Abstract
Chikungunya fever is caused by Chikungunya virus (CHIKV) and is generally considered a self-limiting disease. However, severe clinical presentations with a high mortality rate have been reported in association with underlying medical conditions. This study reports the molecular characterization of the virus and an abnormal pattern of circulating cytokines in a unique lethal CHIKV case during the 2017 outbreak in Italy, which involved an elderly patient with underlying cardiac disease. Analysis of inflammatory cytokines revealed a strong increase of interferon (IFN)-α and IFN-β, as well as interleukin-6, suggesting a possible role of type-I IFN in the cytokine storm, which may be correlated with unfavorable prognosis of CHIKV infection.
Keywords: Chikungunya virus, Italy, lethal, cytokine, type I IFN
Chikungunya virus (CHIKV) is a mosquito-borne alphavirus belonging to the family Togaviridae. CHIKV is transmitted by Aedes aegypti mosquitoes in tropical and subtropical regions, but the potential exists for further spread because of genetic adaptation of the virus to Aedes albopictus, a species that thrives in temperate regions [1]. In Europe, autochthonous outbreaks were reported in 2007 in Italy [2] and in 2010, 2014, and 2017 in France [3]. Recently, a Chikungunya fever (CF) epidemic occurred in Italy, involving 270 confirmed cases in the Lazio and Calabria regions (http://www.salute.gov.it/portale/temi/documenti/chikungunya/bollettino_chikungunya_ULTIMO.pdf). CF is usually not life-threatening, although atypical and severe forms can occur during large-scale epidemics [4, 5]. In addition, CHIKV represents a substantial health burden to affected populations, with symptoms that include severe joint and muscle pain, rashes, and fever, as well as prolonged periods of disability in some patients [1]. We report an extensive virus characterization based on the whole genome sequence and an abnormal pattern of circulating cytokines in a lethal case of CF during the 2017 Lazio region outbreak.
METHODS
The Patient
The patient was a 77-year-old male with relevant cardiac comorbidities who lived in Anzio (Lazio, Italy), in a territory within the 2017 CHIKV outbreak epicenter (http://www.salute.gov.it/portale/temi/documenti/chikungunya/bollettino_chikungunya_ULTIMO.pdf). He was hospitalized at the Santa Maria Goretti Hospital in Latina 2 days after symptoms onset and died after 10 hours the hospitalization, before virological diagnosis was made at the Regional Reference Laboratory for arboviral infections at the National Institute for Infectious Diseases “Lazzaro Spallanzani” (INMI) in Rome. The analysis was part of a larger study entitled “Study of Factors (Microbial, Viral, Host-Derived and Therapeutic) Involved in the Clinical Evolution of Emerging and Severe Viral Diseases,” approved by the INMI Ethical Committee, issue No. 14/2015. Written informed consent was not required as the activities described were performed within the framework of the Regional Surveillance plan.
Virological Investigation
Molecular diagnosis was based on real-time reverse transcription PCR (RT-PCR; RealStar Chikungunya RT-PCR Kit 2.0, Altona Diagnostics GmbH, Germany). Serological diagnostic investigation was based on indirect immunofluorescence assay (Arbovirus Fever Mosaic 2 IgG and IgM, Euroimmun, Germany). Virus isolation from patient’s serum was performed on BHK-21 cells. The near-complete genome sequence was obtained by Sanger sequencing: an 11 467-nt-long sequence was built based on 23 overlapping amplicons, as previously described [6]. A maximum likelihood phylogenetic tree was built with the near-complete genome sequence of the isolate, based on the General Timer Reversible substitution model.
IFN and Cytokine Detection
Interferon (IFN) response (IFN-α, IFN-β, and IFN-γ) and inflammatory cytokine release (interleukin [IL]-6 and tumor necrosis factor [TNF]–α) were evaluated in this fatal case. Circulating mediators were quantified using an enzyme-linked immunosorbent assay (ELISA; VeriKine-HS Human IFN-α, VeriKine-HS Human IFN-β, and VeriKine Human IFN-γ, purchased from PBL Assay Science, Piscataway, New Jersey; Human IL-6 DuoSet ELISA and Human TNF-alpha DuoSet ELISA, purchased from R&D System, Minneapolis, Minnesota), and the levels were compared with those detected in plasma samples obtained during the very early stages of the infection (<3 days after onset of symptoms) in 4 nonfatal cases belonging to the same outbreak (Supplementary Table 1).
RESULTS
Clinical History
In September 2017, a 77-year-old male, living in Anzio, was admitted to the Emergency Room of Santa Maria Goretti Hospital in Latina (Lazio region, Italy). The patient had a history of hypertension, ischemic heart disease, and coronary artery bypass graft surgery (CABG), chronic obliterative arteriopathy of the inferior limbs, and bilateral carotid atherosclerosis; in July, he underwent contrast cardiac ventriculography for acute myocardial infarction.
The patient had a high fever (up to 39°C) and widespread arthromyalgia during the previous 2 days and was admitted to the hospital for acute neurological syndrome that had started in the previous 3 hours. He was conscious and presented deviation of the angle of the mouth toward the right side, hypostenia of the left inferior limb, and dysartria. The vital signs were the following: artery pressure (PA) of 120/60 mmHg, heart rate (HR) of 87 beats per minute (bpm) with a normal rhythm, body temperature (BT) of 37.5°, respiratory rate of 18 breath per minute, normal oxygen saturation (99%) in ambient air (AA), and Glasgow Coma Scale (GCS) of 15.
Thirty minutes after hospital admission, the neurological deficit disappeared and neurological examination was negative. The BT was 38.5°. Chest x-ray showed an accentuation of bronchovascular marking, indicating an interstitial pulmonary edema and ischemic chronic vascular disease, without acute event at computed tomography. The electrocardiogram showed a right bundle branch block and widespread abnormalities of ventricular repolarization. The echocardiogram showed a dilated left cardiomyopathy with a reduced ejection fraction (35%), with mild aortic and mild to moderate mitralic regurgition and normal right heart function and pericardium.
The blood tests showed a white blood cell count of 6.290 × 103 uL (neutrophils 90%, lymphocytes 2.1%); red blood cell count of 3.666 × 106 uL; hemoglobin of 11.2 gr; and a platelet count of 179.000 × 103 uL. Moreover, he had normal liver function and coagulation tests, no electrolyte alterations, creatinine of 1.10 mg/dL, and C-reactive protein of 1.37 mg/dL (normal range, 0–0.5). Blood cultures were negative. Markers of myocardial injury were above the normal range, in particular, high-sensitive Troponin (hs-cTn) of 0.352 pg/mL (normal range, 0–0.34 pg/mL) and Creatine kinase-MB (CK-MB) of 8.40 ng/mL (normal < 7.2 ng/mL). The patient was transferred to a subintensive care unit in stable condition with a fever (39.8°C) and was started on a therapy with furosemide, clodiprogel, acetylsalicylic acid, bisoprolol, paracetamol, ceftriaxone, and azytromicin. After 9-hour admission in subintensive unit care, the patient died of acute cardiac arrest.
Due to the concomitant presence of CHIKV cases in the patient’s family, a blood sample was sent to the INMI for virological investigation. CHIKV infection was diagnosed on the basis of positive RT-PCR test, in the absence of CHIKV-specific IgM/IgG antibodies (Supplementary Table 1).
Virus Molecular Characterization
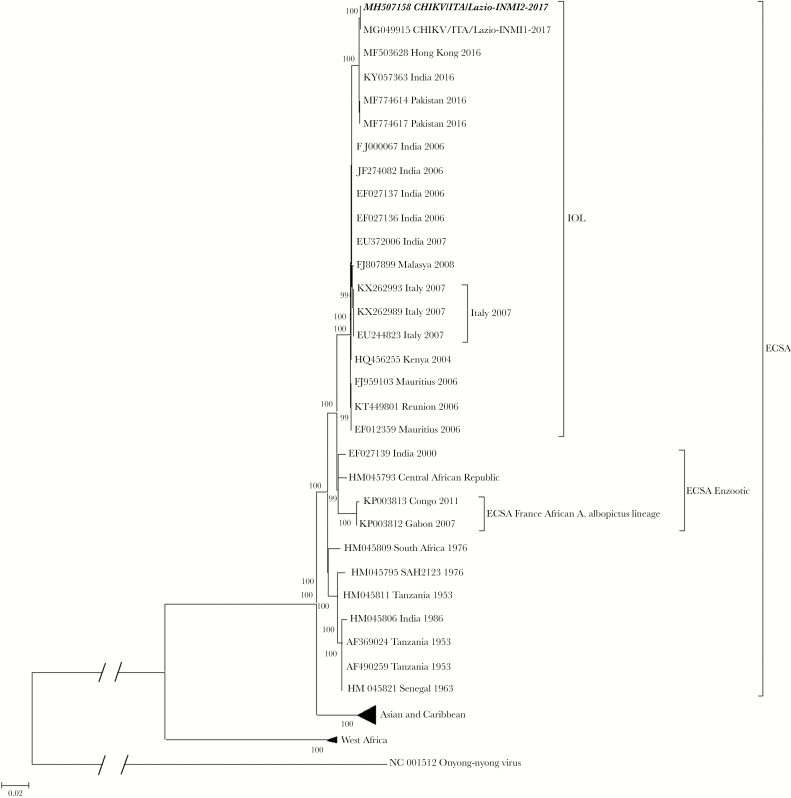
The virus was isolated (CHIKV/ITA/Lazio-INMI2-2017), and its near-complete-length genome sequence was obtained (GenBank accession number MH507158). The sequence clustered with recent isolates belonging to the Indian Ocean sublineage of ECSA (Figure 1) in the maximum likelihood phylogenetic tree and showed only 1 nucleotide difference (synonymous substitution at position nt 3126 of nonstructural polyprotein), as compared with the outbreak prototype strain (CHIKV/ITA/Lazio-INMI1-2017).
Figure 1.
Phylogenetic tree of the whole-genome sequence of the Chikungunya virus (CHIKV) strain involved in a fatal case from Anzio, Italy, 2017. Maximum-likelihood phylogenetic tree is based on General Timer Reversible substitution model of the complete genome sequence of the isolate CHIKV/ITA/Lazio-INMI2-2017 (bold, italics), obtained from a fatal case that occurred during the 2017 CHIKV outbreak, Lazio region, Italy. The tree also includes CHIKV/ITA/Lazio-INMI1-2017 (MG049915), that is, the prototype sequence representing the same outbreak [6], in the context of whole-genome sequences representing the 3 major CHIKV lineages: ECSA (including the Indian Ocean lineage [IOL]), Asia-Caribbean, and West Africa, indicated with their accession numbers, geographic origins, and years of sampling. Sixteen sequences of the Asia-Caribbean (n = 12) and West Africa (n = 4) lineages are collapsed to increase the clarity of the figure. Bootstraps were generated using 1000 replicates; only those >80 are shown. The bar represents the genetic distance (substitutions per nucleotide position).
Cytokine Response
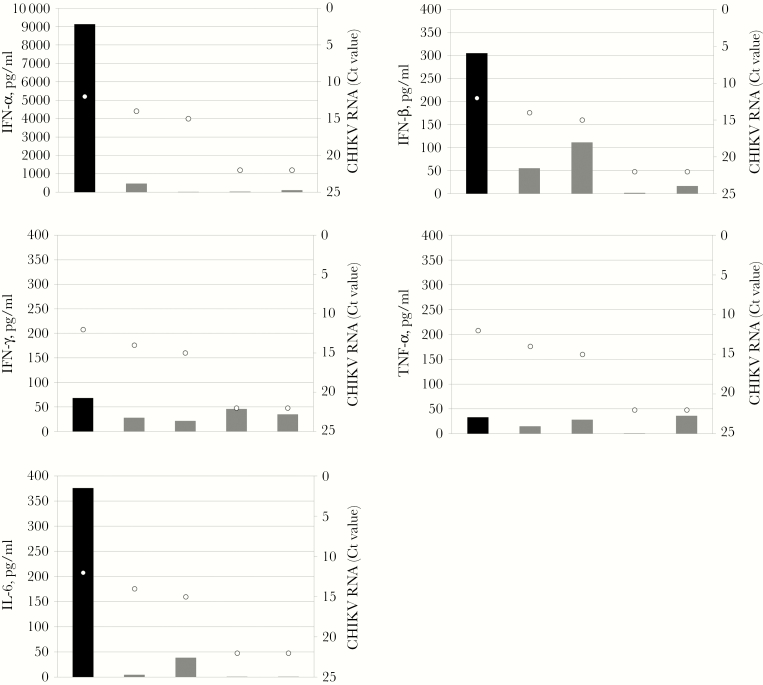
Serum levels of IFN-α, IFN-β, IFN-γ, TNF-α, and IL-6 observed in the deceased patient are shown in Figure 2, in comparison with the pattern observed in a small group of nonfatal cases sampled in the early phases of the infection. CHIKV RNA levels detected in the corresponding patients are also shown. As can be seen, the IFN-α, IFN-β, and IL-6 levels were extremely high in this fatal case as compared with nonfatal cases, whereas IFN-γ and TNF-α levels were in the same range. Although the small number of cases did not allow adequate statistical power, the levels of IFN-α, IFN-β, and IL-6 seemed not to be directly correlated to the levels of CHIKV RNA, as expressed by Ct values (Figure 2).
Figure 2.
Cytokine levels in plasma from a fatal case of Chikungunya virus (CHIKV) infection, Anzio, Italy, 2017. Levels of circulating interferon (IFN)-α, IFN-β, IFN-γ, tumor necrosis factor (TNF)–α, and interleukin (IL)-6 were determined in plasma samples from the lethal CHIKV infection (black bars) and in 4 nonlethal (gray bars) cases diagnosed during the 2017 outbreak in Lazio, sampled during the very early infection stages (within 3 days of symptom onset). White dots indicate the corresponding CHIKV RNA levels expressed as Ct values, determined by reverse transcriptase polymerase chain reaction.
DISCUSSION
CF is generally considered a self-limiting and nonfatal disease, although the accompanying symptom of severe incapacitating arthralgia is often persistent and can result in long-lasting disability [5]. This belief was debunked by the CHIKV outbreak on the Reunion Island in 2005–2006 when there were 266 000 cases and 254 deaths [7, 8]. CHIKV has been strongly suspected to have neurologic, hepatic, and myocardial tropism, leading to dramatic complications and severe clinical presentations with a high mortality rate (48%) [7, 9–11]. In fact, severe clinical forms are associated with the presence of several underlying medical conditions in about 90% of the cases, especially in elderly patients, and preexisting respiratory or cardiovascular diseases and hypertension have been recognized as risk factors of developing severe illness [7, 9].
Here, we report the unique lethal case among all CF cases during the 2017 outbreak in Italy, involving an elderly patient with underlying cardiac disease. Surprisingly, the analysis of inflammatory cytokines revealed a remarkable and strong increase of circulating type-I IFN, as well as of the IL-6 pro-inflammatory cytokine. Generally, elevated pro-inflammatory cytokine/chemokine response contributes to the pathogenesis of several viral infections, and severe inflammation can result in systemic cytokine storm, which may lead to more serious pathological changes and multi-organ dysfunctions [12, 13].
Inflammatory cytokines and chemokines are thought to be involved in the pathogenesis of CHIKV and are associated with severe clinical presentations as well as with development of abrupt and persistent arthralgia. Ng et al. previously reported a significant increase of IL-1β and IL-6 levels in severe compared with nonsevere CF cases, which can raise oxygen demand and have negative inotropic effects on the human myocardium, suggesting their possible role as biomarkers to distinguish between mild and more severe forms of CF [14].
IFNs are antiviral cytokines produced during viral infections and have a well-known role in restricting virus replication and promoting virus clearance. In particular, IFN-α is produced during CHIKV infection and has been shown to strongly inhibit CHIKV in vitro [14]. Therefore, the overproduction of type-I IFN is rather surprising in the lethal CHIKV infection here described. Elevated type-I IFN levels are known to be associated with clinical severity and poor therapy response in chronic diseases of viral (ie, hepatitis C virus and HIV) and autoimmune (ie, systemic lupus erythematosus) etiology [15, 16], whereas overproduction in acute viral infections is rarely reported in humans. One report showed that in patients with severe fever with thrombocytopenia syndrome virus (SFTSV) infection, the concentration of IFN-α and other cytokines, including IL-6, is correlated with the severity of the disease, suggesting that high levels of IFN-α could not control SFTSV in humans but may be involved in the cytokine storm and in the virus pathogenesis [17]. Furthermore, a few studies have shown that abnormally elevated type-I IFN levels can cause morbidity and mortality, as opposed to protection, during influenza virus infection in animal models [18].
Previous evidence suggests that patients with coronary artery disease show increased Th1 cytokine levels in the general population [19, 20]. Our findings are consistent with a possible role of type-I IFN in the CHIKV-driven cytokine storm that, in turn, may have contributed to enhanced tissue injury and unfavorable prognosis in a patient with predisposing factors. However, due to the extremely small number of CHIKV-infected patients and to the lack of comparison with uninfected patients with cardiac disease, from the present study it is not possible to establish whether CHIKV or the underlying cardiac dysfunction was the actual cause of the altered cytokine profile; it is also likely that infection, underlying morbidity, and elderly condition all contributed.
To investigate the relationship between clinical severity and host factors like older age, comorbidities, genetic susceptibility, and inflammatory and immune responses in CHIKV infection and to adequately address the role of CHIKV-driven cytokine dysregulation in worsening cardiovascular disease, a wider observation, possibly designed as a case–control study, would be necessary.
Enhanced attention to unusually severe clinical presentation of CHIKV infection is endorsed by the World Health Organization (http://www.who.int/wer/2015/wer9033.pdf?ua=1), and public health managers should remain cautious about the possible impact, from both human and economic standpoints, when facing a possible spread of the disease, as an increase in patient mortality could remain a plausible scenario.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. This work was supported by funds from the Italian Ministry of Health (Ricerca Corrente, RF-2016-02364155 and GR-2016-02362110).
Potential conflicts of interest. The authors declare that no conflicting financial interests or other competing relationships exist. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Burt FJ, Chen W, Miner JJ, et al. Chikungunya virus: an update on the biology and pathogenesis of this emerging pathogen. Lancet Infect Dis 2017; 17:e107–17. [DOI] [PubMed] [Google Scholar]
- 2. Rezza G, Nicoletti L, Angelini R, et al. ; CHIKV study group Infection with Chikungunya virus in Italy: an outbreak in a temperate region. Lancet 2007; 370:1840–6. [DOI] [PubMed] [Google Scholar]
- 3. Calba C, Guerbois-Galla M, Franke F, et al. Preliminary report of an autochthonous Chikungunya outbreak in France, July to September 2017. Euro Surveill 2017; 22(39):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Economopoulou A, Dominguez M, Helynck B, et al. Atypical Chikungunya virus infections: clinical manifestations, mortality and risk factors for severe disease during the 2005–2006 outbreak on Réunion. Epidemiol Infect 2009; 137(4):534–41. [DOI] [PubMed] [Google Scholar]
- 5. Torres JR, Leopoldo Códova G, Castro JS, et al. Chikungunya fever: atypical and lethal cases in the Western hemisphere: a Venezuelan experience. IDCases 2015; 2:6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carletti F, Marsella P, Colavita F, et al. Full-length genome sequence of a Chikungunya virus isolate from the 2017 autochthonous outbreak, Lazio region, Italy. Genome Announc 2017; 5(49):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lemant J, Boisson V, Winer A, et al. Serious acute Chikungunya virus infection requiring intensive care during the Reunion Island outbreak in 2005–2006. Crit Care Med 2008; 36(9):2536–41. [DOI] [PubMed] [Google Scholar]
- 8. Renault P, Balleydier E, D’Ortenzio E, et al. Epidemiology of Chikungunya infection on Reunion Island, Mayotte, and neighboring countries. Med Mal Infect 2012; 42:93–101. [DOI] [PubMed] [Google Scholar]
- 9. Crosby L, Perreau C, Madeux B, et al. Severe manifestations of Chikungunya virus in critically ill patients during the 2013–2014 Caribbean outbreak. Int J Infect Dis 2016; 48:78–80. [DOI] [PubMed] [Google Scholar]
- 10. Maiti CR, Mukherjee AK, Bose B, Saha GL. Myopericarditis following Chikungunya virus infection. J Indian Med Assoc 1978; 70:256–8. [PubMed] [Google Scholar]
- 11. Rampal, Sharda M, Meena H. Neurological complications in Chikungunya fever. J Assoc Physicians India 2007; 55:765–9. [PubMed] [Google Scholar]
- 12. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol 2017; 39:529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Srikiatkhachorn A, Mathew A, Rothman AL. Immune-mediated cytokine storm and its role in severe dengue. Semin Immunopathol 2017; 39:563–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ng LF, Chow A, Sun YJ, et al. IL-1beta, IL-6, and RANTES as biomarkers of Chikungunya severity. PLoS One 2009; 4(1):e4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McNab F, Mayer-Barber K, Sher A, et al. Type I interferons in infectious disease. Nat Rev Immunol 2015; 15:87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bengtsson AA, Rönnblom L. Role of interferons in SLE. Best Pract Res Clin Rheumatol 2017; 31:415–28. [DOI] [PubMed] [Google Scholar]
- 17. Liu MM, Lei XY, Yu H, et al. Correlation of cytokine level with the severity of severe fever with thrombocytopenia syndrome. Virol J 2017; 14:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Davidson S, Crotta S, McCabe TM, Wack A. Pathogenic potential of interferon αβ in acute influenza infection. Nat Commun 2014; 5:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fernandes JL, Mamoni RL, Orford JL, et al. Increased Th1 activity in patients with coronary artery disease. Cytokine 2004; 26:131–7. [DOI] [PubMed] [Google Scholar]
- 20. Madhumitha H, Mohan V, Deepa M, et al. Increased Th1 and suppressed Th2 serum cytokine levels in subjects with diabetic coronary artery disease. Cardiovasc Diabetol 2014; 13:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.