ABSTRACT
Neprilysin, a widely expressed peptidase upregulated in type 2 diabetes, is capable of cleaving and inactivating the insulinotropic glucagon-like peptide-1 (GLP-1). Like dipeptidyl peptidase-4 (DPP-4), inhibition of neprilysin activity under diabetic conditions is associated with increased active GLP-1 levels and improved glycemic control. While neprilysin expression has been demonstrated in islets, its local contribution to GLP-1-mediated insulin secretion remains unknown. We investigated in vitro whether islet neprilysin inhibition enhances insulin secretion in response to glucose and/or exogenous GLP-1, and whether these effects are mediated by GLP-1 receptor (GLP-1R). Further, we compared the effect of neprilysin versus DPP-4 inhibition on insulin secretion. Isolated islets from wild-type (Glp1r+/+) and GLP-1 receptor knockout (Glp1r−/−) mice were incubated with or without the neprilysin inhibitor thiorphan and/or the DPP-4 inhibitor sitagliptin for 2.5 hours. During the last hour, insulin secretion was assessed in response to 2.8 mmol/l or 20 mmol/l glucose alone or plus exogenous active GLP-1. In Glp1r+/+ islets, neprilysin inhibition enhanced 2.8 mmol/l and 20 mmol/l glucose- and GLP-1-mediated insulin secretion to the same extent as DPP-4 inhibition. These effects were blunted in Glp1r−/− islets. In conclusion, inhibition of islet neprilysin in vitro increases glucose-mediated insulin secretion in a GLP-1R-dependent manner and enhances the insulinotropic effect of exogenous active GLP-1. Thus, neprilysin inhibitors may have therapeutic potential in type 2 diabetes by preserving islet-derived and circulating active GLP-1 levels.
KEYWORDS: DPP-4, GLP-1, insulin secretion, islet, neprilysin
Introduction
Increased activity of the peptidase neprilysin in type 2 diabetes and obesity is associated with impaired glucose homeostasis.1,2 Neprilysin is produced in islets, where its upregulation mediates insulin secretory dysfunction.3 Like dipeptidyl-peptidase-4 (DPP-4), neprilysin cleaves and inactivates the insulinotropic glucagon-like peptide-1 (GLP-1).4 We and others have reported, both in animal models and humans, a glucose-lowering effect of neprilysin inhibition or deletion which is associated with increased circulating active GLP-1 levels.2,5,6 Moreover, dual systemic inhibition of neprilysin and DPP-4 is more effective at maintaining levels of exogenously infused active GLP-1 and enhancing insulin secretion than inhibition of either enzyme alone5, raising the possibility that this combination has therapeutic potential for type 2 diabetes.
Local GLP-1 production by islet alpha cells has been suggested to regulate beta-cell function through paracrine action.7 Both neprilysin and DPP-4 are produced by islets3,8, and inhibition of islet DPP-4 increases insulin secretion by preserving locally produced active GLP.-18 However, the contribution of islet neprilysin in modulating beta-cell function remains unknown. Here, we examine in vitro whether inhibition of islet neprilysin enhances insulin secretion in response to glucose and exogenous GLP-1, and if such effects are mediated by GLP-1 receptor (GLP-1R). Since combined neprilysin and DPP-4 inhibition might further stabilize and preserve active GLP-15, we also evaluated whether this combination has additive effects on insulin secretion.
Results
Islet neprilysin and DPP-4 activities
Incubation of islets with thiorphan alone inhibited neprilysin activity by 96% (Figure 1A) without altering DPP-4 activity (Figure 1B). Similarly, sitagliptin alone decreased DPP-4 activity by 90% (Figure 1B), with no effect on neprilysin activity (Figure 1A). With combined thiorphan and sitagliptin, neprilysin and DPP-4 activities were reduced to levels comparable to those seen with each inhibitor alone (Figure 1A, B).
Figure 1.
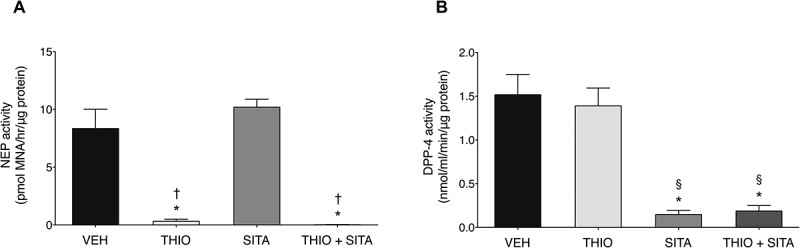
Islet neprilysin and DPP-4 activities. (A) Neprilysin and (B) DPP-4 activities in Glp1r+/+ islets treated acutely (2.5 hours) with vehicle (VEH), 20 μmol/l thiorphan (THIO), 100 μmol/l sitagliptin (SITA) alone or combined (THIO + SITA). Data are mean± S.E.M of 4 independent experiments. *p < 0.05 vs vehicle, †p < 0.05 vs sitagliptin, §p < 0.05 vs thiorphan (ANOVA).
Neprilysin inhibition improves basal and glucose-stimulated insulin secretion (GSIS) in a Glp-1r-dependent manner
In Glp1r+/+ islets, under basal (2.8 mmol/l) glucose conditions thiorphan increased insulin secretion compared to vehicle (Figure 2A). Sitagliptin alone and thiorphan plus sitagliptin increased basal insulin secretion to the same extent as thiorphan alone. Thiorphan alone also significantly enhanced GSIS compared to vehicle, with sitagliptin alone doing so to the same degree (Figure 2A). Since the inhibitors significantly increased basal insulin secretion, we determined whether the increment in insulin secretion from 2.8 to 20 mmol/l glucose for these experiments was in fact greater with thiorphan and/or sitagliptin. Table 1 shows that this is the case, confirming that GSIS is increased with thiorphan or sitagliptin compared to vehicle. Further, the combination of thiorphan plus sitagliptin enhanced GSIS above that observed with thiorphan, but not sitagliptin alone (Figure 2A, Table 1).
Figure 2.
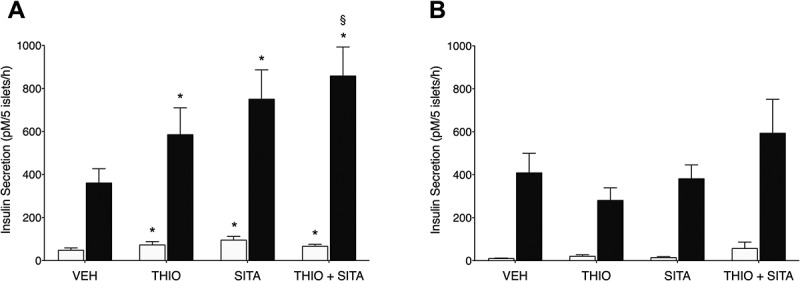
Islet neprilysin and/or DPP-4 inhibition enhance glucose-stimulated insulin secretion in a GLP-1 receptor-dependent manner. Insulin secretion in response to 2.8 mmol/l (white bars) and 20 mmol/l (black bars) glucose in (A) Glp1r+/+ and (B) Glp1r−/− islets following incubation with vehicle (VEH), 20 μmol/l thiorphan (THIO) and 100 μmol/l sitagliptin (SITA) alone or combined (THIO + SITA) in conditions. (A) Data are mean± S.E.M of 8–9 independent experiments. *p < 0.05 vs vehicle within same condition (2.8 mmol/l or 20 mmol/l glucose), §p < 0.05 vs thiorphan at 20 mmol/l glucose (ANOVA). (B) Data are mean± S.E.M of 4–5 independent experiments.
Table 1.
Incremental change in insulin secretion from response at 2.8 mmol/l glucose to 20 mmol/l glucose alone (GSIS) or plus 10 nmol/l GLP-1 in Glp1r+/+ and Glp1r−/− islets following incubation with vehicle (VEH), 20 μmol/l thiorphan (THIO) and 100 μmol/l sitagliptin (SITA) alone or combined (THIO+ SITA). Data are mean± S.E.M of 3–8 independent experiments. *p < 0.05 vs vehicle within same genotype, §p < 0.05 vs thiorphan within same genotype (ANOVA).
|
Glp1r+/+ (n = 8) |
Glp1r−/− (n = 3–4) |
|||||||
|---|---|---|---|---|---|---|---|---|
| VEH | THIO | SITA | THIO + SITA | VEH | THIO | SITA | THIO + SITA | |
| GSIS(pmol/l/5 islets/h) | 305 ± 67 | 496 ± 134* | 643 ± 147* | 799 ± 151*§ | 325 ± 77 | 218 ± 49 | 359 ± 90 | 413 ± 188 |
| GLP-1(pmol/l/5 islets/h) | 1486 ± 340 | 2024 ± 427* | 1932 ± 288 | 2050 ± 237* | 246 ± 35 | 463 ± 103 | 433 ± 70 | 590 ± 65 |
To evaluate whether enhanced GSIS with neprilysin and/or DPP-4 inhibition was mediated by increased GLP-1R signaling, we assessed insulin responses in Glp1r−/− islets. In contrast to Glp1r+/+ islets, neither thiorphan nor sitagliptin significantly enhanced basal or GSIS in Glp1r−/− islets (Figure 2B, Table 1). Of note, total insulin content in Glp1r+/+ and Glp1r−/− islets was comparable (123.9 ± 19.4 vs 116.7 ± 23.2 nmol/L/5 islets; n = 5, p = 0.87).
Neprilysin inhibition increases exogenous Glp-1-mediated insulin secretion
To determine whether islet neprilysin inhibition alone or with DPP-4 inhibition enhances exogenous GLP-1-mediated insulin secretion, islets were incubated with 20 mmol/l glucose plus GLP-1 in the presence or absence of thiorphan and/or sitagliptin (Figure 3). In Glp1r+/+ islets, thiorphan alone and sitagliptin alone both increased insulin secretion in response to exogenous GLP-1 (Figure 3). When insulin secretion was expressed as incremental change from 2.8 mmol/l glucose, only thiorphan alone had a significant effect to increase GLP-1-mediated insulin secretion (Table 1). No further increase in insulin release was observed when thiorphan was combined with sitagliptin (Figure 3). As expected, in Glp1r−/− islets, insulin secretion in response to exogenous GLP-1 was significantly blunted in all groups (Figure 3). However, insulin secretion remained higher in Glp1r−/− islets incubated with thiorphan and/or sitagliptin versus vehicle.
Figure 3.
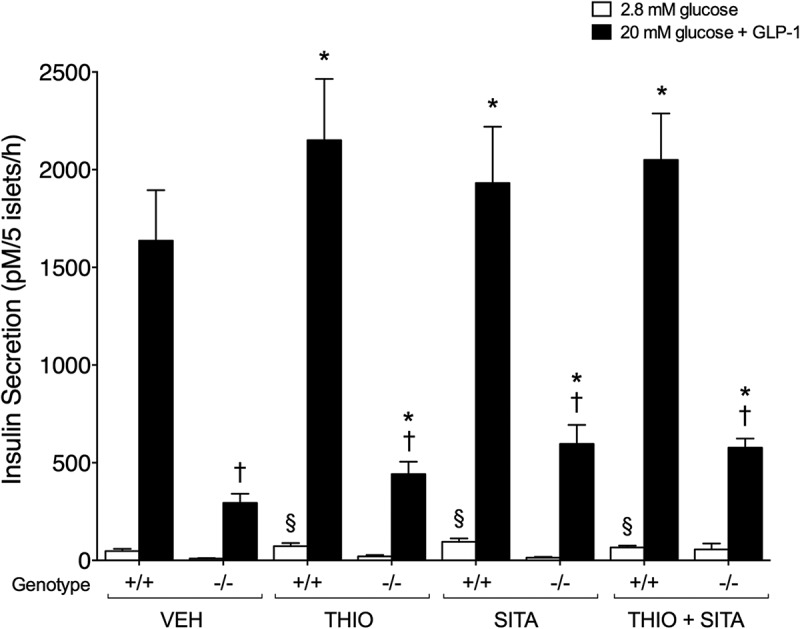
Islet neprilysin and/or DPP-4 inhibition enhance exogenous GLP-1-mediated insulin secretion. Insulin secretion was assessed in Glp1r+/+ (+/+; n = 9–11) and Glp1r−/− (-/-; n = 4–7) islets following incubation with 2.8 mmol/l glucose (white bars) or 20 mmol/l glucose and 10 nmol/l GLP-1 (back bars) in the presence of vehicle (VEH), 20 μmol/l thiorphan (THIO), 100 μmol/l sitagliptin (SITA) alone or combined (THIO + SITA). Data are presented as mean± S.E.M. §p < 0.05 vs vehicle at 2.8 mmol/l glucose within same genotype (ANOVA), *p < 0.05 vs vehicle at 20 mM glucose within same genotype (ANOVA), †p < 0.05 vs Glp1r+/+ islets within same treatment (t-test).
Discussion
We show for the first time that islet neprilysin inhibition enhances glucose- and exogenous GLP-1-mediated insulin secretion, both in a GLP-1R-dependent manner. These data support a role for islet neprilysin in modulating the incretin effect.
There is growing evidence that increased neprilysin activity is associated with impaired glucose homeostasis1,2 and that global deletion or systemic inhibition of neprilysin enhances glycemic control9, in part via improved beta-cell function.2 One potential mechanism by which the latter may occur is preservation of active GLP-1 and its insulinotropic effects.2,5,6 In keeping with this, we showed that neprilysin inhibition enhanced GSIS in Glp1r+/+ islets but not Glp1r−/− islets, strongly suggesting that increased insulin release with neprilysin inhibition occurs due to preservation of locally-produced active GLP-1. Indeed, alpha-cell-derived GLP-1 has been shown to be crucial for glucose homeostasis.7 Moreover, islet neprilysin inhibition in Glp1r+/+ islets also increased insulin secretion in response to exogenous active GLP-1 and this effect was blunted in Glp1r−/− islets, suggesting that islet neprilysin is also important in degrading non-islet-derived active GLP-1.
As shown previously8, islet DPP-4 inhibition enhanced insulin secretion in response to glucose and exogenous GLP-1, in a GLP-1R-dependent manner. We found the magnitude of the sitagliptin effect did not differ from thiorphan alone, suggesting islet neprilysin is at least as important as islet DPP-4 in inactivating both islet-derived and circulating active GLP-1. Importantly, we excluded any compensatory changes in neprilysin and DPP-4 activities following incubation with sitagliptin and thiorphan, respectively. Since neprilysin inhibitors are now approved for use in heart failure9, they may be a better option to treat type 2 diabetic patients with heart failure, given that there are suggestions of increased heart failure risk with DPP-4 inhibitors.10
The combination of thiorphan and sitagliptin enhanced GSIS (in the absence of exogenous GLP-1) in Glp1r+/+ islets to a greater extent than thiorphan alone. Such an effect may be due to enhanced stabilization and/or protection from proteolysis of islet-derived active GLP-1.5 Interestingly, thiorphan plus sitagliptin did not have a similar effect to further augment insulin secretion in response to exogenous GLP-1. These data may be explained by a maximal effect of thiorphan or sitagliptin alone to preserve GLP-1 activity, resulting in maximal insulin secretion that cannot be further increased by dual inhibition.
To date, only two studies have investigated whether combined systemic neprilysin and DPP-4 inhibition could synergize the glucose-lowering effect of either treatment alone, and their results have been conflicting.11 While co-administration of neprilysin and DPP-4 inhibitors was more effective at enhancing insulin secretion and glucose tolerance than either alone when administered acutely to pigs5, it failed to further improve glycemic control above that of DPP-4 inhibition alone when administered for 12 weeks to Goto-Kakizaki rats.11 It is possible such findings are explained by incomplete inhibition of neprilysin activity (only 40%) or use of an atypical non-obese and spontaneous type 2 diabetes animal model.11 Therefore, additional studies are needed to determine whether dual neprilysin and DPP-4 inhibition confers added benefit for glycemic control in vivo.
While our data suggest that inhibition of neprilysin contributes to increased insulin release via increased GLP-1R signaling, other mechanisms may also underlie these effects. Neprilysin, like DPP-4, has broad substrate specificity and can degrade insulinotropic peptides other than GLP-1. Involvement of intra-islet gastric inhibitory polypeptide or peptide YY, which are neprilysin and DPP-4 substrates, cannot not be excluded. Also, an intriguing finding of ours is that thiorphan and sitagliptin, alone and combined, increased insulin secretion in Glp1r−/− islets with addition of exogenous GLP-1. Since this effect was not observed in the absence of exogenous GLP-1, these data suggest that GLP-1 could modulate insulin secretion independent of GLP-1R signaling. To our knowledge, no previous studies have investigated whether GLP-1 actions on insulin release in vitro can be mediated through a GLP-1R independent pathway. Accumulating evidence points to the existence of an alternative GLP-1R in tissues such as myocardium, adipose tissue and bone. In these locales, either the classical GLP-1R is not expressed or GLP-1R antagonists fail to block the actions of GLP-1.12 Whether a GLP-1R independent pathway mediates the insulinotropic action of GLP-1 in the islet remains to be determined.
In conclusion, this study delineates a role for islet neprilysin in modulating insulin secretion in response to both islet-derived and exogenous GLP-1. Thus, neprilysin inhibition promotes insulin secretion via a direct effect on the islet and may have therapeutic potential for type 2 diabetes.
Methods
Animals
Glp1r+/- mice were provided by Daniel Drucker (University of Toronto, Canada) to generate Glp1r+/+ and Glp1r−/− mice on a C57BL/6J background at VA Puget Sound Health Care System (VAPSHCS). Islets from 10–12-week old female and male mice (3 mice of each genotype per n) were isolated by collagenase digestion, then recovered in RPMI 1640 media overnight. Studies were approved by the VAPSHCS Institutional Animal Care and Use Committee.
Insulin secretion and content
Isolated islets recovered overnight were incubated for 90 minutes in Krebs buffer containing 2.8 mmol/l glucose plus vehicle, thiorphan (20 µmol/l, NEP inhibitor; Sigma-Aldrich, St. Louis, MO, USA) and/or sitagliptin (100 µmol/l DPP-4 inhibitor; Selleckchem, Houston, TX, USA). Triplicate batches of 5 islets were then transferred to Krebs buffer containing 2.8 mmol/l or 20 mmol/l glucose alone or plus 10 nmol/l active GLP-1 (Sigma-Aldrich, St. Louis, MO, USA) and incubated for 60 minutes with the vehicle or inhibitors. Supernatant was collected and islets extracted for insulin content using acid-ethanol. Insulin in supernatant and islet extracts was assayed using the Insulin (Mouse) Ultrasensitive ELISA (Alpco, Salem, NH, USA).
Neprilysin and DPP-4 activity assays
Enzyme activities were determined in Glp1r+/+ islets following insulin secretion. In the neprilysin assay, glutaryl-ala-ala-phe-4-methoxy-2-naphthylamine is broken down by neprilysin in islets to Phe-4-methoxy-2-naphthylamine, and then by aminopeptidase M to the fluorescent methoxy-2-naphthylamine. Each sample was assayed in both the absence and presence of thiorphan to differentiate neprilysin activity from nonspecific endopeptidase activity. Fluorescence was compared against a methoxy-2-naphthylamine standard curve. In the DPP-4 assay, non-fluorescent H-Gly-Pro-7-amino-4-methylcoumarin is cleaved by DPP-4 in islets to generate fluorescent 7-amino-4-methylcoumarin. DPP-4 activity was compared against a 7-amino-4-methylcoumarin standard curve.
Statistical analyses
Data are presented as mean± SEM for the number of experiments indicated. Statistical significance was determined using ANOVA with Fisher’s LSD post hoc analysis for comparison between the 4 different treatment groups or Student’s t-test for comparison between the 2 different genotypes. A p < 0.05 was considered statistically significant.
Funding Statement
This work was supported by the National Institutes of Health grants DK-098506 to S.Z. and P30 DK-017047 (University of Washington Diabetes Research Center, Cell Function Analysis Core), and the United States Department of Veterans Affairs. N.E. is supported by the Baillet-Latour Fund and the Belgian American Educational Foundation, the Belgian Association of Diabetes, the French Society of Diabetes, the Horlait-Dapsens Foundation, and the Leon Fredericq Foundation.
Abbreviations
- DPP-4
dipeptidyl peptidase-4
- GLP-1
glucagon-like peptide-1
- GLP-1R
GLP-1 receptor
- GSIS
glucose-stimulated insulin secretion
Disclosure of potential conflicts of interest
S.Z. receives research support from Novartis Pharmaceuticals Corporation for preclinical studies that are not related to the work described in this manuscript.
Acknowledgments
We thank D. Hackney, S. Mongovin, C. Schmidt and J. Willard from Seattle Institute for Biomedical and Clinical Research, USA, for excellent technical support. Also, thanks to Daniel Drucker (University of Toronto, Canada) for providing Glp1r+/- mice to generate the wild-type and knockout mice used in this study.
References
- [1].Standeven KF, Hess K, Carter AM, Rice GI, Cordell PA, Balmforth AJ, Lu B, Scott DJ, Turner AJ, Hooper NM, et al. Neprilysin, obesity and the metabolic syndrome. Int J Obes. 2011;35:1031–1040. doi: 10.1038/ijo.2010.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Willard JR, Barrow BM, Zraika S.. Improved glycaemia in high-fat-fed neprilysin-deficient mice is associated with reduced DPP-4 activity and increased active GLP-1 levels. Diabetologia. 2017;60:.701–708. doi: 10.1007/s00125-016-4172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zraika S, Koh DS, Barrow BM, Lu B, Kahn SE, Andrikopoulos S.. Neprilysin deficiency protects against fat-induced insulin secretory dysfunction by maintaining calcium influx. Diabetes. 2013;62:.1593–1601. doi: 10.2337/db11-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hupe-Sodmann K, McGregor GP, Bridenbaugh R, Göke R, Göke B, Thole B,T, Hole H, Zimmermann B, Voigt K. Characterisation of the processing by human neutral endopeptidase 24.11 of GLP-1(7–36) amide and comparison of the substrate specificity of the enzyme for other glucagon-like peptides. Regul Pept. 1995;58:149–156. doi: 10.1016/0167-0115(95)00063-H. [DOI] [PubMed] [Google Scholar]
- [5].Plamboeck A, Holst JJ, Carr RD, Deacon CF. Neutral endopeptidase 24.11 and dipeptidyl peptidase IV are both mediators of the degradation of glucagon-like peptide 1 in the anaesthetised pig. Diabetologia. 2005;48:1882–1890. doi: 10.1007/s00125-005-1847-7. [DOI] [PubMed] [Google Scholar]
- [6].Vodovar N, Nougué H, Launay JM, Socal AC, Logeart D. Sacubitril/valsartan in PARADIGM-HF. Lancet Diabetes Endocrinol. 2017;5:.495–496. doi: 10.1016/S2213-8587(17)30177-8. [DOI] [PubMed] [Google Scholar]
- [7].Chambers AP, Sorrell JE, Haller A, Roelofs K, Hutch CR, Kim KS, Gutierrez-Aguilar R, Li B, Drucker DJ, D’Alessio DA, et al. The role of pancreatic preproglucagon in glucose homeostasis in mice. Cell Metab. 2017;25:927–934.e3. doi: 10.1016/j.cmet.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Omar BA, Liehua L, Yamada Y, Seino Y, Marchetti P, Ahrén B. Dipeptidyl peptidase 4 (DPP-4) is expressed in mouse and human islets and its activity is decreased in human islets from individuals with type 2 diabetes. Diabetologia. 2014;57:.1876–1883. doi: 10.1007/s00125-014-3299-4. [DOI] [PubMed] [Google Scholar]
- [9].Seferovic JP, Claggett B, Seidelmann SB, Seely EW, Packer M, Zile MR, Rouleau JL, Swedberg K, Lefkowitz M, Shi VC, et al. Effect of sacubitril/valsartan versus enalapril on glycaemic control in patients with heart failure and diabetes: a post-hoc analysis from the PARADIGM-HF trial. Lancet Diabetes Endocrinol. 2017;5:333–340. doi: 10.1016/S2213-8587(17)30087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Udell JA, Cavender MA, Bhatt DL, Chatterjee S, Karkouh ME, Scirica BM. Glucose-lowering drugs or strategies and cardiovascular outcomes in patients with or at risk for type 2 diabetes: a meta-analysis of randomised controlled trials. Lancet Diabetes Endocrinol. 2015;3:.356–366. doi: 10.1016/S2213-8587(15)00044-3. [DOI] [PubMed] [Google Scholar]
- [11].Simonsen L, Pilgaard S, Carr RD, Kanstrup AB, Holst JJ, Deacon CF. Inhibition of neutral endopeptidase 24.11 does not potentiate the improvement in glycemic control obtained with dipeptidyl peptidase-4 inhibition in diabetic Goto–kakizaki rats. Horm Metab Res. 2009;41:.851–853. doi: 10.1055/s-0029-1225609. [DOI] [PubMed] [Google Scholar]
- [12].Cantini G, Mannucci E, Luconi M. Perspectives in GLP-1 research: new targets, new receptors. Trends Endocrinol Metab. 2016;27:.427–438. doi: 10.1016/j.tem.2016.03.017. [DOI] [PubMed] [Google Scholar]


