Abstract
P53 is a transcription factor very often mutated in malignancies. It functions towards the regulation of important cellular activities, such as cell cycle, senescence and apoptosis. Since inflammation and cancer are strongly associated through common pathways, P53 can suppress inflammation in a plethora of human tissues. Growth Hormone - Releasing Hormone is a hypothalamic peptide with a great capacity to affect the complex networks of cellular regulation via GHRH - specific receptors. GHRH antagonistic and agonistic analogs have been developed for clinical applications, including treatment of benign prostatic hyperplasia, breast, prostate and lung cancers, diabetes and neurodegenerative diseases. The epicenter of the current manuscript is the protective role of P53 against inflammation and cancer and emphasizes the p53 – mediated beneficial effects of GHRH antagonists in various human diseases.
Keywords: Growth factors, Oncology, Barrier function
Highlights
-
•
Inflammation is tightly associated with cancer.
-
•
GHRH antagonists induce P53 expression.
-
•
P53 exerts a protective effect against cancer and inflammation.
1. Introduction
1.1. P53: a prologue
The “guardian of the genome” was first discovered in 1979 in a complex with the simian virus 40 (SV40) large T antigen. Since p53 was highly abundant in cancers, it was concluded that it was promoting malignancies. A plethora of studies suggested that P53 could collaborate synergistically with established oncogenes for the “conversion” of non - cancerous cells to tumors and acceleration of metastasis “in vivo”. Hence, P53 was initially declared to be an oncogene responsible for malignant transformations [1].
Preliminary evidence that P53 might be a tumor suppressor was first provided in 1984, when it was revealed by several independent groups that this protein was essential for cancer suppression [2]. Surprisingly, when different labs compared their sequences of cDNAs clones, it was realized that each clone was different. After laborious efforts it was concluded that those experiments were actually performed not with the wild type P53, but with its mutated version [3]. The mutant p53 is highly abundant in cancers, and it promotes them by disabling the function of the wild type P53, as well as by “gain-of-function” procedures [4]. The latter terms refers to the accumulation of those P53 mutations, which augment the oncogenic capacity of the mutated P53 and deliver a stronger tumoral resistance against anticancer treatments [5].
P53 exerts transcriptional activities, due to the ability to strongly bind to specific DNA regions [6]. Wild-type P53 senses a plethora of cellular stimuli and stresses, such as oncogenic transformations, as well as violations of DNA structure and integrity. Following the activation, wild-type P53 possess the capacity to exert transcriptional activities that may slow the progression of cell cycle, to “signal” the promotion of senescence and/or trigger cellular death by apoptosis [7]. This “guardian of the genome” has now become an attractive topic of interest in the grounds of cancer biology. The ability of that molecule to control cancers has triggered intense efforts to develop P53 - based cancer therapies. Such therapies include P53 based vaccines, as well as the activation of P53 by transcriptional activation and nucleolar disruption [8].
1.2. P53 suppresses both inflammation and cancer
It is now well established that inflammation may contribute towards the initiation and maintenance of unregulated cellular proliferation, which may turn to tumor formation and growth. The association between cancer and inflammation was first reported in the XIX century. A strong body of evidence substantiated the aforementioned relation, since chronic inflammation establishes the appropriate beneficial conditions for cancer growth [9]. Although the concept that cancer and inflammation are tightly connected was not initially very attractive, several lines of evidence are now suggesting otherwise.
Worldwide, the factors that are involved in the persistence of chronic inflammation (i.e. microbial infections and autoimmune diseases) are found to elevate the acceleration of tumorigenesis [10]. Interestingly, drugs that present a strong activity against inflammation, are associated with a low number of incidents related to carcinogenesis [11]. The evidence of cancer-related inflammation in malignancies includes the existence of inflammatory factors, as well as tissue recovery and remodeling similar to inflammatory conditions. Furthermore, recent studies are focused on the similarities between wound healing and cancer, since the second stage of wound healing, is the inflammation [12].
Genetic events may lead to tumoral transformations by mutation or suppression of anti-cancer genes, such as P53 [13]. Transformed cells may produce inflammatory mediators that form an inflammatory niche for which there is no prior inflammatory condition. Infections and inflammation may increase the incidence of carcinogenesis at targeted tissues through the activation of Nuclear Factor-κB (NF-κB) and Hypoxia - Inducible Factor 1α (HIF1α) [9]. These proteins regulate the transcription of inflammatory mediators, which activate the same key intracellular components in stromal and tumoral cells. These alterations result in the generation of a newly established cancer - related inflammatory microenvironment [14].
The signal transducer and activator of transcription 3 (STAT3) is strongly involved in the development of carcinogenesis, since it can induct immunosuppression. STAT3 is subjected to various post translational modifications (i.e. phosphorylation) and homo-dimerization. Further, it has the ability to translocate from the cytoplasm to nucleus and strongly bind to specific DNA regions. All those activities increase the production of intracellular components which affect malignancies [15]. It was recently revealed in thyroid cancers, that STAT3 is paradoxically a negative regulator of tumor growth. Thus, the ambivalent role of this transcription factor in that type of cancer indicates that the suppression of that molecule in malignancies should be considered with caution [16]. In particular, the new theurapeutical approaches towards cancer should not be focused exclusively on the inhibition of STAT3, but on those post translational modifications which have the established property to “trigger” oncogenesis [17].
Two of the major types of cardiovascular disease (CVD), namely the Chronic Obstructive Pulmonary Disease (COPD) and emphysema are now considered to be associated with high incidence of pulmonary malignancies. The common risk factors for all these pathologies are smoking, exposure to similar environmental toxic elements, and unhealthy addictions (i.e. smoking). Various investigators have demonstrated that COPD contributes to the development of tumors, independent of inhaling smoke. COPD patients demonstrate a much greater risk to be diagnosed with lung malignancies compared to smokers without CVD [18].
Since cancer and inflammation are coexisting conditions connected by a positive autoregulatory loop, it is not surprising that P53 is extremely efficient in suppressing inflammatory responses through multiple ways. A large number of studies has focused on the exact mechanisms by which P53 operates in order to suppress inflammation. Remarkably, P53 was found to suppress the major inflammatory transcription factor NF-κΒ [19].
Both P53 and NF-κB are pathways that are “streaming” intracellular responses to external and internal stimuli. Under unstressed conditions, they appear to be bound to their suppressors/negative regulators [20]. However, under stress, those proteins are released from their corresponding negative inhibitors and are being translocated to the nucleus. This is where they exercise their transcriptional capacity, by modulating the transcription of numerous responsive genes [21]. Both pathways are deregulated in cancer, but their activation exerts opposite effects. NF-κB protects the cells from apoptosis and promotes of cellular growth. On the other hand, activation of P53 is responsible for tumor suppression [22]. A growing body of experimental data have revealed a reciprocal antagonistic relationship between P53 and NF-κB. Proinflammatory NF-κB-induced cytokines can suppress transcriptional activity of P53 and reagents that lower NF-κB activity induce P53 – mediated effects [23].
Inflammatory infiltration of the lung due to DNA modifications is more severe in P53 -null mice compared the wild type mice. Moreover, mice expressing mutant P53 are more prone to skin inflammation than the wild-type mice [24]. Furthermore, P53 null mice are more sensitive to gastroenteritis and myocarditis than the controls, and P53 was found to be a general inhibitor of inflammation, since it antagonizes NFkB [25]. In an experimental model of LPS - induced lung injury, inflammatory mediators from P53 – null mice showed more robust responses to LPS and were more prone to that endotoxin as compared to wild-type mice [26]. P21 is a direct downstream target of P53. P21 null mice exert an inflammatory responses which is similar to that of the P53 null mice. In particular, these mice are highly susceptible to LPS and demonstrate high levels of NFκΒ activity. Moreover, there is an increased production of cytokines [27].
It was recently shown both in vivo and in vitro in a diverse variety of cells of different origins that the mutant P53 induced tumoral growth by increasing cellular invasion triggered by TNF-a. Furthermore, the mutated p53 “orchestrated” the TNF induced activation of both NF-kB and JNK inflammatory signaling cascades [28]. The wild type P53 has been shown to suppress the excessive production of the intracellular Reactive Oxygen Species, which may result to both inflammation and cancer acceleration. In such cases, P53 act as an anti-oxidant transcription factor, which elevates the production of those proteins and eliminate the intracellular production of the free radicals [29]. P53 has been associated with the tumor suppressor miRNA miR-34, which is transcriptionally activated by P53. That miR-34 is able to counteract cancer development and infiltration of immune cells when in experimental subjects infected with a lentivirus that augments miR-34 expression [30].
1.3. The effects of GHRH antagonists against inflammation and cancer involve P53
Growth Hormone Releasing Hormone [GHRH (1–44) NH2] is a hypothalamic hormone consisted of 44 peptides, and its biological activity is retained in the first 29 peptides [GHRH(1–29)NH2] [31]. Antagonists of Growth Hormone Releasing Hormone (GHRH) are peptide analogs with robust anticancer and antiinflammatory activities. These GHRH antagonists represent the most advanced therapeutic approach towards a diverse variety of malignancies, since they “target” tumor cells and virtually eliminate their growth potential both “in vivo” and “in vitro” [32].
GHRH antagonists suppress the in vivo growth of various experimental cancers such as prostatic, mammary, ovarian and renal cell carcinomas, lung carcinomas, pancreatic and colorectal carcinomas, endometrial, gastric cancer, osteogenic sarcomas, thyroid cancer, malignant glioblastomas as well as acute myeloid leukemia [33]. Furthermore, the therapeutic capacity of GHRH antagonists is enhanced by their robust anti - inflammatory activity [34,35], which has been shown to be associated with P53. In LNCaP prostate cancers, the GHRH antagonist JMR-132 was shown to exert its effect through the induction of the P53. These cancer cells were treated with two doses of the GHRH antagonist JMR-132, as well as GHRH(1–29)NH2 [36]. The P53 protein levels was increased in the cells treated with 0.1 μΜ and 1 μΜ GHRH antagonist JMR-132 and suppressed in those that were incubated with 0.1 μΜ and 1 μΜ GHRH (1–29)NH2 as compared to controls [36]. GHRH agonist JI-32 was shown to suppress P53 in both 0.1 μΜ and 1 μΜ doses [36,37]. Furthermore, the activated NFκB was decreased in the cells treated with GHRH antagonist JMR-132 (1 μΜ) and increased in those tumors that were challenged with 1 μΜ GHRH(1–29)NH2 [36]. These observations confirm with the reciprocal regulation between P53 and NFκB. Induction of the tumor suppressor p53 suppresses NF-κB, whereas NF-κΒ activation results to P53 suppression [38].
In another study, the suppression of proliferation in A549 cells by GHRH antagonist MZ-5-156 (1 μΜ, 0.1 μΜ) was associated with induction of P53, as well as with the suppression of the iNOS, COX-2 and active NFkB. In bold contrast, 1 μΜ and 0.1 μΜ GHRH(1–29)NH2 exerted its mitogenic effects on those cancers cells by suppressing all these inflammatory mediators [39]. The group of Kiaris has previously shown that p21, a downstream target of P53 is essential for the anticancer activities of GHRH antagonists [40]. Stangelberger et al. provided evidence that the protective effects of GHRH antagonist MZ-J-7-138 on prostate cancers are associated with the induction of the wild-type p53. In particular, this antagonist suppressed the growth of PC-3, DU-145, and MDA-PCa-2b xenografts in nude mice via P53 induction. Remarkably, all three cancers express GHRH – specific receptors [41]. In another study, the most advanced MIAMI series GHRH antagonists prevented the growth of RWPE-1, LNCaP, and PC3 cancer cell lines by recruiting the “guardian of the genome” and tumor suppressor P53 [42]. Indeed, P53 and its downstream target p21 mediated the effect of the GHRH antagonist JMR-132 in the endometrial cancer cell lines Ishikawa and ECC-1 [43].
The significance of P53 against human disease, is underlined by the fact that P53 also appears to partially mediate the robust effects of Cetrorelix (LHRH antagonist) in crossover conditioned media from epithelial and stromal prostate cells. WPMY-1 human prostate stromal cells and BPH-1 human benign prostatic hyperplasia cells were subjected to treatment with Cetrorelix. This antagonist induced P53 expression and inhibited the growth of both cell lines, while it suppressed the STAT-3 pathway [44].
The development of novel and specifically targeted therapeutical approaches against various human diseases and pathophysiological conditions is of the highest priority in translational science and medicine. A strong body of evidence supports the protective role of P53 against human disease, including inflammation and cancer. Antagonists of GHRH represent a class of peptide analogs which induce P53 in the affected cells to restore human health and enhance cellular defenses. Because GHRH agonistic and antagonistic analogs regulate expression levels of P53, we speculate that these peptides may be used in a diverse variety of disease states which are characterized by dysregulation of P53. Future studies may focus on the exact mechanisms by which GHRH analogs govern P53 expression (P53 phosphorylation), since these posttranslational modifications affect the activation and the proteasomal P53 degradation. Furthermore, in vivo experiments which will employ genetically modified mice that do not express, or overexpress P53 will reveal the exact role of that molecule on the action of GHRH antagonists against inflammation and cancer.
1.4. Vascular hyperpermeability: a hallmark of inflammation and cancer
The vascular system functions to supply tissues with nutrients and remove waste products. The vasculature must be permeable to permit the transportation of a plethora of intracellular and intercellular components. Vascular permeability has been shown to be greatly elevated during inflammatory processes, malignancies, and wound healing [45]. Cancers have certain growth characteristics, including unregulated proliferation and vascular invasion. To support growth, tumors express a plethora of vascular mediators, that control extravasation of plasma components and vascular hyperpermeability. The vascular hyperpermeability found in malignancies is essential for the increased nutritional needs of tumors and the supply of oxygen [46].
The endothelium is a dynamic structure that shapes a barrier responsible for the transportation of blood fluid, electrolytes and proteins through the vascular wall. Deviations of normal endothelial barrier function may occur during the initiation as well as establishment of inflammation. Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) result from the dysfunction of the vascular barrier [47]. The microvascular permeability is elevated by agents that cause and mediate inflammation (histamine, growth factors, cytokines, free radicals, leukocytes). These factors may trigger signaling events which violate the barrier integrity by modulating junction proteins, proteins located on the surface of the cells and are responsible for binding with other cells or with the extracellular matrix, or the integrity of cytoskeleton [48]. Such responses may be triggered by agonists, which bind to specific receptors and activate key molecular factors that regulate major intracellular regulatory components. Such factors are kinases, phosphatases and GTPases. These molecular cascades are the regulators of cytoskeletal integrity and contractility, which cause the variations in the function of the vascular barrier [49].
1.5. P53 suppresses vascular hyperpermeability of cancers
Angiogenesis contributes to solid tumor growth and expansion of metastatic colonies. Vascular endothelial growth factor (VEGF) is a dimeric glycoprotein which cause mitosis in a diverse variety of cells, including endothelium. The tumor suppressor protein P53 was found to suppress the VEGF transcription induced by v-Src. On the other hand, the mutated forms of P53 cannot block the ability of v-Src to induce VEGF [50]. It was also revealed that P53 represses VEGF transcription by forming with the E2F transcription factor a transcriptional repressor complex for VEGF expression [51]. Furthermore, it was demonstrated in MCF7 and MDA-MB 435 s breast cancer cells that P53 can strongly inhibit the VEGF overexpression and activation [52]. Our group has previously shown that GHRH antagonists inhibit VEGF secretion from endothelial cells [53], decrease focal adhesion kinase and VEGF expression in the A549 non-small cell lung cancer cells as well as in H727 bronchial carcinoid cells [54].
Integrins appear to regulate tumoral metastatic potential. The intracellular amounts of the α5β1, α6β4, α4β1, αvβ3, αvβ5, αvβ6, and α2β1 integrines is associated with cancer prognosis [55]. It was discovered that p53 regulates the expression of α5, β1, β3, and β4 integrins. The P53 inductor and MDM2 antagonist Nutlin-3a decreased the abundance of integrin α5 in cancers, while P53 overexpression due to genetic manipulations or stress resulted to the reduction of Integrin β4 [56].
The deletion of the P53 tumor suppressor in HCT116 human cancer cells induced the vascularization and growth of tumor xenografts in nude mice. Via the activation of the hypoxia –inducible factor 1 [57]. In 11 endometrial cancer cell lines, P53 overexpression suppressed the intracellular VEGF expression as well as its presence in the cultured media [58]. P53 has been shown to limit angiogenesis by suppressing the production of proangiogenic factors, and by increasing the abundance angiogenetic inhibitors. All these activities permit P53 to counteract and limit the angiogenic potential and metastatic growth of cancer cells [59]. The suspension of the P53 anticancer actions, which is present in the great majority of human cancers, is able to setback these effects. The absence of functional P53 during tumorigenesis is crucial for tumor aggression [60]. The loss of senescence and apoptosis due to lack of P53 is responsible for tumor establishment. The absence of checkpoint control enable the uncontrolled tumors growth, and establishes an environment susceptible to the development of new mutations. The enhanced metastatic potential benefits the extravavasation of the cancer cells to nearby tissues and the development of new metastatic locations [61].
Activated RasV12 and impaired P53 activities can synergistically promote cellular reallocation, which is an effect tightly associated with malignancies. RasV12 and P53 suppression induces RhoA activity, elucidating the function of P53 in cancer elimination. The “guardian of the genome” inhibits the RhoA mediated activities, which in turn results to the suppression of the RasV12 - mediated cell motility [62].
On the other hand, suppression of VEGF enhances P53 expression in BEL7402 cells. Inhibition of PKCα by inhibitors or genetic manipulations decreased the growth and metastatic potential of urinary bladder carcinoma cells and human hepatocellular carcinoma cells. All these genetic manipulations were in line with the consistent increase of p53 and its downstream target p21(WAF1/CIP1) [63].
1.6. P53 and vascular permeability in non-cancerous tissues
Although the role of the P53 in the tumoral permeability has been well described, the effects of that molecule towards the permeability of the inflamed vasculature were not clear until recently. In 2015 we have shown that P53 is important for the maintenance of vascular integrity, and is protective again LPS-induced endothelial barrier hyperpermeability. Further, it was revealed that P53, at least in part, “is streaming” the effects of the anti-inflammatory effects of Hsp90 inhibitors in human tissues [64]. Inhibition of P53 by genetic manipulations decreased transendothelial resistance, which reflects the status of endothelial barrier function. Pifithrin, a P53 inhibitor, exerted a similar effect, that elevated the LPS-induced hyperpermeability in human lung microvascular endothelial cells (HLMVEC). Nutlin - induced P53 overexpression weakened the LPS-induced violation of the barrier function. In an in vivo experimental model of Acute Lung Injury, LPS decreased the intracellular pulmonary P53 expression levels. That effect was diminished by pretreatment with Hsp90 inhibitors in human cells and mice. Remarkably, inhibition of Hsp90 suppressed the LPS-induced induction of the P53 negative regulator MDMX, as well as the p53 and MDM2 phosphorylation in HLMVEC. MDM2 and MDMX, which are the major P53 negative regulators, were both downregulated by LPS in vivo. Overexpression of P53 resulted in the corruption of the inflammatory RhoA/MLC2 activation, while P53 suppression produced the inverse effect [64].
In 2018 another recent study from our group reported that p53 is involved in the defense of the vasculature towards LPS, by regulating the opposing effects of Rac1 and RhoA in lung tissues both in vivo and in vivo [65]. HLMVEC exposed to HSP90 inhibitors activated both Rac1 and P21 activated kinase, which serve as a crucial component of vascular barrier function. Hsp90 inhibition enhanced the activation of LIMK and the deactivation of cofilin by phosphorylation. On the other hand, LPS exerted the opposing actions. Mouse lung microvascular endothelial cells treated with LPS showed a downregulation of the deactivated form of cofilin. The Hsp90 inhibitor 17AAG suppressed the intracellular abundance of the non - phosphorylated cofilin. In addition, the suppression of the pyridoxal phosphate phosphatase (PDXP) by siRNA means reduced the LPS - triggered hyperpermeability. Hsp90 inhibitors upregulated P190RHOGAP and resulted to a profound decrease of the toxin - induced pMLC2 upregulation “in vivo”. Pulmonary endothelium cells isolated from “super p53” mice were less susceptible to LPS insult than the control animals [65]. Moreover, another study from our group which explored the beneficial effects of hydrocortisone and ascorbic acid against sepsis, revealed that pretreatment of these cells with both agents prevented the LPS induced - P53 downregulation as well as the cofilin activation by dephosphorylation [66].
The Src kinase is essential for the VEGF - induced hyperpermeability due to VEGF, tumor necrosis factor (TNF-α), and reactive oxygen and nitrogen species in the lungs cells [67]. Src kinase mediates the neutrophil - induced barrier dysfunction in human dermal microvascular cells. In HLMVEC, src activates MLCK. The phosphorylation of the latter molecule signals the production of actin stress fiber. All these src - mediated effects which result to the barrier dysfunction, are exerted through induction of the major P53 negative regulator MDM2 [68].
The “guardian of the genome” as well as the Src kinase were shown to have an antagonistic relation in an experimental in vivo model [69]. In particular, the induction of P53 suppressed the Src - mediated formation of podosomes. P53 increased PTEN levels and reduced STAT3 activation. P53 downregulation strongly enhanced invasion due to the Src activities [70]. Caldesmon is able to bind to actin, and in turn suppresses the development of podosomes. It was revealed that Src suppresses expression of p53 and caldesmon, but enhances that of STAT3. Hsp90 inhibition reduced the Src mediated and LPS-induced Hsp90 phosphorylation [71]. P53 also affects cell migration. Knocking down P53 in mouse fibroblasts resulted to a change in their shape. In particular, they turned from an elongated form to a spherical shape, with subsequent more powerful invasive abilities. The RhoA activation was crucial for that change, which was was opposed by the RhoE [72].
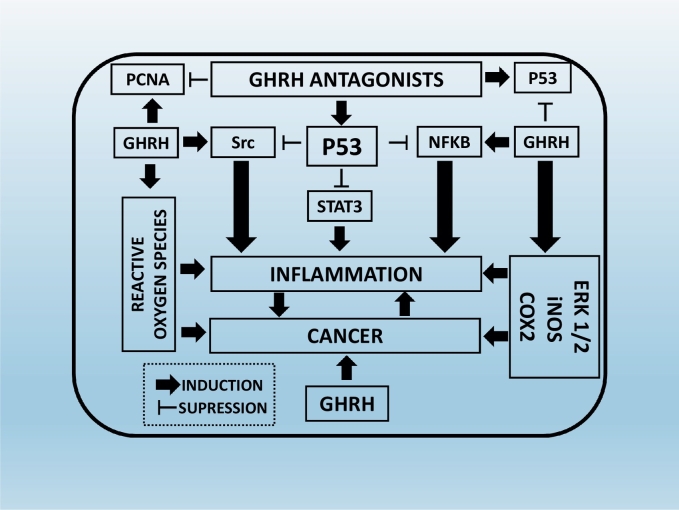
In mouse endothelial cells, P53 protects the animals from heart injury due to irradiation. Those animals that were carriers of an endothelial deletion of p53 did not resist to heart failure after irradiation. The heart failure resulted to the impaired function and morphology of the myocardium, which turned to severe hypoxia [73]. In vitro studies suggested that p53 supported the normal endothelium function after radiation. Those animals that were lacking p21, a downstream p53 target, were vulnerable to myocardial injury after irradiation [74]. Another study suggested that P53 reduced vascular permeability due to an urinary trypsin inhibitor [75]. Fig. 2 provides a summary of the intracellular cascades involved in the regulation of cancer and inflammation by GHRH antagonists and P53.
Fig. 2.
Mechanisms which are involved on the suppression of cancer and inflammation by Growth Hormone Releasing Hormone Antagonists and P53: GHRH antagonists ‘trigger’ P53 induction, which in turn suppresses the activation of the inflammatory mediators Src, Stat3 and NFKB. Those mediators are also involved in carcinogenesis. On the other hand, GHRH is a mitogenetic growth factor “in charge” of the production of ERK1/2, iNOS, COX2 which in turn promote both cancer and inflammation.
2. Conclusions
There is an ongoing laborious effort to “expand” our knowledge on the unexplored properties of the P53 in both inflammation and cancer, since the former condition has long been associated with the latter pathology. The “guardian of the genome” opposes the development of both conditions, by suppressing the induction of key inflammatory and carcinogenesis mediators, as well as by eliminating by apoptosis those cells that are not feasible to be impaired due to excessive damage. The manipulation of that important transcription factor consists the goal of the most advanced experimental theurapeutical approaches, such as the development and application of GHRH antagonists in those diseases that are linked to advanced inflammatory processes. Remarkably, P53 suppresses the vascularization and metastatic potential of a plethora of cancer. Moreover, it counteracts the toxin – induced hyperpermeability responses by strengthening the vascular barrier integrity. Since GHRH - related analogs govern the regulation of P53 expression levels in the intracellular niche of both normal and malignant tissues, we believe that the continuous development of those compounds represent an attractive and exciting strategy to counteract and prevent the development of the most common and severe inflammatory diseases and restore human function to the prior healthy state. Fig. 1 highlights the conclusion of the current work.
Fig. 1.
Remarks on the relation between Growth Hormone Releasing Hormone, P53, Inflammation and Cancer.
2.1. Outstanding questions
The current review highlights the connection between GHRH, P53, inflammation and cancer. Future studies are needed in order to delineate the exact signaling pathways that are involved in the suppression of cancer and inflammation by GHRH antagonists and P53, as well as on the carcinogenetic and inflammatory role of GHRH towards human disease. Genetically modified mice that do not express or overexpress P53 should be treated with GHRH and GHRH antagonists in order to evaluate whether the responses of those experimental subjects will differ after treatment with GHRH and GHRH antagonists. Furthermore, advanced in vitro experimental techniques should be employed in order to evaluate the effect of inflammatory factors (i.e. GHRH and LPS) on the vascular permeability of Human Microvascular Endothelial Cells as well as Bovine Pulmonary Aortic Endothelial Cells. Since GHRH antagonists induce P53, and P53 has been shown to protect human cells against toxin induced hyper - permeability responses, future experiments will be conducted on the possible effect of those compounds on the brain injury following stroke and cerebral ischemia-reperfusion.
2.2. Search strategy and selection criteria
Data for this Review were identified by searches of PubMed, and references from relevant articles using the search terms “GHRH”, “GHRH antagonists”, “P53”, “cancer” and “inflammation”. Only articles published in English between 1989 and 2018 were included.
Acknowledgements
This work was supported by 1) Start - up funds (5RSPEC 300010 271008) to N·B from the College of Pharmacy, University of Louisiana Monroe, Monroe LA 71201 2) The Faculty Research Support Program from Dean's Office (5CALHN-260615) to N·B from the College of Pharmacy, University of Louisiana Monroe, Monroe LA 71201 3) The Malcolm Feist Partners Across Campuses Seed Program (Grant Index # BRS005) to N·B (co-PI), Center for Cardiovascular Diseases and Sciences, Louisiana State University Health Shreveport, Shreveport, LA 71103 4) The Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health (5 P20 GM103424-15 and 3 P20 GM103424-15S1) to N.B.
References
- 1.Freed-Pastor W.A., Prives C. Mutant p53: one name, many proteins. Genes Dev. 2012;26(12):1268–1286. doi: 10.1101/gad.190678.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine A.J. The road to the discovery of the p53 protein. The Steiner Cancer Prize Award Lecture. Int J Cancer. 1994;56(6):775–776. doi: 10.1002/ijc.2910560602. [DOI] [PubMed] [Google Scholar]
- 3.Hinds P., Finlay C., Levine A.J. Mutation is required to activate the p53 gene for cooperation with the ras oncogene and transformation. J Virol. 1989;63(2):739–746. doi: 10.1128/jvi.63.2.739-746.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yue X., Zhao Y., Xu Y., Zheng M., Feng Z., Hu W. Mutant p53 in cancer: accumulation, gain-of-function, and therapy. J Mol Biol. 2017;429(11):1595–1606. doi: 10.1016/j.jmb.2017.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oren M., Rotter V. Mutant p53 gain-of-function in cancer. Cold Spring Harb Perspect Biol. 2010;2(2):a001107. doi: 10.1101/cshperspect.a001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Humpton T.J., Vousden K.H. Regulation of cellular metabolism and hypoxia by p53. Cold Spring Harb Perspect Med. 2016;6(7) doi: 10.1101/cshperspect.a026146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muller P.A., Vousden K.H. p53 mutations in cancer. Nat Cell Biol. 2013;15(1):2–8. doi: 10.1038/ncb2641. [DOI] [PubMed] [Google Scholar]
- 8.Lane D.P., Cheok C.F., Lain S. p53-based cancer therapy. Cold Spring Harb Perspect Biol. 2010;2(9):a001222. doi: 10.1101/cshperspect.a001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mantovani A. The inflammation—cancer connection. FEBS J. 2018;285(4):638–640. doi: 10.1111/febs.14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mantovani A., Garlanda C., Allavena P. Molecular pathways and targets in cancer-related inflammation. Ann Med. 2010;42(3):161–170. doi: 10.3109/07853890903405753. [DOI] [PubMed] [Google Scholar]
- 11.Sica A., Allavena P., Mantovani A. Cancer related inflammation: the macrophage connection. Cancer Lett. 2008;267(2):204–215. doi: 10.1016/j.canlet.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 12.Peiseler M., Kubes P. Macrophages play an essential role in trauma-induced sterile inflammation and tissue repair. Eur J Trauma Emerg Surg. 2018;44(3):335–349. doi: 10.1007/s00068-018-0956-1. [DOI] [PubMed] [Google Scholar]
- 13.Yi H.S., Chang J.Y., Kim K.S., Shong M. Oncogenes, mitochondrial metabolism, and quality control in differentiated thyroid cancer. Korean J Intern Med. 2017;32(5):780–789. doi: 10.3904/kjim.2016.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mantovani A., Allavena P., Sica A., Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 15.Tsai Y.P., Wu K.J. Epigenetic regulation of hypoxia-responsive gene expression: focusing on chromatin and DNA modifications. Int J Cancer. 2014;134(2):249–256. doi: 10.1002/ijc.28190. [DOI] [PubMed] [Google Scholar]
- 16.Couto J.P., Daly L., Almeida A., Knauf J.A., Fagin J.A., Sobrinho-Simoes M. STAT3 negatively regulates thyroid tumorigenesis. Proc Natl Acad Sci U S A. 2012;109(35):E2361–E2370. doi: 10.1073/pnas.1201232109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avalle L., Camporeale A., Camperi A., Poli V. STAT3 in cancer: a double edged sword. Cytokine. 2017;98:42–50. doi: 10.1016/j.cyto.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Mouronte-Roibas C., Leiro-Fernandez V., Fernandez-Villar A., Botana-Rial M., Ramos-Hernandez C., Ruano-Ravina A. COPD, emphysema and the onset of lung cancer. A systematic review. Cancer Lett. 2016;382(2):240–244. doi: 10.1016/j.canlet.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Kawauchi K., Araki K., Tobiume K., Tanaka N. Activated p53 induces NF-kappaB DNA binding but suppresses its transcriptional activation. Biochem Biophys Res Commun. 2008;372(1):137–141. doi: 10.1016/j.bbrc.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 20.Nesic D., Grumont R., Gerondakis S. The nuclear factor-kappaB and p53 pathways function independently in primary cells and transformed fibroblasts responding to genotoxic damage. Mol Cancer Res. 2008;6(7):1193–1203. doi: 10.1158/1541-7786.MCR-07-2125. [DOI] [PubMed] [Google Scholar]
- 21.Gudkov A.V., Gurova K.V., Komarova E.A. Inflammation and p53: a Tale of two Stresses. Genes Cancer. 2011;2(4):503–516. doi: 10.1177/1947601911409747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Napetschnig J., Wu H. Molecular basis of NF-kappaB signaling. Annu Rev Biophys. 2013;42:443–468. doi: 10.1146/annurev-biophys-083012-130338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowe J.M., Menendez D., Bushel P.R., Shatz M., Kirk E.L., Troester M.A. p53 and NF-kappaB coregulate proinflammatory gene responses in human macrophages. Cancer Res. 2014;74(8):2182–2192. doi: 10.1158/0008-5472.CAN-13-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tavana O., Benjamin C.L., Puebla-Osorio N., Sang M., Ullrich S.E., Ananthaswamy H.N. Absence of p53-dependent apoptosis leads to UV radiation hypersensitivity, enhanced immunosuppression and cellular senescence. Cell Cycle. 2010;9(16):3328–3336. doi: 10.4161/cc.9.16.12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komarova E.A., Krivokrysenko V., Wang K., Neznanov N., Chernov M.V., Komarov P.G. p53 is a suppressor of inflammatory response in mice. FASEB J. 2005;19(8):1030–1032. doi: 10.1096/fj.04-3213fje. [DOI] [PubMed] [Google Scholar]
- 26.Liu G., Park Y.J., Tsuruta Y., Lorne E., Abraham E. p53 Attenuates lipopolysaccharide-induced NF-kappaB activation and acute lung injury. J Immunol. 2009;182(8):5063–5071. doi: 10.4049/jimmunol.0803526. [DOI] [PubMed] [Google Scholar]
- 27.Scatizzi J.C., Mavers M., Hutcheson J., Young B., Shi B., Pope R.M. The CDK domain of p21 is a suppressor of IL-1beta-mediated inflammation in activated macrophages. Eur J Immunol. 2009;39(3):820–825. doi: 10.1002/eji.200838683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooks T., Harris C.C. p53 mutations and inflammation-associated cancer are linked through TNF signaling. Mol Cell. 2014;56(5):611–612. doi: 10.1016/j.molcel.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooks T., Harris C.C., Oren M. Caught in the cross fire: p53 in inflammation. Carcinogenesis. 2014;35(8):1680–1690. doi: 10.1093/carcin/bgu134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kasinski A.L., Slack F.J. miRNA-34 prevents cancer initiation and progression in a therapeutically resistant K-ras and p53-induced mouse model of lung adenocarcinoma. Cancer Res. 2012;72(21):5576–5587. doi: 10.1158/0008-5472.CAN-12-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barabutis N., Schally A.V. Knocking down gene expression for growth hormone-releasing hormone inhibits proliferation of human cancer cell lines. Br J Cancer. 2008;98(11):1790–1796. doi: 10.1038/sj.bjc.6604386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiaris H., Chatzistamou I., Papavassiliou A.G., Schally A.V. Growth hormone-releasing hormone: not only a neurohormone. Trends Endocrinol Metab. 2011;22(8):311–317. doi: 10.1016/j.tem.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Barabutis N., Schally A.V. Growth hormone-releasing hormone: extrapituitary effects in physiology and pathology. Cell Cycle. 2010;9(20):4110–4116. doi: 10.4161/cc.9.20.13787. [DOI] [PubMed] [Google Scholar]
- 34.Popovics P., Schally A.V., Salgueiro L., Kovacs K., Rick F.G. Antagonists of growth hormone-releasing hormone inhibit proliferation induced by inflammation in prostatic epithelial cells. Proc Natl Acad Sci U S A. 2017;114(6):1359–1364. doi: 10.1073/pnas.1620884114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schally A.V. Endocrine approaches to treatment of Alzheimer's disease and other neurological conditions: part I: some recollections of my association with Dr. Abba Kastin: a tale of successful collaboration. Peptides. 2015;72:154–163. doi: 10.1016/j.peptides.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Barabutis N., Schally A.V. Antioxidant activity of growth hormone-releasing hormone antagonists in LNCaP human prostate cancer line. Proc Natl Acad Sci U S A. 2008;105(51):20470–20475. doi: 10.1073/pnas.0811209106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barabutis N., Siejka A., Schally A.V. Effects of growth hormone-releasing hormone and its agonistic and antagonistic analogs in cancer and non-cancerous cell lines. Int J Oncol. 2010;36(5):1285–1289. doi: 10.3892/ijo_00000613. [DOI] [PubMed] [Google Scholar]
- 38.Perkins N.D. NF-kappaB: tumor promoter or suppressor? Trends Cell Biol. 2004;14(2):64–69. doi: 10.1016/j.tcb.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Barabutis N., Siejka A., Schally A.V. Growth hormone releasing hormone induces the expression of nitric oxide synthase. J Cell Mol Med. 2011;15(5):1148–1155. doi: 10.1111/j.1582-4934.2010.01096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Volakaki A.A., Lafkas D., Kassi E., Schally A.V., Papavassiliou A.G., Kiaris H. Essential role of p21/waf1 in the mediation of the anti-proliferative effects of GHRH antagonist JMR-132. J Mol Endocrinol. 2008;41(5):389–392. doi: 10.1677/JME-08-0106. [DOI] [PubMed] [Google Scholar]
- 41.Stangelberger A., Schally A.V., Rick F.G., Varga J.L., Baker B., Zarandi M. Inhibitory effects of antagonists of growth hormone releasing hormone on experimental prostate cancers are associated with upregulation of wild-type p53 and decrease in p21 and mutant p53 proteins. Prostate. 2012;72(5):555–565. doi: 10.1002/pros.21458. [DOI] [PubMed] [Google Scholar]
- 42.Popovics P., Cai R., Sha W., Rick F.G., Schally A.V. Growth hormone-releasing hormone antagonists reduce prostatic enlargement and inflammation in carrageenan-induced chronic prostatitis. Prostate. 2018;78(13):970–980. doi: 10.1002/pros.23655. [DOI] [PubMed] [Google Scholar]
- 43.Wu H.M., Schally A.V., Cheng J.C., Zarandi M., Varga J., Leung P.C. Growth hormone-releasing hormone antagonist induces apoptosis of human endometrial cancer cells through PKCdelta-mediated activation of p53/p21. Cancer Lett. 2010;298(1):16–25. doi: 10.1016/j.canlet.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 44.Siejka A., Schally A.V., Barabutis N. The effect of LHRH antagonist cetrorelix in crossover conditioned media from epithelial (BPH-1) and stromal (WPMY-1) prostate cells. Horm Metab Res. 2014;46(1):21–26. doi: 10.1055/s-0033-1349127. [DOI] [PubMed] [Google Scholar]
- 45.Nagy J.A., Benjamin L., Zeng H., Dvorak A.M., Dvorak H.F. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis. 2008;11(2):109–119. doi: 10.1007/s10456-008-9099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maeda H., Fang J., Inutsuka T., Kitamoto Y. Vascular permeability enhancement in solid tumor: various factors, mechanisms involved and its implications. Int Immunopharmacol. 2003;3(3):319–328. doi: 10.1016/S1567-5769(02)00271-0. [DOI] [PubMed] [Google Scholar]
- 47.Bhattacharya J., Matthay M.A. Regulation and repair of the alveolar-capillary barrier in acute lung injury. Annu Rev Physiol. 2013;75:593–615. doi: 10.1146/annurev-physiol-030212-183756. [DOI] [PubMed] [Google Scholar]
- 48.Vandenbroucke E., Mehta D., Minshall R., Malik A.B. Regulation of endothelial junctional permeability. Ann N Y Acad Sci. 2008;1123:134–145. doi: 10.1196/annals.1420.016. [DOI] [PubMed] [Google Scholar]
- 49.Lum H., Malik A.B. Regulation of vascular endothelial barrier function. Am J Physiol. 1994;267(3):L223–L241. doi: 10.1152/ajplung.1994.267.3.L223. Pt 1. [DOI] [PubMed] [Google Scholar]
- 50.Mukhopadhyay D., Tsiokas L., Sukhatme V.P. Wild-type p53 and v-Src exert opposing influences on human vascular endothelial growth factor gene expression. Cancer Res. 1995;55(24):6161–6165. [PubMed] [Google Scholar]
- 51.Qin G., Kishore R., Dolan C.M., Silver M., Wecker A., Luedemann C.N. Cell cycle regulator E2F1 modulates angiogenesis via p53-dependent transcriptional control of VEGF. Proc Natl Acad Sci U S A. 2006;103(29):11015–11020. doi: 10.1073/pnas.0509533103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pal S., Datta K., Mukhopadhyay D. Central role of p53 on regulation of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) expression in mammary carcinoma. Cancer Res. 2001;61(18):6952–6957. [PubMed] [Google Scholar]
- 53.Siejka A., Lawnicka H., Komorowski J., Schally A.V., Stepien T., Krupinski R. GH-RH antagonist (MZ-4-71) inhibits VEGF secretion and proliferation of murine endothelial cells. Life Sci. 2003;72(22):2473–2479. doi: 10.1016/s0024-3205(03)00164-4. [DOI] [PubMed] [Google Scholar]
- 54.Siejka A., Barabutis N., Schally A.V. GHRH antagonist inhibits focal adhesion kinase (FAK) and decreases expression of vascular endothelial growth factor (VEGF) in human lung cancer cells in vitro. Peptides. 2012;37(1):63–68. doi: 10.1016/j.peptides.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 55.Desgrosellier J.S., Cheresh D.A. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10(1):9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ebata T., Hirata H., Kawauchi K. Functions of the Tumor Suppressors p53 and Rb in Actin Cytoskeleton Remodeling. Biomed Res Int. 2016;2016 doi: 10.1155/2016/9231057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ravi R., Mookerjee B., Bhujwalla Z.M., Sutter C.H., Artemov D., Zeng Q. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev. 2000;14(1):34–44. [PMC free article] [PubMed] [Google Scholar]
- 58.Fujisawa T., Watanabe J., Kamata Y., Hamano M., Hata H., Kuramoto H. Effect of p53 gene transfection on vascular endothelial growth factor expression in endometrial cancer cells. Exp Mol Pathol. 2003;74(3):276–281. doi: 10.1016/s0014-4800(03)00020-0. [DOI] [PubMed] [Google Scholar]
- 59.Blagosklonny M.V. P53: an ubiquitous target of anticancer drugs. Int J Cancer. 2002;98(2):161–166. doi: 10.1002/ijc.10158. [DOI] [PubMed] [Google Scholar]
- 60.Teodoro J.G., Evans S.K., Green M.R. Inhibition of tumor angiogenesis by p53: a new role for the guardian of the genome. J Mol Med (Berl.) 2007;85(11):1175–1186. doi: 10.1007/s00109-007-0221-2. [DOI] [PubMed] [Google Scholar]
- 61.Powell E., Piwnica-Worms D., Piwnica-Worms H. Contribution of p53 to metastasis. Cancer Discov. 2014;4(4):405–414. doi: 10.1158/2159-8290.CD-13-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xia M., Land H. Tumor suppressor p53 restricts Ras stimulation of RhoA and cancer cell motility. Nat Struct Mol Biol. 2007;14(3):215–223. doi: 10.1038/nsmb1208. [DOI] [PubMed] [Google Scholar]
- 63.Zhang L., Wang J.N., Tang J.M., Kong X., Yang J.Y., Zheng F. VEGF is essential for the growth and migration of human hepatocellular carcinoma cells. Mol Biol Rep. 2012;39(5):5085–5093. doi: 10.1007/s11033-011-1304-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barabutis N., Dimitropoulou C., Birmpas C., Joshi A., Thangjam G., Catravas J.D. p53 protects against LPS-induced lung endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2015;308(8):L776–L787. doi: 10.1152/ajplung.00334.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barabutis N., Dimitropoulou C., Gregory B., Catravas J.D. Wild-type p53 enhances endothelial barrier function by mediating RAC1 signalling and RhoA inhibition. J Cell Mol Med. 2018;22(3):1792–1804. doi: 10.1111/jcmm.13460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barabutis N., Khangoora V., Marik P.E., Catravas J.D. Hydrocortisone and ascorbic acid synergistically prevent and repair lipopolysaccharide-induced pulmonary endothelial barrier dysfunction. Chest. 2017;152(5):954–962. doi: 10.1016/j.chest.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kevil C.G., Okayama N., Alexander J.S. H(2)O(2)-mediated permeability II: importance of tyrosine phosphatase and kinase activity. Am J Physiol Cell Physiol. 2001;281(6):C1940–C1947. doi: 10.1152/ajpcell.2001.281.6.C1940. [DOI] [PubMed] [Google Scholar]
- 68.Batuello C.N., Hauck P.M., Gendron J.M., Lehman J.A., Mayo L.D. Src phosphorylation converts Mdm2 from a ubiquitinating to a neddylating E3 ligase. Proc Natl Acad Sci U S A. 2015;112(6):1749–1754. doi: 10.1073/pnas.1416656112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mukhopadhyay U.K., Mooney P., Jia L., Eves R., Raptis L., Mak A.S. Doubles game: Src-Stat3 versus p53-PTEN in cellular migration and invasion. Mol Cell Biol. 2010;30(21):4980–4995. doi: 10.1128/MCB.00004-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mukhopadhyay U.K., Eves R., Jia L., Mooney P., Mak A.S. p53 suppresses Src-induced podosome and rosette formation and cellular invasiveness through the upregulation of caldesmon. Mol Cell Biol. 2009;29(11):3088–3098. doi: 10.1128/MCB.01816-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barabutis N., Handa V., Dimitropoulou C., Rafikov R., Snead C., Kumar S. LPS induces pp60c-src-mediated tyrosine phosphorylation of Hsp90 in lung vascular endothelial cells and mouse lung. Am J Physiol Lung Cell Mol Physiol. 2013;304(12):L883–L893. doi: 10.1152/ajplung.00419.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gadea G., de Toledo M., Anguille C., Roux P. Loss of p53 promotes RhoA-ROCK-dependent cell migration and invasion in 3D matrices. J Cell Biol. 2007;178(1):23–30. doi: 10.1083/jcb.200701120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee C.L., Min H., Befera N., Clark D., Qi Y., Das S. Assessing cardiac injury in mice with dual energy-microCT, 4D-microCT, and microSPECT imaging after partial heart irradiation. Int J Radiat Oncol Biol Phys. 2014;88(3):686–693. doi: 10.1016/j.ijrobp.2013.11.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee C.L., Moding E.J., Cuneo K.C., Li Y., Sullivan J.M., Mao L. p53 functions in endothelial cells to prevent radiation-induced myocardial injury in mice. Sci Signal. 2012;5(234):ra52. doi: 10.1126/scisignal.2002918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou N., Xu T., Bai Y., Prativa S., Xu J.Z., Li K. Protective effects of urinary trypsin inhibitor on vascular permeability following subarachnoid hemorrhage in a rat model. CNS Neurosci Ther. 2013;19(9):659–666. doi: 10.1111/cns.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]