ABSTRACT
We investigated the relationship between maternal HIV status and uptake of the full series of three doses of diphtheria-tetanus-pertussis containing vaccines (DTP3) in sub-Saharan African children. We used data obtained from demographic and health surveys conducted in sub-Saharan Africa. We conducted meta-analysis and calculated pooled odds ratios (OR) for the association between maternal HIV status and DTP3 vaccination status for each country. A total of 4,187 out of 5,537 children of women living with HIV received DTP3 (75.6%), compared to 71,290 of 113,513 (62.8%) children of HIV negative women. National DTP3 coverage among children of HIV-positive women varied between 24% and 96% while among children of HIV negative women it was between 26% and 92%. Overall pooled result showed no significant difference in DTP3 coverage between the two groups (OR = 1.05; 95% confidence interval 0.91 – 1.22), with statistically significant heterogeneity (Chi2 = 91.63, P = 0.000, I2 = 71.6%). There was no significant association between DTP3 coverage and maternal HIV status in sub-Saharan Africa. However, DTP3 coverage for both HIV-exposed and non-exposed children were below the required target. Meta-regression revealed no significant association between DTP3 coverage and country characteristics (e.g. HIV prevalence among women, antiretroviral therapy coverage, gross domestic product per capita, human development index, adult literacy rate and sub-region). Improved prevention of mother-to-child transmission services might have contributed to some extent to the higher DTP3 vaccination coverage among the HIV-exposed children. There is also need to address barriers impeding uptake of vaccination among HIV-exposed and non-exposed children.
KEYWORDS: Immunisation coverage, HIV, vaccine-preventable diseases, sub-Saharan Africa, demographic and health surveys
Introduction
Human immunodeficiency virus (HIV) infection remains a major public health challenge and a foremost cause of disability-adjusted life years in sub-Saharan Africa.1,2 Sub-Saharan Africa accounts for about 75% of global burden of HIV.3,4 It is estimated that about 1.5 million HIV-infected children live in sub-Saharan African countries and these countries also account for the highest burden of vaccine-preventable diseases.4,5
Vaccination has been demonstrated to be a cost-effective and beneficial public health intervention in protecting children.6 Vaccination is essential in HIV-infected individuals because of their increased risk of developing various infectious diseases due to defective immune system.7 Unfortunately, the coverage of routine vaccinations for children in many African countries is still low and inadequate to meet the Global Vaccine Action Plan (GVAP) targets.8-10 Studies have also shown that HIV-exposed and infected children have significant increase risk of morbidity and mortality from various vaccine-preventable diseases when compared with HIV-unexposed and uninfected children.11,12 The low vaccination coverage may result in increased susceptibility to infection by various vaccine-preventable diseases among the infants of women living with HIV.
Exploring the effect of maternal HIV status on childhood vaccination among children in sub-Saharan Africa is critical to inform vaccine-preventable diseases prevention strategy. A study on vaccination coverage in HIV-infected patients shows that patients with given indication for specific vaccination have better vaccination coverage, however, adherence to vaccination guidelines is not likely to be a determinant for vaccination among the HIV-infected patients.13 Knowledge gap in research findings with respect to the coverage of childhood vaccination and the impact of maternal HIV status among children necessitate the need to conduct a study to provide the needed evidence.13
This study examined the vaccination coverage of the three doses of diphtheria-tetanus-pertussis containing vaccines (DTP3) among children with respect to the maternal HIV status. The study also assessed the relationship between various country-level characteristics and the coverage of DTP3 among sub-Saharan African children by pooling available survey data
Results
Description of included countries
This study included only 27 countries based on the availability of the required demographic and health surveys (DHS) HIV and immunisation data sets. The surveys were conducted between 2003 and 2016 in the included countries. The years 2013 and 2014 had the highest number of surveys totalling five each. The included countries, year of survey, coverage of antiretroviral drugs use during prevention of mother-to-child transmission (PMTCT), HIV prevalence in female, gross domestic product (GDP) per capital, human development index (HDI), adult literacy rate and the sample sizes are shown in Table 1. A total number of 119,050 respondents were included in this study. A total of 4,187 out of 5,537 (75.6%) children of mothers living with HIV received DTP3, compared to 71,290 out of 113,513 (62.8%) children of HIV negative mothers.
Table 1.
Maternal HIV status, childhood DTP3 uptake, and other characteristics of 27 included countries.
| Population sample size |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Children of HIV seropositive mothers |
Children of HIV seronegative mothers |
|||||||||
| Country | Year of survey | HIV prevalence | ARV coverage during PMTCT (%) | GDP per capital (US$) | HDI | Adult literacy rate | DTP3 uptake* | Total number of children | DTP3 uptake* | Total number of children |
| Angola | 2016 | 2.2 | 44 | 3110.8 | 0.533 | 66.0 | 21 (24) | 88 | 1143 (30) | 3757 |
| Burkina Faso | 2010 | 1.1 | 83 | 649.7 | 0.402 | 34.6 | 49 (83) | 59 | 5397 (79) | 6794 |
| Burundi | 2011 | 1.3 | 84 | 285.7 | 0.404 | 61.6 | 78 (88) | 89 | 3035 (88) | 3458 |
| Cameroon | 2011 | 5.1 | 74 | 1032.6 | 0.518 | 71.3 | 158 (73) | 216 | 3019 (65) | 4678 |
| Chad | 2015 | 1.6 | 63 | 664.3 | 0.396 | 22.3 | 27 (40) | 67 | 1410 (26) | 5337 |
| Congo DR | 2014 | 1 | 70 | 444.5 | 0.435 | 77.0 | 38 (42) | 91 | 4238 (49) | 8571 |
| Cote d'Ivoire | 2012 | 3.5 | 73 | 1526.2 | 0.474 | 43.9 | 67 (58) | 115 | 1879 (57) | 3270 |
| Ethiopia | 2003 | 1.3 | 69 | 706.8 | 0.448 | 39.0 | 71 (57) | 124 | 3510 (35) | 10094 |
| Gabon | 2012 | 5.3 | 76 | 7179.3 | 0.697 | 82.3 | 59 (35) | 167 | 1309 (36) | 3641 |
| Gambia | 2013 | 2 | 69 | 473.2 | 0.452 | 42.0 | 40 (70) | 57 | 2726 (77) | 3528 |
| Ghana | 2014 | 2.1 | 56 | 1513.5 | 0.579 | 71.5 | 43 (77) | 56 | 2129 (77) | 2763 |
| Guinea | 2012 | 1.9 | 43 | 508.1 | 0.414 | 32.0 | 27 (52) | 52 | 1440 (44) | 3309 |
| Kenya | 2009 | 6.9 | 80 | 1455.4 | 0.555 | 78.7 | 147 (71) | 206 | 1758 (73) | 2412 |
| Lesotho | 2014 | 29.8 | 66 | 998.1 | 0.497 | 76.6 | 309 (80) | 386 | 850 (79) | 1078 |
| Liberia | 2013 | 2 | 70 | 455.4 | 0.427 | 42.9 | 22 (47) | 47 | 1930 (57) | 3373 |
| Malawi | 2016 | 11.2 | 84 | 300.8 | 0.476 | 62.1 | 199 (77) | 258 | 2413 (83) | 2893 |
| Mali | 2013 | 1.2 | 35 | 780.5 | 0.442 | 33.1 | 27 (60) | 45 | 2707 (57) | 4754 |
| Namibia | 2012 | 16.6 | 96 | 4140.5 | 0.64 | 88.3 | 17 (77) | 22 | 3206 (60) | 5365 |
| Niger | 2013 | 0.5 | 52 | 363.2 | 0.353 | 15.5 | 295 (79) | 375 | 1412 (78) | 1817 |
| Rwanda | 2015 | 3.8 | 82 | 702.8 | 0.498 | 68.3 | 131 (96) | 137 | 3370 (92) | 3659 |
| Sao T&P | 2009 | n/a | n/a | 1756.1 | 0.574 | 90.1 | 12 (63) | 19 | 1471 (82) | 1787 |
| Senegal | 2011 | 0.6 | 55 | 958.1 | 0.494 | 42.8 | 21 (66) | 32 | 3048 (75) | 4071 |
| Sierra Leone | 2013 | 2 | 87 | 496 | 0.42 | 32.4 | 44 (61) | 72 | 3486 (70) | 4952 |
| Swaziland | 2007 | 34.7 | 95 | 2775.2 | 0.541 | 83.1 | 676 (82) | 822 | 1303 (85) | 1542 |
| Togo | 2014 | 2.7 | 86 | 578.5 | 0.487 | 63.8 | 61 (87) | 70 | 2496 (77) | 3241 |
| Zambia | 2014 | 14.5 | 83 | 1178.4 | 0.579 | 83.0 | 1177 (85) | 1378 | 8533 (80) | 10673 |
| Zimbabwe | 2015 | 16.1 | 93 | 1008.6 | 0.516 | 88.7 | 371 (76) | 487 | 2072 (77) | 2696 |
ARV: anti-retroviral drugs; Congo DR- Congo Democratic Republic, DTP3: three doses of diphtheria-tetanus-pertussis containing vaccines, GDP: gross domestic product, HDI: human development index, n/a: not available, PMTCT: prevention of mother-to-child transmission, Sao T&P: Sao Tome and Principe.
GDP – Low-income economies are defined as those with a GDP per capita of $1,025 or less; lower middle-income economies: $1,026 – $4,035; upper middle-income economies: $4,036 – $12,475; high-income economies: ≥ $12,476. HDI – low: <0.549; medium: 0.550 – 0.770. Percentage coverage of anti-retroviral drugs use during PMTCT – low: ≤ 50.0; ≥ 50.1. HIV prevalence – low: ≤ 10.0; ≥ 10.1. Adult literacy rate – low: ≤ 50.0; ≥ 50.1.
The values for DTP3 uptake are absolute counts (percentage).
(Source: Demographic and Health Surveys, Joint United Nations Programme on HIV/AIDS, United Nations Development Programme).
The percentage coverage of pregnant women who received antiretroviral drugs for PMTCT varied from 35% in Mali to 96% in Namibia. The HIV prevalence among female aged 15–49 years old widely ranged from 0.5% in Niger to 34.7% in Swaziland. Among the 27 countries, two are upper middle-income countries (Gabon and Namibia), eight are lower middle-income countries (Angola, Cameroun, Cote d'Ivoire, Ghana, Kenya, Senegal, Swaziland and Zambia) and the rest are low-income countries. Adult literacy rate ranged from 15.5% in Niger to 88.7% in Zimbabwe. The DTP3 coverage among the children of HIV positive mothers varied between 24% in Angola and 96% in Rwanda while among the children of HIV negative mothers it was 26% in Angola and 92% in Rwanda.
Meta-analysis
The odds ratio (OR) and 95% confidence interval (CI) from the included countries and pooled result are shown in Fig. 1. In most countries (n = 20), there was no significant difference in the proportion of children of mothers living with HIV that received DTP3 and the children of HIV negative mothers. In Sao Tome and Principe, and Malawi, children of mothers living with HIV were significantly less likely to receive DTP3 compared to the HIV negative ones. However, in four countries, Cameroon, Chad, Ethiopia and Zambia, children of mothers living with HIV were significantly more likely to have received DTP3. Nonetheless, when the data from the 27 countries were pooled together using DerSimonian-Laird method, there was no significant difference in DTP3 coverage between the two groups of women (OR = 1.05; 95% CI 0.91 – 1.22), with statistically significant heterogeneity (Chi2 = 91.63 on 26 degree of freedom, P = 0.000, I2 = 71.6%).
Figure 1.
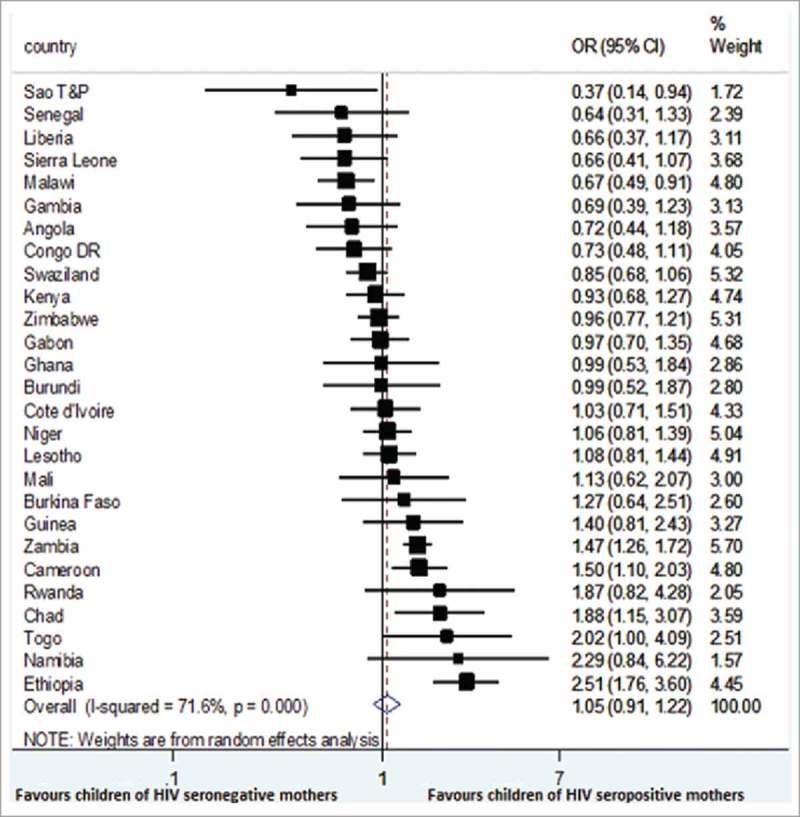
Forest plot showing the association between maternal HIV status and DTP3 coverage in 27 sub-Saharan Africa countries.
Sub-group analyses
Sub-group analysis was conducted in order to access the effect of coverage of pregnant women who received antiretroviral drugs during PMTCT on the pooled estimates. The pooled estimates for countries with low and high coverage were calculated using the DerSimonian-Laird method. The pooled effect estimates for the low coverage countries was (OR = 1.04; 95% CI 0.90 – 1.21). The calculated pooled effect estimates for the high coverage countries was (OR = 1.07; 95% CI 0.85 – 1.33) (Table 2). Sub-group analyses showed that differences between countries in terms of coverage for antiretroviral drugs, HIV prevalence, HDI, GDP, adult literacy rate, years of survey and sub-regions did not explain the heterogeneity of effect estimates on coverage for DTP3 vaccine among the sub-Saharan Africa children (Table 2).
Table 2.
Sub-group analysis and univariate meta-regression analysis results.
| Univariate meta-regression |
||||||
|---|---|---|---|---|---|---|
| Characteristics | No of studies | Subgroup odds ratio | 95% CI | I-squared | β # | 95% CI |
| Year of survey | 0.97 | 0.92,1.02 | ||||
| 2003-2010 | 5 | 1.1 | 0.9, 1.2 | 87.4* | ||
| 2011–2016 | 22 | 1.0 | 0.9, 1.2 | 64.8* | ||
| HIV prevalence female | 1.00 | 0.98, 1.02 | ||||
| Low | 20 | 1.1 | 0.9, 1.3 | 65.4* | ||
| High | 6 | 1.0 | 0.8, 1.4 | 84.2* | ||
| Pregnant women who receive anti-retroviral (%) | 1.00 | 1.00, 1.01 | ||||
| Low | 12 | 1.0 | 0.9, 1.2 | 17.9 | ||
| High | 12 | 1.1 | 0.9, 1.3 | 76.7* | ||
| Gross domestic product per capita (US$) | 1.00 | 1.00, 1.00 | ||||
| Low | 9 | 1.1 | 0.9, 1.3 | 72.3* | ||
| Middle | 18 | 1.0 | 0.8, 1.3 | 73.5* | ||
| Human development index | 0.83 | 0.10, 6.86 | ||||
| Low | 21 | 1.0 | 0.9, 1.2 | 70.1* | ||
| Medium | 6 | 1.1 | 0.9, 1.2 | 72.7* | ||
| Adult literacy rate | 1.00 | 0.99, 1.01 | ||||
| Low | 11 | 1.1 | 0.8, 1.5 | 73.4* | ||
| High | 16 | 1.0 | 0.9, 1.2 | 71.8* | ||
| Regions | 1.01 | 0.83, 1.23 | ||||
| Western Africa | 13 | 1.0 | 0.8, 1.2 | 51.5* | ||
| Central/Eastern Africa | 7 | 1.2 | 0.9, 1.7 | 79.5* | ||
| Southern Africa | 7 | 1.0 | 0.8, 1.3 | 82.5* | ||
| Size | 1.00* | 1.00, 1.00 | ||||
| Smaller studies | 20 | 1.0 | 0.9, 1.1 | 44.3* | ||
| Larger studies | 7 | 1.4 | 1.0, 1.9 | 81.0* | ||
p < 0.05.
β- Beta coefficient.
Sensitivity analyses
Figure 2 shows leave-one-country out sensitivity analyses used to assess the stability of the meta-analysis. For the analyses, the overall estimate was calculated by removing one of the study countries each time. The changes in the confidence intervals with this exclusion remain within the 95% confidence interval for the overall estimate for all the included countries. The analyses show that no one country survey had an undue influence on the estimate of the association between maternal HIV status and DTP3 uptake. The outcome of these sensitivity analyses indicated the stability of the result.
Figure 2.
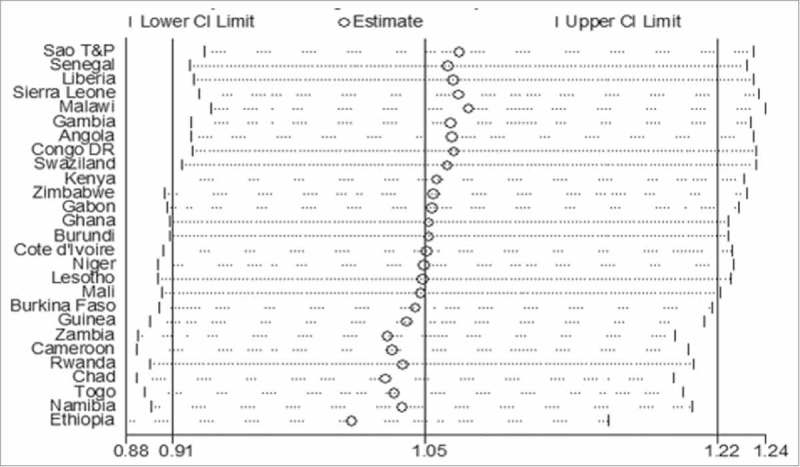
A plot showing the influence of each country on the overall pooled result using "leave-one-country-out" sensitivity analysis.
Meta-regression analysis
Meta-regression analysis was performed to explore factors that might account for heterogeneity with respect to the country-level characteristics. We found that the coverage of antiretroviral drugs use during PMTCT was not significantly associated with the childhood DTP3 vaccination coverage (p = 0.543). Likewise other characteristics were not significantly associated with the DTP3 coverage except the sample size (p = 0.020) (Table 2).
Meta-regression plot of the natural logarithm of the odds ratio of DTP3 coverage regressed against the coverage of pregnant women who received antiretroviral drugs for PMTCT in each country (Fig. 3). The circles represent a country and the size of each circle mirrors the effect of that country on the model. Although there was no significant relationship between natural logarithm of the odds ratio of DTP3 coverage and the coverage of pregnant women who received antiretroviral drugs, however, the plot shows that the DTP3 coverage slightly increases as PMTCT coverage increases (β = 1.00, 95% Cl 0.99 – 1.01, p = 0.543) (Fig. 3). Fig. 4 also shows a bubble plot of DTP3 coverage slightly reducing as HIV prevalence increases.
Figure 3.
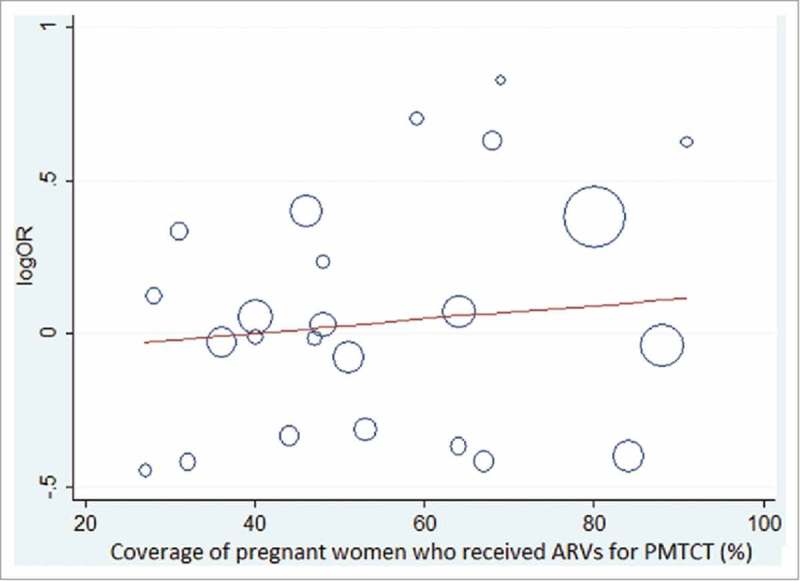
showing the association between natural logarithm of the odds ratio for DTP3 coverage and coverage of women who received anti-retroviral drugs for PMTCT in each included country.
Figure 4.
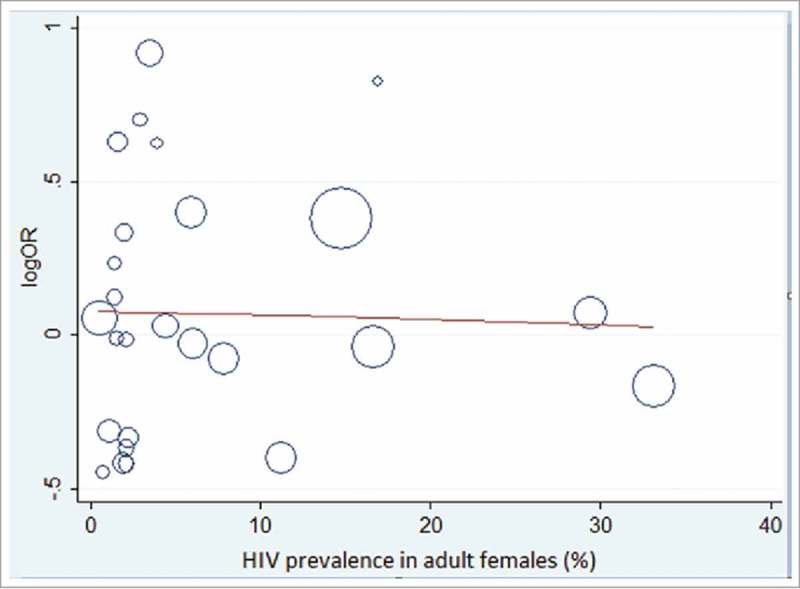
showing the association between natural logarithm of the odds ratio for DTP3 coverage and adult female HIV prevalence in each included country.
Discussion
The overall findings of the meta-analysis involving data from 27 sub-Saharan African countries showed that there is no significant difference in the DTP3 coverage among the infants of women living with HIV and those who are not HIV-infected. The findings from this socio-ecological study shows that there is low coverage of DTP3 vaccination among the HIV-exposed children, however, the DTP3 coverage among this group of children is higher than the HIV non-exposed children.
Studies by Eley and Seste et al. show that HIV-infected and HIV-exposed uninfected ..children are at risk for low immunisation coverage14,15 while another study shows that the children of women living with HIV actually had more than two-fold likelihood of been under-immunised than the children of mothers who are not HIV-infected.16 A Nigerien study shows that children of HIV-positive women were less likely to receive second and third doses of DTP-containing vaccines while another study conducted in South Africa shows that HIV-exposed children are also less likely to complete the uptake of routine childhood vaccinations in comparison with the children of HIV-negative women.9,17 These African studies were conducted in rural and urban poor communities in Uganda, South Africa, Zambia and Niger with small sample sizes. Most of these studies were conducted prior to the era of widespread PMTCT programmes in Africa. This period witnessed unnecessary delays in implementing treatment policies, non-integration of services, Acquired Immunodeficiency Syndrome (AIDS) denialism views, lack of political will, widespread stigma and discrimination.17,18 There were also issues of weak health system and inadequate information about immunisation in most of the African countries at the time.9,18,19
It is worthy to note that the meta-regression analytic findings were not significant for most of the country characteristics apart from the sample size for individual country but the bubble plots show certain patterns that may explain some of the findings of the meta-analysis. DHS are large size cross sectional surveys and likely to have significant relationship with DTP3 coverage, however, the survey sample sizes do not have direct policy implication on the country DTP3 coverage. Meta-regression plot shows that DTP3 coverage slightly increases as the coverage of the pregnant women who received antiretroviral drugs for PMTCT increases (Fig. 4). Likewise the DTP3 coverage slightly reduces as the adult female HIV prevalence increases. Coverage of antiretroviral drugs for PMTCT and HIV prevalence for female are both important PMTCT indicators and their performance might be contributory to the slight edge in DTP3 coverage recorded by the HIV-exposed children and explains the result of the meta-analysis.
The included DHS were mostly conducted post-2009 after the launch “Global Plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive”. Global Plan has the goal of reducing new HIV infections in children, decreasing final mother-to-child transmission rate of HIV infection and increasing the coverage of antiretroviral therapy in mothers and children.19,20 The integration of PMTCT services into a broad-based maternal, newborn, and child health services in many African countries in recent years has improved the quality and coverage of mother-child pair care support given to women living with HIV during the post-delivery and breastfeeding periods. There was a dramatic increase of about five folds in the percentage of mothers or infants on antiretroviral therapy during breastfeeding period from 2009 to 2013.21 The mother-child pairs are now seen together and this gives easier access for early infant diagnosis, scheduled immunisation, timely initiation of antiretroviral treatment, cotrimoxazole prophylaxis etc.22 As shown by the findings of the meta-analysis and meta-regression, all these interventions targeted at the women living with HIV and their children has not yielded significant difference in terms of vaccination coverage among this group. It is expected that HIV-exposed children level of DTP3 coverage would be significantly higher especially with frequent routine clinic visit and scheduled immunisation. However, the reality on ground shows that the mothers and care-givers of the HIV-exposed children are faced with various challenges such as poor socioeconomic status, stigma, discrimination especially at community level, poor awareness of immunisation benefits and negative attitudes of healthcare workers.15,16,21,22 Just as the case with the general population, low coverage of vaccination in HIV-exposed children could also be as a result of various barriers that emanated from the national healthcare system, healthcare providers and caregivers.23
As of 2016, Nigeria, Central African Republic, Niger, Chad, South Sudan, Democratic Republic of Congo, Mozambique, Madagascar, Uganda, Kenya, Somalia and Ethiopia were among the seriously challenged countries that needed Global Alliance for Vaccines and Immunisation (GAVI) support to meet up with their national targets for immunisation.24 The GVAP goal of achieving nationwide DTP3 vaccination coverage of at least 90% was not achieved in many of the included countries at the time of their individual surveys.10 Only Rwanda achieved DTP3 uptake of more than 90% for the children of both HIV-seropositive and –seronegative mothers. At the time of their individual surveys, Angola, Chad, Congo Democratic Republic, Gabon, Guinea and Ethiopia recorded less than 50% coverage either for one or both of the two groups. DTP3 coverage been the proxy indicator of performance of the national immunisation programmes, is relatively low in sub-Saharan Africa recording just 74% by the end of 2016.25
Efforts should be made to remove the barriers of vaccination uptake for the HIV-exposed and non-exposed children.18,21 Implementing public health interventions such as having home visits specifically to identify under-vaccinated or non-vaccinated children and regular immunisation outreach together with giving household incentives will help in improving the DTP3 coverage. This on the long run will translate to having a successful national immunisation programme. Social determinants could help in transforming the DTP3 uptake irrespective of maternal HIV status by exploring the behavioural pattern of individuals and communities via well-structured interventions.26 Interventions like integration of immunisation activities into various existing public healthcare programmes such as maternal and child health, antenatal care, PMTCT, antiretroviral treatment, HIV counselling and testing, and women empowerment programmes will go a long way to improve DTP3 coverage.27 Improving DTP3 coverage among HIV-exposed and non-exposed children especially those living in the rural and urban poor areas requires a multidimensional collaboration.22,23 This entails vaccinating eligible children at every opportunity, address community stigma, mapping out low-coverage areas localities, community mobilisation and referral for immunisation.22,28
Limitations and strengths
Conducting this meta-analysis permits the synthesis of findings and allows comparison across numerous studies.29 DHS being a nationally represented survey has a lot of advantages over primary studies that are limited to just certain geographical locations. This study has several limitations such as non-inclusion of data from South Africa and Nigeria that are the two countries with the largest population of women living with HIV and HIV-infected children. The two countries were excluded due to lack of DHS HIV dataset. As an ecological study with cross-sectional design and likeness of ecological fallacy, care must be taken in attributing causal relationship detected in this research.
Conclusion
In conclusion, the findings from this study are valuable information that will be beneficial to the public healthcare system in terms of improving the vaccination coverage of HIV-exposed children in sub-Saharan Africa. The study indicates that there is no significant difference in the DTP3 coverage among infants with respect to maternal HIV status in sub-Saharan Africa, however, there is a significant variation in terms of the estimates among the sub-Saharan African countries. The DTP3 coverage for both HIV-exposed children and non-exposed children are still below the required target. Improved PMTCT and maternal child health services across board might have contributed to the better uptake of DTP3 vaccination among the HIV-exposed children than the non-exposed children. There is also the need to address various barriers impeding successful uptake of vaccination among HIV-exposed and non-exposed children. A collaborative endeavour is required to improve vaccination coverage of HIV-exposed children and resultant reduction in the risk of contracting various vaccine-preventable diseases.
Methods
Data
The study used data obtained from DHS conducted in sub-Saharan African countries.30 The DHS data are cross-sectional and population-based representative sample surveys of households. The sampling frame for each of the survey was made up of a list of enumeration areas covering the whole country. The survey involved a two-stage sample design with standardised questionnaires which were administered to the selected participants in each country. DHS were implemented by respective National Ministry of Health or Research/Statistical agencies with technical support from MEASURE DHS, ICF International, Calverton, Maryland, USA and with funding from various development partners such as the Global Fund to Fight AIDS, Tuberculosis and Malaria, United States Agency for International Development etc. The included countries were selected based on the availability of DHS surveys with data on the uptake of three doses of DTP and HIV test in the mothers as of November, 2017. Based upon the inclusion criteria, 27 sub-Saharan African countries were included. The included countries were as follows: Angola, Burkina Faso, Burundi, Cameroun, Chad, Democratic Republic of the Congo, Cote d'Ivoire, Ethiopia, Gabon, Gambia, Ghana, Guinea, Kenya, Lesotho, Liberia, Malawi, Mali, Namibia, Niger, Rwanda, Sao Tome and Principe, Senegal, Sierra Leone, Swaziland, Togo, Zambia and Zimbabwe.
Eligible women within the age range of 15–49 years old were encouraged during the interview to voluntarily test for HIV. The trained interviewers then collected finger-prick dried blood spot specimens for HIV test. The sample collection and analysis protocol is based on the anonymous linked protocol. This DHS programme protocol makes room for the merging of results of the HIV test with other data collected per each respondent's individual questionnaires.
Main variable
We recorded the uptake of three doses of diphtheria-tetanus-pertussis containing vaccines among children aged 12–23 months at any time before the survey; as reported on their vaccination cards or by their mothers during interview. The mother's HIV status was recorded as either HIV seropositive or HIV seronegative.
Country-level variable
Country-level data were matched within the same time frame when DHS were conducted using various reports published by the United Nations Development Programme,31 World Bank32 and Joint United Nations Programme on HIV/AIDS (UNAIDS).4 The country-level characteristics included in this study were percentage coverage of pregnant women who received antiretroviral drugs for PMTCT, HIV prevalence, childhood vaccination coverage level, gross domestic product (GDP) per capita, adult literacy rate, and human development index (HDI). We divided the country-level variables into low, medium or high categories in order to provide results that were more easily interpretable for policy purposes.
These country-level variables were some of the maternal and developmental factors that are likely determinants of vaccination coverage especially among HIV-exposed children.16,18 We expected the study to show the associations between vaccination coverage and country-level factors by identifying differences in country characteristics that might be responsible for variations in the coverage in sub-Saharan Africa.
Ethical consideration
This study used existing DHS data from various countries with the study data identifier information removed. The surveys were approved by the Institutional Review Boards of the ICF International, and the Centers for Disease Control and Prevention, Atlanta, USA and by respective National Ethics Committees of the included countries. All the study respondents gave their informed consent for participation with confidentiality maintained as per the collected information for each of the participants.
Statistical analysis
Meta-analysis
We calculated odds ratios (OR) for the association between maternal HIV status and childhood DTP3 vaccination status for each country. DerSimonian-Laird method (random-effects model)33 was used to calculate pooled odds ratio across countries. We evaluated the homogeneity of the results by means of the Chi-square test and used the I2 to describe the percentage variation across countries.34-36 We explored substantial heterogeneity (I2>50%) by subgroup analysis.
To investigate sources of heterogeneity, sub-group analysis was conducted using DerSimonian-Laird method. The sub-groups were designed using the following categories:
-
i.
GDP (low-income: ≤ $1,025; middle-income: $1,026 – $12,475). GDP middle class consists of both lower-middle and upper middle classes.
-
ii.
HDI (low: ≤0.549; medium: 0.550 – 0.770), based on the classification by United Nations Development Programme.
-
iii.
Coverage of pregnant women who received antiretroviral drugs for PMTCT (low: ≤ 50.0; high: ≥ 50.1).
-
iv.
HIV prevalence aged 15–49 years old (low: ≤ 10.0; high: ≥ 10.1). The-cut off of 10.0 was used based on the range of the prevalence for different countries included in this study.
-
v.
Adult literacy rate (low: ≤ 50.0; high: ≥ 50.1).
-
vi.
Sample size (small size if ≤5000 or large size if ≥ 5001). The cut-off point of 5000 was before it is the average sample size for the participating countries.
-
vii.
Sub-region (Central/Eastern, Southern and Western Africa).
-
viii.
Year of survey (2003-2010 and 2011–2016). The cut-off point for the years of survey was 2010 because 2011 marks the start of the Global Vaccine Action Plan.
We performed leave-one-country-out sensitivity analysis in order to determine the stability of the results. This analysis evaluated the influence of individual country results by estimating the weighted average odds ratio in the absence of each country.
Meta-regression analysis
We investigated the impact of various country characteristics on odds ratio estimates through an inverse-weighted linear meta-regression analysis. The independent variable was the natural logarithm of the odds ratio. The explanatory factors included pre-defined country characteristics such as coverage of antiretroviral drugs use during PMTCT, HIV prevalence for females, sample size, sub-region and the calendar year of the survey. Other included factors were HDI, GDP and the adult literacy rates.
All the analyses were two sided with p < 0.05 considered significant. Stata 14 (Stata Corp, College Station, TX, USA) software was used for analysis.37
Funding Statement
Olatunji O. Adetokunboh and Charles S. Wiysonge are supported by the National Research Foundation of South Africa (Grant Numbers: 106035 and 112800) and the South African Medical Research Council. Olalekan A. Uthman is supported by the National Institute of Health Research using Official Development Assistance funding. The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, National Institute for Health Research.
Abbreviations
- AIDS
Acquired Immunodeficiency Syndrome
- ARV
antiretroviral drugs
- CI
Confidence intervals
- DHS
Demographic health survey
- DTP
Diphtheria-tetanus-pertussis
- GAVI
Global Alliance for Vaccines and Immunisation
- GDP
Gross domestic product per capita
- GVAP
Global Vaccine Action Plan
- HDI
Human development index
- HIV
Human immunodeficiency virus
- OR
Odds ratio
- PMTCT
Prevention of mother-to-child transmission
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful for the MEASURE DHS for releasing the data for this study.
Contributions
OOA and OAU conceived the study. OOA did the data analysis, interpreted the results and wrote the initial manuscript. OAU assisted with the data analysis. OAU and CSW reviewed and edited the manuscript. All authors read and approved the final version of the manuscript.
References
- 1.Ortblad KF, Lozano R, Murray CJL. The burden of HIV: insights from the Global Burden of Disease Study 2010. AIDS. 2013;27(13):2003–17. doi: 10.1097/QAD.0b013e328362ba67. PMID:23660576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al.. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 2012;380(9859): 2095–128. doi: 10.1016/S0140-6736(12)61728-0. PMID:23245604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joint United Nations Programme on HIV/AIDS How AIDS changed everything—MDG6: 15 years, 15 lessons of hope from the AIDS response. Geneva, Switzerland: UNAIDS; 2015. http://www.unaids.org/sites/default/files/media_asset/MDG6Report_en.pdf (accessed December16, 2017). [Google Scholar]
- 4.Joint United Nations Programme on HIV/AIDS AIDSInfo. 2017. [url http://aidsinfo.unaids.org/] (accessed December16, 2017).
- 5.World Health Organization Estimates of disease burden and cost effectiveness. [url http://www.who.int/immunization/monitoring_surveillance/burden/estimates/en/]. 2017. (accessed December20, 2017).
- 6.Szucs TD. Cost-Effectiveness of Vaccinations. Novel vaccination strategies. Weinheim, FRG: Wiley-VCH Verlag GmbH & Co. KGaA; 2004. [Google Scholar]
- 7.Afran L, Garcia Knight M, Nduati E, Urban BC, Heyderman RS, Rowland-Jones SL. HIV-exposed uninfected children: A growing population with a vulnerable immune system? Clin Exp Immunol. 2014;176(1):11–22. doi: 10.1111/cei.12251. PMID:24325737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tao W, Petzold M, Forsberg BC. Routine vaccination coverage in low-and middle-income countries: Further arguments for accelerating support to child vaccination services. Global Health Action 2013;6(1):0–8. doi: 10.3402/gha.v6i0.20343. PMID:23639178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tchidjou HK, Vescio MF, Sanou Sobze M, Souleyman A, Stefanelli P, Mbabia A, Moussa I, Gentile B, Colizzi V, Rezza G. Low vaccine coverage among children born to HIV infected women in Niamey, Niger. Hum Vaccin Immunother. 2016;12(2):540–4. doi: 10.1080/21645515.2015.1069451. PMID:26237156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization Global Vaccine Action Plan 2011–2020. Geneva, Switzerland: WHO; 2013. http://www.who.int/immunization/global_vaccine_action_plan/GVAP_doc_2011_2020/en/ (accessed December27, 2017). [Google Scholar]
- 11.Koyanagi A, Humphrey JH, Ntozini R, Nathoo KJ, Moulton LH, Iliff PJ, Mutasa K, Ruff A, Ward BJ, ZVITAMBO Study Group, et al.. Morbidity among human immunodeficiency virus-exposed but uninfected, human immunodeficiency virus-infected, and human immunodeficiency virus-unexposed infants in Zimbabwe before availability of highly active antiretroviral therapy. Pediatr Infect Dis J. 2011;30(1):45–51. doi: 10.1097/INF.0b013e3181ecbf7e. PMID:21173675. [DOI] [PubMed] [Google Scholar]
- 12.Brahmbhatt H, Kigozi G, Wabwire-Mangen F, Serwadda D, Lutalo T, Nalugoda F, Sewankambo N, Kiduggavu M, Wawer M, Gray R. Mortality in HIV-lnfected and Uninfected Children of HIV-lnfected and Uninfected Mothers in Rural Uganda. J Acquir Immune Defic Syndr. 2006;41(4):504–8. doi: 10.1097/01.qai.0000188122.15493.0a. PMID:16652060. [DOI] [PubMed] [Google Scholar]
- 13.Valour F, Cotte L, Voirin N, Godinot M, Ader F, Ferry T, Vanhems P, Chidiac C. Vaccination coverage against hepatitis A and B viruses, Streptococcus pneumoniae, seasonal flu, and A (H1N1) 2009 pandemic influenza in HIV-infected patients. Vaccine. 2014;32(35):4558–64. doi: 10.1016/j.vaccine.2014.06.015. PMID:24951870. [DOI] [PubMed] [Google Scholar]
- 14.Setse RW, Cutts F, Monze M, Ryon JJ, Quinn TC, Griffin DE, Moss WJ. HIV-1 infection as a risk factor for incomplete childhood immunization in Zambia. J Trop Pediatr. 2006; 52(5):324–8. doi: 10.1093/tropej/fmk002. PMID:16401614. [DOI] [PubMed] [Google Scholar]
- 15.Eley B. Immunization in patients with HIV infection: Are practical recommendations possible? Drugs. 2008;68(11):1473–81. doi: 10.2165/00003495-200868110-00001. PMID:18627205. [DOI] [PubMed] [Google Scholar]
- 16.Mast TC, Kigozi G, Wabwire-Mangen F, Sewankambo N, Serwadda D, Gray R, Wawer M, Black R. Immunisation coverage among children born to HIV-infected women in Rakai district, Uganda: Effect of voluntary testing and counselling (VCT). AIDS care. 2006;18(7):755–63. doi: 10.1080/09540120500521053. PMID:16971285. [DOI] [PubMed] [Google Scholar]
- 17.Burton R, Giddy J, Stinson K. Prevention of mother-to-child transmission in South Africa: an ever-changing landscape. Obstet Med.2015; 8(1):5–12. doi: . PMID:27512452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ndirangu J, Bärnighausen T, Tanser F, Tint K, Newell ML. Levels of childhood vaccination coverage and the impact of maternal HIV status on child vaccination status in rural KwaZulu-Natal, South Africa. Trop Med Int Health. 2009;14(11):1383–93. doi: 10.1111/j.1365-3156.2009.02382.x. PMID:19737375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joint United Nations Programmes on HIV/AIDS Countdown to zero. Global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. 2011—2015 Geneva, Switzerland: UNAIDS; 2011; http://www.unaids.org/sites/default/files/media_asset/20110609_JC2137_Global-Plan-Elimination-HIV-Children_en_1.pdf (accessed December20, 2017). [Google Scholar]
- 20.Adetokunboh OO, Oluwasanu M. Eliminating mother-to-child transmission of the human immunodeficiency virus in sub-Saharan Africa: The journey so far and what remains to be done. J Infect Public Health. 2016;9(4):396–407. doi: 10.1016/j.jiph.2015.06.010. PMID:26194038. [DOI] [PubMed] [Google Scholar]
- 21.Sensarma P, Bhandari S, Kutty VR. Barriers to immunization among children of HIV-infected mothers in Kolkata, India: A qualitative study. Asia Pac J Public Health. 2015;27(2):NP1362–71. doi: 10.1177/1010539513486177. PMID:23666833. [DOI] [PubMed] [Google Scholar]
- 22.Goodson JL, Finkbeiner T, Davis NL, Lyimo D, Rwebembera A, Swartzendruber AL, Wallace AS, Kimambo S, Kimario CJ, Wiktor SZ, et al.. Evaluation of using routine infant immunization visits to identify and follow-up HIV-exposed infants and their mothers in Tanzania. J Acquir Immune Defic Syndr. 2013;63(1):9–15. doi: 10.1097/QAI.0b013e31828a3e3f. PMID:23406977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esposito S, Principi N, Cornaglia G, The ESCMID Vaccine Study Group (EVASG) . Barriers to the vaccination of children and adolescents and possible solutions. Clin Microbiol Infect. 2014;20(Suppl 5):25–31. doi: 10.1111/1469-0691.12447. PMID:24354949. [DOI] [PubMed] [Google Scholar]
- 24.Global Alliance for Vaccines and Immunisation GAVI Annual Report 2016. 2017; http://www.gavi.org/progress-report/ (accessed December27, 2017)
- 25.World Health Organization 2017 Assessment report of the global vaccine action plan. Geneva, Switzerland: WHO; 2017; http://www.who.int/immunization/sage/meetings/2017/october/1_GVAP_Assessment_report_web_version.pdf (accessed December20, 2017) [Google Scholar]
- 26.Glatman-Freedman A, Nichols K. The effect of social determinants on immunization programs. Hum Vaccin Immunother. 2012;8(3):293–301. doi: 10.4161/hv.19003. PMID:22327490. [DOI] [PubMed] [Google Scholar]
- 27.Chamla DD, Essajee S, Young M, Kellerman S, Lovich R, Sugandhi N, Amzel A, Luo C. Integration of HIV in child survival platforms: A novel programmatic pathway towards the 90-90-90 targets. J Int AIDS Soc. 2015;18(Suppl 6):20250. doi: 10.7448/IAS.18.7.20250. PMID:26639111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cutts FT. Strategies to improve immunization services in urban Africa. Bull World Health Organ. 1991;69(4):407–14. PMID:1934234. [PMC free article] [PubMed] [Google Scholar]
- 29.Arriola KR, Louden T, Doldren MA, Fortenberry RM. A meta-analysis of the relationship of child sexual abuse to HIV risk behavior among women. Child Abuse Negl. 2005;29(6):725–46. doi: 10.1016/j.chiabu.2004.10.014. PMID:15979712. [DOI] [PubMed] [Google Scholar]
- 30.Measure DHS. Publications by country. [url http://dhsprogram.com/Publications/Publications-by-Country.cfm] 2017. (accessed December21, 2017)
- 31.United Nations Development Programme The Human Development Index (HDI). [url http://hdr.undp.org/en/statistics/indices/hdi/] 2017. (accessed December20, 2017)
- 32.World Bank The World Bank Data. [url http://data.worldbank.org/] 2017. (accessed December22, 2017)
- 33.DerSimonian R, Laird N. Meta-Analysis in Clinical Trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. PMID:3802833. [DOI] [PubMed] [Google Scholar]
- 34.Mantel N. WH. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–48. PMID:13655060. [PubMed] [Google Scholar]
- 35.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. doi: 10.1002/sim.1186. PMID:12111919. [DOI] [PubMed] [Google Scholar]
- 36.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. PMID:12958120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.StataCorp Stata Statistical Software: Release 14. College Station, TX: StataCorp LP; 2015 [Google Scholar]


