ABSTRACT
Objective: To map the different personal positions of Guinean people regarding vaccination against Ebola.
Methods: From January to April 2016, 304 adults in Guinea were presented with 48 vignettes depicting situations in which getting vaccinated would be possible. These situations varied as a function of the constructs of health-protective behavior theories. The participants indicated the likelihood they would get vaccinated in each case.
Results: Seven qualitatively different positions were found: Always Vaccinate (38%), Never Vaccinate (25%), Hesitant (19%), Depends on Cost Only (7%), Depends on Neighbors' Attitude and Cost (5%), Mainly Depends on Risk (4%), and Mistrust of Cheap Vaccines (2%).
Conclusion: The diversity of Guinean people's positions implies that Ebola vaccination campaigns in Guinea, and probably in other sub-Saharan African countries, must not be “one size fits all,” but must be multifaceted and tailored in design and implementation to match the diversity of these people's needs and views.
KEYWORDS: Acceptability, Africa, Ebola vaccine, guinea, vaccination, willingness
Introduction
The 2014–2016 Ebola epidemic in West Africa resulted in over 28,000 cases and over 11,000 deaths.1 Guinean people were among the most affected by the pandemic-with 3,811 cases and 2,543 deaths.2 A vaccine against Ebola was tested in Guinea during the epidemic and demonstrated remarkable efficacy, at least in the short term.3,4 Such a vaccine, if its protection persists, would be the most effective long-term strategy for preventing epidemics. However, the phenomenon of vaccine hesitancy5-7 defined as “delay in acceptance or refusal of vaccines despite the availability of vaccination services”5 strongly suggests that the advent of this vaccine would not guarantee its immediate uptake in spite of its recent success. Acceptance of the vaccine among Guinean people might be hindered by the widespread misconceptions and rumours found in 2015 regarding the nature of the Ebola virus and the origin of the epidemic.8,9 Furthermore, recent studies about participation in the vaccine trials reported that some Ebola-affected communities refused participation due to mistrust of the Ebola surveillance team.3,10
Vaccination for public health can be either a response to an imminent threat, i.e. to the appearance of new cases of the illness, or a preventive measure, to be ready in advance to stop any spread if a new case should appear. The report by Gsell and colleagues4 of the first type of vaccination campaign demonstrates that vaccination can indeed be acceptable in rural Guinea under two conditions. First, the threat was perceived as real and immediate, i.e. not only had an epidemic occurred recently but new cases had turned up. Second, the community had to agree as a whole, so that where this happened, virtually all residents were vaccinated, but where it did not, no one was vaccinated; the need to act at the community level was one of the key lessons of the initial epidemic.9-11
The purpose of the current study was to investigate the acceptability of, and attitudinal barriers to, the second type of vaccination, a more general immunization program to prevent future outbreaks of Ebola. The study was carried out, therefore, not among people directly exposed to or threatened by Ebola-though most had acquaintances who had been affected by the recent epidemic-but among people who would need to get vaccinated to prevent the spread beyond any initial points of infection. Its purpose was not epidemiological, i.e. not to provide an accurate estimate of what percent of the population would accept vaccination or not and for what reasons. Instead its purpose was psychological, i.e. to map the cognitive positions taking by different groups of people and, thereby, to suggest the different types of efforts public health authorities would need to make for a successive vaccination campaign.
The study was thus a response to the World Health Organization's Strategic Advisory Group of Experts on Immunization (SAGEI) assertion that “[vaccine] hesitancy is not uniform across a population”12 and its recommendation to address vaccine hesitancy as soon as possible because “the specific factors leading to hesitancy in the subgroup need to be identified so that the most appropriate intervention options can be applied”.12 Following these recommendations, Kpanake and his team examined the acceptability in Togo of vaccination in two situations. First, they asked Togolese parents about getting their infants vaccinated against malaria and found five qualitatively different positions, which were labeled Depends on neighbors' attitude only (5%), Depends on cost only (21%), Depends on neighbors and cost (22%), Depends on risk and cost (33%), and Always vaccinate (20%).13 Second, they found a similar diversity of positions regarding the acceptability to Togolese adults of an HIV vaccine, with Always vaccinate and Depends on cost/effectiveness ratio as the most frequent positions.14 The team has now used the same methods to study the willingness to get vaccinated against Ebola among people in Guinea.
Results
The patterns of data that correspond to five of the seven clusters are shown in Fig. 1. The detailed results of the corresponding ANOVAs are available from the corresponding author.
Figure 1.
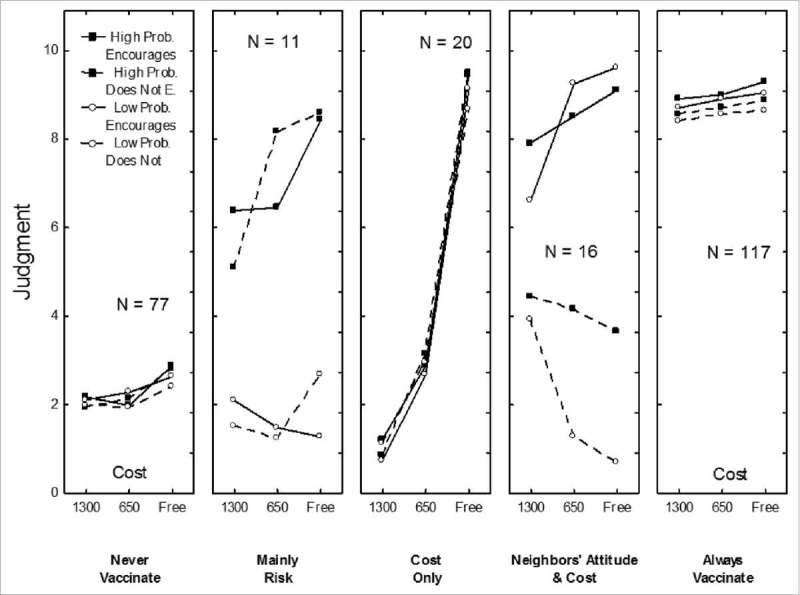
Patterns of results corresponding to five of the seven clusters: “Never Vaccinate”, “Mainly Depends on Risk”, “Depends on Cost Only”, ”Depends on Neighbors' Attitude and Cost“ and “Always Vaccinate”. Each panel corresponds to one cluster. In each panel, willingness to receive Ebola vaccine is on y-axis, the three levels of the cost are on the x-axis and each curve corresponds to social approval and perceived susceptibility. For example, in the “Cost Only” cluster, the four curves are strongly ascending (ratings were higher when vaccination was free than when it was costly) and not separated at all (ratings were not different whether the chances of becoming infected were high or low and whether neighbors agreed with this kind of vaccination or not).
For 117 participants (38%), ratings were always high (M = 8.81). They depended only slightly on neighbors' attitude (8.90 vs. 8.64) and cost (8.97 vs. 8.65). This was the Always Vaccinate cluster found in earlier studies.
For 77 participants (25%), the ratings were always low (M = 2.28). This cluster was the expected Never Vaccinate cluster.
For 58 participants (19%, not shown in Fig. 1), the ratings were always close to the center of the response scale (M = 5.79), and no significant effect was detected. This cluster was the expected Hesitant cluster.
For 20 participants (7%), the ratings were very high (M = 9.20) when the vaccine was free, low (M = 2.92) when the cost was US$50, and very low (M = 1.00) when it was US$100, F(2,38) = 172.75, p < .001, η2p = .90. This was the Depends on Cost Only cluster found in previous studies.
For 16 participants (5%), the ratings were high (M = 8.51) when neighbors encouraged vaccination and low (M = 3.03) when neighbors did not encourage it, F(1,15) = 91.77, p < .001, η2p = .86. In addition, (a) when neighbors did not encourage vaccination, willingness decreased even further as the cost decreased (as in the Mistrust of Cheap Vaccines cluster described below) and (b) when neighbors encouraged vaccination, willingness increased even further as the cost decreased (as in the Depends on Cost Only cluster), F(2,30) = 10.32, p < .001, η2p = .41. This cluster was called Depends on Neighbors' Attitude and Cost.
For 11 participants (4%), the ratings varied as a function of the risk of infection. When the risk of infection was high, vaccination intention was much higher (M = 7.20) than when the risk was low (M = 1.72), F(1,10) = 186.09, p < .001, η2p = .95. The impact of cost was stronger when the risk was high than when it was low, but the interaction was not significant at the chosen threshold. This cluster was called Mainly Depends on Risk.
Finally, for the remaining 5 participants (2%, not shown in Fig. 1), the pattern of ratings was opposite to that of the Depends on Cost Only cluster; that is, ratings were low (M = 2.96) when the vaccine was free and much higher when the cost was US$50 (M = 6.14) or US$100 (M = 7.14), F(2,8) = 9.76, p < .01, η2p = .71. This cluster was called Mistrust of Cheap Vaccines.
As shown in Table 1, the clusters differed significantly regarding age, educational level, religion, and income. Oldest participants (40 years +), participants with elementary education only, and animists were more frequently members of the Never Vaccinate cluster and less frequently members of the Always Vaccinate cluster. Participants with low income (those earning less than US$100 per month) were more frequently members of the Depends on Cost Only cluster and less frequently members of the Never Vaccinate cluster.
Table 1.
Demographic characteristics of the sample and composition of the clusters.
| Cluster |
||||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic and Level | Never Vaccinate | Mainly Depends on Risk | Depends on Cost Only | Mistrust of Cheap Vaccines | Depends on Neighbors' Attitude and Cost | Always Vaccinate | Hesitant | Total |
| Gender | ||||||||
| Male | 34 (24) | 6 (4) | 11 (8) | 1 (1) | 10 (7) | 51 (37) | 26 (19) | 139 |
| Female | 43 (26) | 5 (3) | 9 (6) | 4 (2) | 6 (4) | 66 (40) | 32 (19) | 165 |
| Age | ||||||||
| 18–22 Years | 16 (12)a | 3 (2) | 6 (5) | 2 (2) | 2 (2)a | 59 (47)a | 38 (30)ab | 126 |
| 23–39 Years | 21 (22)a | 6 (6) | 5 (5) | 3 (3) | 11 (12)a | 38 (40)b | 11 (12)a | 95 |
| 40+ Years | 40 (48)a | 2 (2) | 9 (11) | 0 (0) | 3 (4) | 20 (24)ab | 9 (11)b | 83 |
| Educational Level | ||||||||
| Elementary | 50(53)abc | 5 (5) | 9 (9) | 1 (1) | 7 (7) | 9 (9)ab | 14 (15)a | 95 |
| Middle | 17 (22)ab | 4 (5) | 8 (11) | 3 (4) | 6 (8) | 14 (18)cd | 24 (32)ab | 76 |
| High | 4 (10)c | 0 (0) | 1 (3) | 0 (0) | 2 (5) | 26 (67)ac | 6 (15) | 39 |
| College | 6 (6)b | 2 (2) | 2 (2) | 1 (1) | 1 (1) | 68 (73)bd | 14 (15)b | 94 |
| Religious Affiliation | ||||||||
| Christians | 26 (26)a | 3 (3) | 7 (7) | 1 (1) | 9 (9) | 40 (40)ac | 14 (14) | 100 |
| Muslims | 38 (22)b | 8 (5) | 10 (6) | 4 (2) | 6 (3) | 67 (38)bd | 41 (24) | 174 |
| Animists | 12 (57)ab | 0 (0) | 3 (14) | 0 (0) | 1 (5) | 3 (14)ab | 2 (10) | 21 |
| Atheists | 1 (11) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 7 (78)cd | 1 (1) | 9 |
| Income | ||||||||
| < US$100 | 14 (13)ab | 4 (3) | 13(12)ab | 3 (3) | 4 (4) | 49 (45) | 22 (20) | 109 |
| > US$100 | 36 (35)a | 3 (3) | 3 (3)a | 1 (1) | 8 (8) | 37 (36) | 14 (14) | 102 |
| Unknown | 27 (29)b | 4 (4) | 4 (4)b | 1 (1) | 4 (4) | 31 (34) | 22 (24) | 93 |
| Total | 77 (25) | 11 (4) | 20 (7) | 5 (2) | 16 (5) | 117 (38) | 58 (19) | 304 |
Table 1 shows the number and percentage (in parentheses) of participants in each cluster, as a function of gender, age, educational level, religious affiliation, and income. Figures with the same exponent in each column are significantly different, p < .05. For example, in the second column,
= significantly higher number of older people in the Never Vaccinate cluster than of younger people. Clusters differed significantly as a function of age, educational level, religious affiliation, and income.
Discussion
This study was the first to map the personal positions of Guinean people regarding vaccination against Ebola. As expected, we found several qualitatively different positions, in accordance with the SAGEI's statement regarding the heterogeneity of attitudes to vaccination12 and with previous empirical findings.13,14
Firstly, it appears that vaccination acceptance has already been achieved for a plurality (38%) of participants. They were willing to receive the vaccine irrespective of its cost, its level of effectiveness, the perceived consequences of Ebola on them, or their neighbors' attitude about it. This result was consistent with previous findings from studies conducted in Sierra-Leone15 and Nigeria.16 The only previous study on Guinean people's attitudes towards a vaccine against Ebola showed a higher proportion of participants (85.8%) who “agreed or somewhat agreed that their families would accept safe, effective, preventive Ebola vaccines”.17 However, comparing those two studies' results would be misleading because, unlike the material used in the present study, participants in the study of Irwin and colleagues were presented with hypothetical vaccines that did not reflect the main factors influencing vaccine acceptability-such as cost of the vaccine, its level of effectiveness, and its social approval-as suggested by health protective-behavior theories.
Secondly, and as also expected, a non-negligible proportion (25%) of participants would be totally unwilling to get the vaccine, whatever the situation. This finding echoes that of Henao-Restrepo and colleagues, who found that 34% of contacts of Ebola patients in Guinea refused participation in vaccine trials due to mistrust of the Ebola surveillance team.3 During the 2014–2016 Ebola epidemic, various forms of community resistance were reported in Guinea. Many people believed that Ebola virus “was invented by Westerners in order to exterminate African populations”,8 and that “Ebola epidemic was the result of bioterrorism experiments”.8 As a result, many people fled at the sight of medical responders to the epidemic, while crying “Ebola, Ebola!”.18 Several incidents of violence against medical responders were also reported, including the murder of an eight-member team and mob attacks on Ebola treatment facilities.19 The Guinean government and other organizations engaged heavily in educational interventions through flyers, meetings, and local media to increase knowledge about the transmission of Ebola virus and its symptoms.8 While all these efforts had merit, they did little to ameliorate the public's mistrust.8 This study's findings strongly suggest that Ebola vaccine promotion campaigns in Guinea should contain strategies aimed at rebuilding public trust in the institutions involved with vaccination. One possible approach may be the inclusion in these campaigns of trusted and credible community figures (e.g. spiritual and traditional leaders) and political leaders, as well as popular musicians.
Thirdly, for 18% of participants, willingness to get vaccinated would depend on specific factors, whether the cost of the vaccine (for 7%), their neighbors' attitudes (for 5%), their perceived susceptibility to Ebola (for 4%), or their trust in the characteristics of the vaccine (for 2%). Previous studies on the acceptability in West Africa of future vaccines—e.g. against malaria and HIV-have already shown that these factors impact vaccination decision-making.13,14 Thus, rather than suggesting a one-size-fits-all approach that presumes shared barriers to vaccination across the Guinean population, this study's findings point to distinct concerns among those who do not reject vaccination absolutely. This suggests that differential strategies tailored to each sub-group's specific concerns may be necessary to ensure the success of vaccination promotion. For some, those interventions should focus on the reduction of financial hurdles (e.g., the Guinean government could subsidize the cost of the vaccine). For those whose vaccination decision is based on their perceived susceptibility, community education interventions emphasizing the high level of transmissibility of Ebola may increase their willingness to be vaccinated. Finally, for people whose vaccination decision is influenced by others” attitudes, it would be appropriate to develop community-based vaccine promotion that is engaging and persuasive to people.
Finally, many participants (19%) were unsure about receiving the vaccine, regardless of circumstances. This echoed the finding of Ughasoro and colleagues16 in Nigeria that, while most of their participants were willing to get vaccinated against Ebola, they also feared adverse consequences of the vaccine. The Nigerian participants indicated that they would prefer to observe the outcome on others who have received the vaccine before deciding whether to accept it or not. Thus, doubts about vaccination in Guinea would likely decrease as an increasing number of people receive the vaccine and benefit from it.
This study has some limitations. First, it used a sample of only moderate size and restricted to people living in Conakry. Second, although the factors investigated in this study were those suggested by health protective-behavior theories and also those examined in previous studies, other factors could potentially influence vaccination decision-making.20 Any generalization of the findings must, therefore, be done with care. Third, the experimenter did not ask further questions to the respondents to elucidate the reasons underlying their positions. Future follow-up studies using qualitative methods are needed to understand the respondents' justifications. Fourth, the people who chose to participate may have been more likely than the population at large to have a firm opinion about vaccination, either for or against, and to be willing to express it. Thus, the cluster of people whose responses indicated indetermination, labeled Hesitant (19% of the sample), may in fact be larger. This cluster both in the study and in the larger society is likely to include people who oppose vaccination but are reluctant to express their oppositions openly. We were not able to measure the extent of these potential biases.
Despite these limitations, our findings have important implications for promotion of an Ebola vaccine in Guinea, and probably elsewhere in western Africa. Since (as shown in Table 1) demographic variables such as educational level or religious affiliation affect the distribution of people's positions, the proportion of people holding different positions on vaccination would likely vary from one country to another across West Africa. Nonetheless, their qualitative positions are likely to be similar to those of people in Guinea. This diversity of positions strongly suggests that, when the vaccine is fully tested and becomes available for widespread vaccination, no one single intervention strategy to promote vaccination will be appropriate. The present study's findings are the first that can help to inform public health authorities in Guinea about the design and implementation of the tailored interventions that could ensure a widespread uptake of the vaccine. These include both community-level strategies, as suggested above, and individual-level strategies. Indeed, our findings can help health professionals in their efforts to convince people to get vaccinated. Aware of the limited set of positions about vaccination, those professionals should be able, through face to face discussion, to identify the main motives underlying reluctance to get vaccinated. They should, for example, be able to distinguish quickly between a) those who are concerned above all with cost (to whom some kind of free-of-charge vaccination could be proposed), b) those who underestimate their risk of an Ebola infection (who might be reminded that even if risk is low, the consequences of the illness are severe), and c) those who seem to be irreducibly hostile to vaccination, (for whom the use of influential others as role models would likely decrease their reluctance).
Methods
Study area
The study site was Conakry, the capital and largest city in Guinea, with its population of 1.7 million persons.21 The city had been the Ebola epicenter in Guinea.21 From January 1, 2014, to March 29, 2015, a total of 553 Ebola cases were reported in the city and 802 in the 4 surrounding prefectures.21 Sustained transmission of Ebola in Conakry was attributed to multiple factors including limited awareness of the disease and continued refusal by some families to accept Ebola interventions.22,23
Participants
We used a random stratified sampling procedure (strata based on gender, age, educational level, religious affiliation and income), which allowed us to examine whether those demographic characteristics have an impact on participants' responses. From January to April 2016, 400 adults walking along the main sidewalks of Conakry were invited to participate in the study. After having received full explanations regarding the study and the procedure, 304 (139 men and 165 women) agreed to participate. Those who declined to participate evoked lack of available time. The participants received no incentive. Their demographic characteristics are shown in Table 1.
Material
The material consisted of 48 vignettes, composed of all combinations of the five main constructs of health-protective behavior theories24: Cost of the vaccine (free, 650,000 GNF [approximately US$50], or 1,300,000 GNF [approximately US$100]), Effectiveness of the vaccine (50% or at least 75%), Perceived susceptibility to Ebola virus (one chance in 10 or in 50), Perceived severity of Ebola (lethal or not lethal owing to effective treatment), and Social approval of vaccination (neighbors encourage or not do not encourage). The question under each vignette was, “If you were in this case, how likely would you be to get vaccinated?” The 11-point response scale ranged from “Certainly NO” (0) to “Certainly YES” (10). Two examples of scenarios are given in the appendix.
Procedure
For each participant, the researchers arranged for a quiet place to administer the experiment. The site was either a vacant classroom in a local school or the participant's private home, depending on what was the most convenient for the participant. Testing was individual according to the procedure recommended by Anderson.25 The researchers explained to participants what was expected, i.e., that in each case they were to indicate how likely they would be to get vaccinated. They gave ratings at their own pace, and the researchers made certain that the participants understood all relevant information before they gave ratings. When the experiment was completed, the interviewers converted the participants' marks into numbers they entered into the database; they then double-checked the accuracy of these entries.
Ethics approval for the study was obtained from the Guinean National Review Board for Health Research, the Guinean National Review Board for Research on Ebola, and the Institutional Review Board of University of Quebec (Teluq). Full anonymity was provided to all participants.
Statistical analyses
As expected, we detected strong individual differences in responses during data gathering. Accordingly, we performed cluster analysis on the raw data using the K-means method, as recommended by Hofmans and Mullet.26 A seven-cluster solution was retained based on the technique advocated by Schepers and Hofmans.27 We then conducted an overall ANOVA on the raw data with a design of Cluster × Susceptibility × Severity × Effectiveness × Cost × Others' approval, 7 × 2 × 2 × 2 × 3 × 2. As the Cluster factor and three two-way interactions involving this factor were significant, six separate ANOVAs were conducted on the data of each cluster. Owing to the multiple comparisons, the significance threshold was set at .001. Finally, we performed Chi2 tests to examine the effects of demographic characteristics.
Appendix A
Two examples of scenario
I
Mr. Camara, for the moment, is not infected by the Ebola virus.
However, he has good chance of getting infected (about 1 chance out of 10) because he lives in a village in which half of the inhabitants are infected with the virus and the epidemic is continuing to expand.
If Mr. Camara gets infected with the Ebola virus, he will not have access to any effective treatment and will very likely die of it.
Mercier Laboratory has marketed a vaccine against the Ebola virus. This vaccine is quite effective, preventing 2 cases out of 3.
This vaccine is free.
Several of Mr. Camara's neighbors have already been vaccinated and encourage him to get it.
If you were Mr. Camara in this case, how likely would you be to get vaccinated?
Certainly NO o—-o—-o—-o—-o—-o—-o—-o—-o—-o—-o Certainly YES
II
Mr. Ifono, for the moment, is not infected by the Ebola virus.
He has a small chance of getting infected (about 1 chance out of 50) because he lives in a village in which only 2 cases of Ebola have been reported.
If Mr. Ifono should get infected with the Ebola virus, he would have access to an experimental treatment against Ebola which has been found to be effective in several patients. He would have a good chance of being cured.
Sun Laboratory has marketed a vaccine against the Ebola virus. It is moderately effective, preventing 1 case out of 2.
The vaccine costs 1,300,000 GNF [US$100].
None of Mr. Ifono's neighbors have gotten vaccinated nor encourage him to do so.
If you were Mr. Ifono in this case, how likely would you be to get vaccinated?
Certainly NO o—-o—-o—-o—-o—-o—-o—-o—-o—-o—-o Certainly YES.
Funding Statement
This study was funded by a grant from Canada Research Chairs program awarded to LK. Grant number: 950–230745. The funding body had no role in the study or the decision to submit the paper for publication.
Disclosure of potential conflicts of interest
The authors report no conflict of interest. The authors certify that: (a) they have complied with American Medical Association Ethical Principles in the collection of the data, (b) the manuscript contains original work, and (c) the work is not simultaneously submitted for review elsewhere.
References
- 1.World Health Organization (2016) Ebola situation report - 30 March 2016. Geneva: WHO; Available from: http://apps.who.int/ebola/current-situation/ebola-situation-report-30-march-2016. (Accessed June6, 2017). [Google Scholar]
- 2.World Health Organization (2017) Guinea: statistics. Geneva: WHO; Available from: http://www.who.int/countries/gin/en/. (Accessed June6, 2017). [Google Scholar]
- 3.Henao-Restrepo AM, Camacho A, Longini IM, Watson CH, Edmunds WJ, Egger M, Carroll MW, Dean NE, Diatta I, Doumbia M, et al.. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!). Lancet. 2017;389:505–18. doi: 10.1016/S0140-6736(16)32621-6. PMID:28017403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gsell P-S, Camacho A, Kucharski AJ, Watson CH, Bagayoko A, Nadlaou SD, Dean NE, Diallo A, Diallo A, Djidonou AH, et al.. Ring vaccination with rVSV-ZEBOV under expanded access in response to an outbreak of Ebola virus disease in Guinea, 2016: an operational and vaccine safety report. Lancet Infect Dis. 2017;17:1276–84. doi: 10.1016/S1473-3099(17)30541-8. PMID:29033032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubé E, Laberge C, Guay M, Bramadat P, Roy R, Bettinger J. Vaccine hesitancy: an overview. Hum Vaccin Immunother. 2013;9:1763–73. doi: 10.4161/hv.24657. PMID:23584253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacDonald NE, the SAGE Working Group on Vaccine Hesitancy . Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33:4161–4. doi: 10.1016/j.vaccine.2015.04.036. PMID:25896383. [DOI] [PubMed] [Google Scholar]
- 7.Eskola J, Duclos P, Schuster M, MacDonald NE, the SAGE working group on vaccine hesitancy . How to deal with vaccine hesitancy? Vaccine. 2015;33:4215–7. doi: 10.1016/j.vaccine.2015.04.043. PMID:25896378. [DOI] [PubMed] [Google Scholar]
- 8.Kpanake L, Gossou K, Sorum PC, Mullet E. Misconceptions about Ebola virus disease among lay people in Guinea: lessons for community education. J Public Health Policy. 2016;37:160–72. doi: 10.1057/jphp.2016.1. PMID:26865320. [DOI] [PubMed] [Google Scholar]
- 9.Hofman M, Sokhieng A. The politics of fear: Médecins Sans Frontières and the West African Ebola epidemics. New York, NY: Oxford University Press; 2017. [Google Scholar]
- 10.Schieffelin JS. An effective and safe vaccine will not be enough to prepare us for the next Ebola outbreak. Lancet Infect Dis. 2017;17:1224–5. doi: 10.1016/S1473-3099(17)30575-3. PMID:29033035. [DOI] [PubMed] [Google Scholar]
- 11.Coltart CEM, Lindsey B, Ghinai I, Johnson AM, Heymann DL. The Ebola outbreak, 2013–2016: old lessons for new epidemics. Philos Trans R Soc Lond B Biol Sci. 2017;372(1721):20160297. doi: 10.1098/rstb.2016.0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butler R, MacDonald NE, the SAGE Working Group on Vaccine Hesitancy . Diagnosing the determinants of vaccine hesitancy in specific subgroups: the guide to tailoring immunization programmes (TIP). Vaccine. 2015;33:4176–9. doi: 10.1016/j.vaccine.2015.04.038. PMID:25896376. [DOI] [PubMed] [Google Scholar]
- 13.Kpanake L, Sorum PC, Mullet E. The potential acceptability of infant vaccination against malaria: a mapping of parental positions in Togo. Vaccine. 2016;34:408–12. doi: 10.1016/j.vaccine.2015.12.008. PMID:26706273. [DOI] [PubMed] [Google Scholar]
- 14.Kpanake L, Gbandey S, Sorum PC, Mullet E. Acceptability of vaccination against HIV: a mapping of Togolese people's positions. J Health Psychol. 2018;23:800–6. doi: 10.1177/1359105316639440. PMID:27611628. [DOI] [PubMed] [Google Scholar]
- 15.Huo X, Shi G, Li X, Lai X, Deng L, Xu F, Chen M, Wei Q, Samba T, Liang X. Knowledge and attitudes about Ebola vaccine among the general population in Sierra Leone. Vaccine. 2016;34:1767–72. doi: 10.1016/j.vaccine.2016.02.046. PMID:26928073. [DOI] [PubMed] [Google Scholar]
- 16.Ughasoro MD, Esangbedo DO, Tagbo BN, Mejeha IC. Acceptability and willingness-to-pay for hypothetical Ebola virus vaccine in Nigeria. PLoS Negl Trop Dis. 2015;9(6):e0003838. doi: 10.1371/journal.pntd.0003838. PMID:26076007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irwin KL, Jalloh MF, Corker J, Alpha Mahmoud B, Robinson SJ, Li W, James NE, Sellu M, Jalloh MB, Diallo AA, et al.. 2015 Guinean Household Survey of Ebola Virus Disease Project Group. Attitudes about vaccines to prevent Ebola virus disease in Guinea at the end of a large Ebola epidemic: Results of a national household survey. Vaccine. 2017;35:6915–23. doi: 10.1016/j.vaccine.2017.06.026. PMID:28716555. [DOI] [PubMed] [Google Scholar]
- 18.Nossiter A. Fear of Ebola breeds a terror of physicians. The New York Times. 27July2014 Available: https://www.nytimes.com/2014/07/28/world/africa/ebola-epidemic-west-africa-guinea.html (accessed, April22, 2018).
- 19.World Health Organization Guinea: The Ebola virus shows its tenacity. One year into the Ebola epidemic - January 2015. Available: http://www.who.int/csr/disease/ebola/one-year-report/guinea/en/. (accessed on April10, 2018).
- 20.Larson HJ. Negotiating vaccine acceptance in an era of reluctance. Hum Vaccin Immunother. 2013;9:1779–81. doi: 10.4161/hv.25932. PMID:23896582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rico A, Brody D, Coronado F, Rondy M, Fiebig L, Carcelen A, Deyde VM, Mesfin S, Retzer KD, Bilivogui P, et al.. Epidemiology of epidemic Ebola virus disease in Conakry and surrounding prefectures, Guinea, 2014–2015. Emerg Infect Dis. 2016;22:178–83. doi: 10.3201/eid2202.151304. PMID:26812047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faye O, Boëlle PY, Heleze E, Faye O, Loucoubar C, Magassouba NF. Chains of transmission and control of Ebola virus disease in Conakry, Guinea, in 2014: an observational study. Lancet. 2015;15:320–6. doi: 10.1016/S1473-3099(14)71075-8. PMID:25619149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barry M, Traoré F, Sako F, Kpamy D, Bah E, Poncin M. Ebola outbreak in Conakry, Guinea: epidemiological, clinical, and outcome features. Med Mal Infect. 2014;44:491–4. doi: 10.1016/j.medmal.2014.09.009. PMID:25391486. [DOI] [PubMed] [Google Scholar]
- 24.Weinstein ND. Testing four competing theories of health-protective behavior. Health Psychol. 1993;12:324–33. doi: 10.1037/0278-6133.12.4.324. PMID:8404807. [DOI] [PubMed] [Google Scholar]
- 25.Anderson NH. Unified social cognition. New York, NY: Psychology Press; 2008. [Google Scholar]
- 26.Hofmans J, Mullet E. Towards unveiling individual differences in different stages of information processing: A clustering-based approach. Qual Quant. 2013;47:455–64. doi: 10.1007/s11135-011-9529-7. [DOI] [Google Scholar]
- 27.Schepers J, Hofmans J. TwoMP: A MATLAB graphical user interface for two-mode partitioning. Behav Res Methods. 2009;41:507–14. doi: 10.3758/BRM.41.2.507. PMID:19363191. [DOI] [PubMed] [Google Scholar]


