Abstract
Molluscum contagiosum (MC) manifests as small, umbilicated papules caused by the molluscum contagiosum virus (MCV). The extent of clinical misdiagnosis of MC is unknown. Combined clinical, histopathological, and virological evaluation of 203 consecutive patients with clinical diagnosis of MC treated at a university hospital during a 5-year period showed the correct clinical diagnosis in 188 of 203 (92.6%) patients. All 15 clinically misdiagnosed MC lesions were histopathologically and virologically confirmed as either common or anogenital warts caused by different human papillomaviruses. The MCV1/MCV2 subtypes ratio was 1.54:1, and the distribution of MCV subtypes differed across patients’ age and anatomical location of lesions.
Keywords: diagnosis, human papillomaviruses, molluscum contagiosum, molluscum contagiosum virus
Molluscum contagiosum (MC) is a common skin disease caused by the molluscum contagiosum virus (MCV), the only poxvirus with humans as sole natural hosts after the successful eradication of smallpox. Molluscum contagiosum is primarily transmitted by direct contact with infected skin, (non)sexual contacts, and autoinoculation, or contaminated fomites, and manifested clinically by the eruption of umbilicated papules and nodules (reviewed in 1 and 2). In children, lesions are usually widespread and distributed on the face, torso, and extremities, whereas in immunocompetent adults, in whom the infection is predominantly transmitted sexually, lesions often occur in the anogenital region [3]. Despite a rather typical clinical picture, MC may be misdiagnosed as other skin lesions, most commonly as common or anogenital warts, etiologically associated with human papillomaviruses (HPVs), followed by herpes simplex, varicella, cutaneous cryptococcosis, and several other benign and malignant cutaneous neoplasms (reviewed in 1). It is interesting to note that the exact extent of clinical misdiagnosis of MC is unknown because no comprehensive study has yet assessed the accuracy of the clinical diagnosis of MC using reference methods: histopathological assessment of the lesions and/or virological detection of MCV.
Molluscum contagiosum virus is the only representative of the genus Molluscipoxvirus, with approximately 60% of its genes homologous to genes of variola and vaccinia viruses (reviewed in 1 and 4). Molluscum contagiosum exists as 4 genetic entities: subtypes MCV1, MCV2, MCV3, and MCV4 with several viral variants, characterized predominantly using genomic restriction-fragment length polymorphism analyses (reviewed in 2, 5, 6). Although the distribution of MCV subtypes differs across different geographic regions, infections with MCV1 are most common overall, accounting for 76% to 97% of all MCV isolates, followed by MCV2, whereas MCV3 and MCV4 are extremely rare (reviewed in 2). To date, only complete genomes of MCV1 and MCV2 have been deposited to the GenBank database (5 isolates: U60315 and KY040274−KY040277). Therefore, current MCV subtyping assays can only reliably distinguish infections with the MCV1 and MCV2 subtypes [7, 8]. Although some studies suggested that the distribution of MCV subtypes depends on patients’ age, gender, and immune status [9−11], further studies using reliable MCV subtyping assays and performed on comprehensive isolate collections are warranted.
Between October 2009 and April 2015, we enrolled 203 consecutive patients with clinically suspected MC lesions [3]. Clinical and demographic characteristics of a subset of 188 patients with histopathologically confirmed MC and their treatment outcomes were described previously [3]. In this study, we present the results of MCV molecular testing and subtyping of lesions obtained from all 203 enrolled patients and histopathological, clinical, and virological evaluation of lesions with histopathological findings not consistent with MC. To the best of our knowledge, this is the first combined clinical, histopathological, and virological study to objectively determine the accuracy of clinical diagnosis of MC in children and sexually active adults using 2 MC reference diagnostic methods: histopathological assessment of lesions and virological detection of the etiological agent, MCV. In addition, the distribution of MCV subtypes among MC patients was assessed for the first time in central/eastern Europe.
METHODS
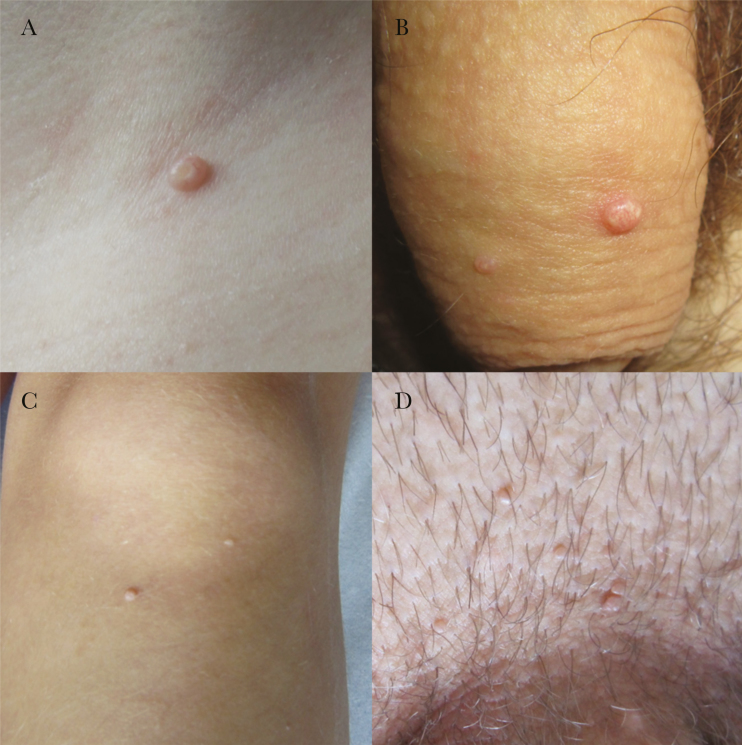
The present prospective study included 203 consecutive patients with a clinical diagnosis of MC-like skin lesion(s)—umbilicated papules located on the torso, extremities, and anogenital region—treated from October 2009 to April 2015 at the Department of Dermatology and Venereal Diseases, University Medical Centre Maribor, Slovenia (Figure 1). The most clinically representative cutaneous lesion that had been removed by curettage was cut in half using a sterile scalpel. One half of the tissue sample was immediately fixed in formalin for histopathological assessment, and the other half was frozen in liquid nitrogen for further virological testing. In addition, before removal, all skin lesions were photographed using a high-resolution digital camera for subsequent clinical macroscopic re-evaluation if needed (Figure 1) [3].
Figure 1.
Benign cutaneous lesions of the torso, extremities, and anogenital region clinically diagnosed as molluscum contagiosum (MC). Examples of lesions histopathologically and virologically confirmed as MC, located on the neck of the 6-year-old child and penis of the 43-year-old adult patient, are shown in A and B, respectively. Examples of lesions misdiagnosed as MC: human papillomavirus (HPV)2-positive cutaneous wart obtained from an 8-year-old child’s leg (C) and HPV6a-positive anogenital wart obtained from a 23-year-old adult patient’s pubic region (D).
Formalin-fixed tissue samples were subsequently embedded in paraffin, stained with hematoxylin and eosin, and examined under a microscope by a pathologist specialized in dermatopathology. While the pathologist was blinded to virological results, all personnel involved in virological testing were blinded to histopathological results.
For detecting and subtyping MCV, total deoxyribonucleic acid (DNA) was extracted from fresh-frozen tissue samples using the commercially available QIAamp DNA Mini Kit (QIAGEN, Hilden, Germany), following the manufacturer’s instructions. The integrity of the extracted DNA was verified by amplification of a 268-base pair fragment of the human beta globin gene, as described previously [12]. Presence of MCV DNA was determined using MCV fluorescence resonance energy transfer (FRET)-based real-time polymerase chain reaction (MCV RT-PCR), allowing very sensitive (at least 3.3 viral copies/reaction) and specific detection of MCV, as well as a reliable differentiation of MCV1 and MCV2 in a single reaction [8]. All MCV DNA-negative isolates obtained from the torso and extremities were further tested for the presence of HPV2, HPV27, and HPV57, which are etiologically associated with more than 65% of common (skin) warts, using multiplex type-specific quantitative HPV2/27/57 RT-PCR, allowing sensitive and specific concurrent detection and differentiation of the 3 HPV types in a single PCR reaction [13]. All MCV-negative DNA isolates obtained from the anogenital region were further tested for the presence of HPV6 and HPV11, which are etiologically associated with more than 90% of anogenital warts, using a FRET-based RT-PCR (HPV6/11 RT-PCR) [14].
Due to unbalanced data, statistical analyses for categorical variables were performed using the Fisher’s exact test with a Monte Carlo approximation, included in the statistical software IBM SPSS, version 22 (SPSS Inc., Chicago, IL). The significance level was set at α = 0.05.
The detailed protocol of the study including all research procedures was approved by the Slovenian Ministry of Health’s Medical Ethics Committee (consent no. 101/12/09) and Institutional Review Boards of the Institute of Microbiology and Immunology and the Institute of Pathology. All samples were obtained in compliance with the Helsinki Declaration. Written informed consent covering all standard diagnostic and therapeutic procedures as well as all research procedures used in this study was obtained from all patients 18 and older and from parents of children 17 or younger.
RESULTS
The 203 patients with a clinical diagnosis of MC included 130 males (64.1%) and 73 females (35.9%). Patients’ ages ranged from 0 to 62, and the majority (72.4%) were adults.
Histopathological assessment showed typical lobulated crateriform lesions with acanthotic epidermis containing intracytoplasmic viral inclusions known as Henderson–Paterson bodies in 188 of 203 (92.6%) of specimens assessed. Virological testing of the second half of each lesion, using MCV RT-PCR, showed the presence of MCV in exactly the same 188 specimens as histological assessment and absence of MCV in the remainder (15 of 203; 7.4%).
All 15 MCV-negative tissue samples were histopathologically defined as common warts (n = 3; all diagnosed in children) or anogenital warts (n = 12; all diagnosed in adults) and were HPV-positive using either HPV2/27/57 RT-PCR (common warts) or HPV6/11 RT-PCR (anogenital warts). Specifically, among 3 children with MCV-negative lesions, HPV57 was detected in lesions of 2 patients, obtained from the inguinal and perianal region, respectively, and HPV2 was detected in a lesion from the third child’s leg (Figure 1C). In contrast, infection with HPV6 was determined in all 12 MCV-negative tissue samples from adult patients; HPV6a in 11 of 12 lesions and HPV6b in a single lesion obtained from the penile region. The pubic region was the most common anatomical location of anogenital warts of adult patients misdiagnosed as MC (n = 5), followed by vaginal (n = 4) and penile regions (n = 3). Figure 1D shows the clinical presentation of the HPV6a-positive anogenital wart in the pubic region misdiagnosed as MC.
The distribution of MCV subtypes by patients’ age, gender, and anatomical location of MC lesions is presented in Table 1. Molluscum contagiosum virus subtype 1 was predominant in our patients, with an MCV1/MCV2 ratio of 1.54:1. The MCV1/MCV2 ratio favored MCV1 in females in comparison to males (1.91:1 vs 1.37:1), but the difference was not statistically significant. However, our results showed that, in comparison to adults, children were more commonly infected with MCV1 (P = .005). Although male patients did not exhibit any statistically significant differences in MCV subtype distribution by age, in contrast to female patients under age 17, adult women were more frequently infected with MCV2 than MCV1 (MCV1/MCV2 ratio of 15:1 vs 0.67:1; P < 0.001). Altogether, 137 and 51 MC lesions from the anogenital and nongenital regions, respectively, were included in this study. In comparison to the anogenital region, the MCV1/MCV2 ratio was higher in favor of MCV1 infections in nongenital tissue samples (2.92:1 vs 1.25:1; P = .019). Although no statistically significant differences were observed when comparing the distribution of MCV subtypes in anogenital lesions of both genders, in comparison to males, MCV1 infections were more frequent in nongenital lesions of females (P = .002). No statistically significant differences were observed when comparing MCV subtype distribution by age of patients with anogenital and nongenital lesions.
Table 1.
Distribution of MCV Subtypes by Patient Age and Gender and Anatomical Location of MC Lesions
| Patients’ Characteristics | No. of MCV1 Positives (%) | No. of MCV2 Positives (%) | Ratio | P Value |
|---|---|---|---|---|
| All patients (N = 188) | 114 (60.6) | 74 (39.4) | 1.54:1 | |
| Gender | ||||
| Male (n = 121) | 70 (57.9) | 51 (42.1) | 1.37:1 | .350 |
| Female (n = 67) | 44 (65.7) | 23 (34.3) | 1.91:1 | |
| Age | ||||
| <17 years (n = 53) | 41 (77.4) | 12 (22.6) | 3.42:1 | .005 |
| ≥17 years (n = 135) | 73 (54.1) | 62 (45.9) | 1.18:1 | |
| Age: males | ||||
| <17 years (n = 21) | 11 (52.4) | 10 (47.6) | 1.10:1 | .631 |
| ≥17 years (n = 100) | 59 (59.0) | 41 (41.0) | 1.44:1 | |
| Age: females | ||||
| <17 years (n = 32) | 30 (93.8) | 2 (6.2) | 15:1 | <.001 |
| ≥17 years (n = 35) | 14 (40.0) | 21 (60.0) | 0.67:1 | |
| Anatomical location | ||||
| Anogenital (n = 137) | 76 (55.5) | 61 (44.5) | 1.25:1 | .019 |
| Nongenitala (n = 51) | 38 (74.5) | 13 (25.5) | 2.92:1 | |
| Anogenital lesions | ||||
| Males (n = 101) | 60 (59.4) | 41 (40.6) | 1.46:1 | .171 |
| Females (n = 36) | 16 (44.4) | 20 (55.6) | 0.80:1 | |
| Nongenital lesionsa | ||||
| Males (n = 20) | 10 (50.0) | 10 (50.0) | 1:1 | .002 |
| Females (n = 31) | 28 (90.3) | 3 (9.7) | 9.33:1 | |
| Anogenital lesions | ||||
| <17 years (n = 4) | 3 (75.0) | 1 (25.0) | 3:1 | .629 |
| ≥17 years (n = 133) | 73 (54.9) | 60 (45.1) | 1.22:1 | |
| Nongenital lesionsa | ||||
| <17 years (n = 49) | 38 (77.6) | 11 (22.4) | 3.45:1 | .419 |
| ≥17 years (n = 2) | 1 (50.0) | 1 (50.0) | 1:1 |
Abbreviations: MC, molluscum contagiosum; MCV, molluscum contagiosum virus.
aMC lesions of the torso and extremities. The statistical analyses for categorical variables were performed using the Fisher’s exact test with a Monte Carlo approximation; the significance level was set at α = 0.05. Statistically significant ρ values are presented in bold.
DISCUSSION
Due to a similar clinical presentation, common and anogenital warts, herpes simplex, varicella, cutaneous cryptococcosis, and several other cutaneous diseases may be clinically misdiagnosed as MC (reviewed in 1), but data concerning the extent of clinical misdiagnoses of MC are limited. In a recent study, we enrolled 203 consecutive patients treated at a university hospital during a 5-year period with clinically suspected MC lesions. Clinical and demographic characteristics of a subset of 188 patients with histologically confirmed MC as well as their treatment outcomes were described previously [3]. After additional MCV testing of all enrolled patients and detailed histopathological, clinical, and virological evaluation of 15 patients with histopathological findings not consistent with MC, we showed that in our setting clinical diagnosis of MC was correct in 92.6% patients. All 15 clinically misdiagnosed MC lesions were histopathologically and virologically confirmed as common or anogenital warts caused by different HPV types. It is interesting to note that our study showed identical diagnostic values of histopathological assessment and virological testing in confirmation or exclusion of clinically diagnosed MC.
Our results indicate that, in adult immunocompetent patients with typical MC lesion(s) located outside the anogenital area, more objective diagnostic methods such as histopathology assessment or virological testing of lesions cannot be justified and recommended. However, histopathological assessment and/or virological testing of suspicious lesions could be of additional value when evaluating adult immunocompromised patients with MC-like lesion(s) in the anogenital region, especially human immunodeficiency virus-positive individuals with a history of other sexually transmitted diseases and those not vaccinated with quadrivalent or nonavalent HPV vaccines. Likewise, it is highly recommended to confirm a clinical diagnosis of MC-like lesions located in children’s anogenital area using more objective methods (preferably virological). Namely, MC-like lesions could represent anogenital warts etiologically linked with sexually transmitted HPV6/11, which in many countries requires investigation into suspected sexual abuse and consequent legal action (reviewed in 13). Virological testing can also reliably distinguish between anogenital and common warts in children’s anogenital area, indicating no further investigation or legal action.
Molluscum contagiosum virus subtype 1 was the predominant MCV subtype determined in 60.6% of our patients. The ratio between MCV1 and MCV2 was 1.54:1, which is the lowest ratio observed so far, varying in previous studies at 1.75:1 in Australia, 2.45:1 in Iran, 3.32:1 in the United Kingdom, 13:1 in Japan, 28:1 in Germany, 61:0 in Turkey, and 146:1 in Spain, suggesting striking geographical differences in the distribution of MCV subtypes (reviewed in 2, 15). In comparison to adults, children were more commonly infected with subtype MCV1 (P = .005). In addition, adult women were more frequently infected with MCV2 than MCV1 (P < 0.001). Our results are in line with previous studies, suggesting that the distribution of MCV subtypes depends on the age of patients, with the MCV1/MCV2 ratio decreasing with increasing age of patients [9−11]. In comparison to the anogenital region, the MCV1/MCV2 ratio was statistically significantly higher in favor of MCV1 infections in nongenital lesions (P = .019), which were more common in children. It is interesting to note that, in contrast to males, where equal frequency of both MCV types was observed in nongenital lesions, MCV1 was 10 times more frequent than MCV2 in female nongenital lesions (P = .002), suggesting different MCV transmission patterns between genders in nongenital areas.
CONCLUSIONS
In conclusion, this combined clinical, histopathological, and virological study of 203 patients with a clinical diagnosis of MC showed that clinical diagnosis of MC is sufficient and reliable in the great majority of cases, not requiring further testing with more objective methods. All clinically misdiagnosed MC lesions were either common warts (all diagnosed in children) or anogenital warts (all diagnosed in adults). Although MCV1 and MCV2 were rather equally distributed in our study population, distribution of MCV subtypes differed significantly by patient age and anatomical location of lesions.
Acknowledgments
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: DNA Tumour Virus 2017 Conference (DNATV 2017), July 17–22, 2017, Birmingham, UK.
References
- 1. Hanson D, Diven DG. Molluscum contagiosum. Dermatol Online J 2003; 9:2. [PubMed] [Google Scholar]
- 2. Chen X, Anstey AV, Bugert JJ. Molluscum contagiosum virus infection. Lancet Infect Dis 2013; 13:877–88. [DOI] [PubMed] [Google Scholar]
- 3. Trčko K, Poljak M, Križmarić M, Miljković J. Clinical and demographic characteristics of patients with molluscum contagiosum treated at the University Dermatology Clinic Maribor in a 5-year period. Acta Dermatovenerol Croat 2016; 24:130–6. [PubMed] [Google Scholar]
- 4. Mendez-Rios JD, Yang Z, Erlandson KJ, et al. Molluscum contagiosum virus transcriptome in abortively infected cultured cells and a human skin lesion. J Virol 2016; 90:4469–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Porter CD, Archard LC. Characterisation by restriction mapping of three subtypes of molluscum contagiosum virus. J Med Virol 1992; 38:1–6. [DOI] [PubMed] [Google Scholar]
- 6. Senkevich TG, Bugert JJ, Sisler JR, et al. Genome sequence of a human tumorigenic poxvirus: prediction of specific host response-evasion genes. Science 1996; 273:813–6. [DOI] [PubMed] [Google Scholar]
- 7. Trama JP, Adelson ME, Mordechai E. Identification and genotyping of molluscum contagiosum virus from genital swab samples by real-time PCR and pyrosequencing. J Clin Virol 2007; 40:325–9. [DOI] [PubMed] [Google Scholar]
- 8. Hošnjak L, Kocjan BJ, Kušar B, et al. Rapid detection and typing of molluscum contagiosum virus by FRET-based real-time PCR. J Virol Methods 2013; 187:431–4. [DOI] [PubMed] [Google Scholar]
- 9. Scholz J, Rösen-Wolff A, Bugert J, et al. Epidemiology of molluscum contagiosum using genetic analysis of the viral DNA. J Med Virol 1989; 27:87–90. [DOI] [PubMed] [Google Scholar]
- 10. Yamashita H, Uemura T, Kawashima M. Molecular epidemiologic analysis of Japanese patients with molluscum contagiosum. Int J Dermatol 1996; 35:99–105. [DOI] [PubMed] [Google Scholar]
- 11. Thompson CH. Identification and typing of molluscum contagiosum virus in clinical specimens by polymerase chain reaction. J Med Virol 1997; 53:205–11. [DOI] [PubMed] [Google Scholar]
- 12. Jancar N, Kocjan BJ, Poljak M, et al. Distribution of human papillomavirus genotypes in women with cervical cancer in Slovenia. Eur J Obstet Gynecol Reprod Biol 2009; 145:184–8. [DOI] [PubMed] [Google Scholar]
- 13. Hošnjak L, Fujs Komloš K, Kocjan BJ, Seme K, Poljak M. Development of a novel multiplex type-specific quantitative real-time PCR for detection and differentiation of infections with human papillomavirus types HPV2, HPV27, and HPV57. Acta Dermatovenerol Alp Pannonica Adriat 2016; 25:65–71. [DOI] [PubMed] [Google Scholar]
- 14. Kocjan BJ, Seme K, Poljak M. Detection and differentiation of human papillomavirus genotypes HPV-6 and HPV-11 by FRET-based real-time PCR. J Virol Methods 2008; 153:245–9. [DOI] [PubMed] [Google Scholar]
- 15. Taghinezhad SS, Mohseni AH, Keyvani H, Ghobadi N. Molecular screening and single nucleotide polymorphism typing of Molluscum contagiosum virus (MCV) from genital specimens, between 2012 and 2015. Iran Biomed J 2018; 22:129–33. [DOI] [PMC free article] [PubMed] [Google Scholar]