ABSTRACT
Actin is the most abundant protein in our cells, and also one of the most studied. Nevertheless, an important modifier of actin, the N-terminal acetyltransferase (NAT) for actin, remained unknown until now. The recent identification of the enzyme that catalyzes actin acetylation, has opened up for functional studies of unacetylated actin using knockout cells. This enzyme, called NAA80 (Nα-acetyltransferase 80) or NatH, belongs to the NAT family of enzymes, which together provides N-terminal acetylation for around 80 % of the human proteome. In many cases, N-terminal acetylation is essential. In the case of actin, the acetyl group that NAA80 attaches to actin plays an important role in actin’s polymerization properties as well as in actin’s function in cell migration.
KEYWORDS: Acetyltransferase, actin, cell motility, cytoskeleton, NAA80, NatH, N-terminal acetylation
Actin plays an essential role in muscle cell contraction, but is also important for diverse cellular functions like skeleton/structure-function, organelle trafficking, cell migration and transcriptional regulation [1,2]. The ability to polymerize is a key feature for actin to provide these cellular services, which in addition depends on a dynamicity in the actin filaments that is achieved through actin’s constant cycle of polymerization and depolymerization. This dynamicity is regulated by several actin-binding and signaling proteins [3]. Further, actin-modifiers may control actin’s biochemical properties and cellular functions through posttranslational modifications, like N-terminal (Nt)-acetylation which is catalyzed by N-terminal acetyltransferases (NATs) and is increasingly recognized as a highly abundant and essential protein modification [4]. Although Nt-acetylation is mainly a co-translational modification, it actually occurs posttranslationally during actin’s unique Nt-maturation process [5]. To date, there is little knowledge on the biological function of actin Nt-acetylation, mainly due to the lacking identification of the actin-specific enzyme. Recent work, however, identified NAA80 as actin’s NAT, and revealed the cellular functions it exerts through alterations of actin’s biochemical properties [6].
NAA80 acetylates actin’s N-terminus
Through an N-terminal-specialized mass spectrometry analysis called COFRADIC, a previously predicted NAT of unknown substrate specificity (NAT6/FUS2), was identified not only as a true NAT, but as a NAT with specificity for actin [6]. This NAT was thus named NAA80/NatH according to the NAT nomenclature [7] and joins the previously described NAT family (NatA-G) of which most function cotranslationally towards an array of substrates, thus combined covering the majority of the proteome. NAA80/NatH differs in that Nt-acetylation of actin occurs posttranslationally since it constitutes the final step of a unique step-by-step mechanism for Nt-maturation. Posttranslational activity is also suggested for the other more recently identified NATs, NAA60/NatF and NAA70/NatG due to their subcellular localization [8,9]. Contrary to the typical NAT, NAA80/NatH appears to be highly selective with actin as the only substrate in vivo in animals, as demonstrated by the lack of actin Nt-acetylation in NAA80 KO cells by COFRADIC analysis [6]. There are at least two factors underlying this narrow substrate specificity. The crystal structure of Drosophila melanogaster NAA80 (DmNAA80) bound to a bisubstrate inhibitor mimicking the N-terminus of β-actin in complex with acetyl coenzyme A revealed unique features of NAA80 [10]. Although DmNAA80 shares the typical GNAT-fold that is common for all described NATs [4], the binding to its substrate, actin, is mainly accomplished by direct and water-mediated interaction of positively-charged residues with the negatively-charged acidic amino acids at position 3 and 4 of actin (Asp in β-actin and Glu in γ-actin) [10]. Usually, the identity of the substrate amino acids at position 1 and 2 are the major determinants for enzyme binding of the other known NATs [11]. Another factor limiting the cellular substrate pool of NAA80 is the presence of NatB which efficiently acetylate all Met-starting acidic N-termini in a co-translational manner (such as the premature N-terminus of actin), and yeast depleted for NatB are lacking Nt-acetylation at these substrates [12]. However, expression of human NAA80 in a yeast NatB deletion strain partially restored the Nt-acetylation of NatB substrates [10]. Thus, NatB Nt-acetylated a number of substrates that in animal cells otherwise might be targeted by NAA80. Furthermore, NAA80 could potentially act as a back-up system for substrates that fail to be cotranslationally Nt-acetylated by NatB. It will be interesting to compare the DmNAA80 structure with the human NAA80 enzyme, which features some additional domains flanking the catalytic GNAT-fold. These include poly-proline stretches at the C-terminus, which are typical profilin-binding sites [13] as well as a NAT-atypical extension of the N-terminus, and could therefore play additional roles in actin recognition and regulation.
NAA80 acts like a brake to keep cell migration in check
Actin is completely devoid of the Nt-acetyl group in NAA80 knockout cells and these cells are therefore well-suited for studies of cellular functions of actin Nt-acetylation. Surprisingly, the cells lacking NAA80, migrates faster than the control cells as demonstrated by cell culture scratch wound assay and chemotaxis migration [6]. We here further present single-cell analyses demonstrating at least a doubled speed for the random cellular movement (Figure 1). One can thus say that NAA80, by acetylating actin’s N-terminus, acts as a natural brake in the cells in order to ensure normal migration speed. Under the microscope, the NAA80 knockout cells also revealed structural changes in the actin cytoskeleton that matched increased migration speed [6].
Figure 1.
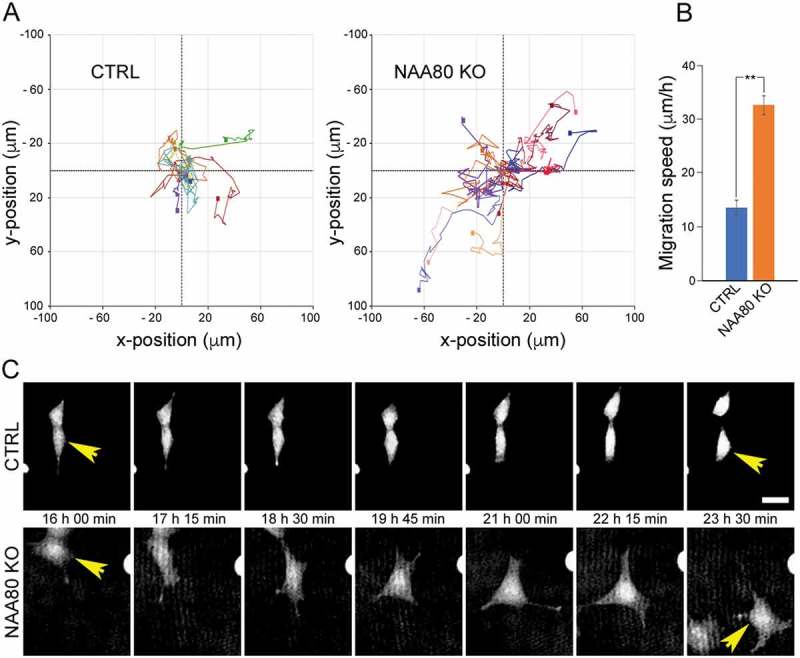
NAA80 KO cells have increased migration speed. HAP1 CTRL and NAA80 KO cells were subjected to live-cell holographic imaging with image acquisition every 15 minutes for 24 hours. A) Rose plot of single-cell trajectories for CTRL and NAA80 KO. B) Migration speed per hour for CTRL (n = 9) and NAA80 KO (n = 9). Error bars show SEM, **P ≤ 0.05, two-sided Student’s t test. C) Example images showing single-cell migration of one representative cell. Migration speed for shown cell (over 24 h): CTRL, 11.8 μm/h; NAA80 KO, 43.5 μm/h. Scale bar, 25 μm.
Actin Nt-acetylation by NAA80 affects polymerization dynamics
Drazic et al. also presented biochemical data that at least in part could explain the increased migration of the NAA80 knockout cells [6]. Purified β/γ-actin from NAA80 KO cells subjected to a pyrene assay revealed that the polymerization rates were altered. This was further visualized here by phalloidin staining of polymerized actin (Figure 2).
Figure 2.
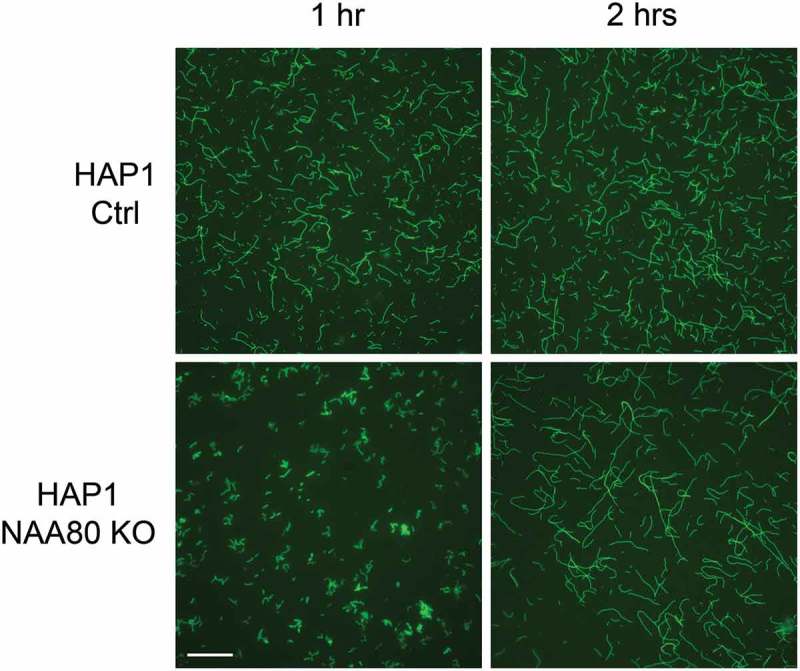
In vitro polymerized actin isolated from CTRL and NAA80 KO cells. A mixture of endogenous β/γ-actin was purified from HAP1 control (Ac-actin) and NAA80 knockout (non-Ac-actin) cells and allowed to polymerize for 1 or 2 hours. F-actin was stained with phalloidin-488 and imaged. Scale bar, 10 μm.
Discussion
The identification of NAA80, an enzyme whose activity has been known for more than 30 years [5], is a milestone in understanding the role which actin Nt-acetylation plays for the cytoskeleton and actin-related cellular processes (Figure 3). We have shown that this posttranslational modification has a significant impact on cytoskeleton dynamics by altered actin polymerization rates (Figure 2), as well as on a cellular level by negatively regulating cell motility (Figure 1). Further effects, which occur when NAA80 is missing, include a reduced globular-to-filamentous actin ratio in cells, increased lamellipodia and filopodia formation (Figure 3) as well as altered polymerization rates in combination with actin-binding proteins such as formin 1 [6]. These studies bring an almost forgotten actin modification back into the spotlight [14], and encourage follow-up studies to unravel how actin’s Nt-acetylation status affect its many roles in a wide range of cellular processes.
Figure 3.
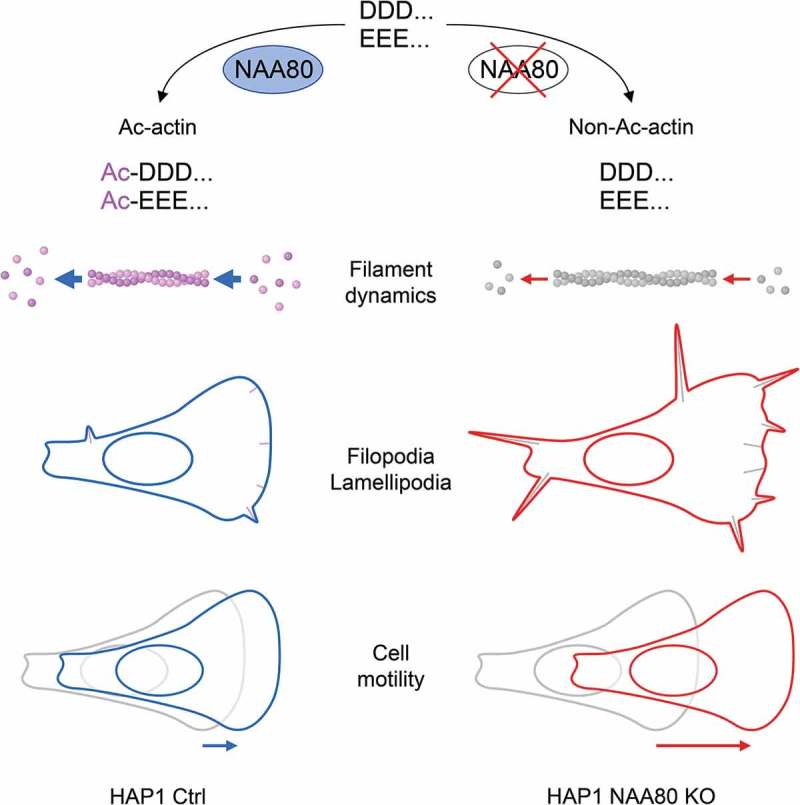
Effects of Nt-acetylation of actin. Nt-acetylation of β/γ-actin is mediated by the animal-specific NAT, NAA80 (left side). The lack of the acetyl group results in altered actin filament dynamics. Polymerization rates are reduced in non-Ac-actin, however, filament stability is increased, leading to elevated formation of filopodia and lamellipodia in NAA80 KO cells (right side). Nt-acetylation of actin acts like a break by keeping cell motility in check in wildtype cells, whereas NAA80 KO cells move faster (bottom).
Material and methods
Holographic microscopy and single-cell tracking
HAP1 CTRL and NAA80 KO cells were seeded on glass-bottom dishes (ibidi, 35 mm) at 40.000 cells/well and monitored on an Holomonitor M4 imaging system (Phase Holographic Imaging PHI AB) with image acquisition every 15 minutes for 24 hours. Single-cell identification and tracking of movement was performed with Hstudio cytometric software.
Visualization of in vitro polymerized actin by phalloidin staining
A mixture of endogenous β/γ-actin was purified from HAP1 control (Ac-actin) and NAA80 knockout (non-Ac-actin) cells by gelsolin affinity chromatography as described in Drazic et al [6].. To observe filamentous actin (F-actin), 1.6 µM globular actin was polymerized by adding polymerization buffer (10 mM Tris/HCl (pH 8.0), 100 mM KCl, 1 mM MgCl2, 1 mM EGTA, 0.2 mM ATP) and incubated at room temperature. After 1 and 2 hours, 5 µL of polymerized actin mixture was diluted with 15 µL polymerization buffer containing 1 µM phalloidin-488 (binds only to F-actin). 15 µL of the sample was then spread on poly-L-lysine coated coverslips and imaged on a Leica DMI6000 B wide field microscope equipped with a Leica DC500 camera and a HCX PL APO 100 × 1.4 NA oil objective.
Funding Statement
This work was supported by the Bergens Forskningsstiftelse; European Research Council [772039]; Helse Vest [912176]; Kreftforeningen;Norges Forskningsråd [249843].
Acknowledgments
This work has received funding from the Research Council of Norway grant 249843 to T.A., the Norwegian Cancer Society to T.A., the Bergen Research Foundation BFS to T.A., the Norwegian Health Authorities of Western Norway (Project 912176), and European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme under grant agreement No 772039.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Sheterline P, Clayton J, Sparrow J.. Actin. Protein Profile. 1995;2:1–103. [PubMed] [Google Scholar]
- [2].Pollard TD, Cooper JA.. Actin, a central player in cell shape and movement. Science. 2009;326:1208–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pollard TD. Actin and actin-binding proteins. Cold Spring Harb Perspect Biol. 2016;8: a018226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Aksnes H, Drazic A, Marie M, et al. First things first: vital protein marks by N-terminal acetyltransferases. Trends Biochem Sci. 2016;41:746–760. [DOI] [PubMed] [Google Scholar]
- [5].Rubenstein PA, Martin DJ. NH2-terminal processing of actin in mouse L-cells in vivo. J Biol Chem. 1983;258:3961–3966. [PubMed] [Google Scholar]
- [6].Drazic A, Aksnes H, Marie M, et al. NAA80 is actin’s N-terminal acetyltransferase and regulates cytoskeleton assembly and cell motility. Proc Natl Acad Sci USA. 2018;115:4399–4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Polevoda B, Arnesen T, Sherman F. A synopsis of eukaryotic Nalpha-terminal acetyltransferases: nomenclature, subunits and substrates. BMC Proc. 2009;3(Suppl 6):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Aksnes H, Van Damme P, Goris M, et al. An organellar nalpha-acetyltransferase, naa60, acetylates cytosolic N termini of transmembrane proteins and maintains Golgi integrity. Cell Rep. 2015;10:1362–1374. [DOI] [PubMed] [Google Scholar]
- [9].Dinh TV, Bienvenut WV, Linster E, et al. Molecular identification and functional characterization of the first Nalpha-acetyltransferase in plastids by global acetylome profiling. Proteomics. 2015;15:2426–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Goris M, Magin RS, Foyn H, et al. Structural determinants and cellular environment define processed actin as the sole substrate of the N-terminal acetyltransferase NAA80. Proc Natl Acad Sci U S A. 2018;115:4405–4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Liszczak G, Goldberg JM, Foyn H, et al. Molecular basis for N-terminal acetylation by the heterodimeric NatA complex. Nat Struct Mol Biol. 2013;20:1098–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Van Damme P, Lasa M, Polevoda B, et al. N-terminal acetylome analyses and functional insights of the N-terminal acetyltransferase NatB. Proc Natl Acad Sci U S A. 2012;109:12449–12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Metzler WJ, Bell AJ, Ernst E, et al. Identification of the poly-L-proline-binding site on human profilin. J Biol Chem. 1994;269:4620–4625. [PubMed] [Google Scholar]
- [14].Rubenstein PA, Wen KK. NATure of actin amino-terminal acetylation. Proc Natl Acad Sci U S A. 2018;115:4314–4316. [DOI] [PMC free article] [PubMed] [Google Scholar]


