;39:522–529. doi: 10.1165/rcmb.2007-0328OC. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L., Li Z., Wan Z., Kilby A., Kilby J.M., Jiang W. Humoral immune responses to Streptococcus pneumoniae in the setting of HIV-1 infection. Vaccine. 2015;33:4430–4436. doi: 10.1016/j.vaccine.2015.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eidenschenk C., Rutz S., Liesenfeld O., Ouyang W. Role of IL-22 in microbial host defense. Curr Top Microbiol Immunol. 2014;380:213–236. doi: 10.1007/978-3-662-43492-5_10. [DOI] [PubMed] [Google Scholar]
- 7.Le Rouzic O., Kone B., Kluza J., Marchetti P., Hennegrave F., Olivier C. Cigarette smoke alters the ability of human dendritic cells to promote anti-Streptococcus pneumoniae Th17 response. Respir Res. 2016;17:94. doi: 10.1186/s12931-016-0408-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Maele L., Carnoy C., Cayet D., Ivanov S., Porte R., Deruy E. Activation of Type 3 innate lymphoid cells and interleukin 22 secretion in the lungs during Streptococcus pneumoniae infection. J Infect Dis. 2014;210:493–503. doi: 10.1093/infdis/jiu106. [DOI] [PubMed] [Google Scholar]
- 9.Pichavant M., Sharan R., Le Rouzic O., Olivier C., Hennegrave F., Remy G. IL-22 Defect During Streptococcus pneumoniae Infection Triggers Exacerbation of Chronic Obstructive Pulmonary Disease. EBioMedicine. 2015;2:1686–1696. doi: 10.1016/j.ebiom.2015.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Commins S., Steinke J.W., Borish L. The extended IL-10 superfamily: IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28, and IL-29. J Allergy Clin Immunol. 2008;121:1108–1111. doi: 10.1016/j.jaci.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 11.Kunz S., Wolk K., Witte E., Witte K., Doecke W.D., Volk H.D. Interleukin (IL)-19, IL-20 and IL-24 are produced by and act on keratinocytes and are distinct from classical ILs. Exp Dermatol. 2006;15:991–1004. doi: 10.1111/j.1600-0625.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- 12.Ouyang W., Rutz S., Crellin N.K., Valdez P.A., Hymowitz S.G. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 13.Wegenka U.M. IL-20: biological functions mediated through two types of receptor complexes. Cytokine Growth Factor Rev. 2010;21:353–363. doi: 10.1016/j.cytogfr.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Myles I.A., Fontecilla N.M., Valdez P.A., Vithayathil P.J., Naik S., Belkaid Y. Signaling via the IL-20 receptor inhibits cutaneous production of IL-1beta and IL-17A to promote infection with methicillin-resistant Staphylococcus aureus. Nat Immunol. 2013;14:804–811. doi: 10.1038/ni.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gough P., Ganesan S., Datta S.K. IL-20 Signaling in Activated Human Neutrophils Inhibits Neutrophil Migration and Function. J Immunol. 2017;198:4373–4382. doi: 10.4049/jimmunol.1700253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutz S., Wang X., Ouyang W. The IL-20 subfamily of cytokines--from host defence to tissue homeostasis. Nat Rev Immunol. 2014;14:783–795. doi: 10.1038/nri3766. [DOI] [PubMed] [Google Scholar]
- 17.Munoz N., Van Maele L., Marques J.M., Rial A., Sirard J.C., Chabalgoity J.A. Mucosal administration of flagellin protects mice from Streptococcus pneumoniae lung infection. Infect Immun. 2010;78:4226–4233. doi: 10.1128/IAI.00224-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong W., Wang X., Zhang Y., Hao J., Xing C., Chu Q. Interleukin-20 promotes airway remodeling in asthma. Inflammation. 2014;37:2099–2105. doi: 10.1007/s10753-014-9944-8. [DOI] [PubMed] [Google Scholar]
- 19.Dessing M.C., Hirst R.A., de Vos A.F., van der Poll T. Role of Toll-like receptors 2 and 4 in pulmonary inflammation and injury induced by pneumolysin in mice. PLoS One. 2009;4 doi: 10.1371/journal.pone.0007993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomlinson G., Chimalapati S., Pollard T., Lapp T., Cohen J., Camberlein E. TLR-mediated inflammatory responses to Streptococcus pneumoniae are highly dependent on surface expression of bacterial lipoproteins. J Immunol. 2014;193:3736–3745. doi: 10.4049/jimmunol.1401413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zahlten J., Steinicke R., Bertrams W., Hocke A.C., Scharf S., Schmeck B. TLR9- and Src-dependent expression of Krueppel-like factor 4 controls interleukin-10 expression in pneumonia. Eur Respir J. 2013;41:384–391. doi: 10.1183/09031936.00196311. [DOI] [PubMed] [Google Scholar]
- 22.Seong R.K., Choi Y.K., Shin O.S. MDA7/IL-24 is an anti-viral factor that inhibits influenza virus replication. J Microbiol. 2016;54:695–700. doi: 10.1007/s12275-016-6383-2. [DOI] [PubMed] [Google Scholar]
- 23.Sakurai N., Kuroiwa T., Ikeuchi H., Hiramatsu N., Maeshima A., Kaneko Y. Expression of IL-19 and its receptors in RA: potential role for synovial hyperplasia formation. Rheumatology (Oxford) 2008;47:815–820. doi: 10.1093/rheumatology/ken061. [DOI] [PubMed] [Google Scholar]
- 24.Scharf S., Zahlten J., Szymanski K., Hippenstiel S., Suttorp N., N'Guessan P.D. Streptococcus pneumoniae induces human beta-defensin-2 and -3 in human lung epithelium. Exp Lung Res. 2012;38:100–110. doi: 10.3109/01902148.2011.652802. [DOI] [PubMed] [Google Scholar]
- 25.De Filippo K., Neill D.R., Mathies M., Bangert M., McNeill E., Kadioglu A. A new protective role for S100A9 in regulation of neutrophil recruitment during invasive pneumococcal pneumonia. FASEB J. 2014;28:3600–3608. doi: 10.1096/fj.13-247460. [DOI] [PubMed] [Google Scholar]
- 26.Raquil M.A., Anceriz N., Rouleau P., Tessier P.A. Blockade of antimicrobial proteins S100A8 and S100A9 inhibits phagocyte migration to the alveoli in streptococcal pneumonia. J Immunol. 2008;180:3366–3374. doi: 10.4049/jimmunol.180.5.3366. [DOI] [PubMed] [Google Scholar]
- 27.Flo T.H., Smith K.D., Sato S., Rodriguez D.J., Holmes M.A., Strong R.K. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material