Abstract
Background
Among the severe malaria syndromes, severe malarial anemia (SMA) is the most common, whereas cerebral malaria (CM) is the most lethal. However, the mechanisms that lead to CM and SMA are unclear.
Methods
We compared transcriptomic profiles of whole blood obtained from Ugandan children with acute CM (n = 17) or SMA (n = 17) and community children without Plasmodium falciparum infection (n = 12) and determined the relationships among gene expression, hematological indices, and relevant plasma biomarkers.
Results
Both CM and SMA demonstrated predominantly upregulated enrichment of dendritic cell activation, inflammatory/Toll-like receptor/chemokines, and monocyte modules, but downregulated enrichment of lymphocyte modules. Nuclear factor, erythroid 2 like 2 (Nrf2)-regulated genes were overexpressed in children with SMA relative to CM, with the highest expression in children with both SMA and sickle cell disease (HbSS), corresponding with elevated plasma heme oxygenase-1 in this group. Erythroid and reticulocyte-specific signatures were markedly decreased in CM relative to SMA despite higher hemoglobin levels and appropriate increases in erythropoietin. Viral sensing/interferon-regulatory factor 2 module expression and plasma interferon-inducible protein-10/CXCL10 negatively correlated with reticulocyte-specific signatures.
Conclusions
Compared with SMA, CM is associated with downregulation of Nrf2-related and erythropoiesis signatures by whole-blood transcriptomics. Future studies are needed to confirm these findings and assess pathways that may be amenable to interventions to ameliorate CM and SMA.
Keywords: cerebral malaria, gene expression profiling, Plasmodium falciparum, severe malarial anemia, transcriptomics
Whole-blood transcriptional profiling revealed downregulation of erythropoietic and Nrf2-regulated signatures in children with cerebral malaria relative to children with severe malarial anemia. Viral sensing/interferon-regulatory factor 2 module expression and plasma interferon-inducible protein-10/CXCL10 negatively correlated with reticulocyte-specific signatures, suggesting interferon-mediated erythropoietic suppression.
In 2016, malaria afflicted ~216 million people globally [1]. Approximately 1%–3% of uncomplicated Plasmodium falciparum malaria cases progress to severe disease that can lead to death [1]. Severe malaria most commonly manifests as severe malarial anemia (SMA), defined in children as hemoglobin <5 g/dL with parasitemia and affects ~30% of children with severe malaria [2]. Malaria can also present as a more lethal syndrome of parasitemia with acute neurological deficits called cerebral malaria (CM), which has an inpatient mortality rate of 15%–20% [3].
The pathologic hallmarks of CM are microvascular obstruction and adherence of infected erythrocytes to cerebrovascular endothelium. Severe malarial anemia is characterized by accelerated erythrocyte destruction via hemolysis and enhanced erythrophagocytosis combined with ineffective erythropoiesis [4]. Both syndromes typically exhibit markedly increased inflammatory cytokines during acute disease with more pronounced inflammation in CM [5, 6]. The role of inflammation and parasite adhesion to the host endothelium in CM pathogenesis has been studied in detail [4]. A recent global analysis of whole-blood gene expression during CM revealed that neutrophil activation was positively associated with malarial retinopathy, a sign indicative of vasculopathy due to cerebral sequestration of infected erythrocytes [7]. However, less is known about the processes that contribute to ineffective erythropoiesis, erythrocyte destruction, and inflammation in SMA [8]. To gain insight on the molecular and cellular processes that differentiate CM and SMA, we compared genome-wide transcriptional profiles of whole blood obtained from Ugandan children with CM or SMA as well as healthy children without P falciparum infection and correlated gene expression with hematological indices and plasma biomarkers.
METHODS
Ethics Statement
The study was reviewed and approved by the Makerere University School of Medicine Research and Ethics Committee, the University of Minnesota Institutional Review Board, and the Ugandan National Council for Science and Technology. Written, informed consent was obtained from parents or guardians of study participants.
Study Site and Participants
From November 2008 through December 2010, 437 children between 18 months to 12 years of age with CM (n = 158) or SMA (n = 140) were enrolled as part of a larger study conducted at Mulago Hospital (Kampala, Uganda) (Supplementary Figure 1). Severe malarial anemia was defined as the presence of P falciparum on blood smear and a hemoglobin level ≤5 g/dL. Cerebral malaria was defined as (1) coma (Blantyre coma score ≤2), (2) P falciparum on blood smear, and (3) no other known cause of coma (eg, meningitis, prolonged postictal state, or hypoglycemia-associated coma). Community children ([CC] n = 139) were healthy participants recruited from the households of children with CM or SMA. Children with malaria were managed according to the Ugandan Ministry of Health treatment guidelines current at the time of diagnosis. Exclusion criteria can be found in the Supplementary Materials. For the present study, to provide the most rigorous definition of CM, we restricted CM to include only children with retinopathy confirmed by ophthalmological evaluation and hemoglobin values >5 g/dL, thus excluding CM children with concurrent SMA. Children with sickle cell disease (HbSS), sickle cell trait, or human immunodeficiency virus (HIV) were not excluded.
Blood Collection and Laboratory Testing
Peripheral blood was collected by venipuncture on hospital admission (CM and SMA) or as an outpatient (CC) for thin and thick smears, hematological testing, plasma, and filter paper blood spots. Whole blood was collected for ribonucleic acid (RNA) using the PAXgene Blood RNA System (PreAnalytiX, Hombrechtikon, Switzerland). Plasma and PAXgene samples were stored at −80°C until further processing. No power outages or other complications affected sample stability during the time of storage. Further details on blood collection and laboratory testing of hematological indices and plasma biomarkers, including plasma cytokines/chemokines, can be found in the Supplementary Materials.
Ribonucleic Acid Processing and BeadChip Microarray Procedures
Total RNA was purified from whole blood, depleted of globin messenger RNA, and processed for gene expression profiling using Illumina HumanHT-12 v4 Expression BeadChips as described in the Supplementary Materials.
Differential Gene Expression Analysis
Data processing, quality control, and differential gene expression analysis were performed in R (version 3.5.1) using the lumi and limma packages as described in the Supplementary Materials.
Statistical Analyses
Statistical analyses were performed in R (version 3.5.1). Significance testing and linear regression models were performed as indicated in figure legends with additional details in the Supplementary Materials.
RESULTS
Demographic and Clinical Factors
Among children who met inclusion criteria and had RNA of sufficient quality, we randomly sampled 20 from each clinical group for microarray processing. After excluding samples with suboptimal expression signal and CC who were siblings of CM/SMA children in the current study and/or who were found to have asymptomatic parasitemia, we analyzed microarray data from 46 children (Supplementary Figure 1). Age and gender distribution were similar between groups (Table 1). Parasite density was similarly elevated in CM and SMA relative to CC. Plasma P falciparum histidine-rich protein-2 (HRP2), a measure of total parasite biomass [9], was increased in severe malaria with significantly higher levels in CM versus SMA (Table 1). Within SMA, 6 children had HbSS, and 1 child was seropositive for HIV (Table 1). Children with HbSS defervesced after antimalarial treatment and had a median parasitemia of 14300 (interquartile range [IQR], 1910–77300) parasites/μL, which was higher than that of CC with asymptomatic parasitemia in the parent study (n = 32; 1240 parasites/μL; IQR, 565–7600), suggesting that parasitemia in HbSS children was not incidental.
Table 1.
Characteristics of Study Participants
| Characteristics | Group (n) | P Value | ||
|---|---|---|---|---|
| CC (12) | CM (17) | SMA (17) | ||
| Age in years, median (IQR) | 4.6 (3.0–4.8) | 3.2 (2.2–4.0) | 2.3 (2.0–4.4) | .19a |
| Female (% of group) | 8 (66.7%) | 6 (35.3%) | 7 (41.2%) | .30b |
| Median weight in kg (IQR) | 14.9 (11.0–16.1) | 12.0 (11.5–13.0) | 12.2 (10.0–14.0) | .28a |
| Parasite density in parasites/µL, median (IQR) | NA | 84000 (17000–340000) | 69000 (12000–200000) | .52c |
| Plasma HRP2 level in ng/mL, median (IQR) | 4.8 (4.8–12.0) | 2500 (1700–4500) | 430 (22–2000) | <.01c |
| HbS genotype (% of group) |
10 AA (83%), 2 AS (17%), 0 SS (0%) | 17 AA (100%), 0 AS (0%), 0 SS (0%) | 11 AA (65%), 0 AS (0%), 6 SS (35%) | .00059b |
| HIV positive (% of group) |
0 (0) | 0 (0)d | 1 (5.9)e | 1.0b |
Abbreviations: CC, community children; CM, cerebral malaria; HbS, sickle hemoglobulin; HIV, human immunodeficiency virus; HRP2, histidine-rich protein 2; IQR, interquartile range; NA, not applicable; SMA, severe malarial anemia.
aKruskal-Wallis test.
bFisher’s test.
cWilcoxon test between CM and SMA; data missing from 2d and 1e individual.
Hematological Indices Differ Between Clinical Groups
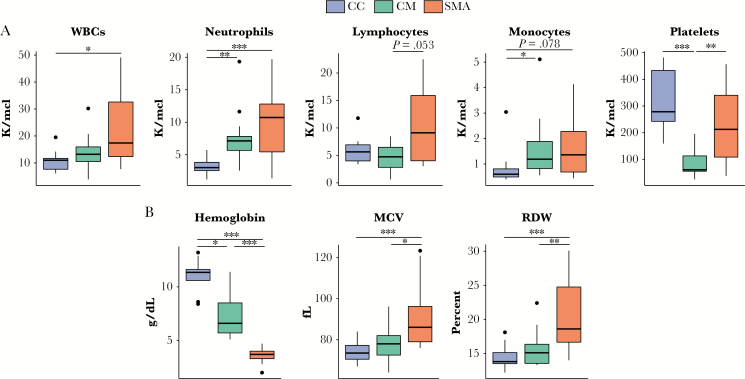
Severe malaria groups demonstrated significant neutrophilia and anemia and modest monocytosis relative to CC (Figure 1A). Children with SMA had modestly increased lymphocyte counts versus CM (Figure 1A). Children with CM exhibited significant thrombocytopenia relative to CC and SMA (Figure 1A). As expected, hemoglobin levels were lowest during SMA (Figure 1B). In SMA, we observed increased red cell distribution width (RDW) and increased red cell mean corpuscular volume (MCV), which, taken together, suggests increased reticulocytosis and thus active erythropoiesis [10] (Figure 1B). By contrast, in CM, RDW and MCV were not significantly different from CC (Figure 1B).
Figure 1.
Hematological parameters by group. (A) Absolute cell counts and (B) red blood cell indices. Samples were obtained at study enrollment (community children [CC]) or at the time of hospital admission (cerebral malaria [CM] and severe malarial anemia [SMA]). Box plots show median and interquartile ranges with outliers as points. Pairwise significance was determined by Kruskal-Wallis with post hoc Dunn’s test and Bonferroni’s adjustment for multiple comparisons. Significance indicated by *, P < .05, **, P < .01, and ***, P < .001; all other comparisons were not statistically significant. Abbreviations: MCV, mean corpuscular volume; RDW, red cell distribution width; WBCs, white blood cells.
Gene Expression Differences Are Broadly Driven by Clinical Syndrome, Parasite Load, and HbSS
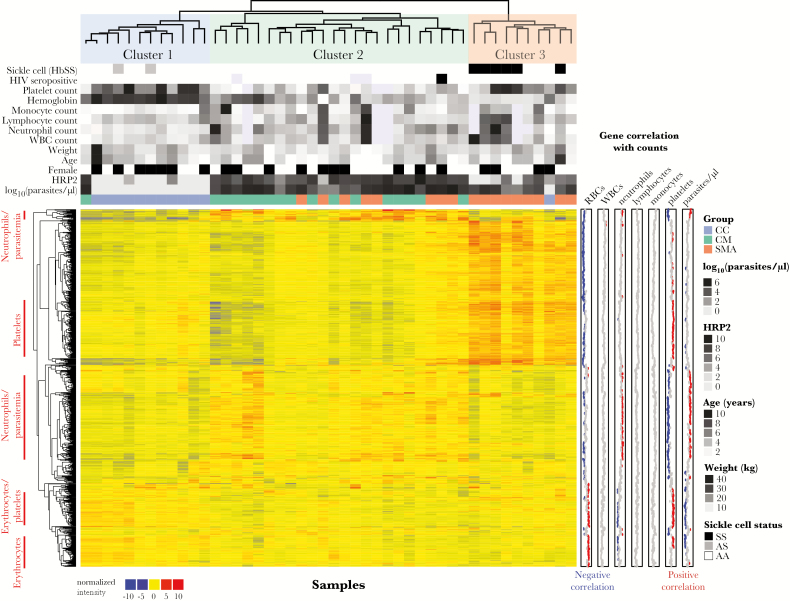
We performed gene expression analysis of peripheral whole blood by microarray to compare global transcription profiles among CM, SMA, and CC. Principal component (PC) analysis of expression data revealed clustering by syndrome (Supplementary Figure 2A). Severe malarial anemia status best explained variation along PC1, with HbSS children appearing as a subcluster within the SMA cluster. Similar to previous studies, children with malaria clustered mainly based on clinical syndrome and parasite load (Supplementary Figure 2B and C) [7, 11, 12]. Unsupervised hierarchical clustering analysis of the most variably expressed genes revealed 3 distinct clusters, with SMA children clustering either within a CM-dominant Cluster 2 or within a SMA-dominant Cluster 3 that included all HbSS children (Figure 2). Platelet count, hemoglobin, and HRP2, but not parasite density, were significantly different between Clusters 2 and 3 (Supplementary Table 1). Variable expression generated distinct gene clusters that correlated with both neutrophil count and parasitemia (Figure 2). Subsequent analyses include statistical adjustments for HbSS and HRP2 to minimize their confounding influence on gene expression. We did not adjust for hemoglobin or platelets given that hemoglobin levels define SMA and that thrombocytopenia possibly plays a role in CM pathogenesis [5, 13].
Figure 2.
Unsupervised hierarchical clustering heat map of most variably expressed genes reveal distinct cell lineage-specific signatures that distinguishes clinical groups. Only the top 1000 most variably expressed genes (determined by median absolute deviation) across all samples are shown. Left row annotations highlight cell lineages that correlate positively with genes within marked rows. Right row annotations show Pearson’s coefficients for correlations between gene expression with the indicated parameter using available data across all samples. Significant correlations (false discovery rate <10%) are shown as red (positive) or blue (negative) points. For column annotations above the heat map that are not listed in the legend, increasing grayscale intensities represent increasing quantiles for continuous variables, and black indicates presence of a binary variable. Missing values are indicated by lavender boxes. Clustering was performed using Pearson correlation and ward.D2. Abbreviations: CC, community children; CM, cerebral malaria; HbSS, sickle cell disease; HIV, human immunodeficiency virus; HRP2, histidine-rich protein 2; RBCs, red blood cells; SMA, severe malarial anemia; WBCs, white blood cells.
Severe Malaria Syndromes Demonstrate Upregulated Enrichment of Innate Inflammatory Modules and Differ in Erythroid-Related Modules
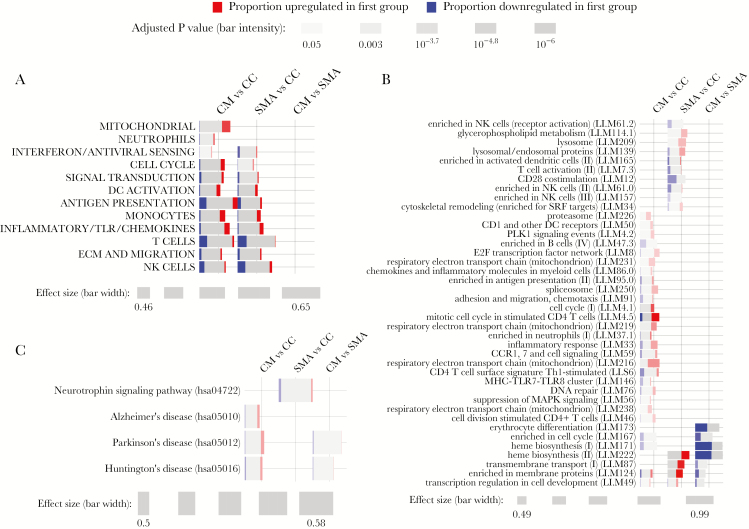
To determine broad differences in immunological and disease processes between blood transcriptomes, we tested for differential enrichment of blood transcription modules (BTMs) [14] and KEGG biological pathways using the tmod package [15] with inclusion of HbSS and HRP2 levels as covariates. We compared CM versus CC and SMA versus CC to evaluate transcriptomic changes relative to healthy controls and CM versus SMA to evaluate differences between the 2 severe malaria syndromes. Using high-level annotation BTMs [16], both CM and SMA demonstrated predominant upregulated enrichment of dendritic cell activation, monocyte, and inflammatory/Toll-like receptor/chemokines modules, but downregulated enrichment of natural killer and T cell modules (Figure 3A). Upregulated enrichment of mitochondrial and neutrophil modules appeared unique to CM (Figure 3A). Differential enrichment of high-level modules did not reach statistical significance in the direct CM versus SMA comparison (Figure 3A). Analysis using 346 low-level BTMs revealed subtle differences in enrichment of specific innate and adaptive modules in CM versus CC and SMA versus CC (Figure 3B). Erythroid-related modules (M171, M173, M222) demonstrated significantly downregulated enrichment in CM relative to SMA (Figure 3B) despite more profound anemia in SMA (Figure 1B).
Figure 3.
Gene enrichment testing between clinical groups using blood transcription modules and KEGG pathways. CERNO testing in Tmod [15] using high-level (A) or low-level (B) annotations for blood transcription modules [14, 16] and (C) KEGG pathways involving neurological processes or diseases for the indicated comparisons between the clinical groups cerebral malaria (CM), several malarial anemia (SMA), and healthy community children (CC) with adjustment for sickle cell disease (HbSS) status and plasma HRP2 levels. Only modules with a Benjamini-Hochberg-adjusted P < .05 are shown. Effect size represents the area under the curve in the CERNO test. For (B), visualized modules were limited to all significant modules for the CM vs SMA comparison and modules that were uniquely significant to the CM versus CC or the SMA versus CC comparisons.
Analysis using KEGG pathways demonstrated enrichment for a greater number of pathways in CM versus CC than in SMA versus CC (Supplementary Figure 3). We observed modest but significant enrichment in 3 neurodegenerative disease pathways in CM relative to CC (Figure 3C). Of these, Parkinson’s and Huntington’s Disease pathways were differentially expressed between CM and SMA.
Cerebral Malaria Induces Greater Transcriptional Activity Than Severe Malarial Anemia
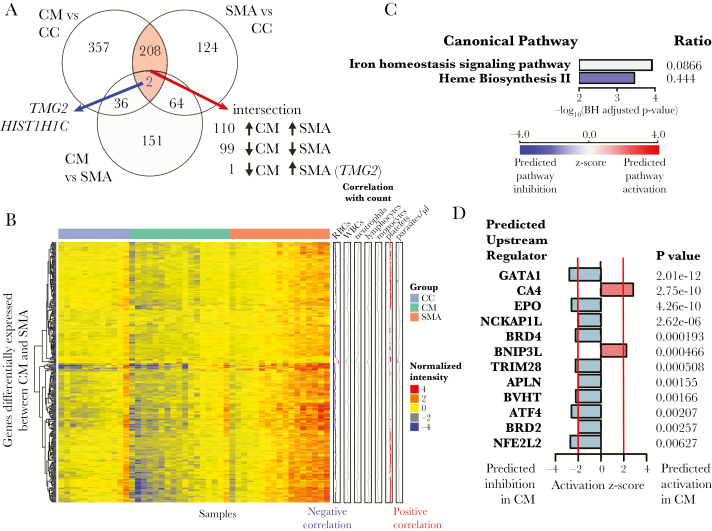
Differential gene expression analysis between groups revealed 603 differentially expressed genes (DEGs) for CM versus CC and 398 DEGs for SMA versus CC (log2-fold change [LFC] threshold 0.585 and false discovery rate [FDR] <10%) (Figure 4A, Supplementary Tables 2 and 3). Of the 210 genes shared between the CM versus CC and SMA versus CC comparisons, 110 genes were concordantly upregulated in both CM and SMA and 99 genes were concordantly downregulated in both CM and SMA (Figure 4A). The only discordantly expressed gene, TGM2 (encodes for transglutaminase 2), was decreased in CM versus CC but increased in SMA versus CC (Figure 4A). Of the 253 DEGs in the CM versus SMA comparison (Figure 4A), 250 were underexpressed in CM, whereas only 3 genes were overexpressed in CM (KLRB1, GZMK, and CXCR6) (Figure 4B, Supplementary Table 4). None of the DEGs positively correlated with leukocytes or parasite density (Figure 4B).
Figure 4.
Differential gene expression and pathways analysis. (A) Differentially expressed genes (DEGs) were determined for the indicated comparisons with adjustment for sickle cell disease (HbSS) and plasma HRP2 levels using an absolute log2 fold-change (LFC) cut-off of 0.585 (1.5 in linear space) and false discovery rate (FDR) of 10%. (B) Heat map of DEGs for the CM vs SMA comparison. Rows are clustered using Pearson correlation and ward.D2. Columns are ordered by group followed by column median intensity. “Correlation with cell count” row annotations show Pearson’s coefficients for correlations between expression for each gene with indicated cell count using data from all samples. (C) Iron homeostasis signaling and heme biosynthesis II were the only differentially expressed canonical pathways using DEGs for the CM vs SMA comparison at a Benjamini-Hochberg (BH)-adjusted P < .05. Ratio indicates the proportion of DEGs that overlap with genes in the pathway. (D) Predicted upstream regulators using DEGs for the CM versus SMA comparison. Only predicted regulators with |activation Z-score| >2 and P < .01 are shown.
Pathways Analysis Reveals Inhibition of the Erythropoietic Response During Cerebral Malaria
We applied Ingenuity pathways analysis using the DEGs (|LFC| > 0.585, FDR <10%) from each HbSS- and HRP2-adjusted comparison. Among the top overrepresented immune pathways between CM and CC, we observed predicted inhibition of T-cell-related signaling pathways in CM, including those involved with OX40, inducible costimulatory (iCOS)-iCOS ligand (iCOSL), and Th1 signaling, and predicted activation of complement (Supplementary Figure 4). In SMA versus CC, T-cell-related pathways, including predicted inhibition of iCOS-iCOSL signaling, also featured prominently (Supplementary Figure 5). Direct comparison of CM versus SMA revealed iron homeostasis signaling and heme biosynthesis II as the only differentially expressed pathways (Figure 4C). Using upstream regulator analysis, which predicts activation states of regulators based on expression of downstream targets, carbonic anhydrase 4 (CA4) and the proapoptotic protein BCL2 interacting protein 3 like (BNIP3L) were predicted to be activated in CM versus SMA (Figure 4D). The erythroid transcription factor GATA1, the erythropoiesis-stimulating hormone erythropoietin (EPO), and nuclear factor, erythroid 2 like 2 ([Nrf2] encoded by NFE2L2), a transcription factor that regulates responses against oxidative stress [17], were predicted to be strongly inhibited in CM versus SMA (Figure 4D).
Nuclear Factor, Erythroid 2 Like 2 Related Gene Signatures in Whole Blood Discriminate Severe Malaria Syndromes
The Nrf2 pathway is considered a therapeutic target for mitigating the oxidative stress and neuroinflammation associated with neurodegenerative diseases [17]. In addition, Nrf2 has been shown to protect against experimental CM (ECM) by inducing heme oxygenase 1 ([HO-1] encoded by HMOX1), an enzyme that catabolizes free heme into ferrous iron, carbon monoxide, and biliverdin [18]. To determine whether Nrf2-related gene signatures in blood could distinguish the severe malarial syndromes, we performed unsupervised clustering using Nrf2-regulated genes from the Ingenuity database. These 23 genes separated children into clusters broadly representing the 3 clinical groups (Supplementary Figure 6). The Nrf2-regulated genes, but not NFE2L2 itself, were increased in SMA relative to CM, with the highest expression in SMA children with HbSS (Supplementary Figure 7A and B). Whole-blood HMOX1 expression and plasma HO-1 levels were increased in all severe malaria groups relative to CC, with variable expression among SMA with HbSS ([SMA HbSS] Supplementary Figure 7C and D). To better ascertain group differences, we assessed plasma HO-1 levels in 546 children from the parent cohort. Consistent with previous findings [19], plasma HO-1 was increased in severe malaria. No difference in HO-1 was observed between CM and SMA non-HbSS; however, children with SMA HbSS had significantly higher HO-1 levels than CM and SMA non-HbSS (Supplementary Figure 7E). We also measured total bilirubin (a catabolite of biliverdin, which itself is a product of HO-1 catalysis) in 645 children. Bilirubin was increased in all severe malaria groups, with the highest levels in CM and SMA HbSS, both of which were significantly greater than SMA non-HbSS (Supplementary Figure 7F). These findings suggest that activation of the Nrf2/HO-1 axis might contribute to the relative protection from CM observed in children with HbSS in the parent cohort [20].
Ineffective Erythropoiesis Is More Pronounced During Cerebral Malaria Than Severe Malarial Anemia
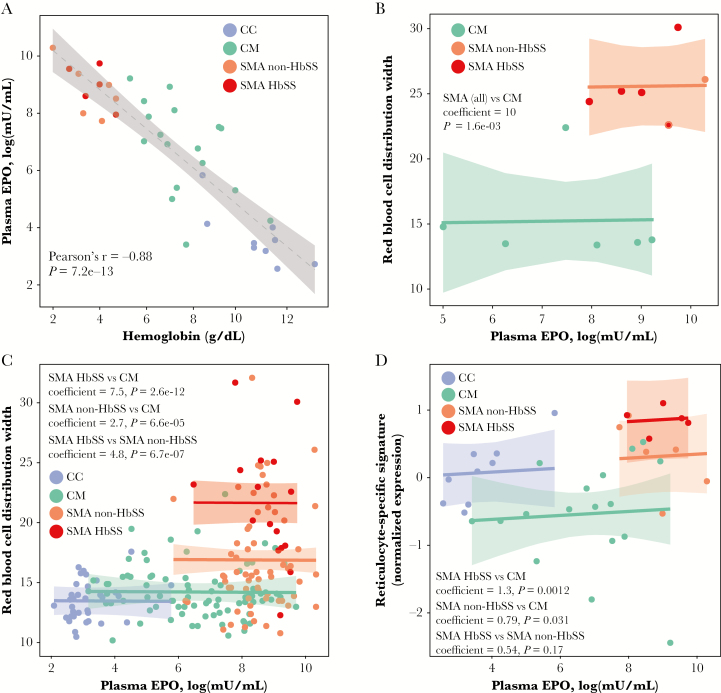
Given the hematological and transcriptional evidence for suppressed erythropoiesis during CM, we investigated plasma EPO levels. The EPO levels increased with decreasing hemoglobin in the expected manner, indicative of adequate EPO production for all groups (Figure 5A). Using data from the parent cohort (n = 196), we assessed erythropoietic response to EPO using RDW as a surrogate for immature erythrocytes and active erythropoiesis [10, 21], taking care to exclude children in whom increased RDW may have been due to microcytosis (MCV <80 femtoliters). The RDW was significantly higher in SMA relative to CM even after adjustment for EPO levels in both the subset of children with microarray data (n = 12; Figure 5B) and in the parent cohort (n = 196) [22], with the highest RDW occurring in SMA HbSS (Figure 5C). Similarly, expression of a previously defined reticulocyte-specific gene signature [23], a more direct measure of erythropoiesis than RDW, was also decreased in CM versus SMA regardless of HbSS status after adjustment for EPO levels (Figure 5D). These data provide in vivo evidence suggestive of ineffective erythropoiesis during severe malaria with greater erythropoietic inhibition during CM compared with SMA.
Figure 5.
Relationship between erythropoietin and erythropoiesis during severe malaria. (A) Correlation between hemoglobin and log-transformed plasma erythropoietin (EPO) levels by group. Dotted line presents linear fit with 95% confidence band. Relationship between log-transformed plasma EPO levels and red blood cell distribution width (RDW) by clinical group (B) in subjects with available data for this study (n = 12) and (C) for the entire cohort (n = 196). (D) Relationship between plasma EPO levels and expression levels for a reticulocyte-specific gene signature by clinical group (n = 37). Reticulocyte-specific gene signature expression was calculated as the mean of z-score-transformed expression values of genes within the reticulocyte gene set from Goh et al [23]. For B–D, shown are actuals (points), fit lines with 95% confidence bands, and the significance for the group comparisons using separate parallel slopes linear regression models in which RDW or reticulocyte-specific gene expression was the response variable and EPO levels and group were predictor variables. For B–C, children with mean corpuscular volume <80 femtoliters were excluded from the models. For B, no CC children had complete data for inclusion in the analysis.
The Interferon Response Negatively Predicts Reticulocyte-Specific Gene Expression in Severe Malaria
To determine molecular signatures of erythropoietic suppression during severe malaria, we correlated expression of the reticulocyte-specific gene signature with parasite density, HRP2 levels, hematological indices, or expression of each of 346 BTMs. As expected, reticulocyte-specific expression positively correlated with variables related to erythropoiesis (Supplementary Figure 8). Reticulocyte-specific expression demonstrated moderate to strong negative correlations with viral sensing and immunity/interferon-regulatory factor 2 (IRF2) targets networks (M111.0 and M111.1); chemokines and receptors (M38); CCR1,7 and cell signaling (M59); and T-cell surface activation (M36) modules (Supplementary Figure 8).
Proinflammatory cytokines elicited during acute malaria can contribute to ineffective erythropoiesis [24] and disease severity in CM [25]. We measured inflammatory cytokines and chemokines in plasma and correlated these levels with the expression of Nrf2-regulated genes and the reticulocyte-specific gene signature. Reticulocyte-specific gene expression negatively correlated with IP-10 levels, a chemokine known to inhibit hematopoiesis [26] (Supplementary Figure 9A). The Nrf2-regulated gene expression did not correlate with any of the cytokines/chemokines measured but highly correlated with reticulocyte-specific gene expression, suggesting that these 2 pathways may be coregulated despite minimal geneset overlap (Supplementary Figure 9B).
To determine whether plasma IP-10 or M111.1, which includes CXCL10 (encodes for IP-10), independently contributed to ineffective erythropoiesis, we performed multiple linear regression using reticulocyte-specific gene expression as the response variable and hemoglobin, leukocyte count, HRP2, clinical group, M111.0 expression, and IP-10 as predictor variables (Table 2). Inclusion of HRP2 was based on its role in formation of hemozoin [27], which, in turn, can inhibit erythropoiesis [28]. Both M111.0 expression and leukocyte count, but not IP-10, negatively predicted reticulocyte-specific expression. Consistent with chronic, compensated hemolysis seen in sickle cell anemia [29], HbSS status predicted higher reticulocyte-gene expression relative to CM after controlling for other factors.
Table 2.
Multiple Linear Regression to Determine the Effect of M111.0 Expression and Plasma IP-10 Levels on Reticulocyte-Specific Gene Expressiona
| Variable | Estimate | Standard Error | t Value | P Value |
|---|---|---|---|---|
| (Intercept) | 0.88 | 1.4 | 0.62 | .54 |
| M111.0: viral sensing and immunity; IRF2 targets network (I) | −0.68 | 0.21 | −3.2 | .0029 |
| Log IP-10 levels (pg/mL) | −0.097 | 0.11 | −0.86 | .40 |
| Absolute white blood cell count (K/mcL) | −0.028 | 0.0095 | −2.98 | .0055 |
| Log hemoglobin (g/dL) | −0.057 | 0.41 | −0.14 | .89 |
| HRP2 (ng/mL) | 6.1E-05 | 3.6E-05 | 1.7 | .10 |
| Clinical Group | ||||
| CM | Reference | |||
| CC | 0.25 | 0.31 | 0.80 | .43 |
| SMA non-HbSS | 0.57 | 0.36 | 1.6 | .13 |
| SMA HbSS | 1.47 | 0.39 | 3.8 | .00071 |
Abbreviations: CC, community children; CM, cerebral malaria; HbSS, sickle cell disease; HRP2, histidine-rich protein 2; IP-10, interferon-inducible protein-10; IRF2, interferon regulatory factor 2; SMA, severe malarial anemia.
aAnalysis was performed on 41 children for whom data were available for all variables.
DISCUSSION
The current study expands on prior studies that have examined the blood transcriptome during human P falciparum infections [7, 11, 12, 30–36]. Consistent with previous studies, we found that parasite load influenced blood transcriptional variation during severe malaria [7, 12, 30]. We also detected enrichment of neurodegenerative disease signatures in blood of children with CM, similar to a previous report [35]. Also of note, our current study provides further insight into malaria pathogenesis by comparing whole-blood transcriptional profiles between the 2 major severe malaria syndromes: retinopathy-positive CM and SMA. We found that both the Nrf2-pathway and erythropoiesis are significantly inhibited in CM relative to SMA, with differential hemolysis and inflammation between these the 2 syndromes being likely contributors to these findings.
The transcription factor Nrf2 regulates the expression of antioxidant and anti-inflammatory proteins in response to an oxidative stress such as infection, hemolysis, or neurodegeneration [37]. During ECM, Nrf2 induces HO-1, an enzyme that catabolizes heme, leading to protection from neurological disease in mice via carbon monoxide production [18, 38]. In humans, HO-1 induced by hemolysis, either from malaria [39] or sickle cell disease [40], can impair neutrophil function, leading to increased host susceptibility to bacterial infection. However, a direct role for HO-1 in malaria pathogenesis is less clear. Similar to a study in Gambia [19], we showed increased plasma HO-1 levels during severe malaria, implicating HO-1 as a marker of malaria pathology. However, we also demonstrated differential expression of Nrf2-regulated genes between CM and SMA, which was found to be influenced by HbSS. Increased Nrf2 pathway activation corresponded with increased plasma HO-1 and the heme catabolite bilirubin in a manner specific to children with both SMA and HbSS—findings that are consistent with increased oxidative stress due to acute or chronic hemolysis. We speculate that, rather than being a predictor of disease severity, elevated HO-1 may specifically protect children with HbSS from CM, a hypothesis supported by mechanistic evidence demonstrating that sickle human hemoglobin induces HO-1 via Nrf2 in mice, leading to protection from ECM [41]. Although the current study excluded children with CM and Hb <5 g/dL, children with HbSS in the parent study were significantly less likely to present with CM than nonsickle cell children [20]. Studies examining the relationship between HO-1 levels and the prospective risk of CM in sickle cell children may help clarify the potential malaria-protective role of HO-1.
Malaria causes bone marrow dyserythropoiesis leading to reduced erythropoiesis [8]. Suggested mechanisms include direct suppression of erythropoiesis by the malaria pigment hemozoin, which is phagocytosed by bone marrow macrophages [42], or by tumor necrosis factor (TNF) produced in abundance during severe malaria [43]. Several studies suggest that the balance of inflammatory cytokines, in particular low plasma interleukin (IL)-10 [44] or low plasma IL-10/TNF ratios [6, 45, 46], contribute to the severity of malarial anemia. We observed significantly suppressed erythropoiesis in CM versus SMA using both transcriptomic and hematological data. Consistent with a study in Ghanaian children [21], we found that the erythropoiesis-surrogate marker RDW did not increase with increasing EPO levels in both CM and SMA on admission, providing further evidence that acute malaria, irrespective of syndrome, inhibits erythropoiesis independently of EPO. However, in contrast to the smaller Ghanaian study (n = 18), which did not find differences in RDW between SMA and CM, we observed significantly greater erythropoiesis in SMA than CM as measured by RDW (n = 196) and reticulocyte-specific gene signatures (n = 37). Implicit in this finding is that anemia during SMA may be driven more by erythrocyte destruction rather than by reduced erythropoiesis, a view supported by other studies [47, 48].
Enhanced proinflammatory responses may provide a convenient explanation for the increased erythropoietic suppression observed in CM. We and others have reliably demonstrated greater plasma levels of proinflammatory cytokines during CM relative to non-CM malaria syndromes [5, 6, 49]. However, we did not detect robust transcriptional differences in inflammatory genes between CM and SMA. One explanation may be that highly variable or increased lymphocyte counts in SMA masked potential transcriptional differences between groups. Differences between CM and SMA may also be influenced by HbSS status, which we adjusted for in our analysis. HbSS not only exacerbates anemia, and thus is disproportionately represented among SMA cases, but also results in diminished proinflammatory responses during malaria [50]. Nevertheless, we observed that expression of the viral-sensing M111.1 module and IP-10/CXCL10 levels negatively correlated with reticulocyte-gene expression, with M111.1, but not IP-10, remaining an independent negative predictor after controlling for relevant confounders. It is notable that M111.1 contains several other interferon-induced genes such as IFIT2, BST2, and DDX58 in addition to CXCL10 [14]. Thus, our data provide in vivo evidence that an interferon-mediated response is an independent predictor of ineffective erythropoiesis during severe malaria.
The current study has several limitations. The cross-sectional design limits causal inference of molecular measures and disease phenotype. In addition, expression differences between CM and SMA ideally should be validated in an independent cohort. Profound differences in blood composition between disease states undoubtedly confound transcriptome profiles of whole blood more so than peripheral blood mononuclear cells (PBMCs). However, this is both a limitation and an asset for this study, because gene signatures related to erythroid and granulocytic lineages would not have been detected in PBMCs. Despite these limitations, our study provides a first assessment of blood transcriptomic differences between CM and SMA.
CONCLUSIONS
In summary, we provide evidence suggesting that oxidative stress and erythropoietic responses differ between CM and SMA. We show that Nrf2-regulated genes, HO-1, and bilirubin are increased in children with SMA and HbSS versus CM. We also demonstrate that erythropoietic suppression during severe malaria may be more prominent during CM than SMA and is independently associated with interferon-response signatures in blood. Future studies are needed to validate these findings and determine their functional and clinical significance.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the study participants and our collaborators at Mulago Hospital (Kampala, Uganda).
Finanical support. This work was funded by the National Institute of Neurological Disorders and Stroke (Grant Number 5R01NS055349; to C. C. J.) and the National Institute of Allergy and Infectious Diseases (Grant Number 5K08AI125682; to T. M. T.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Data availability. Gene expression data is available in the Gene Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/geo/) under the accession number GSE117613.
Presented in part: Central Society of Clinical and Translational Research Annual Meeting on April 27, 2018, Chicago, IL.
References
- 1. World Health Organization. World Malaria Report 2017. Geneva: World Health Organization, 2017. [Google Scholar]
- 2. Opoka RO, Hamre KE, Brand N, Bangirana P, Idro R, John CC. High postdischarge morbidity in Ugandan children with severe malarial anemia or cerebral malaria. J Pediatric Infect Dis Soc 2017; 6:e41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Idro R, Marsh K, John CC, Newton CR. Cerebral malaria: mechanisms of brain injury and strategies for improved neurocognitive outcome. Pediatr Res 2010; 68:267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mackintosh CL, Beeson JG, Marsh K. Clinical features and pathogenesis of severe malaria. Trends Parasitol 2004; 20:597–603. [DOI] [PubMed] [Google Scholar]
- 5. Thuma PE, van Dijk J, Bucala R, et al. Distinct clinical and immunologic profiles in severe malarial anemia and cerebral malaria in Zambia. J Infect Dis 2011; 203:211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mandala WL, Msefula CL, Gondwe EN, Drayson MT, Molyneux ME, MacLennan CA. Cytokine profiles in Malawian children presenting with uncomplicated malaria, severe malarial anemia, and cerebral malaria. Clin Vaccine Immunol 2017; 24:e00533–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feintuch CM, Saidi A, Seydel K, et al. Activated neutrophils are associated with pediatric cerebral malaria vasculopathy in Malawian children. MBio 2016; 7:e01300–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perkins DJ, Were T, Davenport GC, Kempaiah P, Hittner JB, Ong’echa JM. Severe malarial anemia: innate immunity and pathogenesis. Int J Biol Sci 2011; 7:1427–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dondorp AM, Desakorn V, Pongtavornpinyo W, et al. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS Med 2005; 2:e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roberts GT, El Badawi SB. Red blood cell distribution width index in some hematologic diseases. Am J Clin Pathol 1985; 83:222–6. [DOI] [PubMed] [Google Scholar]
- 11. Idaghdour Y, Quinlan J, Goulet JP, et al. Evidence for additive and interaction effects of host genotype and infection in malaria. Proc Natl Acad Sci U S A 2012; 109:16786–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee HJ, Georgiadou A, Walther M, et al. Integrated pathogen load and dual transcriptome analysis of systemic host-pathogen interactions in severe malaria. Sci Transl Med 2018; 10. doi: 10.1126/scitranslmed.aar3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cox D, McConkey S. The role of platelets in the pathogenesis of cerebral malaria. Cell Mol Life Sci 2010; 67:557–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li S, Rouphael N, Duraisingham S, et al. Molecular signatures of antibody responses derived from a systems biology study of five human vaccines. Nat Immunol 2014; 15:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weiner J 3rd, Domaszewska T. tmod: an R package for general and multivariate enrichment analysis. PeerJ Preprints 2016; 4:e2420v1. [Google Scholar]
- 16. Kazmin D, Nakaya HI, Lee EK, et al. Systems analysis of protective immune responses to RTS,S malaria vaccination in humans. Proc Natl Acad Sci U S A 2017; 114:2425–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buendia I, Michalska P, Navarro E, Gameiro I, Egea J, León R. Nrf2-ARE pathway: an emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol Ther 2016; 157:84–104. [DOI] [PubMed] [Google Scholar]
- 18. Jeney V, Ramos S, Bergman ML, et al. Control of disease tolerance to malaria by nitric oxide and carbon monoxide. Cell Rep 2014; 8:126–36. [DOI] [PubMed] [Google Scholar]
- 19. Walther M, De Caul A, Aka P, et al. HMOX1 gene promoter alleles and high HO-1 levels are associated with severe malaria in Gambian children. PLoS Pathog 2012; 8:e1002579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Opoka RO, Bangirana P, Idro R, Shabani E, Namazzi R, John CC. Lack of mortality in 22 children with sickle cell anemia and severe malarial anemia. Pediatr Blood Cancer 2018; 65. doi: 10.1002/pbc.26745. Epub 2017 Aug 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kurtzhals JA, Rodrigues O, Addae M, Commey JO, Nkrumah FK, Hviid L. Reversible suppression of bone marrow response to erythropoietin in Plasmodium falciparum malaria. Br J Haematol 1997; 97:169–74. [DOI] [PubMed] [Google Scholar]
- 22. Shabani E, Opoka RO, Idro R, et al. High plasma erythropoietin levels are associated with prolonged coma duration and increased mortality in children with cerebral malaria. Clin Infect Dis 2015; 60:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goh SH, Josleyn M, Lee YT, et al. The human reticulocyte transcriptome. Physiol Genomics 2007; 30:172–8. [DOI] [PubMed] [Google Scholar]
- 24. Haldar K, Mohandas N. Malaria, erythrocytic infection, and anemia. Hematology Am Soc Hematol Educ Program 2009:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. John CC, Park GS, Sam-Agudu N, Opoka RO, Boivin MJ. Elevated serum levels of IL-1ra in children with Plasmodium falciparum malaria are associated with increased severity of disease. Cytokine 2008; 41:204–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sarris AH, Broxmeyer HE, Wirthmueller U, et al. Human interferon-inducible protein 10: expression and purification of recombinant protein demonstrate inhibition of early human hematopoietic progenitors. J Exp Med 1993; 178:1127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sullivan DJ Jr, Gluzman IY, Goldberg DE. Plasmodium hemozoin formation mediated by histidine-rich proteins. Science 1996; 271:219–22. [DOI] [PubMed] [Google Scholar]
- 28. Lamikanra AA, Merryweather-Clarke AT, Tipping AJ, Roberts DJ. Distinct mechanisms of inadequate erythropoiesis induced by tumor necrosis factor alpha or malarial pigment. PLoS One 2015; 10:e0119836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nsiah K, Dzogbefia VP, Ansong D, et al. The incidence of malaria and the comparison of hematological and biochemical indices of Plasmodium falciparum-parasitemic and aparasitemic sickle cell disease (SCD) patients. Int J Lab Hematol 2010; 32:e197–207. [DOI] [PubMed] [Google Scholar]
- 30. Griffiths MJ, Shafi MJ, Popper SJ, et al. Genomewide analysis of the host response to malaria in Kenyan children. J Infect Dis 2005; 191:1599–611. [DOI] [PubMed] [Google Scholar]
- 31. Ockenhouse CF, Hu WC, Kester KE, et al. Common and divergent immune response signaling pathways discovered in peripheral blood mononuclear cell gene expression patterns in presymptomatic and clinically apparent malaria. Infect Immun 2006; 74:5561–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Franklin BS, Parroche P, Ataide MA, et al. Malaria primes the innate immune response due to interferon-gamma induced enhancement of Toll-like receptor expression and function. Proc Natl Acad Sci U S A 2009; 106: 5789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yamagishi J, Natori A, Tolba ME, et al. Interactive transcriptome analysis of malaria patients and infecting Plasmodium falciparum. Genome Res 2014; 24:1433–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tran TM, Jones MB, Ongoiba A, et al. Transcriptomic evidence for modulation of host inflammatory responses during febrile Plasmodium falciparum malaria. Sci Rep 2016; 6:31291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cabantous S, Doumbo O, Poudiougou B, et al. Gene expression analysis reveals genes common to cerebral malaria and neurodegenerative disorders. J Infect Dis 2017; 216:771–5. [DOI] [PubMed] [Google Scholar]
- 36. Sobota RS, Dara A, Manning JE, et al. Expression of complement and Toll-like receptor pathway genes is associated with malaria severity in Mali: a pilot case control study. Malar J 2016; 15:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 2013; 53:401–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pena AC, Penacho N, Mancio-Silva L, et al. A novel carbon monoxide-releasing molecule fully protects mice from severe malaria. Antimicrob Agents Chemother 2012; 56:1281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cunnington AJ, Njie M, Correa S, Takem EN, Riley EM, Walther M. Prolonged neutrophil dysfunction after Plasmodium falciparum malaria is related to hemolysis and heme oxygenase-1 induction. J Immunol 2012; 189:5336–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Evans C, Orf K, Horvath E, et al. Impairment of neutrophil oxidative burst in children with sickle cell disease is associated with heme oxygenase-1. Haematologica 2015; 100:1508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ferreira A, Marguti I, Bechmann I, et al. Sickle hemoglobin confers tolerance to Plasmodium infection. Cell 2011; 145:398–409. [DOI] [PubMed] [Google Scholar]
- 42. Skorokhod OA, Caione L, Marrocco T, et al. Inhibition of erythropoiesis in malaria anemia: role of hemozoin and hemozoin-generated 4-hydroxynonenal. Blood 2010; 116:4328–37. [DOI] [PubMed] [Google Scholar]
- 43. Grau GE, Taylor TE, Molyneux ME, et al. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med 1989; 320:1586–91. [DOI] [PubMed] [Google Scholar]
- 44. Kurtzhals JA, Adabayeri V, Goka BQ, et al. Low plasma concentrations of interleukin 10 in severe malarial anaemia compared with cerebral and uncomplicated malaria. Lancet 1998; 351:1768–72. [DOI] [PubMed] [Google Scholar]
- 45. Othoro C, Lal AA, Nahlen B, Koech D, Orago AS, Udhayakumar V. A low interleukin-10 tumor necrosis factor-alpha ratio is associated with malaria anemia in children residing in a holoendemic malaria region in western Kenya. J Infect Dis 1999; 179:279–82. [DOI] [PubMed] [Google Scholar]
- 46. Nussenblatt V, Mukasa G, Metzger A, Ndeezi G, Garrett E, Semba RD. Anemia and interleukin-10, tumor necrosis factor alpha, and erythropoietin levels among children with acute, uncomplicated Plasmodium falciparum malaria. Clin Diagn Lab Immunol 2001; 8:1164–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Waitumbi JN, Opollo MO, Muga RO, Misore AO, Stoute JA. Red cell surface changes and erythrophagocytosis in children with severe Plasmodium falciparum anemia. Blood 2000; 95:1481–6. [PubMed] [Google Scholar]
- 48. Fonseca LL, Alezi HS, Moreno A, Barnwell JW, Galinski MR, Voit EO. Quantifying the removal of red blood cells in Macaca mulatta during a Plasmodium coatneyi infection. Malar J 2016; 15:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lyke KE, Burges R, Cissoko Y, et al. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect Immun 2004; 72:5630–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tatfeng YM, Agbonlahor DE, Amegor OF. Measurement of Th1, Th2 cytokines and white cell count in childhood haemoglobinopathies with uncomplicated malaria infection. Hematology 2012; 17:47–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.