ABSTRACT
Immune memory after hepatitis B vaccination among adults is still under investigation. In this study, adults who had normal and high antibody response to the primary series of hepatitis B vaccination (HepB) were followed up at 5 years after the primary immunization. A booster dose was given to those who had low hepatitis B surface antibody (anti-HBs) titers, defined as anti-HBs levels < 10 mIU/mL. Blood samples were collected at two weeks after the booster and anti-HBs levels were measured. We assumed those with ant-HBs levels > = 10 mIU/mL after the booster had anamnestic response. In total, 242 persons completed the booster and the anti-HBs test. The anamnestic response rate was 99.59% (241/242) and geometric mean concentration (GMC) of anti-HBs after the booster was 2989 mIU/mL (95% CI: 255, 35085). Anti-HBs titer after the booster dose had a positive correlation with anti-HBs titers measured right after the primary immunization as well as anti-HBs titers 5 years later just before the booster. After the booster, no significant difference was found in anti-HBs titers between participants who were immunized with the 10μg HepB vaccine and those with the 20μg vaccine. Multivariable analysis showed that 1) vaccine brand used for the primary vaccination, 2) anti-HBs titers after primary vaccination and 3) anti-HBs titers before the booster dose were independently associated with the anti-HBs titers after the booster 1) β = -0.21, 95% CI: -0.33, -0.09, P = 0.001; 2) β = 0.07, 95% CI: 0.05, 0.09, P < 0.001; 3) β = 0.04, 95% CI: 0.02, 0.07, P < 0.001). In summary, anamnestic response exists among almost all adults at five years after HepB primary immunization. Vaccine brand used for primary vaccination, initial anti-HBs titers after primary immunization and anti-HBs titers before the booster were the independent predictive factors of HepB anamnestic response titers.
KEYWORDS: Adults, Booster dose, Hepatitis B vaccine, Immune memory
Introduction
Hepatitis B viral (HBV) infection, which could lead to chronic hepatitis, cirrhosis and hepatocellular carcinoma, continues to be a serious global health problem,1,2 The first Global Health Sector Strategy on viral hepatitis for 2016–2021, which was approved by World Health Assembly in May 2016, introduced the first-ever global targets for viral hepatitis, including a 30% reduction in new cases of hepatitis B and C by 2020. One of the key approaches to achieve the goal is expanded vaccination programs of hepatitis B.3 In the 2006 serologic survey, the prevalence of hepatitis B surface antigen (HBsAg) was 7.2% among Chinese population aged 1–59 years and 8–12% in adults above 20 years.4 In 2011, Chinese Center for Disease Control and Prevention (CDC) and Chinese Prevention Medicine Association (CPMA) released the guidelines for adult Hepatitis B vaccination (HepB) immunization among Chinese, and all unvaccinated adults especially those at high risk for HBV infection should be candidates for vaccination.5
HepB is the most effective and safe method of conferring long-term protection against HBV,6 and has been recommended to infants by the Chinese government since 1992.7 Previous studies showed that adults had persistent immune memory after HepB vaccination.8-11 Anti-HBs ≥10 mIU/mL is usually regarded as adequate (protective) against HBV infection.10-12 The proportion of individuals with anti-HBs ≥10 mIU/mL after a vaccination series varied greatly in healthy populations.13,14 In China, the adult non-responding rate after HepB primary vaccination was estimated at 4.7%–14.22%.15,16 It has been documented that anti-HBs level decays with time after HepB vaccination.17 Although Chinese national guidelines did not recommend persons with adequate response after the primary immunization to receive any boosters,5 some researchers suggested a booster dose to those whose anti-HBs level decreases to 10 mIU/mL or lower.10 The national guidelines were supported by evidence of persistence of immune memory,18,19 and this memory has been documented to last for at least 20 years after HepB primary immunization in infancy.20,21 The factors associated with immune memory after hepatitis B vaccination in adults are still under investigation.8,9,17
We conducted this study to examine the status of immune memory among adults with adequate HepB antibody response at 5 years after the primary immunization. We also explored factors associated with the titer of anti-HBs after a booster dose.
Results
Study population
A total of 242 participants completed the booster and anti-HBs test, and were included in the final analysis. These 242 people were similar in age, sex, anti-HBs titers after the primary immunization with the 249 people who were loss to follow up. However, body mass index (BMI) was significantly higher in those who were lost to follow up (χ2 = 20.23, P < 0.001). The demographic characteristics of the subjects are shown in Table 1.
Table 1.
The characteristic of study population between completed follow-up and not complete follow-up.
| Characteristic | Complete follow-up visit (n = 242) | Not complete follow-up visit (n = 249) | χ2 | P |
|---|---|---|---|---|
| Age at primary immunization (years) | 3.30 | 0.192 | ||
| 18∼29 | 30(12.40) | 19(7.63) | ||
| 30∼39 | 65(26.86) | 75(30.12) | ||
| 40∼49 | 147(60.74) | 155(62.25) | ||
| Sex | 0.97 | 0.360 | ||
| Males | 111(45.87) | 108(43.37) | ||
| Females | 131(54.13) | 141(56.63) | ||
| BMI (kg/m2) | 20.23 | <0.001 | ||
| <23.9 | 136(56.20) | 112(44.98) | ||
| 24.0∼27.9 | 96(39.67) | 97(38.96) | ||
| ≥28 | 10(4.13) | 40(16.06) | ||
| Anti-HBs concentrations after primary immunization (mIU/mL) | 14.50 | 0.106 | ||
| 100∼ | 39(16.12) | 59(23.69) | ||
| 200∼ | 36(14.88) | 38(15.26) | ||
| 300∼ | 32(13.22) | 39(15.66) | ||
| 400∼ | 19(7.85) | 24(9.64) | ||
| 500∼ | 13(5.37) | 11(4.42) | ||
| 600∼ | 14(5.79) | 19(7.63) | ||
| 700∼ | 16(6.61) | 11(4.41) | ||
| 800∼ | 18(7.44) | 7(2.81) | ||
| 900∼ | 14(5.78) | 8(3.21) | ||
| ≥1000 | 41(16.94) | 33(13.25) | ||
| Pre-challenge anti-HBs antibody concentrations (mIU/mL) | 12.75 | 0.174 | ||
| 0∼ | 39(16.12) | 36(14.46) | ||
| 1∼ | 35(14.46) | 31(12.45) | ||
| 2∼ | 40(16.53) | 27(10.84) | ||
| 3∼ | 32(13.22) | 22(8.84) | ||
| 4∼ | 18(7.44) | 31(12.45) | ||
| 5∼ | 19(7.85) | 21(8.43) | ||
| 6∼ | 14(5.79) | 20(8.03) | ||
| 7∼ | 22(9.09) | 24(9.64) | ||
| 8∼ | 13(5.37) | 19(7.63) | ||
| 9∼ | 10(4.13) | 18(7.23) |
Anti-HBs response after the boost dose
After the booster, anamnestic response was observed in 241 subjects and the anamnestic response rate was 99.59% (241/242). The only one person who failed to respond had a post-booster titer of 2 mIU/ml, and his initial titer after the primary immunization was 225 mIU/ml. She was not obese (BMI = 23.44) and had not reported any use of immunotherapy or diabetes. The geometric mean concentrations (GMC) of anti-HBs after the booster dose was 2989 mIU/mL [95% confidence interval (CI) = 255, 35085]. The anti-HBs titer after the booster was positively correlated with both the anti-HBs titer just after the primary series and the anti-HBs titer just before the booster (F = 8.714, P< 0.001; F = 5.427, P< 0.001), and was similar between the subjects with 10μg booster dose and 20μg booster dose (t = 1.533, P = 0.127) (Table 2).
Table 2.
Percentage of subjects with post-booster anti-HBs concentrations 0–9, 10–99, 100–999 and ≥1000 mIU/mL and GMC two weeks after HepB booster, stratified by anti-HBs concentrations after primary immunization and pre-challenge anti-HBs concentrations.
| Subjects (%) Post-booster anti-HBs concentrations (mIU/mL) |
|||||||
|---|---|---|---|---|---|---|---|
| Anti-HBs concentrations (mIU/mL) | N | 0- | 10- | 100- | ≥1000 | Anamnestic response | GMC(95%CI, mIU/ml) |
| Anti-HBs concentrations after primary immunization | |||||||
| 100- | 39 | 0(0.00) | 1(2.56) | 16(41.02) | 22(56.41) | 39(100.00) | 1152(134, 9893) |
| 200- | 36 | 1(2.77) | 1(2.77) | 7(19.44) | 27(75.00) | 35(97.22) | 1505(61, 37292) |
| 300- | 32 | 0(0.00) | 1(3.12) | 3(9.37) | 28(87.50) | 32(100.00) | 2598(236, 28619) |
| 400- | 19 | 0(0.00) | 0(0.00) | 2(10.52) | 17(89.47) | 19(100.00) | 4039(847, 19261) |
| 500- | 13 | 0(0.00) | 0(0.00) | 1(7.69) | 12(92.30) | 13(100.00) | 3703(463, 29648) |
| 600- | 14 | 0(0.00) | 0(0.00) | 0(0.00) | 14(100.00) | 14(100.00) | 4259(1918, 9459) |
| 700- | 16 | 0(0.00) | 0(0.00) | 1(6.25) | 15(93.75) | 16(100.00) | 3201 (544, 18855) |
| 800- | 18 | 0(0.00) | 0(0.00) | 2(11.11) | 16(88.88) | 18(100.00) | 5056(735, 34829) |
| 900- | 14 | 0(0.00) | 0(0.00) | 1(7.14) | 13(92.85) | 14(100.00) | 4905 (825, 29197) |
| ≥1000 | 41 | 0(0.00) | 0(0.00) | 1(2.43) | 40(97.56) | 41(100.00) | 7086(1238, 40569) |
| Total | 242 | 1(0.41) | 3(1.23) | 34(14.04) | 204(84.29) | 241(99.59) | 2989(255, 35086) |
| Pre-booster anti-HBs concentrations | |||||||
| 0- | 39 | 1(2.56) | 1(2.56) | 13(33.33) | 24(61.53) | 38(97.44) | 1186(51, 27571) |
| 1- | 35 | 0(0.00) | 1(2.85) | 9(25.71) | 25(71.42) | 35(100.00) | 2021(144, 28407) |
| 2- | 40 | 0(0.00) | 1(2.50) | 5(12.50) | 34(85.00) | 40(100.00) | 2689 (393, 18410) |
| 3- | 32 | 0(0.00) | 0(0.00) | 2(6.25) | 30(93.75) | 32(100.00) | 4589(493, 42716) |
| 4- | 18 | 0(0.00) | 0(0.00) | 1(5.55) | 17(94.44) | 18(100.00) | 4179 (573, 30473) |
| 5- | 19 | 0(0.00) | 0(0.00) | 0(0.00) | 19(100) | 19(100.00) | 4174(950, 18334) |
| 6- | 14 | 0(0.00) | 0(0.00) | 2(14.28) | 12(85.71) | 14(100.00) | 3398(485, 23787) |
| 7- | 22 | 0(0.00) | 0(0.00) | 1(4.54) | 21(95.45) | 22(100.00) | 4604(654, 32427) |
| 8- | 13 | 0(0.00) | 0(0.00) | 0(0.00) | 13(100.00) | 13(100.00) | 7188(1698, 30424) |
| 9- | 10 | 0(0.00) | 0(0.00) | 1(10.00) | 9(90.00) | 10(100.00) | 5008(706, 35549) |
| Total | 242 | 1(0.41) | 3(1.23) | 34(14.04) | 204(84.29) | 241(99.59) | 2989(255, 35085) |
Factors associated with anti-HBs titer after the booster dose
Multivariable analysis showed that age at the primary immunization, HepB type for the primary immunization, gender, BMI, smoking history, drinking history, and HepB dose for the booster were not significantly associated with anti-HBs titers after the booster, but HepB type for the primary immunization (β = -0.21, 95% CI: -0.33, -0.09, P = 0.001), anti-HBs titers after the primary immunization (β = 0.07, 95% CI: 0.05, 0.09, P< 0.001) and anti-HBs titers just before the booster (β = 0.04, 95% CI: 0.02, 0.07, P< 0.001) were independently associated with anti-HBs titers after the booster dose (Table 3).
Table 3.
Multifactor linear regression model analysis after challenge dose.
| Variables | β (95%CI) | P value |
|---|---|---|
| Age at primary immunization | 0.00(−0.09, 0.08) | 0.963 |
| Vaccine brand used for primary vaccination | −0.21(−0.33, −0.09) | 0.001 |
| Gender | 0.12(−0.02, 0.27) | 0.099 |
| BMI | −0.08(−0.19, 0.02) | 0.106 |
| Smoking history | 0.06(−0.04, 0.17) | 0.245 |
| Drinking history | −0.02(−0.20, 0.16) | 0.826 |
| Revaccination HepB dosage | −0.05(−0.17, 0.07) | 0.399 |
| Anti-HBs concentrations after primary immunization | 0.07(0.05, 0.09) | <0.001 |
| Pre-challenge anti-HBs concentrations | 0.04(0.02, 0.07) | <0.001 |
Discussion
Immune memory can be assessed by anti-HBs level induced by a booster of HepB.22 The present study documented the immune memory for HepB in adults at five years after HepB primary immunization. In the study, although anti-HBs decayed to lower than 10 mIU/mL in these participants, only one participant failed to develop anti-HBs> 10 mIUmL at two weeks after the booster so the anti-HBs response should be is protective against HBV infection. These findings were supported by a study in Canada, where almost 99% people developed protective level immune memory at 15 years after HepB primary immunization.23 In a study in the United States, 49% subjects had anti-HBs titers <10 mIU/mL during 30 years follow-up, but most (88%) persons without seroprotective level of anti-HBs had a rapid rise in titer after a booster, indicating immuno memory.24 Our study is also supported by the previous reports that immune memory outlasted the presence of detectable circulating antibodies.21,25-30 All these studies supported that the booster dose is not needed when anti-HBs is lower than 10 mIU/ml or undetectable.
Our multivariable analysis showed that vaccine brand used for the primary vaccination was an independently predictive factor for anti-HBs titers after the booster dose. The different immunogenicity may be caused by different molecular size and weight.31,32 Anti-HBs titers after the primary immunization was found to be independently associated with anti-HBs titers after the booster dose among adults. Similar results have been reported in some studies among infants or youngsters.21 Furthermore, we also found that pre-booster anti-HBs titers were independently associated with anti-HBs titers post-booster. Chinra et al. reported that pre-booster antibody titers higher than 2 mIU/mL could predict an anamnestic response after HepB booster dose, whereas titers below this value may increase the likelihood of non-response.33 Our study found the factors including age, gender, BMI, smoking history and drinking history had no significant association with anti-HBs titers after the booster, which was in agreement with the study by Middleman et al.34
There are some strengths in our study. First, a large sample size helped obtain reliable results. Second, HepB with different dosage was used for the booster in order to evaluate the influence of HepB dosage on anamnestic response and HepB dosage was known to affect anti-HBs response after the primary immunization.35,36 However, the results of the present study suggested that there is no difference in the immune response to the booster dose of HepB between these two different groups of HepB dosages. There was a high anamnestic response in this study even when a smaller HepB dose was given.
Some limitations in our study should be taken into consideration. First, 249 participants were lost to follow-up after the primary immunization, which might be due to the fact that many people left home and worked in other cities in China. Second, BMI was deferent between the participants include in the study and those who were lost. Given the fact that we did not find BMI was associated with the anamnestic response, we would argue that the difference in BMI might have little impact on the main result.
In conclusions, we documented high anamnestic response among adults with normal and high response to HepB primary vaccination at the 5-years follow-up, confirming that the booster is not needed at least 5 years after the primary vaccination among adults. Anti-HBs titer after the primary immunization, anti-HBs titers pre-booster and HepB types for primary immunization can affect anti-HBs titers after the booster.
Materials and methods
Subjects
HBV seromarkers including HBsAg, anti-HBs and anti-HBc were tested for 24,237 healthy adults who were aged 18–49 years and had no histories of HBV infection and hepatitis B vaccination, recruited from 79 villages of Zhangqiu County, Shandong province, China in 2009. Of these, 11,590 persons were negative for all three indicators and were provided free HepB. Among them, 8,592 subjects completed three doses of HepB and the anti-HBs titers test one month after the last dose. The number of subjects with non-responder, low-responder, normal-responder and high-responder were 900, 1306, 2869 and 3517, respectively.15,37,38 The participants with normal and high response to the primary vaccination were followed up at five years after vaccination, and anti-HBs and anti-HBc were tested. The participants with anti-HBs <10 mIU/mL at follow-up were included in this study. Fig. 1 depicts the study flow chart. The study was approved by the Ethics Committees of Shandong CDC and all subjects signed the informed consent form.
Figure 1.
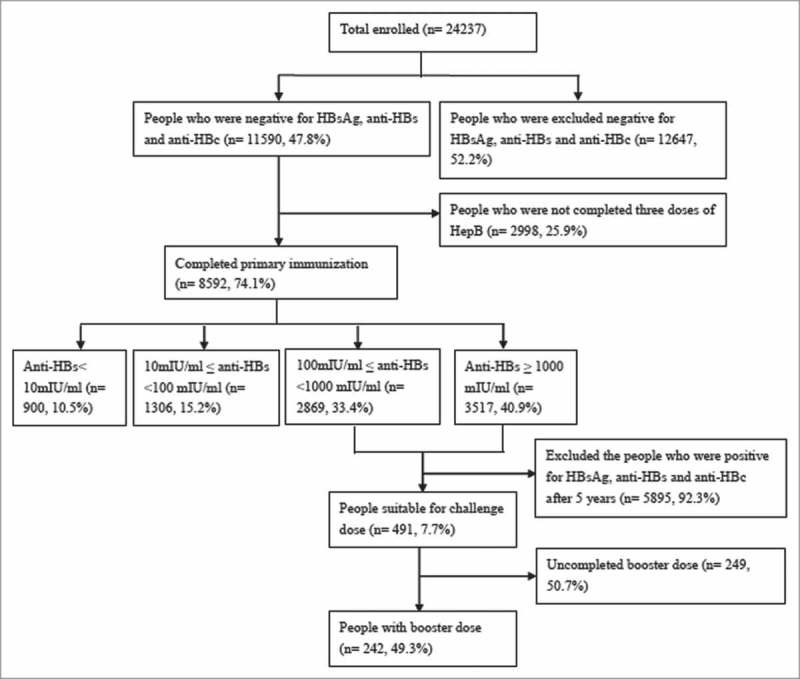
Study flow chart.
Vaccination
Primary immunization was completed with one of the following four types of HepB on 0-1-6 month schedule: 20μg HepB-SC, 20μg HepB derived in Chinese hamster ovary cell (HepB-CHO), 10μg HepB-SC and 10μg HepB derived in Hansenula polymorpha (HepB-HP). At 5 years after the primary immunization, the participants with anti-HBs<10 mIU/mL were grouped using cluster sampling method, and a booster dose was given to the two groups with 10μg HepB-SC and 20μg HepB-SC, respectively.
Serum samples collection and laboratory testing
Serum samples of 3–5 mL were collected before the primary immunization (T0), one month after the primary immunization (T1), five years after the primary immunization (T2) and two weeks after the booster dose (T3). HBsAg, anti-HBs and anti-HBc at T0 were tested by ELISA using reagents kit (HBsAg and anti-HBs were tested using reagents kit produced by Intec Products Inc. (xiamen) (cut-off: ≥1 mIU/mL). Anti-HBc was measured using reagents kit produced by Shanghai Kehua Bio-Engineering co., Ltd (cut-off: <1 mIU/mL)). HBsAg, anti-HBs and anti-HBc at T1, T2 and T3 were measured quantitatively using chemiluminescence microparticle immunoassay (CMIA) (Abbott, USA). All tests were conducted according to the manufacturers' instructions.
Statistical analyses
Data are presented as number and percentage, GMC and 95% CI where appropriate. An anti-HBs titer greater than or equal to 10 mIU/mL was considered protective against HBV infection. The distributions of post-booster anti-HBs titers were examined and then log-transformed to achieve approximately normal distribution for parametric modeling and testing. The percentage of subjects who were seropositive for anti-HBs ≥10 mIU/mL after the booster and their corresponding GMC were tabulated with 95% CI. Factors associated with anti-HBs titers after the booster dose was examined by multivariable linear regression model analysis. The anti-HBs anamnestic response was defined as anti-HBs titers ≥10 mIU/mL at two weeks post-booster in subjects who were seronegative before receiving the booster dose.7 All statistics were two-tailed. P-Values ≤0.05 were considered significant. All statistical analyses were performed using Microsoft Excel 2010 software or R software.
Funding Statement
This study was supported by a grant from the Major Project of National Science and Technology (2012ZX10002001 and 2013ZX10004902), a grant from Taishan Scholar Program of Shandong Province (ts201511105), and a grant from the Shandong Medical Health Science and Technology Development Program (2011HD006 and 2014WS0373).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the Zhangqiu Center for Disease Control and Prevention and relevant personnel for their contribution to this study. We also thank Prof. Fujie Xu for her assistance with word editing.
References
- 1.World Health Organization Hepatitis B vaccines. Wkly Epidemiol Rec. 2009;84:405–19. PMID:19817017. [PubMed] [Google Scholar]
- 2.World Health Organisation Department of Communicable Diseases Surveil- lance and Response. WHO/CDC/CSR/LYO/20022: hepatitis B; 2016 accessed 050116 2016. [Google Scholar]
- 3.World Health Organisation World Hepatitis Day 2016: Know hepatitis – Act now. http://wwwwhoint/campaigns/hepatitis-day/2016/event/en/ 2016.
- 4.Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, et al.. Epidemiological serosurvey of hepatitis B in China–declining HBV prevalence due to hepatitis B vaccination. Vaccine. 2009;27:6550–7. doi: 10.1016/j.vaccine.2009.08.048. PMID:19729084. [DOI] [PubMed] [Google Scholar]
- 5.Cui FQ. Chinese Prevention Medicine Association, National Immunization Program, Chinese Center for Disease Control Prevention. [Technical guide for adult hepatitis B immunization in China]. Zhonghua Liu Xing Bing Xue Za Zhi. 2011;32:1199–203. PMID:22336599. [PubMed] [Google Scholar]
- 6.Marshall H, Nolan T, Diez Domingo J, Rombo L, Sokal EM, Mares J, Casanovas JM, Kuriyakose S, Leyssen M, Jacquet JM. Long-term (5-year) antibody persistence following two- and three-dose regimens of a combined hepatitis A and B vaccine in children aged 1–11 years. Vaccine. 2010;28:4411–5. doi: 10.1016/j.vaccine.2010.04.040. PMID:20434544. [DOI] [PubMed] [Google Scholar]
- 7.Beran J, Van Der Meeren O, Leyssen M, D'Silva P. Immunity to hepatitis A and B persists for at least 15 years after immunisation of adolescents with a combined hepatitis A and B vaccine. Vaccine. 2016;34:2686–91. doi: 10.1016/j.vaccine.2016.04.033. PMID:27105563. [DOI] [PubMed] [Google Scholar]
- 8.Chlibek R, von Sonnenburg F, Van Damme P, Smetana J, Tichy P, Gunapalaiah B, Leyssen M, Jacquet JM. Antibody persistence and immune memory 4 years post-vaccination with combined hepatitis A and B vaccine in adults aged over 40 years. J Travel Med. 2011;18:145–8. doi: 10.1111/j.1708-8305.2010.00499.x. PMID:21366801. [DOI] [PubMed] [Google Scholar]
- 9.Van Damme P, Leroux-Roels G, Crasta P, Messier M, Jacquet JM, Van Herck K. Antibody persistence and immune memory in adults, 15 years after a three-dose schedule of a combined hepatitis A and B vaccine. J Med Virol. 2012;84:11–17. doi: 10.1002/jmv.22264. PMID:22052690. [DOI] [PubMed] [Google Scholar]
- 10.Katoonizadeh A, Sharafkhah M, Ostovaneh MR, Norouzi A, Khoshbakht N, Mohamadkhani A, Eslami L, Gharravi A, Shayanrad A, Khoshnia M, et al.. Immune responses to hepatitis B immunization 10–18 years after primary vaccination: a population-based cohort study. J Viral Hepat. 2016;23:805–11. doi: 10.1111/jvh.12543. PMID:27126365. [DOI] [PubMed] [Google Scholar]
- 11.Roznovsky L, Orsagova I, Kloudova A, Tvrdik J, Kabieszova L, Lochman I, Mrazek J, Hozakova L, Zjevikova A, Pliskova L. Long-term protection against hepatitis B after newborn vaccination: 20-year follow-up. Infection. 2010;38:395–400. doi: 10.1007/s15010-010-0039-7. PMID:20589522. [DOI] [PubMed] [Google Scholar]
- 12.van der Sande MA, Waight P, Mendy M, Rayco-Solon P, Hutt P, Fulford T, Doherty C, McConkey SJ, Jeffries D, Hall AJ, et al.. Long-term protection against carriage of hepatitis B virus after infant vaccination. J Infect Dis. 2006;193:1528–35. doi: 10.1086/503433. PMID:16652281. [DOI] [PubMed] [Google Scholar]
- 13.Wu TW, Lin HH, Wang LY. Chronic hepatitis B infection in adolescents who received primary infantile vaccination. Hepatology. 2013;57:37–45. doi: 10.1002/hep.25988. PMID:22858989. [DOI] [PubMed] [Google Scholar]
- 14.Safary A, Beck J. Vaccination against hepatitis B: current challenges for Asian countries and future directions. J Gastroenterol Hepatol. 2000;15:396–401. doi: 10.1046/j.1440-1746.2000.02002.x. PMID:10824884. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Yan BY, Lyu JJ, Liu JY, Feng Y, Wu WL, Cao CZ, Chen SY, Zhou LB, Liang XF, et al.. [Anti-HBs persistence after revaccination with three doses of hepatitis B vaccine among non-responsive adults: a 4-year of follow-up study]. Zhonghua Yu Fang Yi Xue Za Zhi. 2016;50:497–502. PMID:27256728. [DOI] [PubMed] [Google Scholar]
- 16.Yan BY, Zhang L, Lv JJ, Liu JY, Feng Y, Xu AQ, Chen SY, Gong XH, Cui FQ, Liang XF. [Comparison of antibody response and related influencing factors after primary immunization by 10 mug hepatitis B vaccine made from recombinant DNA techniques in Saccharomyces and Hansenula polymorpha among adults]. Zhonghua Liu Xing Bing Xue Za Zhi. 2012;33:988–9. PMID:23297469. [PubMed] [Google Scholar]
- 17.Sharma R, Ahlm C, Ostergaard L, Dowell A, Tran C, Thomas S, Eymin C. Persistence of immunity in healthy adults aged >/ = 50 years primed with a hepatitis B vaccine 3 years previously. Hum Vaccin Immunother. 2015;11:1709–16. doi: 10.1080/21645515.2015.1019187. PMID:25996838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spradling PR, Kamili S, Xing J, Drobeniuc J, Hu DJ, Middleman AB. Response to challenge dose among young adults vaccinated for hepatitis B as infants: importance of detectable residual antibody to hepatitis B surface antigen. Infect Control Hosp Epidemiol. 2015;36:529–33. doi: 10.1017/ice.2015.6. PMID:25643863. [DOI] [PubMed] [Google Scholar]
- 19.Gilca V, De Serres G, Boulianne N, Murphy D, Ouakki M, De Wals P, Trudeau G, Masse R, Dionne M. Long-term persistence of immunity after vaccination of pre-adolescents with low doses of a recombinant hepatitis B vaccine. Hum Vaccin Immunother. 2013;9:1685–90. doi: 10.4161/hv.25015. PMID:23744506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zanetti A, Parlato A, Romano L, Desole MG, Ferrera G, Giurdanella F, Zuliani M, Richard P, Thomas S, Fiquet A. Challenge with a hepatitis B vaccine in two cohorts of 4-7-year-old children primed with hexavalent vaccines: an open-label, randomised trial in Italy. Vaccine. 2012;30:5770–5. doi: 10.1016/j.vaccine.2012.06.078. PMID:22789511. [DOI] [PubMed] [Google Scholar]
- 21.Behre U, Bleckmann G, Crasta PD, Leyssen M, Messier M, Jacquet JM, Hardt K. Long-term anti-HBs antibody persistence and immune memory in children and adolescents who received routine childhood hepatitis B vaccination. Hum Vaccin Immunother. 2012;8:813–8. doi: 10.4161/hv.19898. PMID:22508412. [DOI] [PubMed] [Google Scholar]
- 22.Fitzsimons D, Francois G, Hall A, McMahon B, Meheus A, Zanetti A, Duval B, Jilg W, Bocher WO, Lu SN, et al.. Long-term efficacy of hepatitis B vaccine, booster policy, and impact of hepatitis B virus mutants. Vaccine. 2005;23:4158–66. doi: 10.1016/j.vaccine.2005.03.017. PMID:15964484. [DOI] [PubMed] [Google Scholar]
- 23.Gilca V, De Serres G, Boulianne N, Murphy D, De Wals P Ouakki M, Trudeau G, Masse R, Dionne M. Antibody persistence and the effect of a booster dose given 5, 10 or 15 years after vaccinating preadolescents with a recombinant hepatitis B vaccine. Vaccine. 2013;31:448–51. doi: 10.1016/j.vaccine.2012.11.037. PMID:23206974. [DOI] [PubMed] [Google Scholar]
- 24.Bruce MG, Bruden D, Hurlburt D, Zanis C, Thompson G, Rea L, Toomey M, Townshend-Bulson L, Rudolph K, Bulkow L, et al.. Antibody Levels and Protection After Hepatitis B Vaccine: Results of a 30-Year Follow-up Study and Response to a Booster Dose. J Infect Dis. 2016;214:16–22. doi: 10.1093/infdis/jiv748. PMID:26802139. [DOI] [PubMed] [Google Scholar]
- 25.Beran J, Kervyn D, Wertzova V, Hobzova L, Tichy P, Kuriyakose S, Leyssen M, Jacquet JM. Comparison of long-term (10 years) immunogenicity of two- and three-dose regimens of a combined hepatitis A and B vaccine in adolescents. Vaccine. 2010;28:5993–7. doi: 10.1016/j.vaccine.2010.06.104. PMID:20637766. [DOI] [PubMed] [Google Scholar]
- 26.Van Damme P Van Herck K. A review of the efficacy, immunogenicity and tolerability of a combined hepatitis A and B vaccine. Expert Rev Vaccines. 2004;3:249–67. doi: 10.1586/14760584.3.3.249. PMID:15176942. [DOI] [PubMed] [Google Scholar]
- 27.Van Damme P, Moiseeva A, Marichev I, Kervyn AD, Booy R, Kuriyakose S, Brockway A, Ng SP, Leyssen M, Jacquet JM. Five years follow-up following two or three doses of a hepatitis B vaccine in adolescents aged 11–15 years: a randomised controlled study. BMC Infect Dis. 2010;10:357. doi: 10.1186/1471-2334-10-357. PMID:21171982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zinke M, Kappes R, Kindler K, Paulus-Koschik A, Goering U, Disselhoff J, Soemantri P, Grunert D, Laakmann KH, Gunasekaran R, et al.. Immune memory to hepatitis B virus in 4-9-year old children vaccinated in infancy with four doses of hexavalent DTPa-HBV-IPV/Hib vaccine. Hum Vaccin. 2009;5:592–8. doi: 10.4161/hv.9051. PMID:19535920. [DOI] [PubMed] [Google Scholar]
- 29.Poovorawan Y, Chongsrisawat V, Theamboonlers A, Crasta PD, Messier M, Hardt K. Long-term anti-HBs antibody persistence following infant vaccination against hepatitis B and evaluation of anamnestic response: a 20-year follow-up study in Thailand. Hum Vaccin Immunother. 2013;9:1679–84. doi: 10.4161/hv.24844. PMID:23732904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bagheri-Jamebozorgi M, Keshavarz J, Nemati M, Mohammadi-Hossainabad S, Rezayati MT, Nejad-Ghaderi M, Jamalizadeh A, Shokri F, Jafarzadeh A. The persistence of anti-HBs antibody and anamnestic response 20 years after primary vaccination with recombinant hepatitis B vaccine at infancy. Hum Vaccin Immunother. 2014;10:3731–6. doi: 10.4161/hv.34393. PMID:25483689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato Y, Ishikawa N, Takagi T. High-performance size-exclusion chromatography and molar mass measurement by low-angle laser light scattering of recombinant yeast-derived human hepatitis B virus surface antigen vaccine particles. J Chromatogr. 1990;507:25–31. doi: 10.1016/S0021-9673(01)84177-7. PMID:2380294. [DOI] [PubMed] [Google Scholar]
- 32.Zhou W, Bi J, Janson JC, Li Y, Huang Y, Zhang Y, Su Z. Molecular characterization of recombinant Hepatitis B surface antigen from Chinese hamster ovary and Hansenula polymorpha cells by high-performance size exclusion chromatography and multi-angle laser light scattering. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;838:71–77. doi: 10.1016/j.jchromb.2006.03.064. PMID:16757217. [DOI] [PubMed] [Google Scholar]
- 33.Chiara F, Bartolucci GB, Cattai M, Piazza A, Nicolli A, Buja A, Trevisan A. Hepatitis B vaccination of adolescents: significance of non-protective antibodies. Vaccine. 2013;32:62–68. doi: 10.1016/j.vaccine.2013.10.074. PMID:24188755. [DOI] [PubMed] [Google Scholar]
- 34.Middleman AB, Baker CJ, Kozinetz CA, Kamili S, Nguyen C, Hu DJ, Spradling PR. Duration of protection after infant hepatitis B vaccination series. Pediatrics. 2014;133:e1500–1507. doi: 10.1542/peds.2013-2940. PMID:24843060. [DOI] [PubMed] [Google Scholar]
- 35.Schonberger K, Riedel C, Ruckinger S, Mansmann U, Jilg W, Kries RV. Determinants of Long-term protection after hepatitis B vaccination in infancy: a meta-analysis. Pediatr Infect Dis J. 2013;32:307–13. doi: 10.1097/INF.0b013e31827bd1b0. PMID:23249904. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L, Liu J, Lu J, Yan B, Song L, Li L, Cui F, Zhang G, Wang F, Liang X, et al.. Antibody response to revaccination among adult non-responders to primary Hepatitis B vaccination in China. Hum Vaccin Immunother. 2015;11:2716–22. doi: 10.1080/21645515.2015.1045172. PMID:26252481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu WL, Yan BY, Lyu JJ, Liu JY, Feng Y, Chen SY, Zhou LB, Liang XF, Cui FQ, Wang FZ, et al.. [Antibody persistence following primary vaccination with hepatitis B vaccine among normal and high-responder adults: a 5-year follow-up study]. Zhonghua Yu Fang Yi Xue Za Zhi. 2016;50:484–90. PMID:27256726. [DOI] [PubMed] [Google Scholar]
- 38.Lyu JJ, Yin XW, Yan BY, Liu JY, Feng Y, Wu WL, Chen SY, Zhou LB, Liang XF, Cui FQ, et al.. [Anti-HBs persistence following revaccination with three doses of hepatitis B vaccine among low-responsive adults after primary vaccination: a 4-year follow-up study]. Zhonghua Yu Fang Yi Xue Za Zhi. 2016;50:491–6. PMID:27256727. [DOI] [PubMed] [Google Scholar]


