ABSTRACT
We describe the existence and functionality of National Immunisation Technical Advisory Groups (NITAGs) in Africa between 2010 and 2016, using data from the WHO-UNICEF Joint Reporting Form. The number of African countries with NITAGs increased from 15 (28%) in 2010 to 26 (48%) in 2016. Countries with a functioning NITAG increased from 5(9%) in 2010 to 16 (30%) in 2016. In 2016, 13 of the 27 (48%) low-income African countries reported having a NITAG; seven (54%) of them functional. Thirteen of the 26 (50%) middle-income countries reported having a NITAG; nine (69%) of them functional. In 2016, six of the seven African countries (86%) in the WHO Eastern Mediterranean Region had a NITAG, with three (50%) functional. In the WHO African Region, 20 of the 47 countries (43%) had NITAGs; 13 (65%) of them functional. Substantial investments should be made to ensure that every African country has a functional NITAG.
KEYWORDS: Global Vaccine Action Plan, National Immunisation Technical Advisory Groups, WHO-UNICEF Joint Reporting Form
Introduction
The importance of vaccination cannot be overstated. It has led to an immense reduction in childhood mortality rates and lowered the incidence of vaccine preventable diseases, with a resultant decrease in health-care costs and health inequities.1 Based on these successes, there has been calls on governments, particularly those of low-income countries to increase ownership of their immunisation programmes so as to harness the benefits of immunisation for their people. Countries are being encouraged to prioritize funding of immunization programmes and build capacity to enable autonomy in immunisation decision making.2
The Global Immunisation Vision and Strategy (GIVS) was launched by the World Health Organization (WHO) and UNICEF in 2006 to ensure the control of morbidity and mortality from vaccine-preventable disease, and to optimize the use of vaccines in people of all ages by 2015.3,4 This strategy was rapidly adopted by many countries, leading to significant improvement in immunisation outcomes.5,6 The success of this strategy set the foundation for the Global Vaccine Action Plan (GVAP). The latter is a framework that was developed by multiple stakeholders involved in immunisation and endorsed by the World Health Assembly in 2012. The main aim of GVAP is to ensure universal access to immunisation by 2020 and beyond. The GVAP consists of six strategic objectives. The first strategic objective which concerns our study is to ensure country ownership of immunisation. Based on this objective, each country is expected to have an independent advisory group, commonly referred to as the National Immunisation Technical Advisory Groups (NITAGs).7
A NITAG is a group comprised of national experts from diverse disciplines whose role is to provide independent evidence-informed advice to national immunisation authorities; taking into consideration local evidence such as disease burden, the impact on the health system and cost effectiveness of vaccines.8 A NITAG is considered to be functional when it meets six defined process indicators, which are: (1) having a legislative or administrative basis, (2) having formal terms of reference, (3) having at least five areas of expertise represented among its membership, (4) having at least one meeting per year, (5) distribution of the agenda and background documents at least one week prior to meetings, and (6) having mandatory disclosure of conflict of interests.9,10 Though these indicators do not guarantee the effective functioning of a NITAG, they ensure a systematic and comparable monitoring of the progress of NITAGs.
Two years away from the GVAP deadline, we assess the progress made by African countries in the creation and functionality of NITAGs.
Methods and analysis
Each year since 2010, countries are expected to report data on the establishment and functionality of NITAGs to WHO and the United Nations Children Fund (UNICEF) using the WHO-UNICEF joint reporting form. The latter is a standardised tool for collecting annual data on country immunisation programme performance.11
In January 2018, we searched the WHO website for country specific immunisation data collected using the WHO-UNICEF Joint Reporting Form. The current form has 12 questions relating to the existence and functioning of country NITAGs. The required data for this study were extracted using a structured data collection form on an Excel spreadsheet. For each African country, we recorded information on the country's income status and existence of a NITAG and its performance on the six process indicators.
Measurement of outcomes
Our outcome measures were the yearly number of countries on the African continent with a NITAG and the yearly number of countries with a functioning NITAG i.e. a NITAG that met all six process indicators since 2010.
The total number of countries with an existing NITAG was obtained by counting the number of countries that responded ‘yes’ to the question “Has the country a standing technical advisory group on immunization (NITAG)?”. The number of countries with a functional NITAG was obtained by counting the number of countries that responded ‘yes’ to questions related to the six process indicators i.e. formal written terms of reference, legislative or administrative basis for the advisory group, at least five different areas of expertise represented among core members, agenda and background documents distributed at least one week prior to the meetings, disclosure of conflict of interest, and meeting at least once a year.
The percentage of countries with a NITAG was calculated by dividing the number of countries reporting an existing NITAG by the number of countries on the continent that year. The annual percentage of countries with a functioning NITAG was calculated by dividing the number of countries reporting a functioning NITAG by the number of countries in the African region that year. We also examined the number of countries with NITAGs and functional NITAGs based on the income status of the country, WHO region and level of development
Results
We obtained data for 53 countries in 2010 and, with South Sudan's independence in 2011, for 54 countries from 2011 to 2016. The country data reported in this study was last updated in the WHO-UNICEF Joint Reporting Form on 12 July 2017.
The number of African countries reporting the existence of a NITAG steadily increased from 15 (28%) in 2010 to 26 (48%) in 2016. The number of countries that reported the existence of a functional NITAG (i.e. one that meets all six process indicators) also increased from 5 (9%) in 2010 to 16 (30%) in 2016 (Fig 1).
Figure 1.
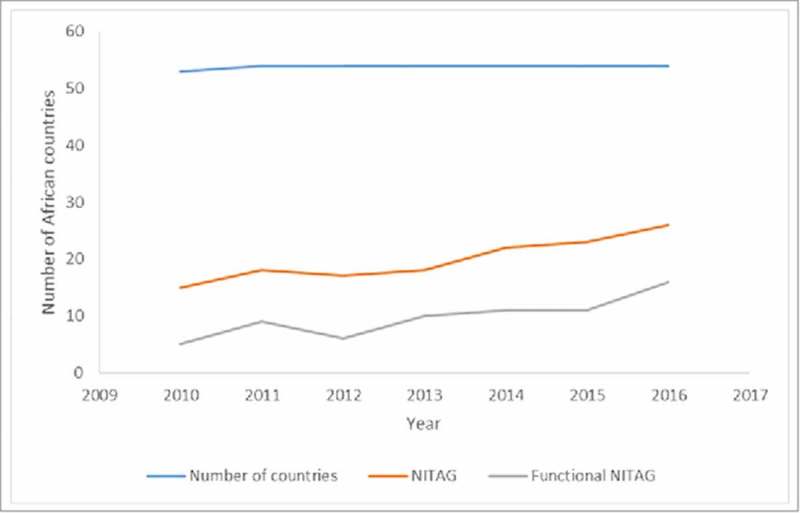
Number of NITAGs and functional NITAGS in Africa 2010–2016.
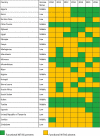
The observed increase in the number of countries with functional NITAGs was not consistent over the six years. Two countries (Cote d'Ivoire, and Tunisia) had functional NITAGs during all the six years included in this analysis. NITAGs in some countries (Burkina Faso, Djibouti, Egypt, Madagascar, Mauritania, Morocco, Mozambique, Niger, Senegal, Sierra Leone, South Africa, Sudan, and Zambia) met all six criteria in one year and dropped out in the following year (Table 1).
Table 1.
Existence of a functional NITAG.
 |
In 2010, the presence of at least five different areas of expertise among core members and mandatory disclosure of conflicts of interest were the two indicators in which NITAGs performed the least. In the period 2014–2016, we observed a shift in the performance across indicators. More countries had NITAGs with formal written terms of reference, legislative mandate, five or more areas of expertise among core members, and mandatory disclosure of conflicts of interest during the period. However, the number of NITAGs who met at least once annually remained low (Table 2).
Table 2.
Number of African countries fulfilling the various NITAG process indicators for each year from 2010 to 2016.
| Criteria | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 |
|---|---|---|---|---|---|---|---|
| Formal written terms of reference | 17 | 16 | 17 | 17 | 23 | 24 | 27 |
| Legislative or administrative basis for the advisory group | 14 | 15 | 12 | 15 | 18 | 22 | 27 |
| At least five different areas of expertise represented among core members | 8 | 12 | 13 | 16 | 19 | 21 | 22 |
| At least one meeting per year | 16 | 18 | 13 | 14 | 15 | 15 | 18 |
| Agenda and background documents circulated at least one week prior to meetings | 16 | 18 | 12 | 14 | 14 | 14 | 17 |
| Mandatory disclosure of any conflict of interest | 9 | 11 | 7 | 14 | 18 | 18 | 22 |
The most recent data on the NITAGs available in January 2018 were for the year 2016. We classified African countries as low, middle, or high-income; according to the World Bank classification.12 For the 2018 fiscal year, the World Bank classifies countries, which in 2016 had gross national income (GNI) per capita: (1) of $1,005 or less as low income; (2) between $1,006 and $12,235 as middle income; and (3) of $12,236 or more as high income. Among the 27 low-income countries in Africa, 13 (48%) reported the existence of a NITAG; with seven (54%) of the NITAGs reported to be functional. Thirteen of the 26 (50%) middle-income countries reported the existence of a NITAG; with 69% (9/13) being functional NITAGs. Seychelles, the only high income country in Africa at the moment, did not report the existence of a NITAG in 2016 (Table 3).
Table 3.
Distribution of NITAGs in 2016 according to country income and developmental status.
| Number of countries | Existence of a NITAG Number (%) | Functional NITAGNumber (%) | |
|---|---|---|---|
| Income status | |||
| Low income | 27 | 13 (48) | 7 (54) |
| Middle income | 26 | 13 (50) | 9 (69) |
| High income | 1 | 0 (0) | 0 (0) |
| WHO Region | |||
| AFR | 47 | 20 (43) | 13 (65) |
| EMR | 7 | 6 (86) | 3 (50) |
AFR, African Region; EMR, Eastern Mediterranean Region.
The 54 countries in Africa fall in two WHO regions: the African Region (47 countries) and the Eastern Mediterranean Region (7 countries). In 2016, six of the seven African countries (86%) in the WHO Eastern Mediterranean Region reported the existence of a NITAG, with 3 of these NITAGs (50%) being functional. In the WHO African region, 20 of the 47 countries (43%) reported the existence of a NITAG, with 13 (65%) meeting all six criteria required for functionality (Table 3).
Discussion
The benefits of having an established and functional NITAG are documented.13,14 For example, the South African NITAG also known as the National Advisory Group on Immunisation (NAGI) has played and continues to play an important role in providing relevant information and advice to the National Department of Health (NDOH) in South Africa on vaccine and vaccine related policies. NAGI has been instrumental in the introduction of new vaccines such as pneumococcal conjugate vaccine (PCV) and rotavirus vaccine (RV) in South Africa.13
The results from this study show steady increase in the number of countries on the African continent that reported having a NITAG between 2010 and 2016. There was also a progressive increase in the number of functional NITAGs during this period. However, these increases are unevenly distributed amongst the countries based on the income and developmental status, and the progress is insufficient if the African continent is aiming to meet the GVAP goals by 2020.
The increase observed in the number of existing and functional NITAGs in the period 2010–2016 could be attributed to increase in regional commitment by governments to ensure country ownership of immunisation programmes in the context of Gavi, the Vaccine Alliance. There has also been constant technical support from partners through initiatives like the Supporting Independent Immunization and Vaccine Advisory Committees (SIVAC) initiative.15 Through this initiative, various immunisation stakeholders worked together to assist countries in establishing and strengthening NITAGs in African countries; mostly Gavi-eligible countries.14,15 In addition, there is an increase in inter-country, regional, and global collaboration and sharing of resources by NITAGs through platforms such as the Global NITAG Network and the NITAG Resource Centre.16,17
Despite the increase in the number of functional NITAGs, efforts need to be accelerated. Several challenges are faced by countries in establishing and ensuring the functioning of NITAGs such as low awareness about NITAGs and their role, poor political commitment, insufficient financial and skilled human resources, and political instability.14 Though some countries have established NITAGs, they struggle to function due to lack of standard operating procedures, challenges with systematic declaration of conflicts of interest, poor understanding of the need for institutional independence, insufficient expertise in evidence-based policy making processes, language barriers, and poor communication between NITAGs amongst others.18
One of the main challenges facing NITAGs is the lack of expertise from different relevant disciplines. Despite the increase in efforts to implement processes for declaration of conflict of interest, professionals who are suitable for NITAG membership are more likely to have relationships with pharmaceutical companies, usually in the form of research grants. As highlighted by Gessner and colleagues, addressing this aspect, even where there are formal processes of declaration of conflict of interest can be challenging. These individuals are experts in their fields, have access to local data and are highly knowledgeable about disease burden, vaccine efficacy and other relevant information crucial for decision making. This situation precludes the existence of fully objective, impartial and completely independent NITAGs in these countries.19
Many countries with small populations such as Lesotho, Botswana, Namibia and Swaziland (of those in the southern region) have not established NITAGs. This is most likely because of the scarcity of the different complements of expertise necessary to establish NITAGs.20 This probably also explains why Seychelles, though a high-income country, does not have a NITAG. It may be that this calls for WHO and partners to support such countries to collaborate with neighbouring countries and from inter-country NITAGs. Experiences with similar committees formed in the context of the Global Polio Eradication Initiative such as the Inter-Country Certification Committee (ICCC) between Swaziland, South Africa and Lesotho should be reviewed to inform decision on establishing inter-country NITAGs.21
The role played by key technical partners such as WHO and UNICEF at country and regional offices in supporting NITAGs needs to be further examined and restructured. According to the “Guidelines on Establishing and Strengthening of NITAG”,8 WHO and UNICEF are key technical partners, yet their exact role and the processes for supporting NITAGs are not clearly outlined. In situations where WHO or UNICEF country representatives and or regional representatives do not attend NITAG meetings, this can be a serious challenge. NITAGs are high level meetings and thus for them to be afforded the significance they deserve, technical partners should give this advisory body the deserved status and consideration.
The consistency and the level of support given to NITAGs by technical partners has a big role to play in ensuring that recommendations of NITAGs are communicated at the appropriate level. Research and reports from countries with NTAGs have pointed to poor channels of communication and or lack of coordination between NITAGs and the Ministries of Health as one of the serious challenges faced. It is understood that some NITAGs face challenges in communicating their recommendations to the appropriate level at Ministries of Health (MoH), where their recommendations can be considered and implemented as desirable.19
Despite financial and technical support from partners such as GAVI, funding is limited and still poses a major challenge to the establishment and sustained functioning of many NITAGs.22 Countries must be encouraged to source sustainable funding to ensure the proper functioning of NITAGs.
Study limitations
This study notes the presence of functional NITAGs through the response to a set of questions that indicate that all six WHO process indicators are met, but was unable to evaluate the performance and effectiveness of the various NITAGs. Also, the answers provided on the JRF by individual countries have not been systematically validated with national counterparts, hence a risk of bias in the study results in that different country representatives could interpret the questions and answers differently.
Conclusion and recommendations
The establishment of functional NITAGs should be encouraged in order to meet the GVAP target for country ownership. Considering the importance of NITAGs, relevant stakeholders at national and global levels need to advocate for the allocation of sufficient resources to the establishment and maintenance of NITAGs. NITAGs need to aim for financial security by devising mechanisms to sustain funding.
There should be further support by relevant organizations including WHO to help ensure that countries with smaller populations establish NITAGs, and this may mean that a group of smaller countries that are geographically closely located and share similar epidemiological profiles could come together to form an inter-country NITAG.
The role played by technical partners such as WHO and UNICEF need to be reviewed and restructured to ensure more meaningful support to NITAGs that will help facilitation of communication between NITAGs and Ministers of Health, and the consideration of NITAG recommendations by the Ministers of Health.
NITAGs need to be strengthened through networking, regional collaborations, tutoring and technical capacity building, so as to enable them perform their duties. Finally, the activities, outputs and outcomes of NITAGs need to be routinely monitored and evaluated to ensure improvements in their performance.
Funding Statement
This work is based on research supported by the South African Medical Research Council and the National Research Foundation of South Africa (Grant Number: 106035).
Disclosure of potential conflicts of interest
The authors declare no conflicts of interest.
Author's contributions
AW and CSW conceived and designed the study. AW and ES analysed the data. AW drafted the manuscript. AW, ES, NN, and CSW revised and approved the final manuscript.
References
- 1.Andre FE, Booy R, Bock HL, Clemens J, Datta SK, John TJ, Lee BW, Lolekha S, Peltola H, Ruff TA, et al.. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull World Health Organ. World Health Organization. 2008. February;86(2):140–6. doi: 10.2471/BLT.07.040089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moxon ER, Das P, Greenwood B, Heymann DL, Horton R, Levine OS, Plotkin S, Nossal G, et al.. A call to action for the new decade of vaccines. Lancet (London, England). Elsevier. 2011. July 23;378(9788):298–302. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization GIVS: Global Immunization Vision and Strategy: 2006–2015. 2005. [Google Scholar]
- 4.Bilous J, Eggers R, Jarrett S, Lydon P, Magan A, Okwo-Bele J-M, Okwo-Bele JM, Salama P, Vandelaer J, Villeneuve P, et al.. A new global immunisation vision and strategy. Lancet. Elsevier. 2006. May 6;367(9521):1464–6. doi: 10.1016/S0140-6736(06)68625-X. [DOI] [PubMed] [Google Scholar]
- 5.Okwo-Bele JM. Integrating Immunization With Other Health Interventions For Greater Impact: The Right Strategic Choice. J Infect Dis. Oxford University Press. 2012. March 1;205(suppl 1):S4–5. [DOI] [PubMed] [Google Scholar]
- 6.Sixty-Fourth World Health Assembly Global immunization vision and strategy Progress report and strategic direction for the Decade of Vaccines Report by the Secretariat; 2011.
- 7.World Health Organization Global Vaccine Action Plan 2011–2020. Global Vaccine Action Plan 2011–2020. 2013. [Google Scholar]
- 8.Duclos P. National Immunization Technical Advisory Groups (NITAGs): Guidance for their establishment and strengthening. Vaccine. Elsevier. 2010. April 19;28:A18–25. doi: 10.1016/j.vaccine.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 9.Blau J, Sadr-Azodi N, Clementz M, Abeysinghe N, Cakmak N, Duclos P, Janusz C, Jauregui B, Mihigo R, Mosina L, et al.. Indicators to assess National Immunization Technical Advisory Groups (NITAGs). Vaccine. 2013. May 28;31(23):2653–7. doi: 10.1016/j.vaccine.2013.01.047. [DOI] [PubMed] [Google Scholar]
- 10.Duclos P, Ortynsky S, Abeysinghe N, Cakmak N, Janusz CB, Jauregui B, Mihigo R, Mosina L, Sadr-Azodi N, Takashima Y, et al.. Monitoring of progress in the establishment and strengthening of national immunization technical advisory groups. Vaccine. 2012. November 26;30(50):7147–52. doi: 10.1016/j.vaccine.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization WHO/UNICEF Joint Reporting Process. 2015[Accessed 2018 Jan 31] http://www.who.int/immunization/monitoring_surveillance/routine/reporting/reporting/en/. [Google Scholar]
- 12.World Bank Country and Lending Groups [Internet]. [cited 2018February1]. Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
- 13.Ngcobo NJ, Cameron NA. The decision making process on new vaccines introduction in South Africa. Vaccine. Elsevier. 2012. September 7;30:C9–13. doi: 10.1016/j.vaccine.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 14.Adjagba A, Senouci K, Biellik R, Batmunkh N, Faye PC, Durupt A, Gessner BD, da Silva A. Supporting countries in establishing and strengthening NITAGs: Lessons learned from 5 years of the SIVAC initiative. Vaccine. 2015. January 29;33(5):588–95. doi: 10.1016/j.vaccine.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 15.Senouci K, Blau J, Nyambat B, Coumba Faye P, Gautier L, Da Silva A, Favorov MO, Clemens JD, Stoeckel P, Gessner BD. The Supporting Independent Immunization and Vaccine Advisory Committees (SIVAC) Initiative: A country-driven, multi-partner program to support evidence-based decision making. Vaccine. Elsevier. 2010. April 19;28:A26–30. doi: 10.1016/j.vaccine.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 16.Adjagba A, Henaff L, Duclos P. The NITAG Resource Centre (NRC): One-stop shop towards a collaborative platform. Vaccine. Elsevier. 2015. August 26;33(36):4365–7. doi: 10.1016/j.vaccine.2015.06.106. [DOI] [PubMed] [Google Scholar]
- 17.Perronne C, Adjagba A, Duclos P, Floret D, Houweling H, Le Goaster C, Lévy-Brühl D, Meyer F, Senouci K, Wichmann O. Implementing efficient and sustainable collaboration between National Immunization Technical Advisory Groups: Report on the 3rd International Technical Meeting, Paris, France, 8–9 December 2014. Vaccine. Elsevier. 2016. March 8;34(11):1325–30. doi: 10.1016/j.vaccine.2016.01.063. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Meeting of the Strategic Advisory Group of Experts on immunization April 2017 – conclusions and recommendations. Relev Epidemiol Hebd . 2017;92(22):301–20. [PubMed] [Google Scholar]
- 19.Gessner BD, Duclos P, Deroeck D, Nelson EAS. Informing decision makers: experience and process of 15 National Immunization Technical Advisory Groups. Vaccine. 2010. April 19;28 Suppl 1:A1–5. doi: 10.1016/j.vaccine.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 20.MacDonald NE, Duclos P, Wichmann O, Henaff L, Harnden A, Alshammary A, Tijerino RA, Hall M, Sacarlal J, Singh RR. Moving forward on strengthening and sustaining National Immunization Technical Advisory Groups (NITAGs) globally: Recommendations from the 2nd global NITAG network meeting. Vaccine. Elsevier. 2017. December 15;35(50):6925–30. doi: 10.1016/j.vaccine.2017.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization WHO Country Cooperation Strategy 2014–2019: Swaziland. 2014. [Google Scholar]
- 22.SAGE April 2017 National Immunization Technical Advisory Groups Background Paper. 2017. [Google Scholar]


