Abstract
α2-Adrenergic receptors (α2ARs) are G-protein–coupled receptors involved in catecholamine signaling by extracellular regulated protein kinase 1 and 2 (ERK1/2) pathways. We examined placental expression and function of α2AR subtypes in women with severe preeclampsia (sPE) with and without intrauterine growth restriction (IUGR). Placental biopsies were analyzed from 52 women with i) sPE (n = 8); ii) sPE + IUGR (n = 9); iii) idiopathic IUGR (n = 8); iv) idiopathic preterm birth (n = 16); and v) healthy term controls (n = 11). Expression of α2AR subtypes (α2A, α2B, α2C) and phospho-ERK1/2 (receptor activation marker) was investigated by immunohistochemistry and/or quantitative real-time RT-PCR. The effects of α2CAR knockdown on syncytialization (syncytin-1 and -2) and β-human chorionic gonadotropin secretion were examined in BeWo cells stimulated with forskolin. The effects of α2AR agonist UK 14,304 and specific α2CAR antagonist were tested, using a trophoblast migration assay. All three α2ARs were expressed and functionally active in human placenta with site-specific localization. Highest α2BAR and α2CAR mRNA expression was identified in sPE + IUGR. α2CAR knockdown increased expression of syncytin-1 and -2 but decreased secretion of β-human chorionic gonadotropin. UK 14,304 impaired trophoblast migration. The observed α2AR expression pattern suggests different function for each subtype. α2CAR modulates trophoblast syncytialization and migration and may carry pathogenic role in sPE + IUGR.
The α-2 adrenoceptors (α2ARs), encoded by ADRA2 genes, are G-protein–coupled receptors with vital roles in cellular physiological processes and adaptation to stress.1 Generally speaking, engagement of α2ARs by catecholamines (preferentially by norepinephrine) results in negative feedback inhibition of adenylate cyclase by G-protein subunit α, group i, resulting in reduced cAMP synthesis.2, 3, 4 α2AR agonists have been widely used for treatment of various medical conditions, including hypertension, attention-deficit/hyperactivity disorder, opioid withdrawal, and, more recently as, anesthesia adjuvants.5 These uses result from the preponderant expression of α2ARs in vasculature and in the central nervous system. However, a number of studies have reported presence of α2AR on other cell types with important sympathetic reactive responses such as adipocytes, pancreatic β-cells, and even cancer epithelial cells where it was found that α2AR stimulation increases metastatic burden in the context of surgical stress.6
The relevance of the α2ARs in reproductive biology was demonstrated through the use of genetically engineered murine models in which the three known receptor subtypes (α2A, α2B, and α2C) were knocked out.7 In mice in which all three α2ARs were deleted, lethality at embryonic day 10.5 was significantly increased because of defective vascular development.7 Furthermore, it was shown that phosphorylation of the extracellular signal-regulated kinases 1 and 2 (ERK1/2) in the placenta was lower than in wild-type animals. Of interest, several lines of evidence pointed toward placental α2BAR and not α2A or α2C AR subtypes as critical for embryonic survivability, at least in the mouse.7 One proposed explanation was that co-expression of α2BAR and type 1 receptor for vascular endothelial growth factor (VEGF) by spongiotrophoblasts and trophoblast giant cells prevents the generation of soluble type 1 receptor for VEGF, the antagonist of VEGF.8 Collectively, the above data provide baseline evidence that perturbation of placental α2AR expression and/or activity may contribute to pregnancy-specific pathologies, including fetal loss and defective angiogenesis.
Aberrant placental growth, maturation, angiogenesis, and invasion are histopathologic hallmarks of both preeclampsia (PE) and intrauterine growth restriction (IUGR); however, the underlying molecular mechanisms remain elusive.9, 10, 11 Trophoblast syncytialization is a highly coordinated cell-to-cell fusion process necessary for formation and function of the syncytiotrophoblast, which produces and secretes many hormones, including β-human chorionic gonadotropin (β-hCG).12, 13, 14, 15 Several proteins, growth factors, and cytokines can modulate trophoblast syncytialization.16, 17 Chief among them are syncytin-1 and syncytin-2, which are considered hallmarks of terminal differentiation of placental trophoblast linage.18 The synthesis and expression of syncytins depends on the cAMP/protein kinase A pathway as demonstrated through experiments that involve pharmacologic stimulation of cytotrophoblast-like (BeWo) cells with the adenylate cyclase activator and intracellular cAMP-elevating agent forskolin.16, 19
From the above experimental data it is tempting to speculate that aberrant expression and activation of α2ARs could disrupt placental development, leading to pathologic processes. This concept has biological plausibility because trophoblast syncytialization is defective in both PE and IUGR.20, 21, 22 These two conditions may occur if heightened expression of α2ARs inhibits adenylyl cyclase, thereby reducing cAMP production and in turn the synthesis of syncytins.
At least two α2AR subtypes are known to be present in human placenta, specifically α2AAR and α2BAR.23 This conclusion was reached from radioligand binding experiments and not by immunohistology, which, before the present study, has not been performed. Here, we tested the hypotheses that PE and IUGR are associated with differential expression of placental α2ARs and that α2ARs are key regulators of trophoblast syncytialization and migration.
Materials and Methods
Patients and Samples
Placental tissues retrieved from 52 women were distributed in the following groups: i) PE with severe features [sPE; n = 8; means ± SEM gestational age (GA), 31 ± 1 weeks]; ii) sPE + IUGR (n = 9; mean GA, 30 ± 1 weeks); iii) idiopathic IUGR (n = 8; mean GA, 31 ± 1 weeks); iv) idiopathic preterm birth (iPTB; n = 16; mean GA, 31 ± 1 weeks), and v) healthy term controls (n = 11; mean GA, 39 ± 1 weeks) were analyzed.
GA in all cases was established from last menstrual period and/or early ultrasound (<20 weeks).24 Preterm birth was defined as delivery before 37 weeks GA. For all iPTB cases intraamniotic infection was excluded based on negative results of amniotic fluid biochemical testing, negative bacterial cultures, and absence of histologic chorioamnionitis. iPTB tissues were the best possible control to identify potential GA regulation in the expression of α2ARs. This control was important because women with sPE and IUGR are frequently delivered at earlier GAs. Because patient recruitment and sample collection were completed before publication of the 2013 Task Force (May 2005 to July 2012), the definition of PE was based on American College of Obstetricians and Gynecologists criteria valid at the time of sample collections.25, 26 IUGR was defined ultrasonographically as an estimated fetal growth <10th percentile for GA.27 A diagnosis of idiopathic IUGR was established in babies without detectable structural anomalies, and when examination of the placenta and the work-up to rule out chromosomal aneuploidy did not provide a cause for lack of growth. Presence of fetal structural anomalies and antenatal viral infections (ie, HIV, hepatitis) were considered exclusion criteria. All women were delivered at Yale-New Haven Hospital and provided signed informed consent under protocols approved by the Human Investigation Committee of Yale University. Within minutes from the time of delivery of the placenta, a full-thickness biopsy was retrieved from the central portion of the placenta. Approximately one-half of the tissue was fixed in formalin. For the second half, decidua basalis was dissected from the villous trophoblast in sterile fashion, rinsed in sterile saline, frozen in liquid nitrogen, and kept at −80°C for RNA studies.
Immunohistochemistry
Purified rabbit polyclonal antibodies were used to stain for α2AAR, α2CAR, and α2BAR (ab91648; Abcam Inc., Cambridge, MA).28 The three polyclonal antisera are specific for their respective α2AR subtype and do not cross-react with other α2AR subtypes. The specificity of these antibodies has been established in several previously published studies.29, 30, 31, 32 The anti-α2CAR antibody recognizes the approximately 70-kDa mature receptor form that translocates from the Golgi compartment to the cell surface.33 A rabbit monoclonal antibody was used to detect phospho-ERK1/2 (p-ERK1/2) (catalog number 4370; Cell Signaling Technology, Danvers, MA).
Five-micron serial paraffin sections of fetal membranes and placental villous tissue were immunostained for α2ARs and p-ERK1/2 as previously described.34 Term human myometrium was used as a positive control. After antigen retrieval with citrate buffer and pretreatment with 1% hydrogen peroxide (15 minutes), sections were incubated overnight (4°C) with affinity-purified rabbit polyclonal antibodies to α2AAR, α2BAR, or α2CAR (dilution 1:100)32 or p-ERK1/2 (dilution 1:100) then at room temperature (60 minutes) with biotinylated donkey anti-rabbit IgG (dilution 1:600; Jackson ImmunoResearch Laboratories, West Grove, PA). Negative controls consisted of tissue samples from the same patient that were processed identically but incubated with non-immune rabbit IgG (Jackson ImmunoResearch Laboratories). Sections were stained by using the Vectastain Elite ABC Kit (Vector Laboratories, Burlingame, CA) with VECTOR NovaRed chromogen, counterstained with hematoxylin, and subjectively scored, as previously described.34 All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless specified. Specific staining in extravillous trophoblasts, villous cytotrophoblasts, syncytiotrophoblast, and wall of placental vascular structures was evaluated semiquantitatively in a blinded fashion by two independent observers (H.K.B.M. and I.A.B) from three random fields per slide. Staining intensity was scored on a scale from 0 (absent) to 5 (intense).
BeWo Cell Culture and Immunofluorescence
The human choriocarcinoma BeWo cell line (gift from Dr. John M. Robinson, The Ohio State University, Columbus, OH) shares morphologic and biosynthetic features with human invasive trophoblasts and is widely used to study placental trophoblast function.17 In this study BeWo cells were used for in vitro manipulation of α2CAR. BeWo cells were cultured in Ham's F12 medium supplemented with 10% fetal bovine serum and l-glutamine (2 mmol/L), containing 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco, Gaithersburg, MD), and were incubated under humidified atmosphere of 5% CO2 in air at 37°C. Cells were seeded at 0.5 to 1 × 106 in T25 25-cm2 flasks and passaged at 60% to 80% confluency by using 0.25% Trypsin (Gibco). For β-hCG measurements and for protein and RNA isolation, cells were seeded at 105 cells per well on 6-well plates.
Cells were incubated overnight in culture media replaced daily with fresh media that contained 20 μmol/L forskolin or dimethyl sulfoxide (DMSO; vehicle control), as appropriate for the planned experiments. α2CAR was visualized with polyclonal rabbit antibody,32 followed by goat anti-rabbit secondary antibody conjugated to Alexa Fluor 594 (A11037; Life Technologies, Carlsbad, CA). E-cadherin (marker of progenitor cytotrophoblasts) was visualized with mouse monoclonal anti–E-cadherin monoclonal antibody (HECD-1; dilution 1:200; Invitrogen, Carlsbad, CA), followed by goat anti-mouse IgG secondary antibody conjugated to Alexa Fluor 488 (A11001; Invitrogen).
Quantitative Real-Time RT-PCR
For gene expression studies, placental tissue was flash frozen in liquid nitrogen immediately after delivery and maintained at −80°C. BeWo cells were washed with ice-cold phosphate-buffered saline, 1 mL TRIzol was added to each well, and plates were stored at −80°C. Placental and BeWo cell RNA was extracted with TRIzol and quantitated and reverse transcribed into cDNA with random hexamer primers by using standard procedures. RT-PCR was performed by using TaqMan (Applied Biosystems, Carlsbad, CA) chemistry in 20-μL reactions composed of 10 μL mastermix (TaqMan Fast Advanced Master Mix), 8 μL water, 1 μL cDNA template, and 1 μL PCR probe set (TaqMan Gene Expression Assays). The following probes (Applied Biosystems) were used: α2AAR (ADRA2A; Hs01099503_s1; reference sequence NM_000681.3), α2BAR (ADRA2B; Hs00265090_s1; reference sequence NM_000682.6), α2CAR (ADRA2C; Hs03044628_s1; reference sequence NM_000683.3), and for the housekeeping genes ribosomal protein L30 (RPL30; Hs00265497_m1; reference sequence NM_000989.3) and β–2-microglobulin (B2M; Hs99999907_m1; reference sequence NM_004048.2). Unique probes for experiments with BeWo cells were syncytin-1 (ERVW-1; Hs00205893_m1; reference sequence NM_001130925.1) and syncytin-2 (ERVFRD-1; Hs01652148_m1; reference sequence NM_207582.2) with RPL30 as housekeeping gene.
Placental mRNA expression of α2ARs was normalized to RPL30 and B2M by using cDNA derived from pools of RNA extracted from iPTB and term control placenta tissue, and BeWo expression was normalized to RPL30. Amplification was performed in duplicate reactions for each target in a two-step cycle (denaturation, 95°C for 15 seconds; annealing/extension at 62°C for 60 seconds) for 40 cycles in the StepOnePlus Real-Time PCR System (Applied Biosystems).
Post-processing calculations were performed by using StepOnePlus Software version 2.1 (Applied Biosystems, Foster City, CA). Ct values for α2AR were normalized to the geometric mean of the endogenous control RNAs by using calculations of dCt (Ct of the target − Ct mean of endogenous controls). Calculation of ddCt (dCt of individual sample − Ct of same target in a reference sample) used as reference sample a cDNA pool of all tissues. For BeWo cell experiments, RNA from cells transduced with the non-human target control vector was used as reference.
Western Blot Analysis
α2CAR expression in BeWo cells was detected with affinity-purified rabbit polyclonal α2CAR antisera (dilution 1:500) from total cellular protein prepared as previously described.32 Cells were lyzed in buffer that contained protease inhibitor cocktail (Complete; Roche Applied Sciences, Indianapolis, IN), and 20 μg of total protein was boiled in reducing sample buffer and separated on 4% to 20% Mini Protean TGX gels (Bio-Rad, Hercules, CA). Intensity of the band that corresponded to α2CAR was quantified by using ImageQuant TL software version 8.1 (GE Healthcare, Pittsburgh, PA) and normalized to glyceraldehyde-3-phosphate dehydrogenase as loading control.
Pharmacologic Targeting of α2ARs
Pharmacologic studies used the nonsubtype selective α2AR receptor agonist UK 14,304 [5-bromo-N-(4,5-dihydro-1H-imidazole-2-yl)-6-quinoxalinamine] or the antagonist MK912 that is more selective for α2CAR.35 After 48 hours, cells were treated in a dose-dependent fashion with UK 14,304 (0.01 to 10 nmol/L) or DMSO for 15 minutes. In another set of experiments, cells were treated with UK 14,304 for 15 minutes after a 30-minute pretreatment with 1 nmol/L MK912 or corresponding volume of water (vehicle control for MK912). β-hCG secretion in cell culture media was assayed by enzyme-linked immunosorbent assay (BioVendor Research and Diagnostic Products, Asheville, NC).
α2CAR shRNA Knockdown Experiments
BeWo cells were seeded 105 per well and incubated overnight. In the morning, culture medium was removed and replaced with fresh solution. BeWo cells were transduced with lentiviral particles at a multiplicity of infection = 1 and were subjected to puromycin selection according to the supplier's protocol (Sigma-Aldrich). Transduced cells underwent three consecutive passages under puromycin selection (9 days total) before use in knockdown assays.
α2CAR expression was knocked down in BeWo cells by using a lentiviral shRNA construct that targeted ADRA2C (TRCN0000008077; 5′-CCGGCAACGACGAGACCTGGTACATCTCGAGATGTACCAGGTCTCGTCGTTGTTTTT-3′; Sigma-Aldrich). BeWo cells transduced with a non-human target control, pLKO.1-puro (Sigma-Aldrich), served as negative control. Cells were incubated in the presence or absence of 20 μmol/L forskolin to induce differentiation. RNA was extracted, and expression of α2CAR and of syncytin-1 and syncytin-2 mRNA was measured by quantitative real-time RT-PCR. Culture media were collected before and at 24, 48, and 72 hours after 15-minute exposure to UK 14,304 or DMSO (vehicle control). Media were stored at −80°C until β-hCG measurement.
Trophoblast Migration Analysis
The migration potential of BeWo cells was assessed by wound healing assay as previously described.36 A single wound gap was induced on each well by scratching the cell monolayer with a pipette tip. BeWo cells were pretreated with 1 nmol/L MK912 or H2O for 15 minutes, followed by incubation with 10 mmol/L UK 14,304 or DMSO for 60 minutes. Images of five wounds for each treatment condition were analyzed at 0 and 24 hours after treatment, and percentage of migration at 24 hours was calculated from three points per wound. ImageJ software version 1.46r (NIH, Bethesda, MD; https://imagej.nih.gov/ij) was used for image analysis.
Statistical Analysis
Statistical analyses were performed with SigmaPlot statistical software version 12.5 (Systat Software, San Jose, CA). After distribution testing by using Shapiro-Wilk test, data were compared with t-tests, one-, two-, or three-way analysis of variance or Kruskal-Wallis analysis of variance on ranks as appropriate. Proportions were compared with χ2 test. P < 0.05 was considered statistically significant.
Results
Demographic and Clinical Characteristics
The demographic, clinical, and outcome characteristics of the patients enrolled in this study are presented in Supplemental Table S1. Women with sPE with and without IUGR had the highest blood pressure levels, proteinuria, and frequency of neurologic manifestations (ie, headache, visual disturbances, etc). Patients diagnosed antenatally with IUGR delivered babies with the lowest birthweights.
Immunolocalization of α2AR Subtypes in Preterm and Term Placenta
Immunoreactivity for all three α2AR subtypes was identified in preterm and term placenta but each with a differing pattern of cellular localization (Supplemental Figure S1). The signal for α2AAR dominated in vascular structures of placental stem villi. This was in contrast to α2B and α2C, which were primarily expressed by trophoblast cells. Term placental specimens showed decreased immunostaining for all α2AR subtypes (Supplemental Figure S1).
Immunolocalization of α2ARs Subtypes in Placenta Affected by sPE and/or IUGR
Figure 1 shows representative micrographs of placental immunostaining for α2AAR, α2BAR, α2CAR, and p-ERK1/2 in patients with iPTB, sPE, sPE + IUGR, and IUGR. α2AAR staining (Figure 1A) predominated in blood vessels of the placental stem and intermediate villi. Terminal villi were almost always free of α2AAR staining. Semiquantitative scoring appreciated no difference in staining with respect to sPE, but significantly weaker staining was seen in blood vessels of placentas affected by IUGR irrespective of sPE status (three-way analysis of variance, P < 0.001 for villous grade, P = 0.007 for IUGR, and P = 0.913 for sPE) (Figure 1B).
Figure 1.
Immunohistochemical staining of α2-adrenergic receptor subtype A (α2AAR), α2BAR, α2CAR, and phospho-extracellular signal-regulated protein kinase (p-ERK)1/2 in human placental tissue. A: Representative micrographs stained for α2AAR from pregnancies complicated by idiopathic preterm birth (iPTB), severe preeclampsia (sPE), sPE with intrauterine growth restriction (sPE + IUGR), and IUGR alone. The imaged zones are representative for the areas scored semiquantitatively. B: α2AAR staining was much stronger in the media of blood vessels in SV and IV than in TV. C: Staining for α2BAR predominated in villous cytotrophoblasts (CYTs) and extravillous trophoblasts (EVTs). There was an irregular pattern of stromal staining in sPE + IUGR. D: Overall no significant differences were observed in signal intensity among groups. E: Staining for α2CAR was most prominent in EVT, CYT, and syncytiotrophoblast (SCT). F: Results of histologic scoring indicated significantly higher α2CAR staining of EVT and SCT associated with sPE + IUGR. Insets display regions of interest at higher magnification. Representative p-ERK1/2 staining from a patient with sPE + IUGR indicated activation in placental vasculature and EVTs. Data are expressed as means ± SEM. n = 6 to 15 patients in each group. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001, determined by three-way analysis of variance. Scale bars: 100 μm (A, C, and E); 25 μm (insets). SV, stem villi; IV, intermediate villi; TV, terminal villi; VS, villous stroma.
Immunostaining for α2BAR was identified primarily in villous cytotrophoblasts (Figure 1C). Overall, no significant changes were found with either sPE or IUGR state for the three scored zones (extravillous trophoblasts, villous cytotrophoblasts, and villous stroma; P = 0.046 for zone, P = 0.329 for sPE, and P = 0.778 for IUGR) (Figure 1D). An interesting irregular granular pattern of staining was seen in stroma of many terminal villi in the sPE + IUGR group.
The most conspicuous staining for α2CAR (Figure 1E) was localized to extravillous trophoblasts, villous cytotrophoblasts, and syncytiotrophoblast of placenta from women with sPE + IUGR (three-way analysis of variance, P = 0.001 for trophoblast types, and P = 0.047 for interaction between sPE and IUGR) (Figure 1F). To support the notion of α2AR functional engagement, adjacent sections were stained for p-ERK1/2. Representative images from a patient with sPE + IUGR are presented in Figure 1, A, C, and E. p-ERK1/2 immunoreactivity was present in the wall of blood vessels of stem villi and in extravillous trophoblasts scattered in the wall of the anchoring villi and abundantly infiltrating within placental islands. The pattern of p-ERK1/2 in groups with sPE and/or IUGR paralleled the cell types stained by α2AAR and α2CAR but not α2BAR.
mRNA Expression of α2AR Subtypes in Human Placenta
To learn if placenta was a site of α2AR synthesis and if that varied with GA, tissues retrieved from women with iPTB and healthy term deliveries were first examined. Quantitative real-time RT-PCR showed that transcripts for α2AAR, α2BAR, and α2CAR were present in both preterm and term tissues (Figure 2A). Transcripts for α2AAR were the most abundant, whereas those for α2BAR were the least represented. For each α2AR subtype, mRNA levels were similar in iPTB and term placenta (two-way analysis of variance, P < 0.001 for receptor subtype and P = 0.327 for GA periods).
Figure 2.
Relative abundance of α2-adrenergic receptor subtype A (α2AAR), α2BAR, and α2CAR mRNA in placenta. A: Relative expression of α2AAR, α2BAR, and α2CAR mRNA in placental tissue of idiopathic preterm birth (iPTB) and term placenta are displayed. Relative quantitation (RQ) of different receptor subtypes was reported relative to expression of housekeeping genes β2-microglobulin and ribosomal protein L30 by using dCt. B–D: Estimate of relative placental mRNA abundance (ddCT) for α2AAR (B), α2BAR (C), and α2CAR (D) for pregnancies complicated by iPTB, severe preeclampsia (sPE), sPE with intrauterine growth restriction (sPE + IUGR), and IUGR, and term controls. ddCt RQ values are reported relative to a reference RNA pool of all tissues. Data are expressed as means ± SEM. n = 4 to 6 samples in each group. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
Next, the placental expression of α2AAR, α2BAR, and α2CAR were compared in sPE, sPE + IUGR, and IUGR by using ddCt. The iPTB and term groups were used for baseline. No difference was found in α2AAR transcript levels across groups (P = 0.137) (Figure 2B). Of interest, placenta of women with sPE, sPE + IUGR, and IUGR had significantly higher expression of α2BAR than with iPTB (P = 0.015 for IUGR and P = 0.012 for sPE) (Figure 2C) but not compared with term tissues. sPE + IUGR had significantly higher α2CAR placental mRNA levels than both the iPTB (P = 0.019) (Figure 2D) and term (P = 0.031) groups.
To the best of our knowledge, this is the first report of α2CAR expression in human placenta; with the use of human trophoblasts, the possible function of this receptor were studied.
Validation of α2CAR Expression by BeWo Trophoblast Cell Line
BeWo cells were chosen as model because of their well-characterized ability to differentiate in vitro in response to adenylate cyclase activation by forskolin.17 By Western blot analysis, it was determined that BeWo cells express constitutively α2CAR as the approximately 50- to 55-kDa band (Supplemental Figure S2A), which was previously reported to represent the core glycosylated form of the receptor.37 When fusion was induced through activation of the cAMP/protein kinase A pathway by forskolin, an increased level of expression of the same band as quantified by densitometric analysis was detected (P < 0.001) (Supplemental Figure S2B), consistent with the previous report of receptor inducibility by cAMP.32 Double immunofluorescence of DMSO-treated BeWo cells confirmed constitutive α2CAR expression, albeit at low levels, whereas expression of E-cadherin, a marker of nonfused trophoblasts, was identified at cellular boundaries (Supplemental Figure S2C). As expected, E-cadherin fluorescence diminished on treatment with forskolin, indicating trophoblast differentiation toward syncytialization. This concurred with increased fluorescence for α2CAR (Supplemental Figure S2D).
Impact of Pharmacologic Modulation of α2CAR on β-hCG Secretion by BeWo Cells
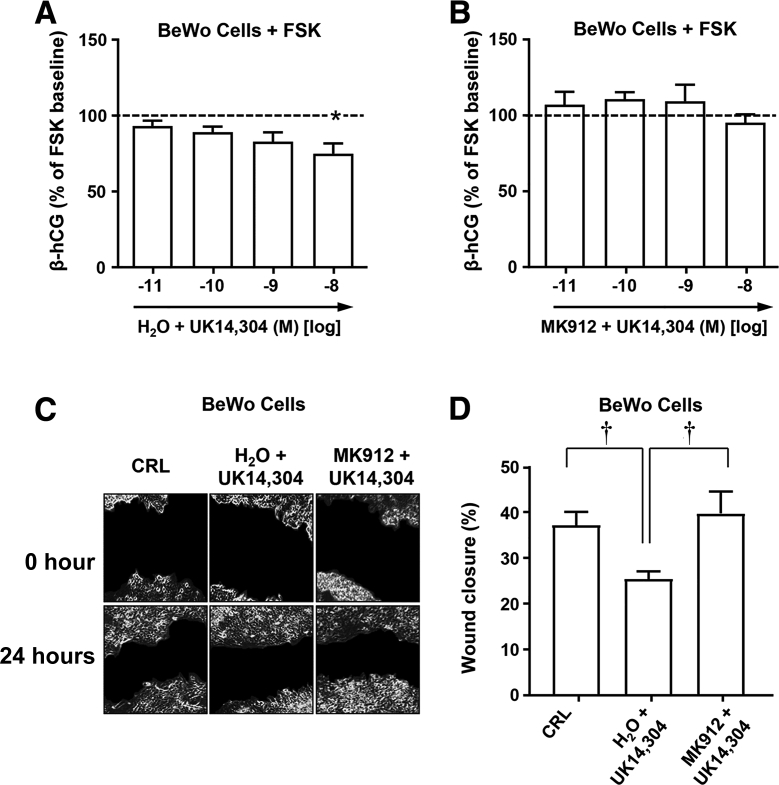
Forskolin-stimulated BeWo cells were treated with increasing concentrations of UK 14,304 (nonselective α2AR receptor agonist) to determine whether general engagement of α2ARs affects β-hCG secretion, an attribute of differentiated trophoblast. Despite the observed lowering trend, only the highest concentration of UK 14,304 produced a statistically significant reduction in β-hCG secretion (P < 0.05) (Figure 3A). This supported some general involvement of α2ARs in the negative regulation of adenylate cyclase. More definitive evidence to implicate α2CAR was the ability to block the UK 14,304–mediated reduction of β-hCG secretion by pretreatment with the α2CAR antagonist MK912 (Figure 3B). These results suggested that stimulation of α2CAR may result in decreased β-hCG secretion and/or decreased syncytialization.
Figure 3.
Effect of α2-adrenergic receptor (α2AR) agonist activation on secretory and migratory function of BeWo cells. A: Levels of β-human chorionic gonadotropin (β-hCG) secreted from BeWo cells stimulated with forskolin (FSK; 20 μmol/L) and exposed to increasing concentrations of α2AR agonist UK 14,304. B: β-hCG secreted from FSK-stimulated BeWo cells treated with UK 14,304 for 15 minutes after pretreatment with the α2CAR antagonist MKI912. C: Representative images of in vitro wound healing scratch assay assessed BeWo cell migration at 24 hours after treatment with α2AR agonist UK 14,304 alone or after pretreatment with α2CAR antagonist MK912. Control wells (CRL) were treated with corresponding volume of vehicle (water and dimethyl sulfoxide). D: Quantification of the gap after 24 hours showed that α2AR activation with UK 14,304 resulted in a significant reduction in cell migration and that pretreatment with α2CAR antagonist MK912 blocked the UK 14,304–mediated inhibition. Dashed lines mark baseline level. Data are expressed as means ± SEM. n = 3 independent experiments expressed as percentage of baseline level before agonist (A); n = 3 experiments (B); n = 3 independent experiments (D). ∗P < 0.05 versus baseline (one-way repeated measures analysis of variance); †P < 0.05 (one-way analysis of variance).
Impact of α2CAR Pharmacologic Modulation on Invasive Potential of BeWo Cells
To examine the potential involvement of α2CAR in modulating processes required for trophoblast cell migration, wounds produced in monolayers of BeWo cells were subjected to α2AR agonist UK 14,304 alone or after pretreatment with MK912. Figure 3C contains images of identical fields in representative wound closure assays photographed at 0 and 24 hours after wounding. BeWo cells treated with the agonist UK 14,304 showed significantly less migration compared with nontreated control cells (P = 0.029) (Figure 3D). Pretreatment of BeWo cells with MK912 blocked the inhibition of cell migration mediated by UK 14,304, thus enhancing wound closure (P = 0.031).
Impact of α2CAR Knockdown on Syncytin mRNA and β-hCG Secretion by BeWo Cells
Because none of the available pharmacologic agents are entirely specific nor have they been previously studied for affinity for trophoblast α2CAR, the gene knockdown approach was used. Lentiviral targeting of ADRA2C resulted in 67% reduction in α2CAR mRNA levels compared with the non-human target control (paired t-test P = 0.019) (Figure 4A). With forskolin stimulation, an increase in mRNA transcripts for syncytin-1 (104-fold) (Figure 4B) and syncytin-2 (280-fold) (Figure 4C) were observed relative to non-human target control.
Figure 4.
Effect of ADRA2C knockdown on expression of syncytins and secretory function of BeWo cells. A: mRNA levels of α2-adrenergic receptor subtype C (α2CAR) after lentiviral knockdown of ADRA2C (KD) or of a non-human target (CRL). B and C: Syncytin-1 (B) and syncytin-2 (C) mRNA after stimulation with forskolin (FSK). D and E: Time course of levels of secreted β-human chorionic gonadotropin (β-hCG) from stably transduced ADRA2C knockdown cells (black bars) and non-human target knockdown cells (control; gray bars) exposed to dimethyl sulfoxide (DMSO; D) as vehicle control or FSK (20 μmol/L; E) to induce syncytialization. F: Levels of β-hCG secreted from FSK-stimulated (20 μmol/L for 48 hours) ADRA2C knockdown cells exposed to increasing concentrations of α2AR agonist UK 14,304. G: Levels of β-hCG secreted from FSK (20 μmol/L) stimulated non-human target knockdown cells treated with UK 14,304. Dashed lines mark baseline level. Data are expressed as means ± SEM. n = 3 stably transduced ADRA2C knockdown cells (D and E); n = 3 non-human target knockdown cells (D and E); n = 3 experiments (F); n = 3 independent experiments (G). ∗P < 0.05 (t-test); †P < 0.05 (two-way repeated measures analysis of variance); ‡P < 0.05 versus baseline (one-way repeated measures analysis of variance).
Compared with the non-human target control, ADRA2C knockdown significantly decreased β-hCG secretion under basal conditions (DMSO control) at 24 (two-way repeated measures analysis of variance, P = 0.012) (Figure 4D), 48 (P = 0.024), and 72 (P = 0.017) hours. Forskolin stimulation increased β-hCG concentration in the medium approximately eightfold at 24 hours. ADRA2C-knockdown cells secreted significantly less β-hCG than control cells on forskolin stimulation at 24 hours (P = 0.046) (Figure 4E). At 48 hours, the difference between knockdown and control cells did not reach significance (P = 0.086) (Figure 4E). At 72 hours, β-hCG secretion was unsustainable and decreased in both ADRA2C knockdown and control with no difference among these two groups (P = 0.276).
Forskolin-stimulated BeWo cells infected with lentivirus that carried the non-human target gene responded to UK 14,304 with decreased secretion of β-hCG (Figure 4F). The direction of this response was similar to that shown in Figure 3A, although the infection itself appeared to render the cells sensitive to lower agonist concentrations. ADRA2C knockdown resulted in loss of this response to UK 14,304 (Figure 4G). This would be consistent with involvement of α2CAR on trophoblast cells in negative regulation of adenylate cyclase. Taken together, these results suggested that BeWo cells expressed α2CARs, which were involved in trophoblast differentiation and/or secretory function through processes that at a minimum included inhibition of syncytin-1 and syncytin-2 synthesis and promotion of β-hCG secretion.
Discussion
In this study, a systematic description has been provided for the pattern of α2AR expression and localization in human placenta. The overlap in p-ERK1/2 staining pattern with that of at least two α2AR subtypes (α2AAR and α2CAR) suggests that α2ARs may be implicated in modulation of multiple regulatory processes essential for fetal and placental development.8 By PCR, transcripts for all α2ARs were identified. The in vitro experiments were designed to provide additional mechanistic evidence that α2CARs, which are primarily known for their critical role in regulating neurotransmitter release from sympathetic nerves and central noradrenergic neurons,38 function in the placenta as local regulators of trophoblast syncytialization and migration. Furthermore, α2BAR and α2CAR were found to be significantly up-regulated at the mRNA and/or protein level in women with sPE and/or IUGR. Our original hypothesis that PE and IUGR associate with altered expression of placental α2ARs was thus confirmed.
Before the present study, the role of α2ARs in reproduction was implied from genetically engineered murine models that showed defective implantation and fetal development.7 Radio-ligand binding and PCR experiments provided evidence that α2A and α2B subtypes are expressed in human placenta.23, 39, 40, 41 In the present study, with the use of PCR and immunohistochemistry, the first systematic description of tissue localization for all three known α2AR subtypes in human placenta is presented. The presence of α2AAR and α2BAR was confirmed, and it was further shown that they are preferentially localized to different structures within the placenta: vascular wall for α2A and trophoblast for α2B and α2C AR subtypes. To our knowledge, identification of α2CARs in the human placenta and description of their localization within the extravillous trophoblast and syncytiotrophoblast are novel findings.
By both PCR and immunohistochemistry, α2BAR was identified in the placenta predominantly in cytotrophoblasts and extravillous trophoblast. If the functional relevance of the α2BAR would be similar to that of mouse placenta, one would expect this AR subtype to be involved in angiogenesis.8 It was suggested that the number of placental α2ARs decline with advancing GA.23 The PCR experiments demonstrated that the relative mRNA levels for each α2AR subtype were similar between iPTB and term placenta, yet the immunohistochemical signal appeared diminished according to the receptor localization (stem, intermediate, and term villi). Collectively, these results imply that involvement of α2ARs in regulation of placental vascular tone and activity of extravillous trophoblast and syncytiotrophoblast is functional from early gestation.
The α2ARs have both presynaptic and postsynaptic functions and are differentially expressed in a variety of tissues, including adrenal gland, vascular endothelium, brain, and kidney.1, 2, 4 In specialized contractile smooth muscle cells in the blood vessel wall, α2ARs mediate constriction of arterioles (referred to as resistance vessels) and veins but not large arteries; α2AR density is inversely related to vessel size.1, 31, 42 The remarkable differences in function of the blood vessels that comprise the vascular bed are due in part to differential α2AR subtype expression within the vascular wall.31 For example, although both α2AAR and α2CAR were localized in human aortas, α2CARs were immunohistochemically localized in the vascular smooth muscle of adventitial arterioles and not aortic media.31 The above-mentioned data confirm the existence of site- and tissue-specific α2AR expression, which enables physiological modulation of specific peripheral vascular resistance (blood flow and pressure). Consistent with other human vascular territories, placenta also displays site-specific α2AR distribution. Our observed immunolocalization of α2AAR within anchoring and terminal placental blood vessels was consistent with an earlier proposed vascular location that was based on the mere existence of smooth muscle cells.41 Remarkably, studies have categorized α2CARs as stress-receptors of the vascular sympathetic system.31However, α2CARs did not have any vascular association in placenta and were localized in extravillous trophoblast and syncytiotrophoblast, potentially signifying different function(s).
Both mRNA levels and immunohistochemistry localization studies showed fundamental differences between human and mouse α2AR placental expression. The relative mRNA levels in human placenta were α2A>>α2C>α2B, and in mouse placenta α2B>>α2A>α2C.8 The newly identified human placental α2CAR was the predominant subtype localized to human syncytiotrophoblast and extravillous trophoblast, whereas α2BARs, the predominant subtype expressed in mouse placenta at embryo day 9.5 to 10.5 localizes to spongiotrophoblasts and giant cells.2, 7 Collectively, this distribution pattern implies a broad spectrum of functions of the placental α2ARs that in addition to vascular growth, development, and blood flow regulation may include roles in implantation, placentation, and endocrine adaptation to pregnancy.
Trophoblast fusion is an essential process for placental development and maturation. Prior studies highlighted forskolin stimulation of cAMP, α2AR negative regulation of adenylyl cyclase, and ability of cAMP to mediate downstream events leading to syncytialization.43, 44 Despite this large body of research, our study is first to demonstrate a regulatory connection between α2ARs and syncitin-1 and syncitin-2, which are essential proteins for trophoblast fusion. Furthermore, we provided a link between α2ARs and cell fusion through experimentation with pharmacologic agents with agonist and antagonist effect. In the cell culture system UK 14,304 reduced secretion of β-hCG, indicating α2ARs are inhibitory on the secretory function of the syncytiotrophoblast. However, an interpretation based entirely on inhibitory G-protein subunit α group i signaling is insufficient to explain α2CAR modulation of trophoblast fusion. The gene knockdown experiments provided additional proof. First, in ADRA2C knockdown cells under both basal and forskolin-stimulated conditions, β-hCG secretion was significantly decreased. This observation is at odds with the experiments demonstrating increased expression of syncitin-1 and syncitin-2. The simplest explanation for these results is that syncytialization and β-hCG are independent processes as previously shown.17 As described, it was shown that, in addition to multinucleated syncytia, β-hCG can also be secreted by mononucleated trophoblast experimentally hindered to undergo fusion. β-hCG may also have a protein-kinase A–independent component.17
We hypothesized that α2ARs may have implications for pathogenesis of PE and IUGR. By PCR significant differences were observed in expression of both α2BAR and α2CAR mRNAs and proteins for both conditions. Significantly elevated α2BAR and α2CAR mRNA levels were associated only with sPE complicated by IUGR, but interestingly, with neither pathologic condition alone. This aspect is remarkable because of our data linking the functional relevance of placental α2ARs and β-hCG secretion. Human data generated from part of the first trimester screening for aneuploidy reported that maternal serum β-hCG is significantly reduced in patients who developed preeclampsia and IUGR later in gestation.45
These data provide new circumstantial evidence of a link between α2AR dysregulation and pathogenesis of PE with IUGR.46 First, the in vitro experiments demonstrated that engagement of the α2AR led to poor syncytialization which is known to be defective in PE.10, 11 Second, the predominant expression of α2CARs in the extravillous trophoblast coupled with the experiments demonstrating less migratory potential in the presence of UK 14,304 agonist suggest that activation of α2CARs may affect extravillous trophoblast migration and remodeling of uterine spiral arteries, which is a pathophysiologic hallmark of PE.47 Finally, the predominant mouse subtype, α2BAR, has a role in placental angiogenesis; thus, it is reasonable to expect that α2BAR may have analogous functions.7, 8 The reason α2BAR and α2CAR are significantly up-regulated only in sPE complicated by IUGR remains unknown. Certainly, the human data are at odds with the observation that embryos of α2BAR-deficient mice were smaller in size compared with wild-type animals, inferring that not only deletion but also overexpression of α2BAR and α2CAR may affect fetal growth.8 Many studies agree on the pivotal role of α2AR in mediating sympathetic tone in response to environmental stress. Genetic association studies between common α2AR variants and phenotypic variability in blood pressure and heart rate responses among individuals have started to emerge.48 Considering the previously reported link between α2AR signaling and regulation of VEGF and soluble type 1 receptor for VEGF,8 these data should stimulate research to investigate a potential link between α2CAR genetic variants and clinical subphenotypes in women with PE. This study demonstrates that the placenta possesses α2ARs and brings into consideration a possible local placental effect of α2AR pharmacologic modulators. Blocking the activity of α2ARs in pregnancies complicated by sPE and IUGR may improve placental blood flow. More studies should be conducted to interrogate the safety and efficacy of αAR blockade in human gestation.
Conclusions
This study provides evidence for differential expression and localization of α2ARs in human placental tissue and found a compelling association between elevated α2CAR levels and the pregnancy-related pathologies of sPE and IUGR.
Acknowledgments
We thank the fellows and residents at Yale University Department of Obstetrics and Gynecology, all patients who provided the samples used in the study, and Dr. John M. Robinson (The Ohio State University) for kindy providing the human choriocarcinoma BeWo cell line.
Footnotes
Supported by NIH/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NIH/NICHD) grant R01HD04732 (I.A.B.); the Center for Perinatal Research at The Research Institute at Nationwide Children's Hospital, Columbus, Ohio; and the graduate training Joint Supervision Program supported by The Egyptian Cultural and Educational Bureau, Washington, DC, and Egyptian Cultural and Educational Sector, Cairo, Egypt (H.K.B.M.).
Disclosures: None declared.
Supplemental material for this article can be found at https://doi.org/10.1016/j.ajpath.2018.08.011.
Supplemental Data
Supplemental Figure S1.
Immunolocalization of α2-adrenergic receptor (α2AR) subtypes in placenta of women with idiopathic preterm birth (iPTB) and uncomplicated deliveries at term. A–F: Representative immunostaining patterns for α2AAR, α2BAR, and α2CAR of placenta from women with iPTB (A–C) or uncomplicated term pregnancy (Term; D–F). In iPTB α2AAR was primarily localized in the wall of the blood vessels of stem villi (sv) and intermediate villi (iv). Terminal villi (tv) were almost free of staining. α2BARs were primarily localized to villous cytotrophoblast (cyt). Villous stroma (vs) and extravillous trophoblast (evt) showed only weak staining. α2CAR immunostaining was most intense in evt, cyt, and syncytiotrophoblast (sct). At term, staining was reduced for all α2AR. G–I: Results of the semiquantitative analysis of staining intensity in iPTB and term placentas for the regions highlighted above. Data are expressed as means ± SEM. n = 5 to 8 patients in each group. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 (two-way repeated measure analysis of variance). Scale bars = 100 μm.
Supplemental Figure S2.
Identification of α2-adrenergic receptor subtype C (α2CAR) by Western blot analysis and immunofluorescence. A: Immunoblot of α2CAR expression in BeWo cells treated with dimethyl sulfoxide (DMSO) or forskolin (FSK; 20 μmol/L). B: Densitometric quantification of the α2CAR band of BeWo cells treated with DMSO or FSK. Data analysis indicated a significant increase in response to FSK stimulation compared with DMSO, normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). C and D: Indirect immunofluorescence of BeWo cells stained with anti-α2CAR (red), anti–E-cadherin (green), and DAPI (blue) treated with DMSO or 20 μmol/L FSK for 72 hours. Data are expressed as means ± SEM. n = 4 FSK stimulation (B); n = 5 DMSO stimulation (B). ∗P < 0.05 (t-test).
References
- 1.Saunders C., Limbird L.E. Localization and trafficking of alpha2-adrenergic receptor subtypes in cells and tissues. Pharmacol Ther. 1999;84:193–205. doi: 10.1016/s0163-7258(99)00032-7. [DOI] [PubMed] [Google Scholar]
- 2.Brede M., Philipp M., Knaus A., Muthig V., Hein L. α2-adrenergic receptor subtypes – novel functions uncovered in gene-targeted mouse models. Biol Cell. 2004;96:343–348. doi: 10.1016/j.biolcel.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Tan C.M., Limbird L.E. The α2-adrenergic receptors: lessons from knockouts. In: Perez D.M., editor. The Adrenergic Receptors: In the 21st Century. Humana Press Inc.; Totowa, NJ: 2006. pp. 241–265. [Google Scholar]
- 4.Gilbach R., Hein L. Are the pharmacology and physiology of α2-adrenoceptors determined by α2-heteroreceptors and autoreceptors respectively? Br J Pharmacol. 2012;165:90–102. doi: 10.1111/j.1476-5381.2011.01533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crassous P.A., Denis C., Paris H., Sénard J.M. Interest of alpha2-adrenergic agonists and antagonists in clinical practice: background, facts and perspectives. Curr Top Med Chem. 2007;7:187–194. doi: 10.2174/156802607779318190. [DOI] [PubMed] [Google Scholar]
- 6.Lavon H., Matzner P., Benbenishty A., Sorski L., Rossene E., Haldar R., Elbaz E., Cata J.P., Gottumukkala V., Ben-Eliyahu S. Dexmedetomidine promotes metastasis in rodent models of breast, lung, and colon cancers. Br J Anaesth. 2018;120:188–196. doi: 10.1016/j.bja.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Philippe M., Brede M., Hadamek K., Gessler M., Lohse M.J., Hein L. Placental alpha(2)-adrenoceptors control vascular development at the interface between mother and embryo. Nat Genet. 2002;31:311–315. doi: 10.1038/ng919. [DOI] [PubMed] [Google Scholar]
- 8.Muthig V., Gilsbach R., Haubold M., Philipp M., Ivacevic T., Gessler M., Hein L. Upregulation of soluble vascular endothelial growth factor receptor 1 contributes to angiogenesis defects in the placenta of alpha2B-adrenoceptor deficient mice. Circ Res. 2007;101:682–691. doi: 10.1161/CIRCRESAHA.107.151563. [DOI] [PubMed] [Google Scholar]
- 9.Reijnders I.F., Mulders A.G.M.G.J., Koster M.P.H. Placental development and function in women with a history of placenta-related complications: a systematic review. Acta Obstet Gynecol Scand. 2018;97:248–257. doi: 10.1111/aogs.13259. [DOI] [PubMed] [Google Scholar]
- 10.Li H., Dakour J., Kaufman S., Guilbert L.J., Winkler-Lowen B., Morrish D.W. Adrenomedullin is decreased in preeclampsia because of failed response to epidermal growth factor and impaired syncytialization. Hypertension. 2003;42:895–900. doi: 10.1161/01.HYP.0000095613.41961.6E. [DOI] [PubMed] [Google Scholar]
- 11.Newhouse S.M., Davidge S.T., Winkler-Lowen B., Demianczuk N., Guilbert L.J. In vitro differentiation of villous trophoblasts from pregnancies complicated by intrauterine growth restriction with and without pre-eclampsia. Placenta. 2007;28:999–1003. doi: 10.1016/j.placenta.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Castellucci M., Scheper M., Scheffen I., Celona A., Kaufmann P. The development of the human placental villous tree. Anat Embryol (Berl) 1990;181:117–128. doi: 10.1007/BF00198951. [DOI] [PubMed] [Google Scholar]
- 13.Castellucci M., Kosanke G., Verdenelli F., Huppertz B., Kaufmann P. Villous sprouting: fundamental mechanisms of human placental development. Hum Reprod Update. 2000;6:485–494. doi: 10.1093/humupd/6.5.485. [DOI] [PubMed] [Google Scholar]
- 14.Loregger T., Pollheimer J., Knofler M. Regulatory transcription factors controlling function and differentiation of human trophoblast--a review. Placenta. 2003;24:S104–S110. doi: 10.1053/plac.2002.0929. [DOI] [PubMed] [Google Scholar]
- 15.Handwerger S. New insights into the regulation of human cytotrophoblast cell differentiation. Mol Cell Endocrinol. 2010;323:94–104. doi: 10.1016/j.mce.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knerr I., Schubert S.W., Wich C., Amann K., Aigner T., Vogler T., Jung R., Dötsch J., Rascher W., Hashemolhosseini S. Stimulation of GCMa and syncytin via cAMP mediated PKA signaling in human trophoblastic cells under normoxic and hypoxic conditions. FEBS Lett. 2005;579:3991–3998. doi: 10.1016/j.febslet.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 17.Orendi K., Gauster M., Moser G., Meiri H., Huppertz B. The choriocarcinoma cell line BeWo: syncytial fusion and expression of syncytium-specific proteins. Reproduction. 2010;140:759–766. doi: 10.1530/REP-10-0221. [DOI] [PubMed] [Google Scholar]
- 18.Soygur B., Sati L. The role of syncytins in human reproduction and reproductive organ cancers. Reproduction. 2016;152:R167–R178. doi: 10.1530/REP-16-0031. [DOI] [PubMed] [Google Scholar]
- 19.Wice B., Menton D., Geuze H., Schwartz A.L. Modulators of cyclic AMP metabolism induce syncytiotrophoblast formation in vitro. Exp Cell Res. 1990;186:306–316. doi: 10.1016/0014-4827(90)90310-7. [DOI] [PubMed] [Google Scholar]
- 20.Lee X., Keith J.C., Jr., Stumm N., Moutsatsos I., McCoy J.M., Crum C.P., Genest D., Chin D., Ehrenfels C., Pijnenborg R., van Assche F.A., Mi S. Downregulation of placental syncytin expression and abnormal protein localization in pre-eclampsia. Placenta. 2001;22:808–812. doi: 10.1053/plac.2001.0722. [DOI] [PubMed] [Google Scholar]
- 21.Roland C.S., Hu J., Ren C.E., Chen H., Li J., Varvoutis M.S., Leaphart L.W., Byck D.B., Zhu X., Jiang S.W. Morphological changes of placental syncytium and their implications for the pathogenesis of preeclampsia. Cell Mol Life Sci. 2016;73:365–376. doi: 10.1007/s00018-015-2069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruebner M., Strissel P.L., Ekici A.B., Stiegler E., Dammer U., Goecke T.W., Faschingbauer F., Fahlbusch F.B., Beckmann M.W., Strick R. Reduced syncytin-1 expression levels in placental syndromes correlates with epigenetic hypermethylation of the ERVW-1 promoter region. PLoS One. 2013;8:e56145. doi: 10.1371/journal.pone.0056145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Falkay G., Kovács L. Expression of two alpha 2-adrenergic receptor subtypes in human placenta: evidence from direct binding studies. Placenta. 1994;15:661–668. doi: 10.1016/s0143-4004(05)80412-6. [DOI] [PubMed] [Google Scholar]
- 24.American College of Obstetricians and Gynecologists ACOG Practice Bulletin No. 101: ultrasonography in pregnancy. Obstet Gynecol. 2009;113:451–461. doi: 10.1097/AOG.0b013e31819930b0. [DOI] [PubMed] [Google Scholar]
- 25.ACOG Committee on Practice Bulletins--Obstetrics ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002;99:159–167. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 26.American College of Obstetricians and Gynecologists. Task Force on Hypertension in Pregnancy Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 27.American College of Obstetricians and Gynecologists ACOG Practice bulletin no. 134: fetal growth restriction. Obstet Gynecol. 2013;121:1122–1133. doi: 10.1097/01.AOG.0000429658.85846.f9. [DOI] [PubMed] [Google Scholar]
- 28.Daunt D.A., Hurt C., Hein L., Kallio J., Feng F., Kobilka B.K. Subtype-specific intracellular trafficking of alpha2-adrenergic receptors. Mol Pharmacol. 1997;51:711–720. doi: 10.1124/mol.51.5.711. [DOI] [PubMed] [Google Scholar]
- 29.Motawea H.K., Jeyaraj S.C., Eid A.H., Mitra S., Unger N.T., Ahmed A.A., Flavahan N.A., Chotani M.A. Cyclic AMP-Rap1A signaling mediates cell surface translocation of microvascular smooth muscle α2C-adrenoceptors through the actin-binding protein filamin-2. Am J Physiol Cell Physiol. 2013;305:C829–C845. doi: 10.1152/ajpcell.00221.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bailey S.R., Eid A.H., Mitra S., Flavahan S., Flavahan N.A. Rho kinase mediates cold-induced constriction of cutaneous arteries: role of alpha2C-adrenoceptor translocation. Circ Res. 2004;94:1367–1374. doi: 10.1161/01.RES.0000128407.45014.58. [DOI] [PubMed] [Google Scholar]
- 31.Chotani M.A., Mitra S., Su B.Y., Flavahan S., Eid A.H., Clark K.R., Montague C.R., Paris H., Handy D.E., Flavahan N.A. Regulation of alpha(2)-adrenoceptors in human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2004;286:H59–H67. doi: 10.1152/ajpheart.00268.2003. [DOI] [PubMed] [Google Scholar]
- 32.Jeyaraj S.C., Unger N.T., Eid A.H., Mitra S., El-Dahdah N.P., Quilliam L.A., Flavahan N.A., Chotani M.A. Cyclic AMP-Rap1A signaling activates RhoA to induce α(2c)-adrenoceptor translocation to the cell surface of microvascular smooth muscle cells. Am J Physiol Cell Physiol. 2012;303:C499–C511. doi: 10.1152/ajpcell.00461.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeyaraj S.C., Chotani M.A., Mitra S., Gregg H.E., Flavahan N.A., Morrison K.J. Cooling evokes redistribution of alpha2C-adrenoceptors from Golgi to plasma membrane in transfected human embryonic kidney 293 cells. Mol Pharmacol. 2001;60:1195–1200. doi: 10.1124/mol.60.6.1195. [DOI] [PubMed] [Google Scholar]
- 34.Oliver E.A., Buhimschi C.S., Dulay A.T., Baumbusch M.A., Abdel-Razeq S.S., Lee S.Y., Zhao G., Jing S., Pettker C.M., Buhimschi I.A. Activation of the receptor for advanced glycation end products system in women with severe preeclampsia. J Clin Endocrinol Metab. 2011;96:689–698. doi: 10.1210/jc.2010-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uhlén S., Porter A.C., Neubig R.R. The novel alpha-2 adrenergic radioligand [3H]-MK912 is alpha-2C selective among human alpha-2A, alpha-2B and alpha-2C adrenoceptors. J Pharmacol Exp Ther. 1994;271:1558–1565. [PubMed] [Google Scholar]
- 36.Ali M., Heyob K., Jacob N.K., Rogers L.K. Alterative expression and localization of profilin 1/VASPpS157 and cofilin 1/VASPpS239 regulates metastatic growth and is modified by DHA supplementation. Mol Cancer Ther. 2016;15:2220–2231. doi: 10.1158/1535-7163.MCT-16-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chotani M.A., Flavahan N.A. Intracellular α(2C)-adrenoceptors: storage depot, stunted development or signaling domain? Biochim Biophys Acta. 2011;1813:1495–1503. doi: 10.1016/j.bbamcr.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neumeister A., Charney D.S., Belfer I., Geraci M., Holmes C., Sharabi Y., Alim T., Bonne O., Luckenbaugh D.A., Manji H., Goldman D., Goldstein D.S. Sympathoneural and adrenomedullary functional effects of alpha2C-adrenoreceptor gene polymorphism in healthy humans. Pharmacogenet Genomics. 2005;15:143–149. doi: 10.1097/01213011-200503000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Bagaméry K., Kovács L., Viski S., Nyári T., Falkay G. Ontogeny of imidazoline binding sites in the human placenta. Acta Obstet Gynecol Scand. 1999;78:89–92. [PubMed] [Google Scholar]
- 40.Falkay G., Melis K., Kovács L. Correlation between beta- and alpha-adrenergic receptor concentrations in human placenta. J Recept Res. 1994;14:187–195. doi: 10.3109/10799899409066030. [DOI] [PubMed] [Google Scholar]
- 41.Sarkar S., Tsai S.W., Nguyen T.T., Plevyak M., Padbury J.F., Rubin L.P. Inhibition of placental 11beta-hydroxysteroid dehydrogenase type 2 by catecholamines via alpha-adrenergic signaling. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1966–R1974. doi: 10.1152/ajpregu.2001.281.6.R1966. [DOI] [PubMed] [Google Scholar]
- 42.Flavahan N.A., Lindblad L.E., Verbeuren T.J., Shepherd J.T., Vanhoutte P.M. Cooling and alpha1- and alpha2-adrenergic responses in cutaneous veins: role of receptor reserve. Am J Physiol Heart Circ Physiol. 1985;249:H950–H955. doi: 10.1152/ajpheart.1985.249.5.H950. [DOI] [PubMed] [Google Scholar]
- 43.Delidaki M., Gu M., Hein A., Vatish M., Grammatopoulos D.K. Interplay of cAMP and MAPK pathways in hCG secretion and fusogenic gene expression in a trophoblast cell line. Mol Cell Endocrinol. 2011;332:213–220. doi: 10.1016/j.mce.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 44.Kudo Y., Boyd C.A., Kimura H., Cook P.R., Redman C.W., Sargent I.L. Quantifying the syncytialisation of human placental trophoblast BeWo cells grown in vitro. Biochim Biophys Acta. 2003;1640:25–31. doi: 10.1016/s0167-4889(03)00004-1. [DOI] [PubMed] [Google Scholar]
- 45.Abdel Moety G.A., Almohamady M., Sherif N.A., Raslana A.N., Mohamed T.F., El Moneam H.M., Mohy A.M., Youssef M.A. Could first-trimester assessment of placental functions predict preeclampsia and intrauterine growth restriction? A prospective cohort study. J Matern Fetal Neonatal Med. 2016;29:413–417. doi: 10.3109/14767058.2014.1002763. [DOI] [PubMed] [Google Scholar]
- 46.Manyonda I.T., Slater D.M., Fenske C., Hole D., Choy M.Y., Wilson C. A role for noradrenaline in pre-eclampsia: towards a unifying hypothesis for the pathophysiology. Br J Obstet Gynaecol. 1998;105:641–648. doi: 10.1111/j.1471-0528.1998.tb10179.x. [DOI] [PubMed] [Google Scholar]
- 47.Lyall F., Robson S.C., Bulmer J.N. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: relationship to clinical outcome. Hypertension. 2013;62:1046–1054. doi: 10.1161/HYPERTENSIONAHA.113.01892. [DOI] [PubMed] [Google Scholar]
- 48.Kohli U., Diedrich A., Kannankeril P.J., Muszkat M., Sofowora G.G., Hahn M.K., English B.A., Blakely R.D., Stein C.M., Kurnik D. Genetic variation in alpha2-adrenoreceptors and heart rate recovery after exercise. Physiol Genomics. 2015;47:400–406. doi: 10.1152/physiolgenomics.00124.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.