ABSTRACT
Cell-based immunotherapy using natural killer (NK) cells, cytokine-induced killer (CIK) cells and dendritic cells (DCs) is emerging as a potential novel approach in the auxiliary treatment of a tumor. However, non-standard operation procedure, small-scale cell number, or human error may limit the clinical development of cell-based immunotherapy. To simplify clinical scale NK cells, CIK cells and DCs expansions, we investigated the use of the WAVE bioreactor, a closed system bioreactor that utilizes active perfusion to generate high cell numbers in minimal volumes. We developed an optimized rapid expansion protocol for the WAVE bioreactor that produces clinically relevant number of cells for our adoptive cell transfer clinical protocols. The high proliferative rate, surface phenotypes, and cytotoxicity of these immune cells, as well as the safety of cultivation were analyzed to illuminate the effect of WAVE bioreactor. The results demonstrated that the benefit of utilizing modern WAVE bioreactors in cancer immunotherapy was simple, safe, and flexible production.
KEYWORDS: Tumor immunotherapy, NK cells, CIK cells, DCs, WAVE bioreactor
Introduction
Many new strategies for cancer treatment have been developed in recent years, including advances in surgical techniques,1 new chemotherapy regimens,2 radiotherapy techniques,3 and molecularly targeted therapies.4 While these advances have significantly improved the outcomes for many cancer patients in terms of curability, overall survival, and tolerance of the treatments, expectations for patients with advanced tumors remain limited.5,6 In the last decade, cancer immunotherapy has emerged as a clinically effective tool in several solid tumors, particularly in combination with more conventional therapies.7 There are a variety of approaches in tumor immunotherapy, including manipulation of the immune system through the use of immune agents, such as vaccines,8 cytokines,9 checkpoint inhibitors (including anti-programmed death 1 [PD-1]/PD-ligand 1 [PD-L1] antibodies and anti-cytotoxic T-lymphocyte-associated antigen (CTLA)-4 antibodies10,11), kinase inhibitors (such as apatinib and gefitinib),12,13 and immune cells.14-20 Among all the possible strategies, cellular immunotherapy has flourished and is widely used in tumor treatment, especially in Asia. CIK cells, NK cells, and DCs are the predominant cells used in cell-based immunotherapy, which are easy to obtain from umbilical cord blood or peripheral blood mononuclear cells (PBMCs). These cells possess higher in vitro proliferation capacity, stronger antitumor activity, and broader antitumor spectrum.21,22 The tumoricidal ability of these cells is implemented by inducing tumor cell apoptosis through direct cell-to-cell contact and secretion of cytokines such as tumor necrosis factor (TNF)-α and interferon (IFN)-γ.23 However, the limited quantity and high-paid labor versus the robust demand have restricted the clinical applications of cell-based therapy. In order to alleviate this predicament, numerous attempts were carried out by our group to rapidly expand cell numbers and genetically modified PBMCs under GMP(Good Manufacturing Practice) conditions for clinical trials.
WAVE bioreactor, a novel easy-to-use, flexible, and cost-efficient alternative to stainless steel bioreactors, is widely used for many purposes as it offers comprehensive options for process monitoring and control.24 Agitation is based on a wave-like movement of the cultivation plate. Important cultivation parameters, like pO2 and pH, can be measured and controlled by a fully automatic system. A cellbag on the platform is a chamber partially filled with media and inflated with air using the integral sterile inlet filter. The disposable contact material eliminates the need for cleaning and validation, thereby significantly reducing costs in cGMP operations. Recent report by Demanga CG25 and his colleagues have shown that the production of gametocytes in the WAVE bioreactor under GMP-compliant conditions will not only facilitate cellular, developmental, and molecular studies of gametocytes, but also the high-throughput screening for new anti-malarial drugs and, possibly, the development of whole-cell gametocyte or sporozoite-based vaccines. Tsai AC et al.26 have demonstrated that the WAVE bioreactor could be utilized in producing human mesenchymal stem cell (hMSC) aggregates with controlled size distribution for therapeutic application. Due to its the characteristics of rapid process development and clinical manufacturing, our group exploited the application of WAVE bioreactor in cell-based immunotherapy.
In this study, our group investigated the use of automatic WAVE Bioreactor (GE Xuri™W25, USA) (Fig. 1) in rapid expansion of CIK cells, NK cells or DCs from PBMCs under GMP conditions for clinical trials. The cell viability and immunological characteristics, such as the surface molecules, cytokines secreted, and tumor-cytotoxicity, were studied. All these observations may enhance the potential application of WAVE bioreactor in clinic tumor immunotherapy.
Figure 1.

Rapid expansion using the WAVE bioreactor. The cultivation of human peripheral blood mononuclear cell by WAVE bioreactor.
Results
Cell viability and counting
The number of viable CIK cells, NK cells, and DCs in total static group or WAVE group was determined and shown in Fig. 2A. The results have shown that the viability of CIK cells in the WAVE group was higher than the static group on day 21. Similarly, the viability of NK cells was significantly higher in the WAVE group on day 10 and day 15. However, no significant difference was found in the DCs viability.
Figure 2.
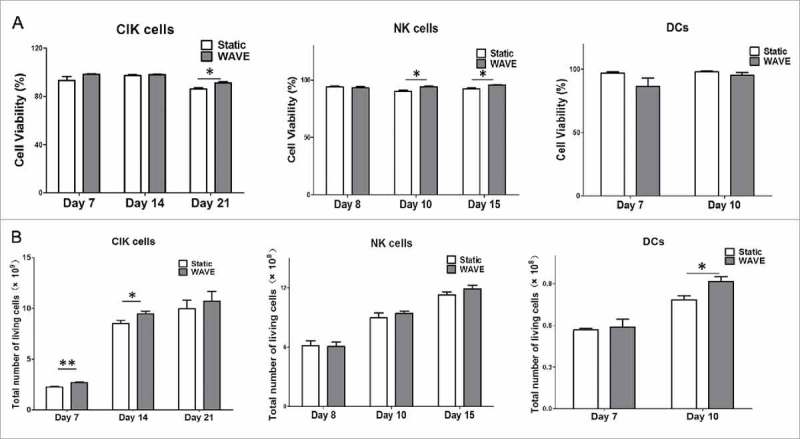
Cell viability and counting. Growth profile of CIK cells, DCs, and NK cells in the traditional group or the WAVE bioreactor were detected and demonstrated in the plots. The significant difference in cell viability between the two groups was monitored by Kruskal-Wallis test(*, P < 0.05). (A) The viable cells percentage in total cells. (B) The viable cell number in the two groups.
Subsequently, the viable cell number of CIK cells, NK cells, and DCs from the two groups were counted and displayed in the Fig. 2B. The WAVE bioreactor improved growth of CIK cells and DCs by day 14 and day 10.
Endotoxin detection
A standard curve was established for each assay in the range between 0.002 EU/ml and 2.0 EU/ml, according to the manufacturer's instructions for the LAL product. Differences between traditional cultivation and the WAVE bioreactor cultivation at endotoxin level were assessed by LAL test, and the results were all below 0.02 EU/ml (Fig. 3). These findings also suggest that, the WAVE bioreactor could be a safe and non-toxic immunotherapy cell culture method.
Figure 3.
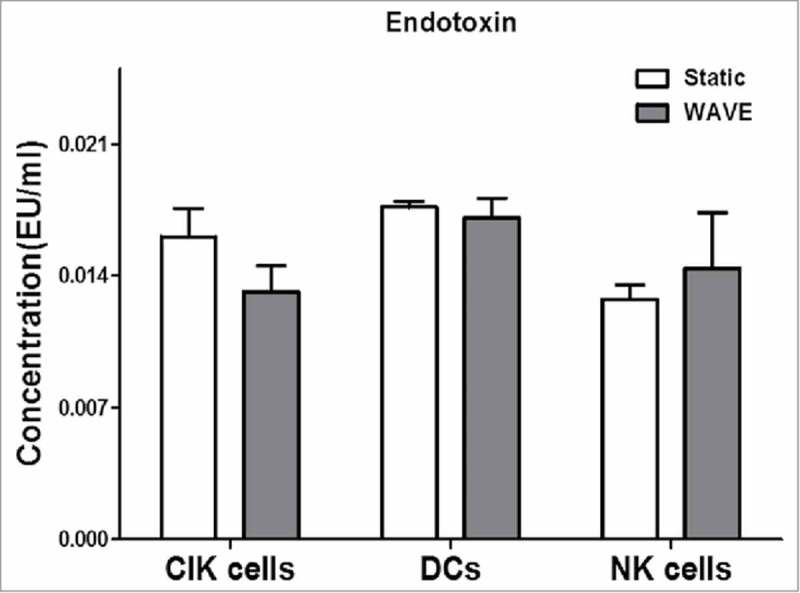
Endotoxins detection The level of endotoxin in the CIK cells, NK cells and DCs were shown in the chart, which imply the security of the WAVE bioreactor.
Composition of CIK cells
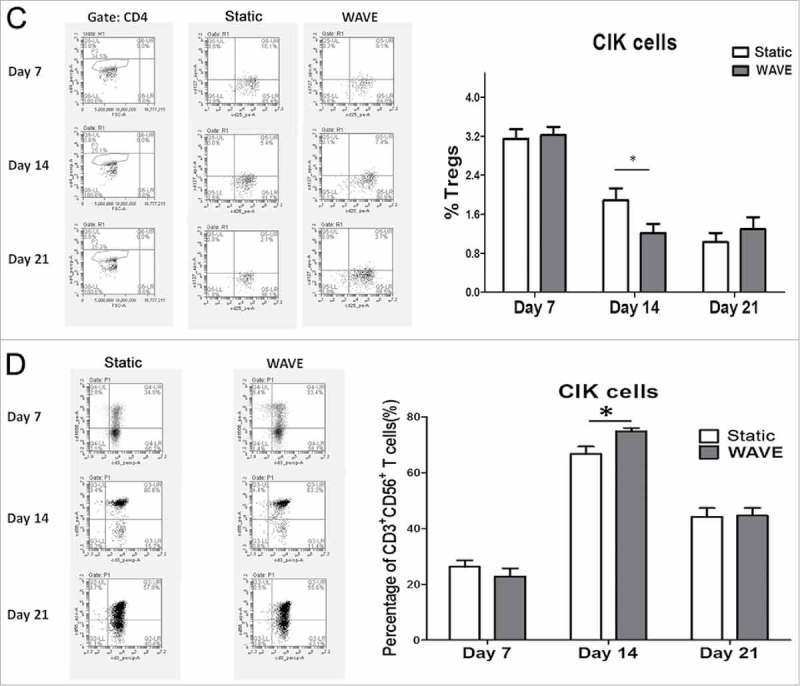
CIK cells were successfully generated from tumor patients from peripheral blood within 3 weeks of cultivation of the two groups that included timed addition of IFN-γ and interleukin (IL)-2. The expression of CD4+ T cell (CD3+CD4+), CD8+ T cell (CD3+CD8+), Tregs (CD4+CD25+CD127−), and CD3+CD56+ T cells (CD3+CD56+) between static vs. WAVE group on day 7, 14, and 21 are shown in Fig. 4. The proportion of CD8+ T cell and natural killer T (NKT) cells in the WAVE group were significantly higher than in the static cultured cells on day 14, meanwhile, the proportion of Tregs declined gradually in WAVE group, which indicated that the WAVE bioreactor might have enhanced the antitumor activity of CIK cells.
Figure 4.

The constitutions of CIK cells. The proportions of CIK cells in different groups were detected by flow cytometry on day 7, day 14 and day 21. The expression of CD4+T cells(A), CD8+T cells(B), Tregs(C) and CD3+CD56+T cells(D) were shown in the figure. The representative flow chart of different group were shown in the left column and the statistical results were shown in the right column. (*,p < 0.05).
Figure 4.
(Continued)
Purity of NK cells
NK cell markers (CD56) in both groups were analyzed on day 8, 10, and 15 by flow cytometry (Fig. 5). The results implied that the WAVE bioreactor could significantly improve the number of NK cells when compared with the static culture method.
Figure 5.

The quantification of NK cells. The phenotype of NK cells was detected by flow cytometry, in addition, the representative flow chart and the data were shown in this plot. (*,p < 0.05).
DC count and maturation
The typical marker for DCs (CD11c) and the matured markers [CD86, CD80, and human leukocyte antigen - antigen D related (HLA-DR)] were detected by flow cytometry (Fig. 6). Although the expression of DC markers improved after culture in different conditions, there was no significant difference between the two groups. These observations implied that the wave formation might have insignificant effect on the growth of adherent DCs.
Figure 6.

DCs numbers and maturation. The DC typical marker (CD11c) plus the matured marker (CD86, CD80 or HLA-DR) were demonstrate in the plot. Representative flow chart was shown in the left and the statistical data were shown in the right. No significantly alteration in the DC surface markers regardless of the cultured formation.
Level of cytokines
To explore the secretome dynamics of CIK cells, NK cells or DCs, the main classes of cytokines, i.e., IFN-γ, TNF-α, and IL-12 were analyzed in the culture groups. We compared the release in different groups, on the day of autologous blood transfusion, by real-time PCR, and the results are shown in the Fig. 7. These observations implied that the WAVE bioreactor might have not significantly modulated the secretome of cells when compared with the static group.
Figure 7.
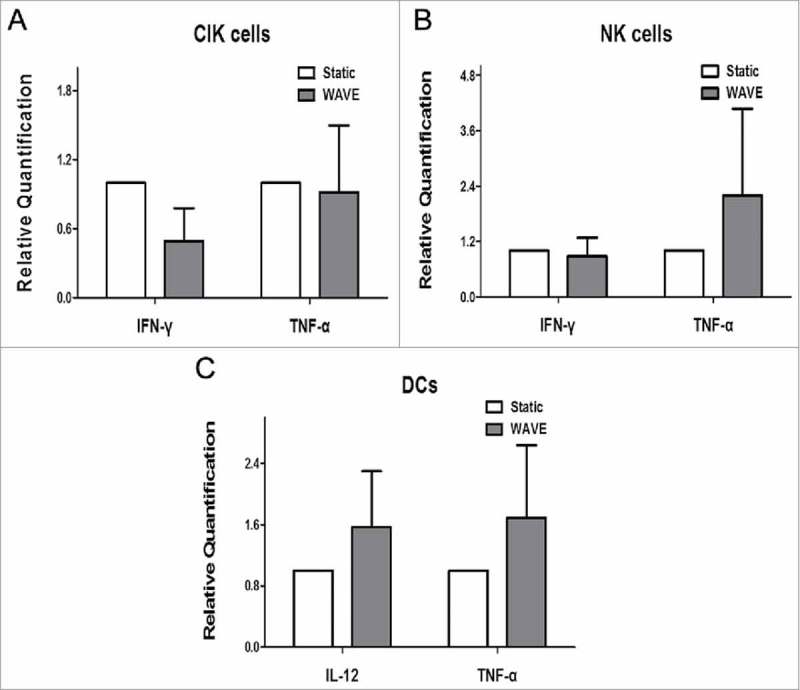
The level of Cytokines. The relative quantities of IFN-γ, TNF-α and IL-12 were detected by Real-time PCR. Unfortunately, there was no difference between the Static group and WAVE group when compared to IFN-γ, TNF-α and IL-12 (p > 0.05).
Cell degranulation marker CD107a
The effector cells (CIK cells or NK cells) and target cells (K562 cells) were co-incubated at 37°C in culture medium at an E:T cell ratio of 5:1, 10:1, 20:1 and 40:1 in the presence of monoclonal mouse anti-human adenomatous polyposis coli (APC)-CD107a to a total volume of 100 µl. The effector cells were then labeled in cytometry tubes with phycoerythrin (PE)-conjugated CD56 monoclonal antibody (mAb) (Fig. 8). Regardless of the E:T cell combinations, the frequency of CD107a-positive cells was higher in the NK cell subset than in the CIK cell subset. The WAVE bioreactor might improve the tumor lysis characteristic of CIK cells or NK cells at the ratio of 5:1 or 10:1.
Figure 8.
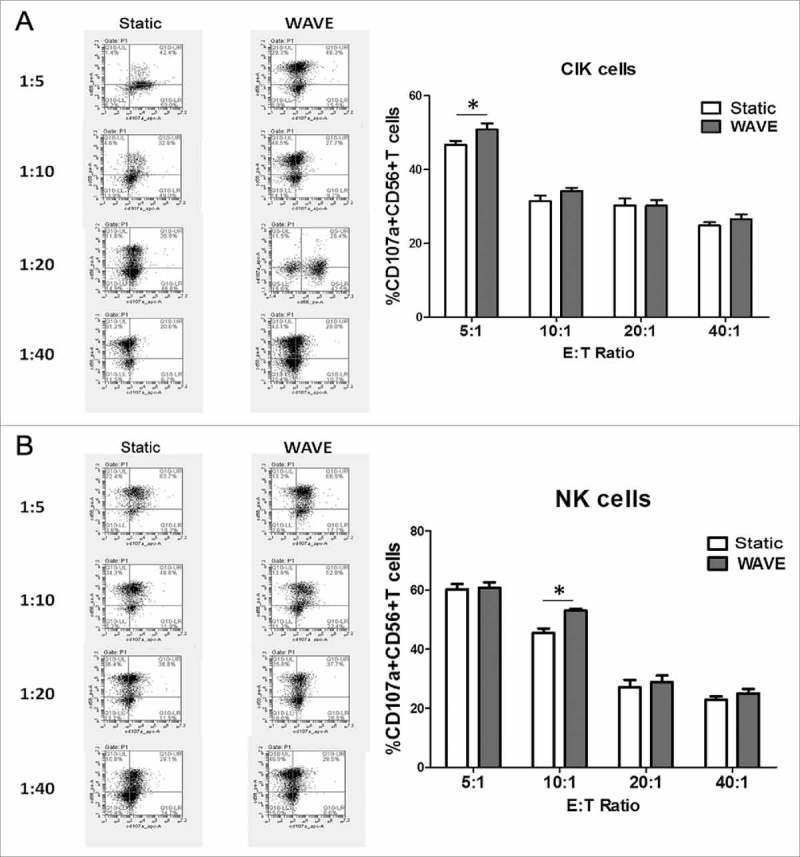
Cell degranulation marker CD107a. CD107a expression in CIK cells (A) or NK cells (B) of different groups cultured in the presence of K562 cells were shown in the plot. The representative flow charts were shown in the left column and the statistical data were displayed in the right column.
Cellular cytotoxicity assay
The results of the cytotoxicity assay demonstrated that CIK cells and NK cells exhibited antineoplastic activities in vitro (Fig. 9). Comparison of the cytotoxic effects between CIK cells and NK cells showed significant differences, in which the cytotoxic effects on K562 cells were significantly higher when cultured in the WAVE bioreactor. In addition, the results were consistent with the observation of CD107a degradation to some extent, which offers further confirmation that the WAVE bioreactor might enhance the anti-tumor activity of CIK cells or NK cells.
Figure 9.
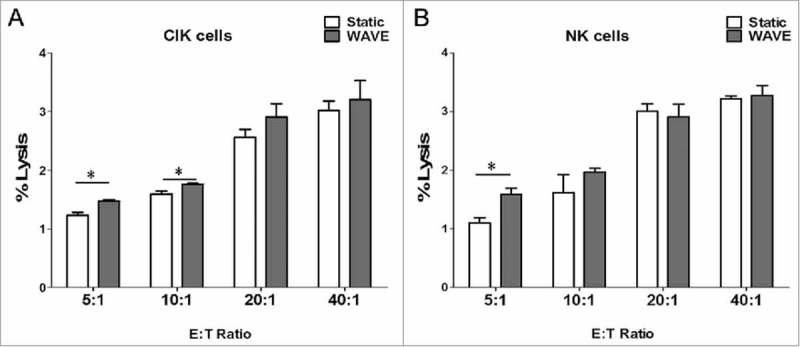
Cellular cytotoxicity assay. Cytotoxic activity of DC-CIK and DC-CTL cells against B16 melanoma cells. In the CCK-8 cytotoxic analysis, K562 cells were used as the target cells at various E:T ratios (5:1, 10:1, 20:1 and 40:1) to evaluate the specific cytotoxic activity. The results indicated that the cytotoxicity increased as the E:T ratio increased between 5:1 and 40:1. However, the cytotoxic effect of the WAVE group and Static group were significantly different (P<0.05) at the ratio of 5:1 of CIK cells and NK cells, plus ratio of 10:1 of CIK cells, with the cytotoxic effects.
Discussion
In recent years, immunotherapy has been rising rapidly and is considered the fourth most effective therapeutic method after surgery, radiotherapy, and chemotherapy.27 Use of adoptive immune cell transfer therapies have been flourishing in cancer treatment in Asia, which based on the infusion of ex vivo expanded and/or activated immune effectors that are able to identify and destroy cancer cells.28,29 The first focuses on T lymphocytes capable of recognizing tumor-associated antigens (TAA) through their specific T cell receptor (TCR). The second focuses on elements of the innate immune system that that do not rely on HLA-mediated recognition of tumor targets. These effectors are NK cells,30,31 CIK cells32 and DCs.33
Traditionally this has been achieved by utilizing static culture methods, wherein diluting products such as cytokines and lactate may limit the growth of immunotherapy cells. In addition, the semi-open system coupled with frequent culture manipulations introduces multiple opportunities for contamination, thus, highly skilled personnel are required.34,35 These technical challenges have proved to be an impediment to a wider dissemination of cell-based immunotherapy. In order to rapidly expand on a large scale by simultaneously, keeping the most comfortable culture circumstances, our laboratory took advantage of the WAVE bioreactor for cultivation. The WAVE bioreactor can be installed and used rapidly for process development and clinical manufacturing, thus minimizing the time to market for biological products. The rocking motion facilitates mixing and promotes oxygen transfer, which makes it highly attractive over traditional systems for animal cell culture such as shake flasks and stirred tanks. Batch cultivation of mammalian, insect, and plant cells have been reported in WAVE Bioreactors.36,37 In this article, we have compared the WAVE bioreactor with static cultivation and summarized the implementation scheme used in both cultivation methods.
In the proliferation assay, WAVE bioreactor could stimulate the growth of immune cells, such as CIK cells and NK cells. Simultaneously, the wave cultivation improved CIK cells and DC viability when compared with the static formation. The observations were correlated with the alterations in cell surface molecules, which were examined by flow cytometry. The WAVE bioreactor could increase the quantities of CD8+ T cells and CD3+CD56+ T cells, simultaneously suppressing the growth of Tregs in expanded CIK cell population in vitro. In addition, the number of NK cells cultured in the WAVE bioreactor increased significantly demonstrating that the antineoplastic effect of in vitro expanded CIK cells and NK cells may be enhanced to some extent. In addition, these effects were further confirmed by the CD107a degradation test and tumor cell lysis experiment. However, there was no significant alteration in the expression of IFN-γ and TNF-α, which were secreted and synthesized by CIK cells and NK cells possibly because the transfused CIK cells and NK cells cultured by either static method or the WAVE bioreactor were not pure but a more heterogeneous cell population. That means with respect to the expanded CIK cells or NK cells that will be transfused back to the tumor patients, there was no distinct difference between the static group and the WAVE group. Moreover, the cultivation of immune cells by the WAVE bioreactor was safe and sterile, which was testified by the endotoxin assay. Although the WAVE bioreactor has shown immense potential in CIK cell or NK cell cultivation (except for a viable cell number) there was no significant difference in DC cultivation between the two groups, most likely because the wave formation might not have been favorable for the cultivation of adherent cells such as DCs. Therefore, all these findings suggest that the WAVE bioreactor was a safe and efficient culture method for non-adherent immune cells.
This article has shown that the WAVE bioreactor is capable of generating efficient antineoplastic CIK cells and NK cells. In addition, the immune cell culture procedure by WAVE bioreactors and static cultivation were summarized simultaneously, which lay the foundation for clinical application at a large scale. In recent times, cell-based immunotherapy has developed rapidly. In the last five years, innovative cell-based immunotherapies such as, chimeric antigen receptor (CAR)-redirected T cell therapy, programmed death 1 (PD-1) or cytotoxic T cell lymphocyte-associated antigen 4 (CTLA-4) treated natural T cells and TCR engineered T cells have emerged from the bench and made splashy headlines in the clinical setting at a number of academic institutions.38-40 In order to rapidly expand these T cells under GMP conditions for clinical trials in the future, the WAVE bioreactor, according to our analysis, might be a preferred method of cell expansion for some cell subsets or phenotypes.
WAVE Bioreactors are attractive for wide range of specialized applications, including production of clinical-grade viral vectors,41 human mesenchymal stem cells,26 plant cell culture,42 and culture of malarial parasites.25,43 However, they have some disadvantages, such as, extra cost of disposable cellbags, size limit of 500 L, and lack of distant-control or monitoring system. The high expense would be a trade-off for less hands-on setup and cleaning labor. Another popular culture technique, three-dimensional (3D) cell culture, could mimic in vivo conditions, such as dynamic interaction between tumor or immune compartments, demonstrating more organotypic properties than static cultures.44 3D cell culture is widely used in laboratory investigations, including in the research on cell differentiation,5 stem-cell-based regenerative medicine,45 skin, tissue, or organoid regeneration,47-49 tumor microenvironment, and drug susceptibility.46,50 Although 3D culture has proven to be superior as compared to its traditional static counterparts; the high cost, challenging setup, low reproducibility, and time-consumption have limited the utilization on clinical scale-up cell immunotherapy.
In summary, the aim of this article was to demonstrate that the rocking culture platform is not only implementable for academic institutions and production of low-value products, but also scalable as demonstrated in the large-scale culture of mammalian cells under cGMP-compliance for therapeutic applications. Meanwhile, more clinical data and follow-up information could clarify the importance of WAVE bioreactor in clinical cell culturing.
Methods
Patients
Participants (aged 49–80 years) whose levels of white blood cells (WBCs) were within the range of 3 × 103 to 8 × 103/μL were enrolled in the study, and written informed consent was obtained. The Ethics Committee of Cancer Hospital of China Medical University approved the study protocol. There were 6 male patients and 5 female tumor patients who were treated with radiotherapy chemotherapy and combined therapy after operation. What's most important was that all the cells cultured by WAVE bioreactor were not transfused back to any tumor patients.
Reagents
LAL reagents (5 mL/vial), endotoxin-free water (5.5 mL/bottle, <0.001 EU/mL), endotoxin-free pipette tips, and endotoxin-free test tubes were purchased from Associates of Cape Cod. All glassware, needles, and syringes used in the RPT test were pyrogen-free. All the flow antibodies were purchased form BD bioscience and all the primers were prepared by Sangon Biotech (Shanghai) Co., Ltd.
Cultivation
Preparation of CIK cell in a static way
Peripheral blood mononuclear cells (PBMCs) from peripheral blood(50 ml) were collected and purity by density gradient centrifugation after venipuncture or apheresis. The cells were incubated for 2 h at 37°C, under 5% CO2, 95% relative humidity using X-VIVO 15 serum-free medium (Longza, Japan). The adherent cells were reserved for further DCs cultivation. Then the cell suspension was counted and divided equally into two cellbags, one cultured on WAVE bioreactor; and the other seeded in another culture flask containing 1000 U/ml IFN-γ (Peprotech, USA). After 24 h, anti-CD3 antibody (at a final concentration of 100 ng/ml, eBioscience, USA), 1 ng/ml IL-1α (Peprotech, USA) and 1000 U/ml recombinant human IL-2 (QuanGang, China) is added to the medium. After 5–7 days of culture, the final expansion levels are usually in the range of hundred-fold and the cells are transferred into a clinical-grade cell culture bag. On days 7–10, cells are counted and 2.5 L of fresh medium containing 1000 U/ml rhIL-2 is added. On day 11, each of the products are sampled for sterility testing. Further, on day 14, autologous CIK cells are re-suspended in 200 ml normal saline and are confirmed to be free of bacterial, mycoplasma, or fungal contamination. After autologous blood transfusion, the remained cells in the cellbag were collected and cultivation for another 7 days in static incubator and WAVE bioreactor.
Preparation of NK cells in a static way
Peripheral blood mononuclear cells (PBMC) were isolated over a Ficoll-Hypaque cushion (GE, Pittsburgh, PA) from EDTA-contained blood(20 ml). Subsequently, the purity cells were resuspended in X-VIVO 15 culture medium supplemented with heat-inactivated autologous plasma (1.0%), IL-2 and OK-432 (Picibanil: Chugai Pharmaceutical Co, Tokyo, Japan). Cells were then transferred to a cell-culture flask placed with CD3 mAb coated culture bottle cultivation. On day 8, the cultured cells were counted and divided equally into two cellbag with 500 ml X-VIVO 15 serum-free medium plus heat-inactivated autologous plasma (1.0%) and IL-2, one cultured in WAVE bioreactor, and the other under the traditional cultured protocol described below: On day 10, Cells were then expanded by adding medium and on day 15, the cultured cells were harvested, washed and re-suspended in 100 mL of a saline based-solution supplemented with 1% of human serum albumin (Albuminar; CSL Behring, PA, USA) then administered to patients immediately.
Preparation of DCs in a static way
After 2 h cultivation, the adherent cells described above would be added with Interleukin-4 (IL-4) and granulocyte-macrophage colony-stimulating factor every other day. At day 8, the DCs were counted and divided equally into two cellbag with 500 ml X-VIVO 15 serum-free medium plus TNF-α, one cultured in WAVE bioreactor, and the other under the traditional cultured protocol described below: After stimulated for 48 h the matured DCs will be transfused back to the patients.
Rapid expansion using the WAVE bioreactor
After transferred to the 3 liter WAVE cellbags, all the CIK cells, NK cells or DCs were attached to the tray of a WAVE bioreactor. The cellbags were inflated with 5%CO2 and the system started. A bag of medium and a 20-liter bag to collect waste were attached to the appropriate ports of the cellbag. Media was added to the cell bag to a final volume of 3 liters. The media was warmed to 37°C and aerated by rocking at 7 rpm at an angle of 6°for 2 hours. Samples were sterilely drawn daily from the needleless ports for determination of cell number. The glucose concentration, PH value and lactate levels in the cell bag was measured automaticly.
Cell viability and counting
All the cellbags from the static group and the WAVE group were sampled (5–10 ml) and the cell concentration and viability were examined on the day of adding culture solution or the last day of cultivation. Cells were counted using a Count Star hemacytometer(IC1000, China). Viable and non-viable cells were determined by trypan-blue exclusion method. Viable cells are impermeable to trypan-blue and therefore the cells are transparent while non-viable cells are blue-dyed.
Endotoxin detection
The samples (5–10 ml) of the static group and the WAVE group were collected on the last day of cultivation. In the limulus amebocyte lysate(LAL) assay, the samples were added into the 10 EU/mL endotoxin, the glass tubes were sealed with a sealing film (Parafilm) and incubated at 37°C for 1 h. Then, 50 μL samples were mixed with 50 μL of LAL reagent, the mixtures were detected using the incubating kinetic tube reader(Lab Kinetics Ltd. Lot. LKM-02-64, UK) at 37°C. The response time was recorded when the optical density(OD) reached 0.02. Afterwards, the clot reaction was monitored continuously and its concentration-response time curve was measured. All samples had between three repeated measurements by the professional researcher.
The level of cytokines
Total RNAs of IFN-γ, TNF-α and IL-12 secreted by CIK cells, NK cells and DCs of different groups were analyzed by Real-Time PCR and extracted using Trizol reagent (TaKaRa, Japan) by following the user manual. Reverse transcription was performed using GoTaq® 2-Step RT-qPCR System(Promega, USA). The housekeeping genes GAPDH was used for normalization of DNA analysis. The human gene IFN-γ was amplified using forward primer: 5′-GTGTGGAGACCATCAAGGAAGAC-3′ and reverse primer: 5′-CAGCTTTTCGAAGTCATCTCGTTT-3′. The TNF-α gene was amplified using forward primer: 5′-ATCTTCTCGAACCCCGAGTGA-3′ and reverse primer: 5′-GGAGCTGCCCCTCAGCTT-3′. The IL-12 gene was amplified using forward primer: 5′-GCAAAACCCTGACCATCCAA-3″ and reverse primer: 5′-TGAAGCAGCAGGAGCGAAT-3′. Human GAPDH was amplified using forward primer: 5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse primer: 5′-CTGGGTGGCAGTGATGGCATGG-3′ as internal control. The real-time RT-PCR assay was performed under the following conditions: stage 1, 3 minutes at 45°C and 5 minutes at 37°C; stage 2, 30 seconds at 95°C, 30 seconds at 37°C, and 30 seconds at 60°C; and stage 3, 60 cycles of 30 seconds at 95°C, 30 seconds at 63°C, and 30 seconds at 72°C. The real-time fluorescence intensity was monitored at each cycle of the third stage and measured by Real-Time PCR System (Prism® 7500, ABI, USA).
Cell phenotype, cytotoxicity of NK cells or CIK cells and DC maturation
Upon completion of the designated treatments and incubations, cells were harvested and assessed for expression of NK (CD56), CIK cells constitution(CD4+T, CD8+T, NKT cells and Tregs) and DC (CD11c) surface markers, degranulation (CD107) and maturation (CD80, CD83, CD86, CD40) as described below. NK cell effector molecules by flow cytometry as described previously. Briefly, all the samples were treated surface stained with PerCP-CD3, APC-CD8, FITC-CD4, PE-CD56, APC-CD127, PE- CD25, APC-CD11c, FITC-CD80, PE-CD86, PerCP-HLA-DR and APC-CD107 antibodies at 4°C for 30 min for surface staining. And then, the excess Ab was removed and the stained cells were washed and analyzed by BD Accuri C6(BD bioscience, USA).
Cellular cytotoxicity assay
Cytotoxicity of NK cells and CIK cells were assessed against cell lines K562 for up to 48 h in various effector-to-target ratios (E/T ratio 1:1; 5:1, 10:1, 20:1, 40:1) of cell suspension. The co-culture cells were placed in 96-well plates with X-VIVO 15 mediums containing 10% fetal bovine serum (FBS). The cellular cytotoxicity was detected using the Cell Counting Kit-8(CCK-8) Assay (Dojindo, Japan). Following incubation, 20 µl CCK-8 was added to each well and the plate was incubated for an additional 4 h at 37°C. The absorbance at 450 nm of each aliquot was determined using a microplate reader (Beckman Coulter DTX880, USA). The stimulation index was calculated as a percentage of the absorbance between the cells from the traditional group and WAVE group.
Statistical analysis
To determine if there were significant differences between the static group and WAVE group, all the data were analyzed with SPSS 11.5 (SPSS, Inc., Chicago, USA) and the results were expressed as Mean±SEM. Statistical comparisons were performed by one-way analysis of variance. P < 0.05 or P < 0.01 was considered to indicate a statistically significant difference between data.
Funding Statement
This research work was supported by Doctoral Scientific Research Foundation of Liaoning Province (201601412 to Yiming Meng) and Excellent Talent Fund of Liaoning Province Cancer Hospital.
Disclosure of potential conflicts of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgments
We appreciate all the tumor patients listed in the article.
References
- 1.Ethun CG, Bilen MA, Jani AB, Maithel SK, Ogan K, Master VA. Frailty and cancer: Implications for oncology surgery, medical oncology, and radiation oncology. CA Cancer J Clin. 2017;67:362–377. doi: 10.3322/caac.21406. PMID:28731537. [DOI] [PubMed] [Google Scholar]
- 2.Valent P, Akin C. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood. 2017;129:1420–7. doi: 10.1182/blood-2016-09-731893. PMID:28031180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Citrin DE. Recent Developments in Radiotherapy. The N Engl J Med. 2017;377:1065–75. doi: 10.1056/NEJMra1608986. PMID:28902591. [DOI] [PubMed] [Google Scholar]
- 4.Gross S, Rahal R, Stransky N, Lengauer C, Hoeflich KP. Targeting cancer with kinase inhibitors. J Clin Invest. 2015;125:1780–9. doi: 10.1172/JCI76094. PMID:25932675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward EM, Flowers CR, Gansler T, Omer SB, Bednarczyk RA. The importance of immunization in cancer prevention, treatment, and survivorship. CA Cancer J Clin. 2017;67:398–410. doi: 10.3322/caac.21407. [DOI] [PubMed] [Google Scholar]
- 6.Reck M. Precision Diagnosis and Treatment for Advanced Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377:849–61. doi: 10.1056/NEJMra1703413. PMID:28854088. [DOI] [PubMed] [Google Scholar]
- 7.Van Limbergen EJ, De Ruysscher DK, Olivo Pimentel V, Marcus D, Berbee M, Hoeben A, Rekers N, Theys J, Yaromina A, Dubois LJ, Lambin P. Combining radiotherapy with immunotherapy: the past, the present and the future. Br J Radiol. 2017;90:20170157. doi: 10.1259/bjr.20170157. PMID:28541096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su Y, Romeu-Bonilla E, Anagnostou A, Fitz-Patrick D, Hearl W, Heiland T. The safety and immunological effects of rAd5-EBV-LMP2 vaccine in nasopharyngeal carcinoma patients: a phase I clinical trial and two-year follow-up. Chem Pharm Bull. 2016;64:1118–23. doi: 10.1248/cpb.c16-00114. PMID:27477649. [DOI] [PubMed] [Google Scholar]
- 9.Passalacqua R, Caminiti C, Buti S, Porta C, Camisa R, Braglia L, Tomasello G, Vaglio A, Labianca R, Rondini E. Adjuvant low-dose interleukin-2 (IL-2) plus interferon-alpha (IFN-alpha) in operable renal cell carcinoma (RCC): a phase III, randomized, multicentre trial of the Italian Oncology Group for Clinical Research (GOIRC). J Immunother. 2014;37:440–7. doi: 10.1097/CJI.0000000000000055. PMID:25304727. [DOI] [PubMed] [Google Scholar]
- 10.Zhang T, Xie J, Arai S, Wang L, Shi X, Shi N, Ma F, Chen S, Huang L, Yang L, Ma W, Zhang B, Han W, Xia J. The efficacy and safety of anti-PD-1/ PD-L1 antibodies for treatment of advanced or refractory cancers: a meta-analysis. Oncotarget. 2016;7:73068–79. PMID:27683031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH Jr, Lao CD. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–84. doi: 10.1016/S1470-2045(15)70076-8. PMID:25795410. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, Liu W, Tong J, Liu Y, Xu R, Wang Z, Wang Q, Ouyang X, Yang Y. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroe- sophageal junction. J Clin Oncol. 2016;34:1448–54. doi: 10.1200/JCO.2015.63.5995. PMID:26884585. [DOI] [PubMed] [Google Scholar]
- 13.Boye M, Wang X, Srimuninnimit V, Kang JH, Tsai CM, Orlando M, Puri T, Kim JS, Rajan N, Yang JC. First-line pemetrexed plus cisplatin followed by gefitinib maintenance therapy versus gefitinib monotherapy in East Asian never-smoker patients with locally advanced or metastatic nonsquamous non-small-cell lung cancer: quality of life results from a randomized phase III trial. Clin Lung Cancer. 2016;17:150–60. doi: 10.1016/j.cllc.2015.12.004. PMID:26809984. [DOI] [PubMed] [Google Scholar]
- 14.Stanculeanu DL, Daniela Z, Lazescu A, Bunghez R, Anghel R. Development of new immunotherapy treatments in different cancer types. J Med Life. 2016;9:240–8. PMID:27974927. [PMC free article] [PubMed] [Google Scholar]
- 15.Peoples GE, Gurney JM, Hueman MT, Woll MM, Ryan GB, Storrer CE, Fisher C, Shriver CD, Ioannides CG, Ponniah S. Clinical trial results of a HER2/neu (E75) vaccine to prevent recurrence in high-risk breast cancer patients. J Clin Oncol. 2005;23:7536–45. doi: 10.1200/JCO.2005.03.047. PMID:16157940. [DOI] [PubMed] [Google Scholar]
- 16.Dudley ME, Gross CA, Somerville RP, Hong Y, Schaub NP, Rosati SF, White DE, Nathan D, Restifo NP, Steinberg SM. Randomized selection design trial evaluating CD8+-enriched versus unselected tumor- infiltrating lymphocytes for adoptive cell therapy for patients with melanoma. J Clin Oncol. 2013;31:2152–9. doi: 10.1200/JCO.2012.46.6441. PMID:23650429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Z, Liao H, Ju Y. Effect of compound Kushen injection on T-cell subgroups and natural killer cells in patients with locally advanced non- small-cell lung cancer treated with concomitant radiochemotherapy. J Tradit Chin Med. 2016;36:14–18. doi: 10.1016/S0254-6272(16)30002-4. PMID:26946613. [DOI] [PubMed] [Google Scholar]
- 18.Chia WK, Teo M, Wang WW, Lee B, Ang SF, Tai WM, Chee CL, Ng J, Kan R, Lim WT, Tan SH, Ong WS, Cheung YB. Adoptive T-cell transfer and chemotherapy in the first-line treatment of metastatic and/or locally recurrent nasopharyngeal carcinoma. Mol Ther. 2014;22:132–9. doi: 10.1038/mt.2013.242. PMID:24297049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jäkel CE, Vogt A, Gonzalez-Carmona MA, Schmidt-Wolf IGH. Clinical studies applying cytokine-induced killer cells for the treatment of gastrointestinal tumors. J Immunol Res. 2014;2014:1–12. doi: 10.1155/2014/897214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi L, Zhou Q, Wu J, Ji M, Li G, Jiang J, Wu C. Efficacy of adjuvant immunotherapy with cytokine-induced killer cells in patients with locally advanced gastric cancer. Cancer Immunol Immunother. 2012;61:2251–9. doi: 10.1007/s00262-012-1289-2. PMID:22674056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun C, Dotti G. Utilizing cell-based therapeutics to overcome immune evasion in hematologic malignancies. Blood. 2016;127:3350–9. doi: 10.1182/blood-2015-12-629089. PMID:27207792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrett D, Fish JD. Autologous and allogeneic cellular therapies for high-risk pediatric solid tumors. Pediatr Clin North Am. 2010;57:47–66. doi: 10.1016/j.pcl.2010.01.001. PMID:20307711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meng Y, Yu Z, Wu Y, Du T, Chen S, Meng F, Su N, Ma Y, Li X, Sun S, Zhang G. Cell-based immunotherapy with cytokine-induced killer (CIK) cells: From preparation and testing to clinical application. Hum Vaccin Immunother. 2017;13:1–9. doi: 10.1080/21645515.2017.1285987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh V. Method for culturing cells using wave-induced agitation. US Patent. 2001;190:913. [Google Scholar]
- 25.Demanga CG, Eng JWL, Gardiner DL, Roth A, Butterworth A, Adams JH, Trenholme KR, Dalton JP. The development of sexual stage malaria gametocytes in a Wave Bioreactor. Parasit Vectors. 2017;10:216. doi: 10.1186/s13071-017-2155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai AC, Liu Y, Yuan X, Chella R, Ma T. Aggregation kinetics of human mesenchymal stem cells under wave motion. Biotechnol J. 2016. doi: 10.1002/biot. PMID:26849021. [DOI] [PubMed] [Google Scholar]
- 27.Burugu S, Dancsok AR. Emerging targets in cancer immunotherapy. Semin Cancer Biol. 2017; pii: S1044-579X(17)30182-7. doi: 10.1016/j.semcancer.2017.10.001. PMID:28987965. [DOI] [PubMed] [Google Scholar]
- 28.Garg AD. Cell death and immunity in cancer: From danger signals to mimicry of pathogen defense responses. Immunol Rev. 2017;280:126–48. doi: 10.1111/imr.12574. PMID:29027218. [DOI] [PubMed] [Google Scholar]
- 29.Singh N, Shi J, June CH. Genome-Editing Technologies in Adoptive T Cell Immunotherapy for Cancer. Curr Hematol Malig Rep. 2017;12:1–8. doi: 10.1007/s11899-017-0417-7. PMID:29039115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nair S. Natural Killer T Cells in Cancer Immunotherapy. Front Immunol. 2017;8:1178. doi: 10.3389/fimmu.2017.01178. PMID:29018445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.López-Soto A, Gonzalez S, Smyth MJ. Control of Metastasis by NK Cells. Cancer Cell. 2017;32:135–54. doi: 10.1016/j.ccell.2017.06.009. PMID:28810142. [DOI] [PubMed] [Google Scholar]
- 32.Villadangos JA. Antigen-specific impairment of adoptive T-cell therapy against cancer: players, mechanisms, solutions and a hypothesis. Immunol Rev. 2016;272:169–82. doi: 10.1111/imr.12433. PMID:27319350. [DOI] [PubMed] [Google Scholar]
- 33.Keller CW, Freigang S. Reciprocal Crosstalk between Dendritic Cells and Natural Killer T Cells: Mechanisms and Therapeutic Potential. Front Immunol. 2017;8:570. doi: 10.3389/fimmu.2017.00570. PMID:28596767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dwarshuis NJ, Parratt K, Santiago-Miranda A. Cells as advanced therapeutics: State-of-the-art, challenges, and opportunities in large scale biomanufacturing of high-quality cells for adoptive immunotherapies. Adv Drug Deliv Rev. 2017;114:222–39. doi: 10.1016/j.addr.2017.06.005. PMID:28625827. [DOI] [PubMed] [Google Scholar]
- 35.Wong YNS, Joshi K, Pule M, Peggs KS, Swanton C, Quezada SA, Linch M. Evolving adoptive cellular therapies in urological malignancies. Lancet Oncol. 2017;18:e341–53. doi: 10.1016/S1470-2045(17)30327-3. PMID:28593860. [DOI] [PubMed] [Google Scholar]
- 36.Scholz J. A re-usable wave bioreactor for protein production in insect cells. MethodsX 2016;3:497–501. doi: 10.1016/j.mex.2016.08.001. PMID:27556015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cirés S, Alvarez-Roa C. First use of the WAVE™ disposable rocking bioreactor for enhanced bioproduct synthesis by N2 -fixing cyanobacteria. Biotechnol Bioeng. 2015;112:621–6. doi: 10.1002/bit.25455. PMID:25219374. [DOI] [PubMed] [Google Scholar]
- 38.Lohmueller J. Current modalities in cancer immunotherapy: Immunomodulatory antibodies, CARs and vaccines. Pharmacol Ther. 2017. doi: 10.1016/j.pharmthera.2017.03.008. doi: 10.1016/j.pharmthera.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim WA. The Principles of Engineering Immune Cells to Treat Cancer. Cell. 2017;168:724–40. doi: 10.1016/j.cell.2017.01.016. PMID:28187291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beatty GL. Chimeric antigen receptor-modified T cells for the treatment of solid tumors: Defining the challenges and next steps. Pharmacol Ther. 2016;166:30–39. doi: 10.1016/j.pharmthera.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Loo JC, Swaney WP, Grassman E, Terwilliger A, Higashimoto T, Schambach A, Baum C, Thrasher AJ, Williams DA, Nordling DL, et al.. Scale-up and manufacturing of clinical-grade self-inactivating γ-retroviral vectors by transient transfection. Gene Ther. 2012;19:246–54. doi: 10.1038/gt.2011.102. PMID:21753795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwon J Y, Yang Y S, Cheon S H, Nam HJ, Jin GH, Kim DI. Bioreactor engineering using disposable technology for enhanced production of hCTLA4Ig in transgenic rice cell cultures. Biotechnol Bioeng. 2013;110:2412–24. doi: 10.1002/bit.24916. [DOI] [PubMed] [Google Scholar]
- 43.Dalton J P, Demanga C G, Reiling S J, Wunderlich J, Eng JW, Rohrbach P. Large-scale growth of the Plasmodium falciparum malaria parasite in a wave bioreactor. Int J Parasitol. 2012;42:215–20. doi: 10.1016/j.ijpara.2012.01.001. PMID:22326740. [DOI] [PubMed] [Google Scholar]
- 44.Andersson TB. Evolution of Novel 3D Culture Systems for Studies of Human Liver Function and Assessments of the Hepatotoxicity of Drugs and Drug Candidates. Basic Clin Pharmacol Toxicol. 2017;121:234–8. doi: 10.1111/bcpt.12804. PMID:28470941. [DOI] [PubMed] [Google Scholar]
- 45.Underhill GH. Bioengineered Liver Models for Drug Testing and Cell Differentiation Studies. Cell Mol Gastroenterol Hepatol. 2018;5:426–39. doi: 10.1016/j.jcmgh.2017.11.012. PMID:29675458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rebelo SP, Pinto C, Martins TR, Harrer N, Estrada MF, Loza-Alvarez P, Cabeçadas J, Alves PM, Gualda E, Sommergruber W, et al.. 3D-3-culture: A tool to unveil macrophage plasticity in the tumour microenvironment. Biomaterials 2018;163:185–97. doi: 10.1016/j.biomaterials.2018.02.030. PMID:29477032. [DOI] [PubMed] [Google Scholar]
- 47.Prévôt ME, Ustunel S. Liquid Crystal Elastomers-A Path to Biocompatible and Biodegradable 3D-LCE Scaffolds for Tissue Regeneration. Materials (Basel) 2018;11. pii: E377. doi: 10.3390/ma11030377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niehues H, Bouwstra JA, El Ghalbzouri A, Brandner JM, Zeeuwen PLJM, van den Bogaard EH. 3D skin models for 3R research: The potential of 3D reconstructed skin models to study skin barrier function. Exp Dermatol. 2018;27:501–511. doi: 10.1111/exd.13531. [DOI] [PubMed] [Google Scholar]
- 49.Ho BX, Pek NMQ. Disease Modeling Using 3D Organoids Derived from Human Induced Pluripotent Stem Cells. Int J Mol Sci. 2018;19:pii: E936. doi: 10.3390/ijms19040936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Costa EC, Moreira AF, de Melo-Diogo D, Gaspar VM, Carvalho MP, Correia IJ. 3D tumor spheroids: an overview on the tools and techniques used for their analysis. Biotechnol Adv. 2016;34:1427–41. doi: 10.1016/j.biotechadv.2016.11.002. PMID:27845258. [DOI] [PubMed] [Google Scholar]


