Abstract
Although antibiotics are useful, they can also bring negative effects. We found that antibiotic-treated mice exhibit an alteration in the gene expression profile of corneal tissues and a decrease in corneal nerve density. During corneal wound healing, antibiotic treatment was found to impair corneal nerve regeneration, an effect that could be largely reversed by reconstitution of the gut microbiota via fecal transplant. Furthermore, CCR2− corneal macrophages were found to participate in the repair of damaged corneal nerves, and a decrease in CCR2− corneal macrophages in antibiotic-treated mice, which could be reversed by fecal transplant, was observed. Adoptive transfer of CCR2− corneal macrophages promoted corneal nerve regeneration in antibiotic-treated mice. The application of probiotics after administration of antibiotics also restored the proportion of CCR2− corneal macrophages and increased the regeneration of corneal nerve fibers after epithelial abrasion. These results suggest that dysbiosis of the gut microbiota induced by antibiotic treatment impairs corneal nerve regeneration by affecting CCR2− macrophage distribution in the cornea. This study also indicates the potential of probiotics as a therapeutic strategy for promoting the regeneration of damaged corneal nerve fibers when the gut microbiota is in dysbiosis.
Antibiotics (Abxs) are used by numerous individuals and farm animals every day. Although Abxs are a cure for many infectious diseases, they also have negative impacts on the physiology of patients.1, 2 An increasing number of studies have revealed that the gut microbiota is strongly associated with human health. It not only participates in nutrient metabolism and maintenance of the gut mucosal integrity, but it is also involved in immunoregulation and defense against pathogen invasion.3, 4 Recent research has indicated that Abx-induced dysbiosis causes pathologic changes in the central nervous system, including impairment of neurodevelopment in newborn mice and neurodegeneration in adult mice.5, 6, 7
Corneal nerves are peripheral nerve fibers that adjoin the central nervous system, the most densely innervated and sensitive tissue in the human body. This serried nerve network is composed of sensory, sympathetic, and parasympathetic nerves8 and can rapidly respond to mechanical and chemical stimulation; thus, it plays a crucial role in protecting the cornea from external injury. In addition, corneal nerves can sense alterations in outside temperature, stimulate the secretion of tears from the lacrimal glands to maintain a humid ocular surface,9 and secrete neuropeptides to promote mitosis of corneal epithelial cells.10, 11, 12 Because of the exposure of the cornea, corneal nerve damage as a result of mechanical or chemical stimulation is possible. Unlike the neurons in the central nervous system, where injured neurons usually cannot regenerate, the damaged nerve axons within the peripheral nervous system can regenerate. However, this process is affected by many factors that may make nerve regeneration incomplete, which, in turn, can lead to corneal diseases, such as loss of corneal sense, dry eye disease, and metabolic disorder of the epithelium.8 Thus, a clear understanding of the mechanisms controlling corneal nerve regeneration is critical to the development of new therapies for curing corneal neuropathic diseases and restoring vision. A recent study showed that a close relationship exists between the gut microbiota and neurogenesis in the brain.5 Therefore, we hypothesized that the gut microbiota may also participate in corneal nerve regeneration.
In peripheral nerve fibers, the axon stump near the neuron cell body can regenerate after injury while the axon stump distal to the site of transection begins to degenerate.13 This degeneration is important for the generation of an appropriate local environment for the regeneration of injured axons,14 and macrophages play a crucial role in this process. When nerve fibers are damaged, many macrophages are recruited to the injury site, where they remove cell debris, thus clearing a path for new axons to regenerate. If this recruitment is inhibited, both the clearing of cell debris and the regeneration of axons are seriously impaired.15 In addition, macrophages can secrete many neurotrophins, such as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin (NTF)–3, and NTF-5, to promote regeneration of damaged axons.16 Many studies have revealed that gut microbiota can affect the function and distribution of immune cells in some tissues, thereby controlling inflammation and the response to infection in these locations.17, 18, 19 For instance, tissue-resident segmented filamentous bacteria in the gut can modulate autoimmune arthritis by affecting the activation of type 17 helper T cells.20 Furthermore, the maturation and function of macrophages in the brain (microglia) are negatively affected by deficiencies in gut microbiota.21 Given the important roles of macrophages in the regeneration of injured nerves, we inferred that macrophages may be the mediators by which the gut microbiota controls the regrowth of damaged axons. Thus, we assessed whether macrophages participate in corneal nerve regeneration and if the gut microbiota controls the distribution of macrophages in the cornea.
In this study, we demonstrate that Abx treatment negatively affects corneal homeostasis and nerve regeneration. Furthermore, reconstitution of the gut microbiota with fecal transplant significantly promotes corneal nerve regeneration in Abx-treated mice. CCR2− macrophages were confirmed to participate in the repair process of damaged corneal nerve fibers, and gut microbiota affected the distribution of these cells in the cornea. Moreover, administration of probiotics to Abx-treated mice was found to reverse the impairment in corneal nerve regeneration.
Materials and Methods
Animals
Specific-pathogen-free female C57BL/6 mice without eye diseases, aged 7 to 8 weeks, were bought from the Medical Experimental Animal Center (Guangdong, China). All animal protocols were approved by the Jinan University Laboratory Animal Committee on Animal Welfare. All the animals were treated in accordance with the Association for Research in Vision and Ophthalmology's Statement for the Use of Animals in Ophthalmology and Vision Research and the guidelines of the Animal Experimental Committee at Jinan University. The animals were anesthetized by inhalation of 2% isoflurane. Animals were euthanized by overdose of carbon dioxide and cervical dislocation.
Antibiotic Treatment
Mice were provided 1 g/L ampicillin (number A1593; Sigma-Aldrich, St. Louis, MO), 500 mg/L vancomycin (number V2002; Sigma-Aldrich), 1 g/L neomycin sulfate (number N0401000; Sigma-Aldrich), and 1 g/L metronidazole (number M3761; Sigma-Aldrich) in drinking water for 4 weeks. Each C57BL/6 mouse was found to consume approximately 5 mL of aqueous antibiotic solution per day in this study. Thus, the amounts of ampicillin, neomycin sulfate, and metronidazole were approximately 5 mg/day per mouse, and the amount of vancomycin was approximately 2.5 mg/day per mouse. On the basis of the pharmacokinetic studies of mice after oral administration of these four antibiotics,22, 23, 24, 25, 26 the estimated antibiotic concentrations in the mouse serum were 0.015 μg/μL for ampicillin, 0 μg/μL for vancomycin (not absorbed in the gastrointestinal tract), 0.001 μg/μL for neomycin sulfate, and 0.028 μg/μL for metronidazole. It is possible that these antibiotics could be absorbed into ocular tissue through the intestinal tract. To exclude the potential effects of this on posttraumatic nerve regeneration, approximately 3 to 5 μL of eye drops of a mixture of ampicillin, neomycin sulfate, and metronidazole at the above serum concentrations were put in each cornea every day for 4 weeks.
Fecal Transplant and Probiotic Administration
After antibiotic treatment, drinking water with antibiotic was replaced by sterile water. Three days later, the antibiotic-treated mice were administered fecal flora by 200-μL gavage, the feces of one donor mouse were given to two recipient mice, or antibiotic-treated mice were orally challenged with unflavored VSL#3 probiotic mixture (VSL Pharmaceuticals Inc., Towson, MD); each mouse was given nearly 1 × 107 probiotic bacteria, once every 3 days for three times. VSL#3 is a commercial probiotic cocktail containing eight bacterial strains, which are Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus paracasei, Lactobacillus bulgaricus, and Streptococcus thermophiles.
16S rRNA Gene Sequencing
The diversity and abundance of the gut microbiota were analyzed with 16S rRNA gene sequencing. DNA of the gut microbiota from stool was obtained by the QIAamp DNA Stool Mini Kit (number 51504; Qiagen, Germantown, MD). Then, a DNA sample was sent to TinyGene Company (Shanghai, China) for 16S rRNA gene sequencing analysis.
mRNA Sequencing Analysis
After the mice were euthanized, their eyeballs were clipped and the cornea was left complete. Corneas were cut into pieces, put into Buffer RZ (number RK145; Tiangen, Beijing, China), and ground by a TissueRuptor (Qiagen). Total RNA of the corneal tissues was obtained with the RNA simple Total RNA Kit (number DP419; Tiangen). The RNA samples were then sent to the BGI Company (Guangdong, China) for mRNA sequencing analysis. Gene ontology analysis was performed using the Gene Ontology Consortium.
Corneal Wound Healing Model
Mouse cornea was mechanically abraded, as previously described.27, 28, 29, 30, 31, 32 Briefly, the animals were anesthetized using an i.p. injection of sodium phenobarbital (25 to 50 mg/kg). The central corneal epithelium was marked with a 2-mm trephine, and this labeled region was removed using a golf club spud (Accutome, Malvern, PA).
Macrophage Depletion
According to our previous study, corneal macrophage populations were depleted using related antibodies or an antagonist.33 In brief, CCR2− macrophages were depleted by subconjunctival injection of 5 μL anti–colony stimulating factor 1 receptor (CSF1R) antibody (number 16-1152-82; eBioscience, San Diego, CA) (0.5 μg/μL; 10 times, once every other day), whereas the control group was treated with isotype control IgG (number 16-4321-82; eBioscience). CCR2+ macrophages were depleted using the CCR2 antagonist, BMS CCR2 22 (number 3129; R&D Systems, Minneapolis, MN) dissolved in ethanol, then diluted with saline. Mice were given this antagonist by i.p. injection (10 μg/20 g) (10 times, once every other day). The control group was treated with the diluent without BMS CCR2 22.
Corneal Sensitivity Measurement
Corneal sensitivity was measured with a Cochet-Bonnet esthesiometer (number 8630-1490-29; Luneau SAS, Prunay-le-Gillon, France), as previously described.34 Briefly, the Cochet-Bonnet esthesiometer has a monofilament at lengths ranging from 0.5 to 6.0 cm. The central cornea was touched by the monofilament at each length four times, with the monofilament contacting the corneal surface vertically. The fraction was recorded when the mice have a blink response.
Immunostaining and Qualitative Analysis
Immunostaining and qualitative analysis were performed as previously described.28 After the mice were euthanized, their eyeballs were fixed in 4% paraformaldehyde for 1 hour and were then clipped in phosphate-buffered saline under a dissecting microscope, leaving the corneas with complete limbi. After washing three times with phosphate-buffered saline, corneas were blocked in 2% bovine serum albumin for 15 minutes and permeabilized with 0.1% Triton X-100/2% bovine serum albumin for 15 minutes. The treated corneas were then incubated with anti–β-III tubulin conjugated with NL557 (number NL1195R; R&D Systems) (1:10) or anti-mouse CD64 fluorescein isothiocyanate (number 139315; BioLegend, San Diego, CA) (1:100) antibodies at 4°C overnight. After staining, corneas were washed with phosphate-buffered saline three times (5 minutes each time) and then placed on glass slides and cut radially to flatten them. A fluorescent mounting medium containing 1 μmol/L DAPI (number 28718-90-3; Sigma-Aldrich) was placed on the corneas. Corneas were imaged by the DeltaVision microscopy imaging system (Applied Precision, Issaquah, WA). Whole mounts of the corneas were evaluated using a 40× objective oil immersion lens to analyze each field of view (150 × 150 μm) across the cornea from limbus to limbus. Digital images of tubulin III–positive nerve fibers were analyzed using Imaris software version 7.4.2 (Bitplane, Zurich, Switzerland), and the length of nerve fibers in each field was measured.
Flow Cytometric Analysis
Corneal tissues were digested with 0.2% collagenase type I (number C0130; Sigma-Aldrich) for 1.5 to 2 hours and were then washed with phosphate-buffered saline and passed through a 75-μm filter to get single cells. These single corneal cells were blocked with Flow Cytometry Staining Buffer (number 00-4222; eBioscience) containing anti-mouse CD16/32 antibody (number 14-0161-85; eBioscience) at room temperature for 10 minutes and were then incubated by a mixture of the following antibodies (dilution 1:100): anti-mouse CD45 antibody conjugated with fluorescein isothiocyanate (number 553080; BD Biosciences, San Jose, CA), anti-mouse CD64 conjugated with Brilliant Violet 421 (number 139309; BioLegend), and anti-mouse CCR2 conjugated with allophycocyanin (number FAB5538A; R&D Systems) at room temperature for 30 minutes. Finally, the stained cells were analyzed using a BD FACSCanto (BD Biosciences, Franklin Lakes, NJ).
Transcript Amplification from Corneal Macrophages
Corneal cells were stained with the following antibody mixture (dilution 1:100), involving anti-mouse CD45 antibody conjugated with fluorescein isothiocyanate, anti-mouse CD64 conjugated with Brilliant Violet 421, and anti-mouse CCR2 conjugated with allophycocyanin, at room temperature for 30 minutes. These stained corneal cells were analyzed and sorted by the BD FACSAria (BD Biosciences) to get CD45+CD64+CCR2− and CD45+CD64+CCR2+ macrophages. After that, whole transcriptomes of the two macrophage populations were amplified by the REPLI-gWTA Single Cell Kit (number 150063; Qiagen).
Adoptive Transfer of Macrophages
The corneal cells were stained with anti-mouse CD45 antibody conjugated with fluorescein isothiocyanate, anti-mouse CD64 conjugated with Brilliant Violet 421, and anti-mouse CCR2 conjugated with allophycocyanin, and then sorted by FACSAria to get CD45+CD64+CCR2− and CD45+CD64+CCR2+ macrophages. The two kinds of sorted cells were then subconjunctivally injected to the Abx-treated mice (104 cells/eye).
qPCR
mRNA levels were quantified by real-time quantitative PCR (qPCR). The corneal tissues were cut into pieces, put into Buffer RZ (number RK145; Tiangen), and ground by the TissueRuptor. Total RNA from the corneal tissues was then extracted by the RNA Simple Total RNA Kit (number DP419; Tiangen). Reverse transcription of mRNA was performed with the ReverTra Ace qPCR RT Kit (number FSQ-101; Toyobo, Osaka, Japan) to obtain cDNA. Finally, expression of the target genes in the cDNA of corneal tissues and the amplified transcriptomes in the sorted CCR2− and CCR2+ corneal macrophages were investigated with the THUNDERBIRD SYBR qPCR Mix (number QPS-201; Toyobo). The qPCR primers used in our study are shown in Table 1.
Table 1.
PCR Primers Used in This Study
| Gene name | Primer pair | |
|---|---|---|
| Ngf | Forward | 5′-ACTCATACTGCACCACGACT-3′ |
| Reverse | 5′-TCAGCCTCTTCTTGTAGCCTT-3′ | |
| Bdnf | Forward | 5′-GACGGTCACAGTCCTAGAGAA-3′ |
| Reverse | 5′-CCTTATGAATCGCCAGCCAAT-3′ | |
| Ntf3 | Forward | 5′-TACGGCAACAGAGACGCTAC-3′ |
| Reverse | 5′-GTGGTGAGGTTCTATTGGCTAC-3′ | |
| Ntf5 | Forward | 5′-AGGCACTGGCTCTCAGAATG-3′ |
| Reverse | 5′-AGCTGTGTCGATCCGAATCC-3′ | |
| GAPDH | Forward | 5′-CAAGGACACTGAGCAAGAG-3′ |
| Reverse | 5′-TGCAGCGAACTTTATTGATG-3′ | |
Statistical Analysis
The results are presented as means ± SD. For comparisons between groups, analysis of variance was performed, followed by the Tukey's honestly significant difference test. Statistical significance was set at P < 0.05.
Results
Abx Treatment Affects Corneal Homeostasis
Using 16S rRNA gene sequencing, it was found that most gut microorganisms were depleted after extended treatment with broad-spectrum Abxs (ampicillin, vancomycin, neomycin sulfate, and metronidazole) in drinking water, which results in gut microbiota dysbiosis (Supplemental Figure S1). Furthermore, RNA sequencing showed that gene transcription in the corneal tissues of Abx-treated mice was markedly changed compared with that of the control mice (treated with sterile water). Among the changed genes, 116 genes involved in neurogenesis were down-regulated (Figure 1A). To observe the effect of Abx treatment on the homeostasis of corneal nerves, corneal nerve density was assessed after anti–β-III tubulin staining in the Abx-treated and control mice. The cornea with complete limbus was examined under the microscope using a 40× objective oil immersion lens and was then divided into five zones from limbus to central cornea. The first zone is in the limbus, and the second, third, fourth, and fifth zones are in the cornea (Figure 1B). Corneal nerves can be classified into stromal nerve fibers, subbasal nerve plexus, and branches projecting from this plexus into the stratified epithelium (Figure 1C). The average nerve density within each zone of the cornea (the second, third, fourth, and fifth zones) was assessed. Thus, within the area under the 40× objective lens, the number of nerve branches in the stratified epithelium or the length of subbasal and stromal nerve fibers was set as the nerve density. Moreover, using a Cochet-Bonnet esthesiometer, corneal sensitivity was detected in the Abx-treated and control mice. Epithelial nerve branch density, subbasal nerve density, and stromal nerve density were all lower in Abx-treated mice than in control mice, whereas the extent of decrease in corneal nerve density did not affect corneal sensitivity (Figure 1, D–F, and Supplemental Figure S2).
Figure 1.
Alteration of corneal homeostasis in mice after antibiotic (Abx) treatment in drinking water. A: RNA sequencing was used to detect changes in gene expression in the corneas from Abx-treated mice compared with those from the control mice (treated with sterile water). Biological processes were assigned to genes by using annotated gene ontology (GO). The bar of the color represents the aggregation degree of genes in one kind of biological process. B: Corneal whole-mount immunostaining of anti–β-III tubulin NL557. The cornea with complete limbus can be divided into five (1–5) zones from limbus to central cornea under the microscope by using a 40× objective oil immersion lens. Left panel: Zone 1 is in the limbus, and zones 2–5 are in the cornea. Right five panels: Enlarged views in the five zones in one direction. C: Definition of nerve fibers located in different corneal layers. D–F: Comparison of corneal nerve density in stratified epithelium, subbasal layer, and stroma between Abx-treated and control mice (sterile water treated). Left panels: Nerve density in each corneal zone from control and Abx-treated mice. Right panels: Total nerve density from the second to fifth zones in the cornea of control and Abx-treated mice. Data are expressed as means ± SD (D–F). n = 2 independent experiments (5 mice per experiment; A); n = 6 mice in each group (D–F). ∗P < 0.05. Scale bars: 200 μm (B, left panel); 25 μm (B, right five panels, and C).
Abx Treatment Impairs Corneal Nerve Regeneration after Epithelial Abrasion
To evaluate the effects of oral Abx treatment on corneal nerve regeneration after corneal abrasion, part of the corneal epithelium was abraded to establish a mouse model of corneal nerve injury, as described previously.28 In this model, epithelial nerve branches and subbasal nerves from the third to fifth zones within the cornea were injured (Figure 2A). RNA-sequencing analysis of corneal tissues showed that the gene transcription in the corneal tissues of Abx-treated mice was markedly changed compared with that of the control mice, and genes involving neurogenesis decreased after Abx treatment at wounding at 24 hours (Figure 2B). This result suggests that the regeneration of damaged corneal nerves may be affected. Two time points, 4 and 8 days after abrasion, were chosen to observe the regeneration of epithelial nerve branches and subbasal nerves. The results showed that the epithelial nerve branches were still not regenerated at 4 days after wounding; although the nerve branches started to regrow at 8 days after wounding, the density was not significantly different between Abx-treated and control mice (Figure 2C). At 4 and 8 days after abrasion, the density of subbasal nerves within the fourth and fifth zones, as well as in the total corneal fields of the Abx-treated mice, decreased, compared with that of the control mice (Figure 2, D and E). Moreover, the Abx-treated mice exhibited less corneal sensitivity than the control mice from 4 to 8 days after being injured (Figure 2F). These data indicate that Abx treatment impairs corneal nerve regeneration. There are two possible reasons that Abx treatment delays the repair of corneal nerve fibers, there is either a toxic effect of the Abx itself on the host or an effect of dysbiosis of gut microbiota after Abx treatment.35 To determine whether antibiotic compounds absorbed in the gastrointestinal tract directly suppress corneal nerve regeneration after abrasion, the antibiotic cocktail used in the animal drinking water was topically administered for 4 weeks at the serum concentrations based on previous pharmacokinetics data.22, 23, 24, 25, 26 Control mice were given sterile saline eye drops once per day for 4 weeks. The results of corneal nerve regrowth showed that there was a slight, but nonsignificant, decrease after topical Abx treatment in the density of corneal subbasal nerve fibers and the corneal sensitivity (Supplemental Figure S3).
Figure 2.
Alteration of corneal nerve regeneration after oral antibiotic (Abx) treatment with or without fecal transplant. A: Nerve fiber distribution after corneal epithelial abrasion. Left panel: A schematic view of the cornea with fields (150 × 150 μm) relative to the abraded area (enclosed by dotted line). Cornea with complete limbus can be divided into five zones (1–5) from limbus to central cornea under the microscope by using a 40× objective oil immersion lens. Zone 1 is in the limbus, and zones 2–5 are in the cornea. Right panels: The subbasal nerves and branches projecting from subbasal nerves are injured. B: RNA sequencing was used to detect the gene expression in the corneas of control mice, Abx-treated mice, and Abx-treated mice with fecal transplant. The upper two volcano plots represent the changes of gene expression in corneas from the Abx-treated mice when compared with those from the control mice at 0 or 24 hours after epithelial abrasion. The heat map is about the expression of the top 30 differentially expressed genes of neurogenesis in the corneas of the control (sterile water–treated) mice, Abx-treated mice, and Abx-treated mice with fecal transplant after wounding at 24 hours (the mean value was used to make the graph). C: Comparison of the density of epithelial nerve branches at 8 days after epithelial abrasion between the control and Abx-treated mice. The two graphs are the density of epithelial nerve branches in each corneal zone (left panel) and in the total corneal fields (from the third to fifth zones; right panel) of the two groups of mice. D and E: Comparison of the density of subbasal nerves at 4 and 8 days after epithelial abrasion among the control mice, Abx-treated mice, and Abx-treated mice with fecal transplant. The two graphs are the density of subbasal nerves in each corneal zone (left panels) and in the total corneal fields (from the third to fifth zones; right panels) of the three groups of mice. F: Comparison of corneal sensitivity after corneal epithelial abrasion among the three groups of mice. Corneal sensitivity was measured using a Cochet-Bonnet esthesiometer. Data are expressed as means ± SD. n = 2 independent experiments (5 mice per experiment; B); n = 6 mice in each group (C–F). ∗P < 0.05, ∗∗P < 0.01 for Abx-treated mice versus control mice; †P < 0.05, ††P < 0.01 for Abx-treated mice with fecal transplant versus Abx-treated mice; ‡P < 0.05 for Abx-treated mice with fecal transplant versus control mice. Scale bar = 25 μm (A, right panels).
Fecal Transplant Promotes Corneal Nerve Regeneration in Abx-Treated Mice
The impairment of corneal nerve regeneration likely results from Abx-induced dysbiosis of the gut microbiota. To confirm this hypothesis, the gut microbiota of Abx-treated mice was reconstituted by fecal transplant. After fecal transplant from normal mice to Abx-treated mice, the abundance of gut microorganisms in Abx-treated mice was similar to that of control mice given sterile water (Supplemental Figure S1). It indicated that the gut microbiota in the Abx-treated mice is restored. Next, a part of the corneal epithelium was abraded (Figure 2A), and the regeneration of subbasal nerves was observed in each group of mice. The results showed that the genes involved in neurogenesis and the density of subbasal nerves in Abx-treated mice after fecal transplant increased compared with that in Abx-treated mice with no transplant at both 4 and 8 days after wounding, whereas they were slightly lower than that in the control mice (Figure 2, B, D, and E). In addition, the corneal sensitivity of the Abx-treated mice with fecal transplant also increased compared with that of the Abx-treated mice with no transplant, although it was slightly lower than that of the control mice at 6 and 8 days after wounding (Figure 2F). These results indicate that the regrowth rate of corneal nerve fibers in the Abx-treated mice increases after fecal transplant, although not to a level equivalent to that of the control mice.
Lack of CCR2− Macrophages Impairs Corneal Nerve Regeneration
After damage of the peripheral nerves, macrophages are not only involved in removal of cell debris, but they also secrete neurotrophins to promote axon regrowth near axotomized neuronal cell bodies.36, 37, 38 The costaining of anti-mouse CD64 antibody, which identifies macrophages, and anti–β-III tubulin showed that macrophages are usually located around corneal nerve fibers (Figure 3A). Our previous study showed that corneal macrophages can be classified into CCR2− and CCR2+ populations by differential gene expression and function.33 With RT-PCR, neurotrophin gene expression was evaluated in flow cytometry–sorted CCR2− and CCR2+ macrophages. These data show that CCR2− macrophages express neurotrophin genes (Ngf, Bdnf, Ntf-3, and Ntf-5), whereas CCR2+ macrophages do not (Figure 3B). CCR2− corneal macrophages are defective in anti–CSF1R antibody–treated mice, and CCR2+ corneal macrophages depend on monocyte contribution and decrease after CCR2 antagonist BMS CCR2 22 treatment.33 Thus, CCR2− corneal macrophages were depleted by injection of anti-CSF1R antibody, and CCR2+ corneal macrophages were depleted by injection of CCR2 antagonist BMS CCR2 22 in this study. The expression of neurotrophins in corneal tissues and the density of subbasal nerves from anti–CSF1R antibody–treated mice were decreased at 8 days after corneal wounding compared with that in the isotype control mice (Figure 3, C and D). In the CCR2 antagonist–treated mice, the expression of neurotrophins in cornea tissues and the density of subbasal nerves were not obviously changed at 8 days after corneal wounding compared with those in the control mice, although the density of subbasal nerves within the fourth zone and total corneal fields of CCR2 antagonist–treated mice decreased slightly (Figure 3, E and F). Moreover, the corneal sensitivity in anti–CSF1R antibody–treated mice was lower than that in the isotype control mice at 6 and 8 days after wounding, whereas CCR2 antagonist–treated mice showed no significant change (Figure 3, G and H). These results suggest that the lack of CCR2− macrophages impairs corneal nerve regeneration after epithelial abrasion.
Figure 3.
Effect of macrophage (MФ) depletion on corneal nerve regeneration. A: Anti–β-III tubulin NL557 and CD64 fluorescein isothiocyanate antibody costaining of cornea. B: RT-PCR evaluation of flow cytometry–sorted CCR2− and CCR2+ corneal macrophages for the expression of neurotrophin genes. C and E: The change of neurotrophin gene expression in corneal tissues at 8 days after epithelial abrasion. CCR2− corneal macrophages were depleted by subconjunctival injection of anti–colony stimulating factor 1 receptor (CSF1R) antibody, and CCR2+ corneal macrophages were depleted by i.p. injection of CCR2 antagonist BMS CCR2 22. D and F: Change in the density of subbasal nerves at 8 days after epithelial abrasion in mice depleted of CCR2− or CCR2+ macrophages. G and H: The change in corneal sensitivity after corneal epithelial abrasion in mice depleted of CCR2− or CCR2+ macrophages. Data are expressed as means ± SD. n = 3 independent experiments [10 mice per experiment (B) and 6 mice per experiment (C and E)]; n = 6 mice in each group (D and F–H). ∗P < 0.05, ∗∗P < 0.01. Scale bar = 100 μm (A).
Gut Microbiota Affects Macrophage Distribution in Cornea
Previous studies revealed that the composition of gut microbiota affects the function and distribution of immune cells in tissues.17, 18, 19 In this study, with RNA-sequencing analysis, the expression of macrophage-related genes in the corneal tissues was down-regulated after Abx treatment at wounding at 24 hours and was mostly reversed by fecal transplant (Figure 4A). It suggests that the gut microbiota may also participate in the regulation of corneal macrophages. Therefore, the distribution of macrophages in the cornea of the control (treated with sterile water), Abx-treated mice, and Abx-treated mice with fecal transplant was studied, and it was found that the proportion of CCR2− macrophages in the Abx-treated mice was significantly decreased at 4 and 8 days after epithelial abrasion compared with that in the control group. After fecal transplant, the proportion of CCR2− macrophages in Abx-treated mice increased and equaled that of control mice at 6 and 8 days after wounding (Figure 4B). Moreover, the proportion of CCR2+ macrophages in Abx-treated mice decreased at 2 days after wounding, whereas it gradually increased and normalized at 8 days after wounding. After fecal transplant, the proportion of CCR2+ macrophages in Abx-treated mice was also restored (Figure 4B). This finding suggests that transplant of gut microbiota can restore the distribution of both CCR2− and CCR2+ macrophages in the cornea of Abx-treated mice. Furthermore, considering that the CCR2+ corneal macrophages in Abx-treated mice were finally normalized, and that these cells mainly rely on monocyte contribution,33 changes in monocytes in peripheral blood were assessed; the proportion of monocytes in peripheral leukocytes of Abx-treated mice was found to be lower than that of control mice at 2 days after epithelial abrasion. However, it was equal to the level in control mice at 8 days after wounding (Supplemental Figure S4). The gating strategies for analyzing macrophages and monocytes in flow cytometry were justified by related isotype controls (Supplemental Figure S5).
Figure 4.
The effects of gut microbiota on the expression of macrophage (MФ)–related genes and distribution of macrophages in cornea. A: Investigation of the expression of macrophage-related genes in the control mice, Abx-treated mice, and Abx-treated mice with fecal transplant by RNA-sequencing analysis after wounding at 24 hours. Forty-seven genes involved in the regulation of macrophages were changed, and the top 30 differentially expressed genes are shown in the heat map (the mean value was used to make the graph). B: Dynamic changes in CCR2− and CCR2+ corneal macrophages at 2, 4, 6, and 8 days after epithelial abrasion in the three groups of mice. The gating strategies were justified by related isotype controls in Supplemental Figure S5. Data are expressed as means ± SD. n = 2 independent experiments (5 mice per experiment; A); n = 3 independent experiments (5 mice per experiment; B). ∗P < 0.05 for antibiotic (Abx)–treated mice versus control mice; †P < 0.05 for Abx-treated mice after fecal transplant versus Abx-treated mice. FSC, forward scatter; SSC, side scatter.
Adoptive Transfer of CCR2− Macrophages Promotes Corneal Nerve Regeneration in Abx-Treated Mice
CCR2− macrophages play an important role in corneal nerve regeneration, and they are affected by the composition of gut microbiota. This information suggests that the gut microbiota participates in corneal nerve regeneration by affecting CCR2− macrophage distribution. To confirm this hypothesis, CCR2− corneal macrophages were transferred from normal mice to Abx-treated mice. A previous study indicated that subconjunctival injection of peritoneal macrophages could promote corneal wound healing.39 Macrophages from normal mice were, therefore, transferred to Abx-treated mice by subconjunctival injection. After epithelial abrasion, the sorted CCR2− corneal macrophages were subconjunctivally injected into Abx-treated mice once every other day. The results showed that the density of subbasal nerves in Abx-treated mice with transplant of CCR2− macrophages increased compared with that in the Abx-treated mice without transplant. CCR2+ corneal macrophages were also transferred to the Abx-treated mice later, but this had no significant effect on corneal nerve regeneration in these mice (Figure 5). These data indicate that the transplant of CCR2− macrophages can promote corneal nerve regeneration in Abx-treated mice.
Figure 5.
Effect of adoptive transfer of CCR2− and CCR2+ macrophages (MФs) on corneal nerve regeneration in antibiotic (Abx)–treated mice. Data are expressed as means ± SD. n = 6 mice in each group. ∗P < 0.05.
Probiotic Treatment Promotes Corneal Nerve Regeneration in Abx-Treated Mice
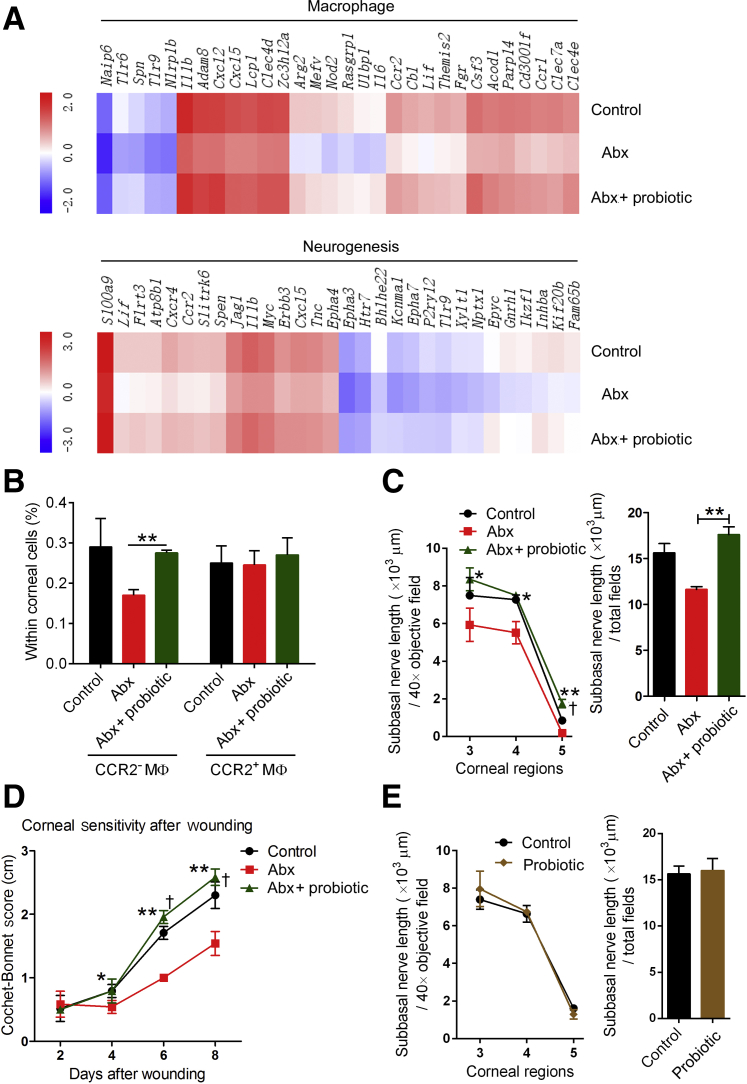
Among the numerous gut microorganisms, some strains are known to be beneficial, and taking appropriate doses of these has been shown to cure certain diseases.40, 41, 42, 43 These gut microorganisms are commonly known as probiotics. VSL#3 is a commercially available probiotic mixture containing eight bacterial strains. Previously, supplementation with VSL#3 was shown to restore hippocampal neurogenesis and brain function in Abx-treated mice.5 Therefore, VSL#3 was given to Abx-treated mice to determine whether probiotics can improve corneal nerve regeneration after Abx treatment. RNA sequencing showed that the genes involved in the regulation of macrophages and neurogenesis both increased in Abx-treated mice after probiotic treatment (Figure 6A). And 8 days after epithelial abrasion, the proportion of macrophages within corneal cells was assessed in each group of mice. Supplementation of Abx-treated mice with probiotic treatment was found to restore the proportion of CCR2− macrophages (Figure 6B). Furthermore, the density of subbasal nerve fibers in Abx-treated mice given probiotics was higher than that in Abx-treated mice, and it was even slightly higher than that in control mice (treated with sterile water) (Figure 6C). In addition, from 6 to 8 days after wounding, corneal sensitivity was increased in Abx-treated mice with probiotic treatment compared with Abx-treated mice without probiotic treatment, and it was even higher than that in the control mice at 6 and 8 days after wounding (Figure 6D). These results suggest that probiotics can restore CCR2− macrophage distribution and markedly promote corneal regeneration in Abx-treated mice. In view of the potent ability of probiotics to promote nerve regeneration in mice with gut microbiota dysbiosis, it was tested whether probiotics could promote regeneration of injured corneal nerves in mice that had normal gut microbiota. The change in corneal nerve regeneration of mice that had normal gut microbiota after probiotic treatment was studied. However, the results showed no difference between the control mice (treated with sterile water) and probiotic-treated mice in the regeneration of damaged corneal nerves (Figure 6E).
Figure 6.
Effect of probiotic treatment on the macrophage (MФ) distribution and nerve regeneration in the cornea. A: Investigation of the expression of genes involved in the regulation of macrophages and neurogenesis in the control mice, antibiotic (Abx)–treated mice, and Abx-treated mice with probiotic treatment by RNA-sequencing analysis 24 hours after wounding. The top 30 differentially expressed genes are shown in the heat map (the mean value was used to make the graph). B: Comparison of the distribution of CCR2− and CCR2+ macrophages in the cornea at 8 days after epithelial abrasion among the control mice, Abx-treated mice, and Abx-treated mice with probiotic treatment. C: Alteration of the density of subbasal nerves at 8 days after epithelial abrasion in the Abx-treated mice given probiotics. The two graphs show the density of the subbasal nerve in each corneal zone and total fields from the third to fifth zone of the cornea in the three groups of mice. D: Comparison of the corneal sensitivity after epithelial abrasion among the three groups of mice. E: Alteration of the density of subbasal nerves at 8 days after epithelial abrasion in mice that have normal gut microbiota given probiotics. Data are expressed as means ± SD. n = 2 independent experiments (5 mice per experiment; A); n = 3 independent experiments (5 mice per experiment; B); n = 6 mice in each group (C–E). ∗P < 0.05, ∗∗P < 0.01 for Abx-treated mice with probiotic treatment versus Abx-treated mice; †P < 0.05 for Abx-treated mice with probiotic treatment versus control mice.
Discussion
Although the use of Abxs has undoubtedly saved countless lives worldwide, these drugs also have adverse impacts on the human body. In this study, the long-term use of Abxs was found to induce dysbiosis of the gut microbiota and affect corneal homeostasis, including the alteration of corneal gene transcription and decrease of corneal nerve density. Investigation of corneal wound healing showed that, although Abx treatment impaired corneal nerve regeneration, reconstitution of the gut microbiota in Abx-treated mice by fecal transplant reversed this effect to a large extent. CCR2− macrophages were known to participate in the regrowth of injured corneal nerves, and Abx treatment decreased these cells in the cornea. Fecal transplant was found to restore the proportion of these cells, and adoptive transfer of CCR2− macrophages could promote corneal nerve regeneration in Abx-treated mice. Moreover, probiotic treatment was confirmed to largely promote the regeneration of corneal nerves in Abx-treated mice.
The long-term use of Abxs is known to negatively affect homeostasis and physiology of the mice, including causing a decrease in the number of nitrergic neurons in intestine,44 inhibiting hippocampal neurogenesis and memory retention,5 delaying of gastrointestinal peristalsis,44 and causing decreased hematopoiesis.45, 46 Consistent with the above-mentioned studies, it was confirmed that Abx treatment significantly affected corneal gene transcription and decreased corneal nerve density. Furthermore, the investigation of corneal wound healing showed that Abx treatment impaired the capacity of corneal nerve regeneration, and reconstitution of the gut microbiota by fecal transplant could mostly restore this capacity. Thus, dysbiosis of the gut microbiota can be argued to be the primary factor causing the impairment of corneal nerve regeneration after oral Abx treatment. Morgun et al35 suggested that Abx-induced changes in the intestine could be explained by two factors: dysbiosis of the gut microbiota and the toxic effects of Abxs on intestinal tissues. Further study showed that topical treatment on injured corneas did not significantly delay corneal nerve regrowth. Because Abxs are known to have a toxic effect on intestinal tissues,35 a cascade effect in intestinal tissues may affect the regeneration of cornea nerves; this needs further investigation.
Regeneration of peripheral nerve fibers after injury is a complicated process involving the interactions of gliocytes, growth factors, cell adhesion molecules, and extracellular matrix, and the recruitment of macrophages.47 When peripheral nerves are damaged, macrophages not only clear cell debris but also secrete neurotrophins to promote axon regeneration.36, 37, 38 Depletion of macrophages around the sciatic nerve by injection of ganciclovir or clodronate was found to result in severe impairment of the regrowth of the injured sciatic nerve and in a deficiency of movement and sensory function.48, 49 Immunostaining of the cornea revealed that several macrophages are located around the corneal nerve fibers, which suggests that macrophages may have a close relationship with corneal nerve growth. However, macrophages are a heterogeneous cell population, and in accordance with their secretion of different cytokines and functions, they can be classified into two populations: types M1 and M2. Investigation of mouse spinal cord injury revealed that M1 macrophages exert neurotoxicity and did not promote axon regrowth, whereas M2 macrophages largely promoted axon regeneration.50 Our previous studies indicated that corneal macrophages can be classified into CCR2− and CCR2+ populations.33 CCR2+ macrophages are similar to M1 macrophages in the gene expression and function, whereas CCR2− macrophages are similar to M2 macrophages.33 Herein, only CCR2− macrophages were found to express neurotrophin genes (Ngf, Bdnf, Ntf-3, and Ntf-5). Furthermore, depletion of these cells resulted in decreased neurotrophin expression in corneal tissues and regeneration of injured corneal nerves. By contrast, depletion of CCR2+ macrophages did not obviously affect the regeneration of corneal nerves. This finding suggests that CCR2− corneal macrophages act in a manner similar to M2 macrophages in the spinal cord and promote the regeneration of damaged corneal nerve fibers.
Although the proportion of CCR2− macrophages within corneal cells decreased in Abx-treated mice, reconstitution of the gut microbiota with fecal transplant could restore their distribution in the cornea. This suggests that the composition of the gut microbiota affects the distribution of macrophages in the cornea. In accordance with the vital roles of CCR2− macrophages in corneal nerve regeneration, we hypothesized that the gut microbiota participates in corneal nerve regeneration, mainly through affecting the distribution of CCR2− macrophages. As expected, the adoptive transfer of CCR2− macrophages could promote the regeneration of corneal nerves in Abx-treated mice. Although the use of Abx may affect other immune cells, it does not hinder us from getting the conclusion that CCR2− macrophages are a crucial mediator of corneal nerve regeneration and are affected by gut microbiota. A recent study indicated that Abx-induced changes affect the immune system, with marked decreases in Ly6Chi monocytes in the hippocampus, bone marrow, and blood after Abx treatment, whereas Ly6Chi cells were found to subsequently normalize in the bone marrow and blood.5 In this study, the decrease in CCR2− corneal macrophages of Abx-treated mice was still evident at 8 days after wounding, whereas CCR2+ macrophage numbers gradually increased and normalized. CCR2− corneal macrophages have a self-renewal capacity and rarely depend on contributions from monocytes, whereas CCR2+ corneal macrophages mainly rely on monocyte input.33 In the present study, the proportion of monocytes in the blood was found to be normalized at 8 days after wounding. Therefore, the subsequent normalization of CCR2+ macrophages in Abx-treated mice is not surprising.
It is becoming evident that supplementation of some strains of gut microorganisms is beneficial to our body. In fact, probiotics have been reported to be useful for the treatment and prevention of infectious diarrhea40 as well as the amelioration of symptoms in Crohn disease, ulcerative colitis, and irritable bowel syndrome.36, 37 VSL#3 is a probiotic mixture containing eight bacterial strains and has been shown to ameliorate inflammation in inflammatory bowel disease.51 Moreover, Bassaganya-Riera et al52 showed that VSL#3 can change the phenotype and distribution of macrophages in dextran sulfate sodium–induced acute colitis and markedly alter inflammation in the colon. In this study, it was found that supplementation with VSL#3 restored the number of CCR2− macrophages and accelerated the regrowth of injured corneal nerves in Abx-treated mice. By contrast, probiotic treatment did not promote the regeneration of injured corneal nerves in mice with normal gut microbiota. Thus, the capacity of probiotics to promote regeneration of injured corneal nerves is limited to conditions causing dysbiosis of the gut microbiota.
Collectively, these results indicate that Abx treatment negatively affects corneal homeostasis, including altering gene expression and decreasing nerve density in the cornea. In addition, the gut microbiota affects the regeneration of corneal nerve fibers by controlling the distribution of CCR2− macrophages in the cornea. More important, probiotic treatment can restore the proportion of CCR2− macrophages in the cornea of Abx-treated mice and accelerate the regeneration of corneal nerve fibers. Although the impairment of corneal nerve regeneration induced by Abx treatment is a complicated process, these findings shed light on the importance of gut microbiota homeostasis during corneal nerve regeneration as well as highlight the important roles of CCR2− macrophages in nerve fiber regeneration.
Acknowledgments
The authors thank Xinqiang Lai (Core Laboratory for Analysis and Test, Jinan University) for instructions on flow cytometry analysis.
Z.L. and J.L. conceived and designed the study; M.W. analyzed immunostaining and performed real-time quantitative PCR; J.H., C.X., and Y.X. helped with treatment of the animals, including drug treatment and corneal epithelial wounding; J.L. performed adoptive transfer of cells and flow cytometry analysis; T.F., C.L., and D.D. conducted the statistical analysis; J.L. drafted the first version of the manuscript; Z.L. critically reviewed and revised the manuscript for intellectual content; and all the authors critiqued the manuscript and approved its submission.
Footnotes
Supported by National Natural Science Foundation of China grants 81470603 (Z.L.), 81770962 (Z.L.), and 81700808 (Y.X.); Ph.D. Start-up Fund of the Natural Science Foundation of Guangdong Province of China grant 2018A030310605 (J.L.); China Postdoctoral Science Foundation grant 2017M622913 (J.L.); and NIH grant 5R01EY018239-08 (C.W.S.).
J.L. and M.W. contributed equally to this work.
Disclosures: None declared.
Supplemental material for this article can be found at https://doi.org/10.1016/j.ajpath.2018.08.009.
Supplemental Data
Supplemental Figure S1.
Investigation of the diversity and abundance of gut microbiota in control mice, antibiotic (Abx)–treated mice, and Abx-treated mice with fecal transplant.
Supplemental Figure S2.

Comparison of corneal sensitivity in antibiotic (Abx)–treated and control mice. Corneal sensitivity was measured using a Cochet-Bonnet esthesiometer. Data are expressed as means ± SD. n = 6 mice in each group.
Supplemental Figure S3.
Alteration of corneal nerve regeneration after topical antibiotic (Abx) treatment. The change in the density of subbasal nerves (A) and corneal sensitivity (B) after topical Abx treatment on the cornea. Data are expressed as means ± SD. n = 6 mice in each group.
Supplemental Figure S4.
Comparison of monocytes in peripheral blood between control and antibiotic (Abx)–treated mice. A: The gate strategies used to identify monocytes. The gating strategies were justified by related isotype controls (Supplemental Figure S5). B: The proportion of monocytes within peripheral blood leukocytes in control and antibiotic-treated mice at 2 and 8 days after wounding. Data are expressed as means ± SD. n = 6 mice in each group (B). ∗P < 0.05. FSC, forward scatter; P1, singlets; P2, monocytes; P3, Ly6C+Ly6G- cells; SSC, side scatter.
Supplemental Figure S5.
Isotype controls for justifying the gating strategies. A: Isotype controls for antibodies [CD45–fluorescein isothiocyanate (FITC), CD64-BV421, and CCR2–allophycocyanin (APC)] used in Figure 4A. B: Isotype controls for antibodies [Ly6G-FITC and Ly6C–phosphatidylethanolamine (PE)–Cy7] used in Supplemental Figure S4A. SSC, side scatter.
References
- 1.Bercik P., Collins S.M. The effects of inflammation, infection and antibiotics on the microbiota-gut-brain axis. Adv Exp Med Biol. 2014;817:279–289. doi: 10.1007/978-1-4939-0897-4_13. [DOI] [PubMed] [Google Scholar]
- 2.Mostafa S., Miller B.J. Antibiotic-associated psychosis during treatment of urinary tract infections: a systematic review. J Clin Psychopharmacol. 2014;34:483–490. doi: 10.1097/JCP.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 3.Jandhyala S.M., Talukdar R., Subramanyam C., Vuyyuru H., Sasikala M., Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21:8787–8803. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Hara A.M., Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Möhle L., Mattei D., Heimesaat M.M., Bereswill S., Fischer A., Alutis M., French T., Hambardzumyan D., Matzinger P., Dunay I.R., Wolf S.A. Ly6C hi monocytes provide a link between antibiotic-induced changes in gut microbiota and adult hippocampal neurogenesis. Cell Rep. 2016;15:1945–1956. doi: 10.1016/j.celrep.2016.04.074. [DOI] [PubMed] [Google Scholar]
- 6.Cryan J.F., Dinan T.G. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 7.Sampson T.R., Mazmanian S.K. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe. 2015;17:565–576. doi: 10.1016/j.chom.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller L.J., Marfurt C.F., Kruse F., Tervo T.M. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76:521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 9.Parra A., Madrid R., Echevarria D., del Olmo S., Morenilla-Palao C., Acosta M.C., Gallar J., Dhaka A., Viana F., Belmonte C. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nat Med. 2010;16:1396–1399. doi: 10.1038/nm.2264. [DOI] [PubMed] [Google Scholar]
- 10.Baker K.S., Anderson S.C., Romanowski E.G., Thoft R.A., SundarRaj N. Trigeminal ganglion neurons affect corneal epithelial phenotype: influence on type VII collagen expression in vitro. Invest Ophthalmol Vis Sci. 1993;34:137–144. [PubMed] [Google Scholar]
- 11.Reid T.W., Murphy C.J., Iwahashi C.K., Foster B.A., Mannis M.J. Stimulation of epithelial cell growth by the neuropeptide substance P. J Cell Biochem. 1993;52:476–485. doi: 10.1002/jcb.240520411. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Hirschfeld J., Lopez-Briones L.G., Belmonte C. Neurotrophic influences on corneal epithelial cells. Exp Eye Res. 1994;59:597–605. doi: 10.1006/exer.1994.1145. [DOI] [PubMed] [Google Scholar]
- 13.Sunderland S. Advances in diagnosis and treatment of root and peripheral nerve injury. Adv Neurol. 1979;22:271–305. [PubMed] [Google Scholar]
- 14.Perry V.H., Brown M.C. Role of macrophages in peripheral nerve degeneration and repair. Bioessays. 1992;14:401–406. doi: 10.1002/bies.950140610. [DOI] [PubMed] [Google Scholar]
- 15.Beuche W., Friede R.L. The role of non-resident cells in Wallerian degeneration. J Neurocytol. 1984;13:767–796. doi: 10.1007/BF01148493. [DOI] [PubMed] [Google Scholar]
- 16.Wu J., Xie H., Yao S., Liang Y. Macrophage and nerve interaction in endometriosis. J Neuroinflammation. 2017;14:53. doi: 10.1186/s12974-017-0828-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dorrestein P.C., Mazmanian S.K., Knight R. Finding the missing links among metabolites, microbes, and the host. Immunity. 2014;40:824–832. doi: 10.1016/j.immuni.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamada N., Seo S.U., Chen G.Y., Nunez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 19.Round J.L., Mazmanian S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu H.J., Ivanov I.I., Darce J., Hattori K., Shima T., Umesaki Y., Littman D.R., Benoist C., Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erny D., Hrabe de Angelis A.L., Jaitin D., Wieghofer P., Staszewski O., David E., Keren-Shaul H., Mahlakoiv T., Jakobshagen K., Buch T., Schwierzeck V., Utermohlen O., Chun E., Garrett W.S., McCoy K.D., Diefenbach A., Staeheli P., Stecher B., Amit I., Prinz M. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishida Y., Kurosaka Y., Murakami Y., Otani T., Yamaguchi K. Therapeutic effect of oral levofloxacin, ciprofloxacin, and ampicillin on experimental murine pneumonia caused by penicillin intermediate Streptococcus pneumoniae for which the minimum inhibitory concentrations of the quinolones are similar. Chemotherapy. 1999;45:183–191. doi: 10.1159/000007181. [DOI] [PubMed] [Google Scholar]
- 23.Rao S., Kupfer Y., Pagala M., Chapnick E., Tessler S. Systemic absorption of oral vancomycin in patients with Clostridium difficile infection. Scand J Infect Dis. 2011;43:386–388. doi: 10.3109/00365548.2010.544671. [DOI] [PubMed] [Google Scholar]
- 24.Hamamoto H., Kurokawa K., Kaito C., Kamura K., Manitra Razanajatovo I., Kusuhara H., Santa T., Sekimizu K. Quantitative evaluation of the therapeutic effects of antibiotics using silkworms infected with human pathogenic microorganisms. Antimicrob Agents Chemother. 2004;48:774–779. doi: 10.1128/AAC.48.3.774-779.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Waaij D., Berghuis-de Vries J.M., Korthals Altes C. Oral dose and faecal concentration of antibiotics during antibiotic decontamination in mice and in a patient. J Hyg (Lond) 1974;73:197–203. doi: 10.1017/s0022172400024025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deutsch G., Foster J.L., McFadzean J.A., Parnell M. Human studies with “high dose” metronidazole: a non-toxic radiosensitizer of hypoxic cells. Br J Cancer. 1975;31:75–80. doi: 10.1038/bjc.1975.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Z., Burns A.R., Miller S.B., Smith C.W. CCL20, gammadelta T cells, and IL-22 in corneal epithelial healing. FASEB J. 2011;25:2659–2668. doi: 10.1096/fj.11-184804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Z., Burns A.R., Han L., Rumbaut R.E., Smith C.W. IL-17 and VEGF are necessary for efficient corneal nerve regeneration. Am J Pathol. 2011;178:1106–1116. doi: 10.1016/j.ajpath.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Z., Burns A.R., Rumbaut R.E., Smith C.W. gamma delta T cells are necessary for platelet and neutrophil accumulation in limbal vessels and efficient epithelial repair after corneal abrasion. Am J Pathol. 2007;171:838–845. doi: 10.2353/ajpath.2007.070008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Byeseda S.E., Burns A.R., Dieffenbaugher S., Rumbaut R.E., Smith C.W., Li Z. ICAM-1 is necessary for epithelial recruitment of gammadelta T cells and efficient corneal wound healing. Am J Pathol. 2009;175:571–579. doi: 10.2353/ajpath.2009.090112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Z., Burns A.R., Smith C.W. Two waves of neutrophil emigration in response to corneal epithelial abrasion: distinct adhesion molecule requirements. Invest Ophthalmol Vis Sci. 2006;47:1947–1955. doi: 10.1167/iovs.05-1193. [DOI] [PubMed] [Google Scholar]
- 32.Liu Q., Smith C.W., Zhang W., Burns A.R., Li Z. NK cells modulate the inflammatory response to corneal epithelial abrasion and thereby support wound healing. Am J Pathol. 2012;181:452–462. doi: 10.1016/j.ajpath.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J., Xue Y., Dong D., Xiao C., Lin C., Wang H., Song F., Fu T., Wang Z., Chen J., Pan H., Li Y., Cai D., Li Z. CCR2(-) and CCR2(+) corneal macrophages exhibit distinct characteristics and balance inflammatory responses after epithelial abrasion. Mucosal Immunol. 2017;10:1145–1159. doi: 10.1038/mi.2016.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chucair-Elliott A.J., Zheng M., Carr D.J.J. Degeneration and regeneration of corneal nerves in response to HSV-1 infection. Invest Ophthalmol Vis Sci. 2015;56:1097–1107. doi: 10.1167/iovs.14-15596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgun A., Dzutsev A., Dong X., Greer R.L., Sexton D.J., Ravel J., Schuster M., Hsiao W., Matzinger P., Shulzhenko N. Uncovering effects of antibiotics on the host and microbiota using transkingdom gene networks. Gut. 2015;64:1732–1743. doi: 10.1136/gutjnl-2014-308820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Griffin J.W., George R., Ho T. Macrophage systems in peripheral nerves: a review. J Neuropathol Exp Neurol. 1993;52:553–560. doi: 10.1097/00005072-199311000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Mueller M., Leonhard C., Wacker K., Ringelstein E.B., Okabe M., Hickey W.F., Kiefer R. Macrophage response to peripheral nerve injury: the quantitative contribution of resident and hematogenous macrophages. Lab Invest. 2003;83:175–185. doi: 10.1097/01.lab.0000056993.28149.bf. [DOI] [PubMed] [Google Scholar]
- 38.Niemi J.P., DeFrancesco-Lisowitz A., Roldan-Hernandez L., Lindborg J.A., Mandell D., Zigmond R.E. A critical role for macrophages near axotomized neuronal cell bodies in stimulating nerve regeneration. J Neurosci. 2013;33:16236–16248. doi: 10.1523/JNEUROSCI.3319-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li S., Li B., Jiang H., Wang Y., Qu M., Duan H., Zhou Q., Shi W. Macrophage depletion impairs corneal wound healing after autologous transplantation in mice. PLoS One. 2013;8:e61799. doi: 10.1371/journal.pone.0061799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allen S.J., Martinez E.G., Gregorio G.V., Dans L.F. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010;2010:CD003048. doi: 10.1002/14651858.CD003048.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meijer B.J., Dieleman L.A. Probiotics in the treatment of human inflammatory bowel diseases: update 2011. J Clin Gastroenterol. 2011;45 Suppl:S139–S144. doi: 10.1097/MCG.0b013e31822103f7. [DOI] [PubMed] [Google Scholar]
- 42.Moayyedi P., Ford A.C., Talley N.J., Cremonini F., Foxx-Orenstein A.E., Brandt L.J., Quigley E.M. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325–332. doi: 10.1136/gut.2008.167270. [DOI] [PubMed] [Google Scholar]
- 43.Marteau P. Probiotics, prebiotics, synbiotics: ecological treatment for inflammatory bowel disease? Gut. 2006;55:1692–1693. doi: 10.1136/gut.2004.051458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anitha M., Vijay–Kumar M., Sitaraman S.V., Gewirtz A.T., Srinivasan S. Gut microbial products regulate murine gastrointestinal motility via toll-like receptor 4 signaling. Gastroenterology. 2012;143:1006–1016.e4. doi: 10.1053/j.gastro.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Josefsdottir K.S., Baldridge M.T., Kadmon C.S., King K.Y. Antibiotics impair murine hematopoiesis by depleting the intestinal microbiota. Blood. 2017;129:729–739. doi: 10.1182/blood-2016-03-708594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khosravi A., Yanez A., Price J.G., Chow A., Merad M., Goodridge H.S., Mazmanian S.K. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe. 2014;15:374–381. doi: 10.1016/j.chom.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horie H., Kadoya T., Hikawa N., Sango K., Inoue H., Takeshita K., Asawa R., Hiroi T., Sato M., Yoshioka T., Ishikawa Y. Oxidized galectin-1 stimulates macrophages to promote axonal regeneration in peripheral nerves after axotomy. J Neurosci. 2004;24:1873–1880. doi: 10.1523/JNEUROSCI.4483-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barrette B., Hebert M.A., Filali M., Lafortune K., Vallieres N., Gowing G., Julien J.P., Lacroix S. Requirement of myeloid cells for axon regeneration. J Neurosci. 2008;28:9363–9376. doi: 10.1523/JNEUROSCI.1447-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen P., Cescon M., Zuccolotto G., Nobbio L., Colombelli C., Filaferro M., Vitale G., Feltri M.L., Bonaldo P. Collagen VI regulates peripheral nerve regeneration by modulating macrophage recruitment and polarization. Acta Neuropathol. 2015;129:97–113. doi: 10.1007/s00401-014-1369-9. [DOI] [PubMed] [Google Scholar]
- 50.Kigerl K.A., Gensel J.C., Ankeny D.P., Alexander J.K., Donnelly D.J., Popovich P.G. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Appleyard C.B., Cruz M.L., Isidro A.A., Arthur J.C., Jobin C., De Simone C. Pretreatment with the probiotic VSL#3 delays transition from inflammation to dysplasia in a rat model of colitis-associated cancer. Am J Physiol Gastrointest Liver Physiol. 2011;301:G1004–G1013. doi: 10.1152/ajpgi.00167.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bassaganya-Riera J., Viladomiu M., Pedragosa M., De Simone C., Carbo A., Shaykhutdinov R., Jobin C., Arthur J.C., Corl B.A., Vogel H., Storr M., Hontecillas R. Probiotic bacteria produce conjugated linoleic acid locally in the gut that targets macrophage PPAR gamma to suppress colitis. PLoS One. 2012;7:e31238. doi: 10.1371/journal.pone.0031238. [DOI] [PMC free article] [PubMed] [Google Scholar]