Abstract
Background
The distinguishing characteristics of extraintestinal pathogenic Escherichia coli (ExPEC) strains are incompletely defined.
Methods
We characterized 292 diverse-source human Escherichia coli isolates (116 from fecal specimens, 79 from urine specimens [of which 39 were from patients with cystitis and 40 were from patients with pyelonephritis], and 97 from blood specimens) for phylogenetic group, sequence type complex (STc), and 49 putative extraintestinal pathogenic E. coli (ExPEC)–associated virulence genes. We then assessed these traits and ecological source as predictors of illness severity in a murine sepsis model.
Results
The study isolates exhibited a broad range of virulence in mice. Most of the studied bacterial characteristics corresponded significantly with experimental virulence, as did ecological source and established molecular definitions of ExPEC and uropathogenic E. coli (UPEC). Multivariable modeling identified the following bacterial traits as independent predictors of illness severity both overall and among the fecal and clinical (ie, urine and blood) isolates separately: fyuA (yersiniabactin receptor), kpsM K1 (K1 capsule), and kpsM II (group 2 capsules). Molecular UPEC status predicted virulence independently only among fecal isolates. Neither ecological source (ie, clinical vs fecal) nor molecular ExPEC status added predictive power to these traits, which accounted collectively for up to 49% of the observed variation in virulence.
Conclusions
Among human-source E. coli isolates, specific accessory traits and phylogenetic/clonal backgrounds predict experimental virulence in a murine sepsis model better than does ecological source.
Keywords: Escherichia coli, virulence, sepsis, mouse models, phylogenetic groups, virulence factors, sequence types, clinical isolates, fecal isolates
Among human-source Escherichia coli isolates, specific accessory traits and phylogenetic/clonal backgrounds predicted experimental illness severity in a murine sepsis model better than did ecological source (ie, fecal vs clinical origin).
Extraintestinal Escherichia coli infections cause considerable morbidity and mortality and increased healthcare costs [1]. Most such infections are due to distinctive E. coli strains, termed extraintestinal pathogenic E. coli (ExPEC) [2] or uropathogenic E. coli (UPEC) on the basis of their enhanced ability to cause extraintestinal disease, including urinary tract infection [3]. However, the distinguishing characteristics of ExPEC or UPEC strains remain incompletely defined.
Molecular epidemiological comparisons of isolates from different ecological sources (eg, fecal vs clinical isolates) can be informative [3–5]. However, they risk confounding by host compromise, which allows low-virulence strains to cause disease [6, 7], and by the intestinal reservoir of ExPEC [8], which creates an ExPEC subset among fecal surveillance isolates [9, 10]. By contrast, animal challenge studies, despite their limitations, provide a direct readout of intrinsic extraintestinal virulence, allowing nonconfounded comparisons of bacterial traits with virulence.
Previous studies used this approach to study collections of isolates and identified various E. coli phylogenetic subsets and accessory traits (ie, putative virulence factors) as statistical correlates of virulence in diverse animal models [3, 6, 11–14]. However, these studies assessed a limited number and source diversity of isolates and range of bacterial traits. Accordingly, we sought to identify bacterial correlates of experimental virulence in a murine sepsis model by using a large set of extensively characterized E. coli isolates from diverse ecological contexts, locales, periods, and host populations.
METHODS
Isolates
The 292 E. coli study isolates were selected from multiple published collections [5, 6, 15–17], with a target number of approximately 20 (or 40, for the veterans fecal collection) presumptive ExPEC and non-ExPEC isolates per collection, as available (Table 1), for 300 total isolates. Collections were chosen to give a broad distribution by year of isolation (1981 through 2000), ecological source (ie, surveillance fecal specimens and clinical specimens [urine specimens from patients with cystitis or pyelonephritis and blood specimens from patients with urosepsis or bacteremia]), host population (ie, male inpatients at Veterans Affairs [VA] medical centers and ambulatory and hospitalized women), and presumptive ExPEC status (based on established molecular criteria) [18]. For the fecal [5, 15], cystitis [15], and pyelonephritis [16] collections, which contained abundant presumptive ExPEC and non-ExPEC isolates, similar numbers of ExPEC and non-ExPEC isolates were selected randomly. For the VA bacteremia collection [5], presumptive ExPEC isolates, which predominated, were selected randomly, whereas all 9 presumptive non-ExPEC isolates were used. For the Seattle urosepsis collection [17], for which experimental virulence data were available [6], all isolates were used, irrespective of presumptive ExPEC status. Of the 300 initially selected isolates, 8 were excluded for technical reasons, leaving 292 isolates as the final study population (Table 1).
Table 1.
Origins of the 292 Escherichia coli Study Isolates
| Source (No. [%]), Syndrome | Context | Locale | Years | Isolates Selected From Source Collection, No. | ExPECJJa Isolates in Source Collection, % | Reference(s) | ||
|---|---|---|---|---|---|---|---|---|
| Total | ExPECJJa | Non-ExPECJJa | ||||||
| Feces (116 [40]) | ||||||||
| Not applicableb | Student health servicec | Minneapolis, MN | 1999–2000 | 39 | 22 | 17 | 37 | [15] |
| Not applicableb | Hospitalized veteransd | Minneapolis, MN | 1996–1999 | 77 | 41 | 36 | 38 | [5] |
| Urine (79 [27]) | ||||||||
| Cystitis | Student health servicec | Minneapolis, MN | 1999–2000 | 39 | 23 | 16 | 41 | [15] |
| Pyelonephritis | Ambulatory women | Multicenter (USA) | 1994–1997 | 40 | 21 | 19 | 69 | [15, 16] |
| Blood (97 [33]) | ||||||||
| Urosepsis | Four hospitalse | Seattle, WA | 1981–1985 | 67 | 59 | 8 | 88 | [6, 17] |
| Bacteremia | Hospitalized veteransd | Minneapolis, MN | 1996–1999 | 30 | 21 | 9 | 81 | [5] |
aIsolates were defined as extraintestinal pathogenic E. coli (ExPECJJ) if ≥2 of the following virulence factor genes were present: papAH and/or papC, sfa/focDE, afa/draBC, iutA, and kpsM II.
bDonors of fecal specimens had no clinical evidence of infection.
cUniversity of Minnesota.
dMinneapolis Veterans Affairs Medical Center.
fHarborview Medical Center, Public Health Service Hospital, Seattle Veterans Affairs Medical Center, and University of Washington Medical Center.
Genome Sequencing
Genomes were sequenced and analyzed as described elsewhere [9]. Pooled paired-end libraries were sequenced on an Illumina MiSeq and Genome Analyzer IIx to a read length of ≥100 base pairs, at a mean coverage depth (±SD) of 58.27-fold ± 35.4-fold. After alignment of short-read sequences to a reference genome by using BWA-mem (v.0.7.12) [19], single-nucleotide polymorphisms (SNPs) were called using GATK (v.3.5) [20], recombinant regions were identified and removed using Gubbins (v.2.1) [21], and the resultant SNP matrix was used to construct phylogenetic trees in PhyML with Smart Model Selection [22].
Sequence Types (STs) and Phylogenetic Groups
We determined STs by extracting from the genomes the 7 ST-defining housekeeping loci used in the Achtman MLST system. By using Enterobase (available at: https://enterobase.warwick.ac.uk/), each sequence variant was assigned an allele designation, and each allele combination was assigned to an ST. STs were grouped by ST complex (STc) according to Enterobase or if they differed by 1 locus.
Phylogroups were provisionally assigned using the updated polymerase chain reaction (PCR)–based method of Clermont et al [23] and were definitively assigned on the basis of each isolate’s placement within the phylogram. Outlier isolates were classified as having an undetermined phylogroup.
Virulence Genotyping
Forty-nine putative or proven extraintestinal virulence genes were sought by either PCR analysis (n = 47) or in silico analysis (n = 2). PCR was done using established protocols [6], duplicate boiled lysates as template DNA, and inclusion of positive and negative controls. For yfcV and chuA, genomes were screened using BLAST analysis, based on 90% similarity to reference sequences [9]. Based on previous epidemiological and experimental validation, isolates were classified as ExPECJJ (per the criteria of J. Johnson) if positive for ≥2 of papAH and/or papC (P fimbriae), sfa/focDE (S and F1C fimbriae), afa/draBC (Dr-binding adhesins), iutA (aerobactin siderophore system), and kpsM II (group 2 capsules) [18]; and as UPECHM (per the criteria of H. Mobley) if positive for ≥2 of chuA (heme uptake), fyuA (yersiniabactin siderophore system), vat (vacuolating toxin), and yfcV (adhesin) [3].
Murine Sepsis Model
An established murine subcutaneous sepsis model was used [6, 11, 14, 24]. The protocol was reviewed and approved by the local institutional animal care and use committee. Approximately 3 × 108 colony-forming units of bacteria in the exponential phase of growth were injected subcutaneously into female outbred Swiss-Webster mice (Harlan; Indianapolis, IN), using 5 mice initially and, if the initial standard error exceeded 20%, 5–10 additional mice. In parallel, laboratory strain MG1655 and pyelonephritis isolate CFT073 were injected into 5 mice each as negative and positive controls, respectively [11, 14, 24].
After inoculation, mice were observed for 72 hours and were scored daily for illness severity by a single experienced observer blinded to strain identity, using a 5-point scale (with a score of 1 denoting healthy; 2, mildly ill; 3, moderately ill; 4, severely ill/moribund; and 5, dead), with standardized criteria for each stage. Mice that died received a score of 5 for any remaining study days. Mice were euthanatized on reaching stage 4 or surviving 72 hours. For mice challenged with a given strain, the mean of the daily illness scores was the strain’s overall illness severity score, and the proportion of mice that died or reached stage 4 was the strain’s lethality score.
Statistical Methods
Statistical testing of dichotomous variables was limited to those with an overall prevalence of 5%–95%. Comparisons of proportions were tested using the Fisher exact test or a χ2 test, as appropriate. Comparisons involving continuous variables were tested using a 2-tailed t test. Because the virulence indicators were dichotomous and the outcomes were continuous, we used Spearman rank correlations to assess the strength of the associations between the potential predictors and outcomes. Concurrent assessment of multiple variables as predictors of a continuous dependent variable was done using multiple regression analysis, with both forced entry and forward and backward stepwise entry.
Because 5–15 mice were tested per strain, analysis at the mouse level conceivably could increase sample size and, thereby, statistical power but also could be confounded by clustering at the strain level. To determine whether outcomes could be analyzed validly at the mouse level and how much additional statistical power this would provide, we assessed the impact of clustering by strain, using an unconditional multilevel model, with severity as the dependent variable. This showed that strain-level effects accounted for 83% of the overall variance in illness severity, evidence that clustering by strain was quite significant and would therefore require adjustment in a by-mouse analysis, and that the increment in statistical power from a by-mouse analysis, once adjusted for by-strain clustering, would be small. Because of this and because adjustment for by-strain clustering would preclude stepwise multivariable modeling, we analyzed the data at the by-strain level.
For multivariable modeling, variables were selected as candidate predictors, using a multistage approach, with different partitions of the data set (ie, total and partial [fecal vs clinical isolates]), to assess consistency of results across source groups. First, within a given data set, all candidate predictor variables were assessed for their strength of correlation with the selected outcome (Table 2), and those with a correlation coefficient of ≥0.30 were assessed further. Next, we examined for intercorrelations among these predictors, and, where 2 predictors had a coefficient of >0.70, we chose the predictor most highly correlated with the outcome as the primary predictor variable. If 2 intercorrelated predictors were correlated equivalently with the outcome, the one that dominated more alternate intercorrelated predictors was used. In the end, all variables for entry into the regression models had coefficients of ≥0.30 for severity and <0.70 for each other. The same selection process was followed for each population partition and was done both with and without considering the composite variables, UPECHM and ExPECJJ, as candidate predictors.
Table 2.
Univariable Correlates of Experimental Virulence in a Murine Sepsis Model
| Category, Traita | All Isolates (n = 292) | Fecal Isolates (n = 116) | Clinical Isolates (n = 176) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. (%) | Mean Illness Severity Score | Correlationb | P b | No. (%) | Mean Illness Severity Score | Correlationb | P b | No. (%) | Mean Illness Severity Score | Correlationb | P b | ||||
| Trait Absent | Trait Present | Trait Absent | Trait Present | Trait Absent | Trait Present | ||||||||||
| Phylogenetic group | |||||||||||||||
| Group A | 30 (10) | 3.6 | 2.6 | –0.19 | .001 | 14 (12) | 3.4 | 2.7 | –0.08 | .26 | 16 (9) | 3.5 | 2.5 | –0.25 | .002 |
| Group B1 | 30 (10) | 3.4 | 2.1 | –0.31 | <.001 | 22 (19) | 3.4 | 2.0 | –0.40 | <.001 | 8 (5) | 3.5 | 2.3 | –0.19 | .03 |
| Group B2 | 167 (57) | 2.6 | 3.8 | 0.46 | <.001 | 61 (53) | 2.4 | 3.8 | 0.52 | <.001 | 106 (60) | 2.8 | 3.8 | 0.40 | <.001 |
| Group D | 41 (14) | 3.3 | 3.0 | –0.10 | .12 | 15 (13) | 3.2 | 2.6 | –0.16 | .06 | 26 (15) | 3.4 | 3.2 | –0.06 | .47 |
| STc | |||||||||||||||
| STc10 | 17 (6) | 3.3 | 2.8 | –0.09 | .12 | 6 (5) | 3.1 | 3.2 | 0.03 | .85 | 11 (6) | 3.5 | 2.7 | –0.17 | .046 |
| STc14 | 17 (6) | 3.3 | 2.8 | –0.09 | .07 | 5 (4) | 3.1 | 2.8 | –0.03 | .61 | 12 (7) | .35 | 2.8 | –0.14 | .06 |
| STc69 | 15 (5) | 3.3 | 3.0 | –0.05 | .41 | 6 (5) | 3.1 | 2.4 | –0.152 | .25 | 9 (5) | 3.4 | 3.4 | –0.02 | 1.0 |
| STc73 | 43 (15) | 3.2 | 3.9 | 0.20 | .001 | 4 (3) | 3.0 | 4.4 | 0.27 | <.001 | 34 (19) | 3.3 | 3.7 | 0.13 | .03 |
| STc95 | 37 (13) | 3.1 | 4.1 | 0.29 | <.001 | 19 (16) | 2.9 | 4.2 | 0.36 | <.001 | 18 (10) | 3.3 | 4.3 | 0.25 | <.001 |
| Adhesin | |||||||||||||||
| papAH | 145 (50) | 2.8 | 3.8 | 0.37 | <.001 | 42 (36) | 2.7 | 3.7 | 0.35 | <.001 | 103 (59) | 2.9 | 3.8 | 0.35 | <.001 |
| papG II | 114 (39) | 3.0 | 3.7 | 0.29 | <.001 | 29 (25) | 2.9 | 3.8 | 0.28 | .001 | 85 (48) | 3.1 | 3.7 | 0.23 | .001 |
| papG III | 44 (15) | 3.2 | 3.6 | 0.13 | .03 | 17 (15) | 3.0 | 3.4 | 0.12 | .24 | 27 (15) | 3.3 | 3.8 | 0.12 | .04 |
| sfa/focDE | 71 (24) | 3.1 | 3.7 | 0.20 | .001 | 22 (19) | 3.0 | 3.4 | 0.12 | .21 | 49 (28) | 3.2 | 3.9 | 0.22 | <.001 |
| sfaS | 25 (9) | 3.2 | 3.8 | 0.13 | .02 | 11 (9) | 3.1 | 3.3 | 0.06 | .69 | 14 (8) | 3.3 | 4.3 | 0.21 | <.001 |
| focG | 34 (12) | 3.2 | 3.6 | 0.08 | .10 | 6 (5) | 3.1 | 3.1 | –0.02 | 1.0 | 28 (16) | 3.4 | 3.7 | 0.08 | .14 |
| afa/draBC | 21 (7) | 3.3 | 3.5 | 0.05 | .29 | 6 (5) | 3.1 | 3.4 | 0.08 | .61 | 15 (9) | 3.4 | 3.6 | 0.05 | .47 |
| iha | 102 (35) | 3.1 | 3.5 | 0.15 | .006 | 30 (26) | 3.0 | 3.4 | 0.15 | .14 | 72 (41) | 3.3 | 3.6 | 0.11 | .07 |
| hra | 86 (29) | 3.2 | 3.5 | 0.13 | .035 | 31 (27) | 3.0 | 3.4 | 0.13 | .13 | 55 (31) | 3.3 | 3.6 | 0.11 | .13 |
| yfcV | 245 (84) | 2.7 | 3.4 | 0.20 | <.001 | 97 (84) | 2.5 | 3.2 | 0.21 | .03 | 148 (84) | 2.9 | 3.5 | 0.18 | .03 |
| Toxin | |||||||||||||||
| hlyD | 90 (31) | 3.1 | 3.7 | 0.25 | <.001 | 22 (19) | 3.0 | 3.5 | 0.18 | .07 | 68 (39) | 3.2 | 3.8 | 0.27 | <.001 |
| hlyF | 19 (7) | 3.3 | 3.7 | 0.09 | .08 | 5 (4) | 3.1 | 4.0 | 0.13 | .08 | 14 (8) | 3.4 | 3.6 | 0.07 | .47 |
| cnf1 | 37 (13) | 3.2 | 3.8 | 0.15 | .009 | 1 (0.8) | 3.1 | 3.1 | –0.004 | 1.0 | 26 (15) | 3.3 | 4.1 | 0.23 | <.001 |
| cdtB | 18 (6) | 3.3 | 3.6 | 0.06 | .27 | 4 (3) | 3.1 | 3.9 | 0.10 | .40 | 14 (8) | 3.4 | 3.5 | 0.01 | .77 |
| sat | 94 (32) | 3.1 | 3.6 | 0.18 | .002 | 26 (22) | 2.9 | 3.7 | 0.25 | .009 | 68 (39) | 3.3 | 3.6 | 0.10 | .10 |
| pic | 31 (11) | 3.2 | 3.8 | 0.14 | .02 | 5 (4) | 3.0 | 4.8 | 0.29 | <.001 | 26 (15) | 3.4 | 3.6 | 0.07 | .38 |
| vat | 154 (53) | 2.7 | 3.8 | 0.47 | <.001 | 56 (48) | 2.35 | 3.9 | 0.57 | <.001 | 98 (56) | 2.9 | 3.8 | 0.37 | <.001 |
| Siderophore | |||||||||||||||
| iroN | 90 (31) | 3.1 | 3.8 | 0.26 | <.001 | 30 (26) | 2.9 | 3.8 | 0.31 | .001 | 60 (34) | 3.2 | 3.8 | 0.22 | .002 |
| fyuA | 210 (72) | 2.1 | 3.7 | 0.57 | <.001 | 71 (61) | 2.1 | 3.8 | 0.63 | < .001 | 139 (79) | 2.2 | 3.7 | 0.47 | <.001 |
| ireA | 68 (23) | 3.2 | 3.6 | 0.13 | .03 | 23 (19) | 2.9 | 3.8 | 0.24 | .006 | 45 (26) | 3.4 | 3.5 | 0.05 | .63 |
| iutA | 126 (44) | 3.1 | 3.6 | 0.15 | .008 | 34 (29) | 2.9 | 3.6 | 0.24 | .01 | 92 (52) | 3.3 | 3.5 | 0.05 | .51 |
| chuA | 220 (75) | 2.3 | 3.6 | 0.43 | <.001 | 79 (68) | 2.2 | 3.5 | 0.44 | <.001 | 141 (80) | 2.4 | 3.7 | 0.41 | <.001 |
| Capsule | |||||||||||||||
| kpsM II | 211 (72) | 2.3 | 3.7 | 0.49 | <.001 | 78 (67) | .21 | .36 | 0.50 | <.001 | 133 (76) | 2.4 | 3.7 | 0.46 | <.001 |
| K1 | 83 (28) | 3.0 | 4.1 | 0.41 | <.001 | 35 (30) | 2.6 | 4.2 | 0.54 | <.001 | 48 (27) | 3.2 | 4.0 | 0.31 | <.001 |
| K2/K100 | 20 (7) | 3.3 | 3.6 | 0.08 | .19 | 1 (0.8) | 3.1 | 2.0 | –0.08 | .36 | 19 (11) | 3.4 | 3.7 | 0.11 | .24 |
| K5 | 32 (11) | 3.2 | 3.7 | 0.11 | .03 | 10 (9) | 3.0 | 3.8 | 0.18 | .049 | 22 (13) | 3.4 | 3.6 | 0.05 | .33 |
| Miscellaneous | |||||||||||||||
| cvaC | 16 (5.4) | 3.3 | 3.7 | 0.09 | .10 | 5 (4) | 3.0 | 4.4 | 0.18 | <.001 | 11 (6) | 3.4 | 3.4 | 0.07 | .94 |
| usp | 169 (58) | 2.6 | 3.8 | 0.48 | <.001 | 64 (55) | 2.2 | 3.8 | 0.59 | <.001 | 105 (60) | 2.8 | 3.8 | 0.40 | <.001 |
| traT | 171 (59) | 2.9 | 3.6 | 0.28 | <.001 | 64 (55) | 2.7 | 3.5 | 0.29 | .001 | 107 (61) | 3.0 | 3.6 | 0.23 | .001 |
| ibeA | 47 (16) | 3.1 | 4.1 | 0.28 | <.001 | 18 (16) | 3.0 | 3.9 | 0.24 | .007 | 29 (16) | 3.3 | 4.2 | 0.27 | <.001 |
| ompT | 208 (71) | 2.8 | 3.5 | 0.26 | <.001 | 81 (70) | 2.6 | 3.3 | 0.27 | .003 | 127 (72) | 2.9 | 3.6 | 0.24 | .002 |
| iss | 21 (7) | 3.2 | 3.8 | 0.11 | .045 | 5 (4) | 3.0 | 4.5 | 0.21 | <.001 | 15 (90) | 3.4 | 3.5 | 0.04 | .65 |
| H7 fliC | 48 (16) | 3.1 | 4.0 | 0.27 | <.001 | 27 (23) | 2.8 | 3.8 | 0.30 | .001 | 21 (12) | 3.3 | 4.3 | 0.29 | <.001 |
| malX | 153 (52) | 2.7 | 3.8 | 0.42 | <.001 | 54 (47) | 2.5 | 3.8 | 0.52 | <.001 | 99 (56) | 2.9 | 3.8 | 0.36 | <.001 |
| clbB/N | 123 (42) | 2.9 | 3.8 | 0.33/0.35 | <.001 | 43 (37) | 2.6 | 3.9 | 0.47/0.48 | <.001 | 79 (45) | 3.2 | 3.7 | 0.22/0.24 | .001 |
| Pathotype | |||||||||||||||
| ExPECJJ | 187 (64) | 2.5 | 3.7 | 0.48 | <.001 | 68 (59) | 2.3 | 3.8 | 0.56 | <.001 | 124 (70) | 2.7 | 3.7 | 0.39 | <.001 |
| UPECHM | 216 (74) | 2.1 | 3.7 | 0.56 | <.001 | 75 (65) | 2.0 | 3.7 | 0.63 | <.001 | 141 (80) | 2.2 | 3.7 | 0.48 | <.001 |
| Source | |||||||||||||||
| Clinical | 176 (60) | 3.1 | 3.4 | 0.12 | .04 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
Abbreviations: E. coli, Escherichia coli; NA, not applicable; STc, sequence type complex.
aTraits shown are those with a 5%–95% overall prevalence. Definitions are as follows: papAH, P fimbriae major subunit (results were similar for papC, papEF, and papG); papG allele II, variant P adhesin; papG allele III, variant P adhesin, sfa/focDE, S and F1C fimbriae; sfaS, S fimbriae; focG, F1C adhesin; afa/draBC, Dr-binding adhesins, iha, adhesin-siderophore; yfcV, chaperone-usher fimbriae; hlyD, hemolysin; hlyF, hemolysin F; cnf1, cytotoxic necrotizing factor 1; cdtB, cytolethal distending toxin; sat, secreted autotransporter toxin; pic, protein associated with intestinal colonization; vat, vacuolating autotransporter toxin; iroN, salmochelin receptor; fyuA, yersiniabactin receptor; ireA, siderophore receptor; iutA, aerobactin receptor; chuA, heme uptake; kpsM II, group 2 capsules; K1, variant group 2 capsule; K2/K100, group 2 capsule variants; K5, group 2 capsule variant; cvaC, colicin (microcin) V; usp, uropathogenic-specific protein; traT, serum resistance-associated surface exclusion protein; ibeA, invasion of brain endothelium; ompT, outer membrane protease; iss, increased serum survival; H7 fliC, variant flagellin; malX, pathogenicity island marker; clbB/N, colibactin synthesis; ExPECJJ, extraintestinal pathogenic E. coli (molecular definition per James Johnson: ≥2 of papA and/or papC, sfa/foc, afa/dra, kpsM II, and iutA); UPECHM, uropathogenic E. coli (molecular definition per Harry Mobley: ≥2 of chuA, fyuA, vat, and yfcV).
bSpearman correlation coefficient (ρ).
cBy a 2-tailed t test. Values <.05 are considered statistically significant.
After selecting sets of candidate predictors, multiple regression models (forced and stepwise entry) were constructed for each population partition. For stepwise models, the criteria for entry and removal were P values of <.01 and <.05, respectively. Bootstrapping (with 500 iterations) was used to assess model stability.
RESULTS
Study Population Characteristics
The 292 study isolates represented diverse ecological sources, clinical contexts, host populations, locales, and periods (Table 1). They were predominantly from phylogroup B2 (167 [57%]), with minor contributions from groups D (41 [14%)], A (30 [10%]), B1 (30 [10%]), C (10 [3.4%]), F (9 [3%]), and E (2 [0.7%]); 3 (1%) had an undefined phylogroup. The 5 STc with a ≥5% prevalence were STc73 (43 [15%]), STc95 (37 [13%]), STc10 (17 [6%]), STc14 (17 [6%]), and STc69 (15 [5%]). Each of the 49 virulence genes sought except clpG (a non-P adhesin; not detected) was identified in from 0.3% (papG allele I and F17 adhesin) to 99% (fimH type 1 fimbria) of isolates. Overall, 186 isolates (64%) qualified molecularly as ExPECJJ, and 216 (74%) qualified as UPECHM; these variables were closely correlated (Spearman ρ = 0.71; P < .001).
Murine Sepsis Model Outcomes
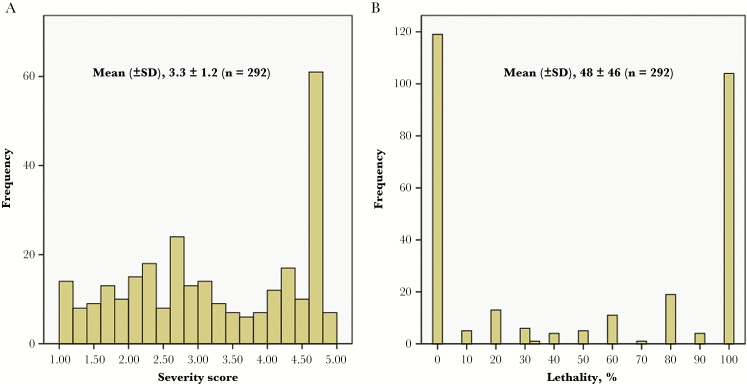
In the murine sepsis model, mean illness severity over the 3-day observation period was distributed fairly evenly across the population (Figure 1). By contrast, the lethality percentage was strongly bimodal, with peaks at 0% and 100%. Mean illness severity and lethality percentage were highly correlated (Spearman ρ = .92; P < .001). For maximal power, illness severity was selected as the representative virulence outcome.
Figure 1.
Distribution of mean illness severity (A) and lethality percentage (B) in the murine sepsis model for 292 clinical (ie, urine and blood) and fecal Escherichia coli isolates.
Univariable Comparisons of Bacterial Characteristics and Source to Virulence
Of the studied bacterial characteristics, 46 qualified for statistical analysis, based on a prevalence of 5%–95% (Table 2). These included 4 phylogenetic groups, 5 STc, 35 accessory traits (ie, 10 adhesin genes, 7 toxin genes, 5 siderophore systems, 4 capsule markers, and 9 miscellaneous traits), and 2 composite variables (ie, ExPECJJ and UPECHM). These bacterial characteristics, plus clinical (vs fecal) source, were compared to illness severity (Table 2).
Overall, 38 analyzed bacterial traits (83%), including multiple individual traits per category plus the composite variables, ExPECJJ and UPECHM, were correlated significantly with illness severity in ≥1 (usually all 3) population partitions (Table 2). Most correlations were positive and highly statistically significant and, when significant among both fecal and clinical isolates, pointed in the same direction in each group. Of the 8 exceptional traits that were uncorrelated with illness severity, 6 exhibited an overall prevalence of <10%.
Clinical source also was correlated significantly with illness severity, albeit weakly (ρ = 0.12; P = .04; Table 2). Mean severity scores (±SD) overlapped considerably between the fecal and clinical isolates (3.1 ± 1.3 and 3.4 ± 1.2, respectively). Other population partitions by source (ie, invasive vs noninvasive and blood vs nonblood) yielded no significant correlations with illness severity (data not shown).
Derived Sets of Predictors of Illness Severity
The multistage process that was used to select variables for multivariable modeling identified, within the total population, 6 bacterial characteristics as primary variables (ρ < .70; Table 3). Neither UPECHM nor ExPECJJ qualified, since both were highly correlated with (stronger predictor) fyuA.
Table 3.
Intercorrelations Among Bacterial Variables Significantly Associated With Illness Severity
| Population | UPECHM/ExPECJJ Considered?a | Variable (ρ for Correlation With Illness Severity) | |
|---|---|---|---|
| Primary Variableb | Correlated Variables,b Dominated by Primary Variable | ||
| Total (n = 292) | No or yes | fyuA (0.57) | Without UPECHM/ExPECJJ, none; with them, UPECHM (0.56), ExPECJJ (0.48) |
| kpsM II (0.49) | chuA (0.43) | ||
| usp (0.48) | vat (0.46), group B2 (0.46), malX (0.43), clbB (0.33), clbN (0.35) | ||
| K1 (0.41) | None | ||
| papAH (0.37) | papEF (0.37), papC (0.37), papG (0.33) | ||
| group B1 (–0.31) | None | ||
| Fecal (n = 116) | No | fyuA (0.63) | usp (0.59), vat (0.57), group B2 (0.52) |
| K1 (0.54) | None | ||
| malX (0.52) | None | ||
| kpsM II (0.50) | chuA (0.45) | ||
| clbN (0.48) | clbB (0.47) | ||
| group B1 (–0.40) | None | ||
| STc95 (0.36) | None | ||
| papAH (0.35) | papEF (0.34), papC (0.33) | ||
| iroN (0.31) | None | ||
| Yes | UPECHM (0.63) | fyuA (0.63), usp (0.59), group B2 (0.52) | |
| vat (0.57) | clbN (0.48), clbB (0.48) | ||
| ExPECJJ (0.56) | kpsM II (0.50) | ||
| malX (0.52) | None | ||
| group B1 (–0.40) | None | ||
| STc95 (0.36) | None | ||
| papAH (0.35) | papEF (0.37), papC (0.33) | ||
| iroN (0.31) | None | ||
| K1 (0.31) | None | ||
| Clinical (n = 176) | No or yes | fyuA (0.49) | Without UPECHM/ExPECJJ: none; with them: UPECHM (0.48), ExPECJJ (0.39) |
| kpsM II (0.46) | chuA (0.41) | ||
| group B2 (0.40)c | usp (0.40),cvat (0.37), malX (0.36) | ||
| papEF (0.36) | papC (0.36), papAH (0.35), papG (0.35) | ||
| K1 (0.31) | None | ||
ρ values of ≥ 0.30 were considered indicative of a statistical association with illness severity.
Abbreviations: ExPECJJ, extraintestinal pathogenic E. coli, as defined using James Johnson’s molecular definition; UPECHM, uropathogenic E. coli, as defined using Harry Mobley’s molecular definition.
aThe analysis was done with and without considering UPECHM and ExPECJJ as candidate predictor variables.
bDefinitions are as follows: fyuA, yersiniabactin receptor; kpsM II, group 2 capsule synthesis; usp, uropathogenic specific protein; K1, group 2 capsule variant; papAH, papC, papEF, papG, P fimbriae structural subunit, assembly, minor tip pilins, and tip adhesin; groups B1 and B2, phylogenetic groups; malX, pathogenicity island marker; clbN and clbB, colibactin synthesis; STc95, sequence type complex 95; iroN, salmochelin receptor; vat, vacuolating toxin; malX, pathogenicity island marker; chuA, heme uptake.
cAmong the clinical isolates, group B2 and usp yielded identical values for rho (0.398), but B2 dominated two alternate correlated variables (vat and malX), whereas usp dominated only one (malX). Accordingly, for parsimony, B2 was selected over usp for inclusion in the model.
Among the fecal isolates, this process identified 9 primary variables, including 5 of 6 from the total population analysis (Table 3). usp, a primary variable in the total population analysis, was excluded here because it was correlated with (ie, was a stronger predictor of) fyuA. When assessed together with the individual bacterial traits, UPECHM and ExPECJJ now qualified as primary variables, along with vat, in place of 3 of the initial primary variables (ie, fyuA, clbN, and kpsM II), which were dominated by the correlated variables (ie, stronger predictors) UPECHM, vat, and ExPECJJ, respectively (Table 3).
Among the clinical isolates, this process identified 5 primary variables, including 3 that had been identified previously as primary variables (ie, fyuA, kpsM II, and K1) and one that was interchangeable with such variables (ie, papEF vs papAH); the only newly identified variable was group B2. As in the total population analysis, neither UPECHM nor ExPECJJ qualified because they were highly correlated with (stronger predictor) fyuA.
Multiple Regression Models
To identify independent predictors of illness severity, these derived sets of variables (Table 3) were used as candidate predictors in multiple regression models (Table 4). Forward and backward stepwise entry consistently arrived at the same final result (data not shown); hence, Table 4 shows the results of forward stepwise entry only.
Table 4.
Multivariable Models to Predict Illness Severity in Mice Challenged with Escherichia coli Isolates
| E. coli Set | UPECHM/ExPECJJ Considered?a | Methodb | Model No. | Adjusted r2 | Variablec | β | P d |
|---|---|---|---|---|---|---|---|
| Total (n = 292) | No or yese | Forced | NAf | .39 | fyuA | 0.35 | <.001 |
| K1 | 0.21 | <.001 | |||||
| kpsM II | 0.11 | .10 | |||||
| papAH | 0.06 | .26 | |||||
| usp | 0.06 | .40 | |||||
| Group B1 | 0.003 | .96 | |||||
| Stepwise | 1 | .33 | fyuA | 0.58 | <.001 | ||
| 2 | .38 | fyuA | 0.49 | <.001 | |||
| K1 | 0.24 | <.001 | |||||
| 3 | .39 | fyuA | 0.40 | <.001 | |||
| K1 | 0.21 | <.001 | |||||
| kpsM II | 0.15 | .02 | |||||
| Fecal (n = 116) | No | Forced | NAf | .46 | K1 | 0.33 | .002 |
| fyuA | 0.30 | .01 | |||||
| malX | 0.13 | .20 | |||||
| papAH | –0.11 | .26 | |||||
| clbN | 0.10 | .36 | |||||
| iroN | 0.06 | .46 | |||||
| kpsM II | 0.06 | .61 | |||||
| Group B1 | –0.02 | .79 | |||||
| STc95 | –0.01 | .90 | |||||
| Stepwise | 1 | .39 | fyuA | 0.63 | <.001 | ||
| 2 | .46 | fyuA | 0.47 | <.001 | |||
| K1 | 0.32 | <.001 | |||||
| Yes | Forced | NAf | .48 | K1 | 0.33 | .003 | |
| UPECHM | 0.28 | .01 | |||||
| ExPECJJ | 0.22 | .08 | |||||
| papAH | –0.16 | .14 | |||||
| malX | 0.10 | .32 | |||||
| iroN | 0.07 | .42 | |||||
| vat | 0.03 | .81 | |||||
| Group B1 | –0.006 | .95 | |||||
| STc95 | –0.002 | .98 | |||||
| Stepwise | 1 | .39 | UPECHM | 0.63 | <.001 | ||
| 2 | .47 | UPECHM | 0.47 | <.001 | |||
| K1 | 0.34 | <.001 | |||||
| 3 | .49 | UPECHM | 0.35 | .001 | |||
| K1 | 0.32 | <.001 | |||||
| ExPECJJ | 0.19 | .05d | |||||
| Clinical (n = 176) | No or yes a | Forced | NAf | .32 | fyuA | 0.29 | .002 |
| K1 | 0.17 | .01 | |||||
| kpsM II | 0.17 | .06 | |||||
| papEF | 0.13 | .10 | |||||
| Group B2 | 0.01 | .89 | |||||
| Step | 1 | .26 | fyuA | 0.51 | <.001 | ||
| 2 | .30 | fyuA | 0.35 | <.001 | |||
| kpsM II | 0.26 | .002 | |||||
| 3 | .31 | fyuA | 0.34 | <.001 | |||
| kpsM II | 0.21 | .01 | |||||
| K1 | 0.16 | .02 |
Abbreviations: ExPECJJ, extraintestinal pathogenic E. coli, as defined using James Johnson’s molecular definition; NA, not applicable; UPECHM, uropathogenic E. coli, as defined using Harry Mobley’s molecular definition.
aThe analysis was done with and without considering UPECHM and ExPECJJ as candidate predictor variables.
bForced variable entry or conditional stepwise variable entry. Forward and backward stepwise entry gave identical final models; for brevity, only the stepwise forward entry models are shown.
dValues <.05 are considered statistically significant. Bootstrapping findings supported all significant P values except that for ExPEC (with fecal isolates, UPECHM/ExPECJJ were considered in a stepwise model).
eResults were the same regardless of whether UPECHM and ExPECJJ were considered, since they did not qualify as predictor variables.
fFor forced entry, there was only 1 model per data set and variable list.
Within the total population, in univariable models UPECHM yielded an r2 of 0.31 and ExPECJJ yielded an r2 of 0.23, whereas the multivariable forced entry model yielded an r2 of 0.39 and identified as significant 2 of 6 candidate predictor variables: fyuA (β = 0.35; P < .001) and K1 (β = 0.21; P < .001; Table 4). By contrast, the stepwise models identified also kpsM II (β = 0.15; P = .02) and achieved an r2 of 0.39 with only 3 predictor variables. Bootstrapping results were confirmatory.
Among the fecal isolates, in univariable models UPECHM yielded an r2 of 0.39 and ExPECJJ yielded an r2 of 0.31, whereas without UPECHM and ExPECJJ the multivariable forced entry model yielded an r2 of 0.46 and identified as significant 2 of 9 candidate predictor variables: K1 (β = 0.33; P < .001) and fyuA (β = 0.30; P = .01; Table 4). The corresponding stepwise models likewise achieved an r2 of 0.46 and identified the same 2 variables as significant predictors, in reverse order of potency. By contrast, with UPECHM and ExPECJJ included, the forced entry model achieved a slightly higher r2 (r2 = 0.48) and identified as significant predictors K1 (β = 0.33; P = .003) and UPECHM (β = 0.28; P = .01), whereas the corresponding stepwise models yielded an r2 of 0.49 and identified ExPECJJ (β = 0.19, P = .05) as a significant predictor, as well. Bootstrapping findings were confirmatory except with regard to the significance of ExPECJJ (P = .08).
Finally, among the clinical isolates, univariable models UPECHM yielded an r2 of 0.23 and ExPECJJ yielded an r2 of 0.15, whereas the multivariable forced entry model yielded an r2 of 0.32 and identified as significant 2 of 5 primary predictor variables: fyuA (β = 0.29; P = .002) and K1 (β = 0.17; P = .01; Table 4). Additionally, kpsM II approached statistical significance (β = 0.17; P = .06). The corresponding stepwise models achieved an r2 of 0.31 and identified all 3 variables as significant. Bootstrapping findings were confirmatory.
For exploratory purposes, clinical source was added to the (total population) multivariable models as a candidate predictor, despite yielding a ρ of <0.30 with illness severity (Table 2). In the forced entry model, it was nonsignificant and did not increase r2, and in the stepwise models it was excluded (data not shown).
DISCUSSION
Here we assessed ecological source and diverse bacterial traits as predictors of virulence in a murine sepsis model for 292 clinical and fecal E. coli isolates. Our findings support 4 main conclusions. First, multiple bacterial characteristics, including specific phylogenetic groups, clonal groups (ie, STc), and accessory traits (ie, virulence genes), significantly predicted experimental virulence. Second, trait combinations were more predictive than any single trait, explaining up to 49% of the total virulence. Third, ecological (ie, clinical vs fecal) source, when considered in isolation, also significantly predicted experimental virulence. Fourth, bacterial traits were much more potent predictors than was ecological source, and similar traits were predictive among clinical and fecal isolates.
These conclusions, which support and extend previously proposed concepts [11, 25], have important implications regarding the relationship between intrinsic virulence, bacterial traits, and ecological source. A common approach for classifying E. coli isolates on the basis of their presumed extraintestinal virulence potential relies on source: clinical isolates presumptively are virulent pathogens (eg, UPEC or ExPEC), whereas fecal isolates presumptively are low-virulence commensal organisms [3, 5]. By contrast, we found a broad range of experimental virulence among clinical and fecal isolates alike, demonstrating that an isolate’s clinical versus fecal origin does not indicate reliably its intrinsic virulence potential. Although on average the clinical isolates (which represented predominantly pyelonephritis and bacteremia) tended to be more virulent than the fecal isolates, these populations overlapped considerably.
We selected our study population by deliberately stratifying isolates according molecular ExPECJJ status to provide sufficient numbers within key subgroups to overcome the statistical power limitations that occur with natural or ad hoc isolate collections. Such biased selection might be considered to preclude a valid assessment of virulence in relation to ecologic source by distorting the authentic relationship between source and ExPECJJ status. However, this concern actually presupposes the validity of the study’s conclusion that genetic content is a more important determinant of experimental virulence than is ecological source. Here, ecological source was so weak a predictor of illness severity that it did not qualify for inclusion in the multivariable models and, when included anyway, proved noncontributory. Additional evidence of virulence commonality across source groups was the finding that similar trait combinations, including the established composite variables, ExPECJJ and UPECHM, predicted experimental virulence among fecal and clinical isolates alike.
Collectively, these findings suggest that the observed overall virulence differences between clinical and fecal isolates can be explained better by these populations’ different admixtures of virulent versus nonvirulent strains, rather than by categorical strain-level virulence differences by source. The most strongly predictive individual traits, both overall and among fecal and clinical isolates, were fyuA (yersiniabactin system) and kpsM II and K1 (group 2 capsule genes), although to what extent they contributed directly to or were simply markers of virulence is unclear.
Nonetheless, even the best-performing trait-based prediction models yielded an r2 of <50%. To the extent that bacterial characteristics determine infection outcomes, this implies the possible importance of traits other than those we tested, perhaps including known virulence genes [26] or as-yet-unknown traits that await discovery, such as by genome [27, 28] or transcriptome [29] analysis. Alternatively, expression levels, trait combinations, multiply determined phenotypes, and/or regulatory pathways may be important, as may be other typing methods or advanced analytical approaches [30].
Host factors also must be considered. In humans, host compromise allows low-virulence E. coli strains to cause severe disease [6, 31]. Likewise, different inbred mouse strains with specific immune polymorphisms exhibit different responses to bacterial challenge [32, 33]. Host-to-host variation may be relevant here since we used outbred mice, for which the host response to bacterial challenge may vary by mouse. To avoid confounding by such host variation, we assigned mice randomly to each test strain. Nonetheless, stochastic effects could have led to some strains being administered to groups of mice that were disproportionately more or less resistant to infection, biasing the results for those strains.
The present findings’ relevance to E. coli infections in humans is unknown. Mice are not humans, and the model is highly artificial. Moreover, given known differences in anatomic site–specific host defenses, nutrient resources, and bacterial receptors, different animal models might yield different results. Still, given the appreciable commonality across anatomic sites with respect to the challenges pathogens face in persisting and causing disease, certain bacterial characteristics likely are important, regardless of the specific model or host species. Notably, our nonurinary tract model identified as significant virulence predictors certain traits, such as pap and usp, that were interpreted initially—and even named (“pyelonephritis-associated pili” and “uropathogenic-specific protein,” respectively)—as urovirulence factors [34, 35] and that have proven urinary tract–specific mechanisms [36, 37]. Likewise, UPECHM, a composite variable derived by molecular epidemiological comparisons of human urine and fecal isolates, here was predictive of systemic virulence, especially so among fecal isolates, in a model that bypasses completely the urinary tract. This provides further evidence of the tenuous and somewhat arbitrary nature of the distinction between UPEC versus ExPEC and between urovirulence factors versus generic extraintestinal virulence factors.
Our use of a broader pool of candidate predictor variables than studied previously identified novel trait combinations that outperformed the established molecular definitions ExPEC [18] and UPEC [3], suggesting the possibility of devising improved molecular definitions. However, for this it would be desirable to test additional isolates, including those from humans, animals [38], food [39], and the environment [40], in multiple infection models, and to supplement such experimental data with epidemiological data that, ideally, would consider host compromise status and clinical presentation.
Study limitations include the reliance on a murine sepsis model, analysis only of selected bacterial traits (and for presence or absence only), inattention to host factors, and small numbers in some subgroups. Study strengths include the comparatively large study population and broad range of traits analyzed, the multistage analytical approach, and the inclusion of the composite variables, ExPECJJ and UPECHM.
In summary, the experimental virulence in mice of human clinical and fecal E. coli isolates corresponded more closely with virulence gene content and phylogenetic background than with source, and even the most predictive combinations of bacterial variables explained less than half of the observed virulence variation. This both confirms the primacy of bacterial traits over ecological origin in predicting the extraintestinal virulence in E. coli and identifies a need to investigate further the host and bacterial determinants of extraintestinal virulence.
Notes
Acknowledgments. We thank the study participants and the contributing clinical microbiology laboratories.
Disclaimer. The opinions expressed are strictly those of the authors and not those of their respective institutions, the Department of Veterans Affairs, or the National Institutes of Health.
Financial support. This work was supported in by the Office of Research and Development, Department of Veterans Affairs (grants 1 I01 CX000920-01 and 2I01CX000920-04 to J. R. J.) and the National Institutes of Health (grant R21AI117654 to L. B. P.).
Potential conflicts of interest. J. R. J. has received research grants from Achaogen, Allergan, Melinta, Merck, Syntiron, and Tetraphase; collaborates with IDGenomics; is a consultant for Crucell/Janssen and Syntiron; and has patent applications for tests to detect specific E. coli strains. L. B. P. has a patent application for tests to detect specific E. coli strains and a grant from Merck. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Russo TA, Johnson JR. Medical and economic impact of extraintestinal infections due to Escherichia coli: an overlooked epidemic. Microbes Infect 2003; 5:449–56. [DOI] [PubMed] [Google Scholar]
- 2. Russo TA, Johnson JR. A proposal for an inclusive designation for extraintestinal pathogenic Escherichia coli: ExPEC. J Infect Dis 2000; 181:1753–4. [DOI] [PubMed] [Google Scholar]
- 3. Spurbeck RR, Dinh PC Jr, Walk ST, et al. Escherichia coli isolates that carry vat, fyuA, chuA, and yfcV efficiently colonize the urinary tract. Infect Immun 2012; 80:4115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moreno E, Andreu A, Pigrau C, Kuskowski MA, Johnson JR, Prats G. Relationship between Escherichia coli strains causing acute cystitis in women and the fecal E. coli population of the host. J Clin Microbiol 2008; 46:2529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sannes MR, Kuskowski MA, Owens K, Gajewski A, Johnson JR. Virulence factor profiles and phylogenetic background of Escherichia coli isolates from veterans with bacteremia versus uninfected control patients. J Infect Dis 2004; 190:2121–8. [DOI] [PubMed] [Google Scholar]
- 6. Johnson JR, Porter S, Johnston B, et al. Host characteristics and bacterial traits predict experimental virulence for Escherichia coli bloodstream isolates from patients with urosepsis. Open Forum Infect Dis 2015; 2:ofv083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Man P, Cläeson I, Johanson IM, Jodal U, Svanborg Edén C. Bacterial attachment as a predictor of renal abnormalities in boys with urinary tract infection. J Pediatr 1989; 115:915–22. [DOI] [PubMed] [Google Scholar]
- 8. Yamamoto S, Tsukamoto T, Terai A, Kurazono H, Takeda Y, Yoshida O. Genetic evidence supporting the fecal-perineal-urethral hypothesis in cystitis caused by Escherichia coli. J Urol 1997; 157:1127–9. [PubMed] [Google Scholar]
- 9. Johnson JR, Davis G, Clabots C, et al. Household clustering of Escherichia coli sequence type 131 clinical and fecal isolates according to whole genome sequence analysis. Open Forum Infect Dis 2016; 3:ofw129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wold AE, Caugant DA, Lidin-Janson G, de Man P, Svanborg C. Resident colonic Escherichia coli strains frequently display uropathogenic characteristics. J Infect Dis 1992; 165:46–52. [DOI] [PubMed] [Google Scholar]
- 11. Johnson JR, Clermont O, Menard M, Kuskowski MA, Picard B, Denamur E. Experimental mouse lethality of Escherichia coli isolates, in relation to accessory traits, phylogenetic group, and ecological source. J Infect Dis 2006; 194:1141–50. [DOI] [PubMed] [Google Scholar]
- 12. Diard M, Baeriswyl S, Clermont O, et al. Caenorhabditis elegans as a simple model to study phenotypic and genetic virulence determinants of extraintestinal pathogenic Escherichia coli. Microbes Infect 2007; 9:214–23. [DOI] [PubMed] [Google Scholar]
- 13. Lavigne JP, Vergunst AC, Goret L, et al. Virulence potential and genomic mapping of the worldwide clone Escherichia coli ST131. PLoS One 2012; 7:e34294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Picard B, Garcia JS, Gouriou S, et al. The link between phylogeny and virulence in Escherichia coli extraintestinal infection. Infect Immun 1999; 67:546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sannes MR, Kuskowski MA, Johnson JR. Antimicrobial resistance among Escherichia coli isolates from women with cystitis and pyelonephritis, healthy human volunteers, and domestic dog feces. J Am Vet Med Assoc 2004; 225:368–73. [DOI] [PubMed] [Google Scholar]
- 16. Sannes MR, Kuskowski MA, Johnson JR. Geographical distribution of antimicrobial resistance among Escherichia coli causing acute uncomplicated pyelonephritis in the United States. FEMS Immunol Med Microbiol 2004; 42:213–8. [DOI] [PubMed] [Google Scholar]
- 17. Johnson JR, Orskov I, Orskov F, et al. O, K, and H antigens predict virulence factors, carboxylesterase B pattern, antimicrobial resistance, and host compromise among Escherichia coli strains causing urosepsis. J Infect Dis 1994; 169:119–26. [DOI] [PubMed] [Google Scholar]
- 18. Johnson JR, Murray AC, Gajewski A, et al. Isolation and molecular characterization of nalidixic acid-resistant extraintestinal pathogenic Escherichia coli from retail chicken products. Antimicrob Agents Chemother 2003; 47:2161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009; 25:1754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McKenna A, Hanna M, Banks E, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 2010; 20:1297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Croucher NJ, Page AJ, Connor TR, et al. Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using Gubbins. Nucleic Acids Res 2015; 43:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guindon S, Lethiec F, Duroux P, Gascuel O. PHYML Online–a web server for fast maximum likelihood-based phylogenetic inference. Nucleic Acids Res 2005; 33:W557–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Clermont O, Christenson JK, Denamur E, Gordon DM. The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo-groups. Environ Microbiol Rep 2013; 5:58–65. [DOI] [PubMed] [Google Scholar]
- 24. Johnson JR, Porter SB, Zhanel G, Kuskowski MA, Denamur E. Virulence of Escherichia coli clinical isolates in a murine sepsis model in relation to sequence type ST131 status, fluoroquinolone resistance, and virulence genotype. Infect Immun 2012; 80:1554–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Johnson JR, Kuskowski M, Denamur E, Elion J, Picard B. Clonal origin, virulence factors, and virulence (letter and reply). Infect Immun 2000; 68:424–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Spurbeck RR, Stapleton AE, Johnson JR, Walk ST, Hooton TM, Mobley HL. Fimbrial profiles predict virulence of uropathogenic Escherichia coli strains: contribution of ygi and yad fimbriae. Infect Immun 2011; 79:4753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Salipante SJ, Roach DJ, Kitzman JO, et al. Large-scale genomic sequencing of extraintestinal pathogenic Escherichia coli strains. Genome Res 2015; 25:119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McNally A, Oren Y, Kelly D, et al. Combined analysis of variation in core, accessory and regulatory genome regions provides a super-resolution view into the evolution of bacterial populations. PLoS Genet 2016; 12:e1006280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kisiela DI, Radey M, Paul S, et al. Inactivation of transcriptional regulators during within-household evolution of Escherichia coli. J Bacteriol 2017:(in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bakhtiarizadeh MR, Moradi-Shahrbabak M, Ebrahimi M, Ebrahimie E. Neural network and SVM classifiers accurately predict lipid binding proteins, irrespective of sequence homology. J Theor Biol 2014; 356:213–22. [DOI] [PubMed] [Google Scholar]
- 31. Lomberg H, Hanson LA, Jacobsson B, Jodal U, Leffler H, Edén CS. Correlation of P blood group, vesicoureteral reflux, and bacterial attachment in patients with recurrent pyelonephritis. N Engl J Med 1983; 308:1189–92. [DOI] [PubMed] [Google Scholar]
- 32. Godaly G, Ambite I, Svanborg C. Innate immunity and genetic determinants of urinary tract infection susceptibility. Curr Opin Infect Dis 2015; 28:88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hagberg L, Hull R, Hull S, McGhee JR, Michalek SM, Svanborg Edén C. Difference in susceptibility to Gram-negative urinary tract infection between C3H/HeJ and C3H/HeN mice. Infect Immun 1984; 46:839–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hull RA, Hull SI, Falkow S. Frequency of gene sequences necessary for pyelonephritis-associated pili expression among isolates of Enterobacteriaceae from human extraintestinal infections. Infect Immun 1984; 43:1064–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kurazono H, Yamamoto S, Nakano M, et al. Characterization of a putative virulence island in the chromosome of uropathogenic Escherichia coli possessing a gene encoding a uropathogenic-specific protein. Microb Pathog 2000; 28:183–9. [DOI] [PubMed] [Google Scholar]
- 36. Korhonen TK, Virkola R, Holthöfer H. Localization of binding sites for purified Escherichia coli P fimbriae in the human kidney. Infect Immun 1986; 54:328–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yamamoto S, Nakano M, Terai A, et al. The presence of the virulence island containing the usp gene in uropathogenic Escherichia coli is associated with urinary tract infection in an experimental mouse model. J Urol 2001; 165:1347–51. [PubMed] [Google Scholar]
- 38. Johnson TJ, Wannemuehler Y, Johnson SJ, et al. Comparison of extraintestinal pathogenic Escherichia coli strains from human and avian sources reveals a mixed subset representing potential zoonotic pathogens. Appl Environ Microbiol 2008; 74:7043–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Johnson JR, Porter SB, Johnston B, et al. Extraintestinal pathogenic and antimicrobial resistant Escherichia coli, including sequence type 131 (ST131), from retail chicken breasts: United States, 2013. Appl Environ Microbiol 2017; 83. doi: 10.1128/AEM.02956-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mohamed M, Owens K, Gajewski A, et al. Extraintestinal pathogenic and antimicrobial-resistant Escherichia coli Contamination of 56 public restrooms in the greater Minneapolis-St. Paul metropolitan area. Appl Environ Microbiol 2015; 81:4498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]