ABSTRACT
For this study, we utilized class-I and class-II preQ1-sensing riboswitches as model systems to decipher the structure-activity relationship of rationally designed ligand derivatives in vitro and in vivo. We found that synthetic preQ1 ligands with amino-modified side chains that protrude from the ligand-encapsulating binding pocket, and thereby potentially interact with the phosphate backbone in their protonated form, retain or even increase binding affinity for the riboswitches in vitro. They, however, led to significantly lower riboswitch activities in a reporter system in vivo in E. coli. Importantly, when we substituted the amino- by azido-modified side chains, the cellular activities of the ligands were restored for the class-I conditional gene expression system and even improved for the class-II counterpart. Kinetic analysis of ligand binding in vitro revealed enhanced on-rates for amino-modified derivatives while they were attenuated for azido-modified variants. This shows that neither high affinities nor fast on-rates are necessarily translated into efficient cellular activities. Taken together, our comprehensive study interconnects in vitro kinetics and in vitro thermodynamics of RNA-ligand binding with the ligands’ in vivo performance and thereby encourages azido- rather than amino-functionalized design for enhanced cellular activity.
KEYWORDS: RNA ligand recognition, modifications, riboswitches, kinetics, thermodynamics, RNA biosensor tools, preQ1 derivatives
Introduction
Riboswitches have emerged as possible targets for the development of alternative antimicrobial approaches [1–8]. They are typically located in the 5ʹ noncoding regions of bacterial mRNA and are able to bind specific metabolites to their aptamers with very high selectivity [9–11]. In a manner that is dependent on metabolite concentration, nascent mRNAs containing riboswitch domains can enter one of two mutually exclusive folding pathways to impart regulatory control [12]. The outcome of the folding pathway corresponds to ligand-bound or -free state. Thereby, the aptamer fold triggers structural cues into the expression platform which, in turn, transduces an ‘on’ or ‘off’ signal for gene expression, predominantly at the transcriptional or translational level [13–15].
One of the most critical steps in riboswitch gene regulation is ligand-sensing by the aptamer. For most riboswitches, the ligand becomes almost completely encapsulated by the RNA scaffold. Besides nucleobase stacking, most riboswitch aptamers involve every possible hydrogen donor or acceptor position of the ligand in hydrogen bond interactions with nucleotides of the binding pocket. This makes the structure-based design of modified ligand analogs and ligand mimics rather challenging. Nevertheless, the identification of novel potent ligands is a topic of intense research because ever since their discovery, riboswitches have been viewed as promising targets for the development of novel antibiotic strategies [16]. Likewise, efforts to engineer riboswitches for imaging purposes [17–19] or as biotechnological tools for the detection of endogenous and non-endogenous small molecules are in the focus of synthetic biologists interested in understanding and reprogramming cellular behavior [20].
In the present study, we examine the structure-activity relationships between 7-aminomethyl-7-deazaguanine (preQ1) sensing riboswitches [21] and chemically functionalized preQ1 ligands, both in vitro and in vivo. In particular, we ask the question how ‘add-on’ functionalities such as aminoalkyl, azidoalkyl, and ethylene glycol moieties that can potentially interact with the phosphate backbone and that are amenable for further derivatization, impact binding thermodynamics and kinetics and how the obtained in vitro parameters translate into riboswitch activity in the cell.
Results and discussion
Structure-based design of functionalized ligands for preQ1-I and -II riboswitches
PreQ1 is an intermediate of the biosynthesis pathway of the hypermodified nucleoside queuosine. Although queuosine is found in specific tRNAs of most eukaryotes and bacteria, it is only synthesized in bacteria. The queuosine modification enhances translational fidelity at the wobble position [22–25], and queuosine deficiency in bacteria can lead to reduced growth fitness and diminished virulence [26,27]. Bacterial riboswitches responsive to preQ1 are currently known to fall into three phylogenetically distinct classes. The preQ1-I (class 1) aptamer is distributed widely and rather compact, comprising not more than 34 nucleotides [28]. The preQ1-II (class 2) riboswitch is about twice this size and has been found in the Firmicutes [29]. Both classes are prevalent among important pathogens, such as Streptococcaceae. By contrast, the preQ1-III (class 3) riboswitch has been found exclusively in Clostridium, and it is the largest of all preQ1 riboswitches [21].
In the present comprehensive study, we have focused on the two most widespread classes of preQ1 riboswitches (I and II). These two classes employ distinct ligand binding modes (Figure 1) [30,31]. The class-I riboswitch recognizes preQ1 with cytosine (C15) through classical Watson–Crick base pairing and additionally through bidentate interaction of the ligand’s N3 and C2-NH2 with the trans Watson–Crick face of adenosine (A29) (Figure 1A,C). Moreover, the N9-H of preQ1 is H-bonding to the carbonyl O4 of uridine (U6) and the 7-aminomethyl moiety is involved in a further H-bond, namely to O6 of guanosine (G5).
Figure 1.
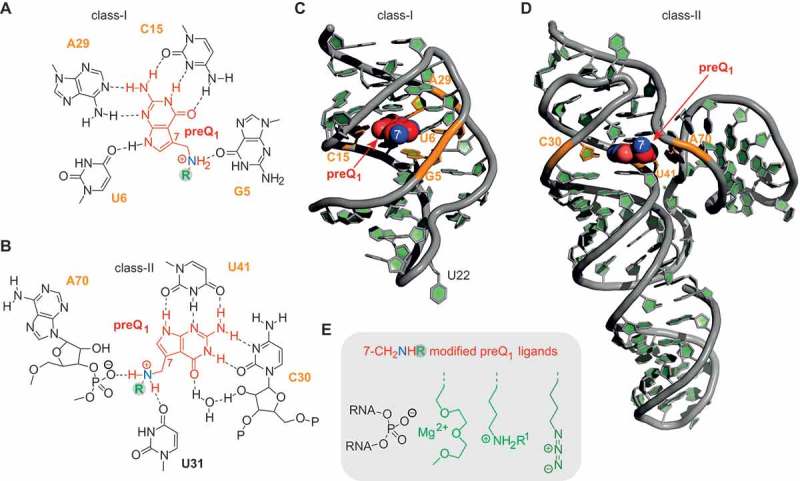
Structural analyses of preQ1 riboswitches. A) H-bond interactions between preQ1 and RNA binding pocket of class-I riboswitch; B) H-bond interactions between preQ1 and RNA binding pocket of class-II riboswitch; C) Three-dimensional structure of the Thermoanaerobacter tengcongensis (Tte) class-I preQ1 riboswitch (PDB ID: 3Q50); D) Three-dimensional structure of the Lactobacillus rhamnosus class-II preQ1 riboswitch (PDB ID: 4JF2); E) ‘Add-on’ functionalities of preQ1 ligands with the potential to interact with phosphate backbone units at the entrance of the ligand pocket to support ligand-RNA binding.
The class-II riboswitch binds preQ1 differently (Figure 1B,D). PreQ1 pairs in bidentate fashion through trans Watson-Crick/Watson-Crick to cytidine (C30) and tridendate via N9-H–N3–C2-NH2 to the trans Watson–Crick face of uridine (U41). The O6 of the preQ1 lactam moiety forms a water-mediated bridge to the 2ʹ-OH of C30. Compared to the class-I riboswitch, the 7-aminomethyl group of the ligand is more strongly involved in interactions with the RNA, namely H-bonding to O6 of U31 and via electrostatic interactions to the phosphate group between A70 and A71.
Although preQ1 binding modes of class I and II riboswitches are different with respect to H-bonding patterns, for both riboswitches the 7-aminomethyl group of the ligand remains solvent-accessible in the bound state. The 7-aminomethyl group therefore appears to be a suitable anchor for tether attachment without disturbing ligand-aptamer recognition. Because we intended to retain the interaction characteristics of the 7-aminomethyl moiety, its alkylation (resulting in secondary amines) rather than acylation (resulting in amides) was considered to provide the most fitting functionality for attachments.
For tethering additional functionalities to the native ligand, we have mainly focused on two types of modifications. First, aminoalkyl tethers as shown for derivative 3 and 4 (Figure 1E, Figure 2) in their protonated form at suitable pH should be able to support binding of the modified ligand, based on specific electrostatic interactions with the phosphate backbone at the entrance of the ligand binding site. Higher affinities can be expected and additionally, binding kinetics are likely influenced due to an apparent increase in concentration because of non-specific interactions of the ammonium groups with the RNA phosphate backbone.
Figure 2.
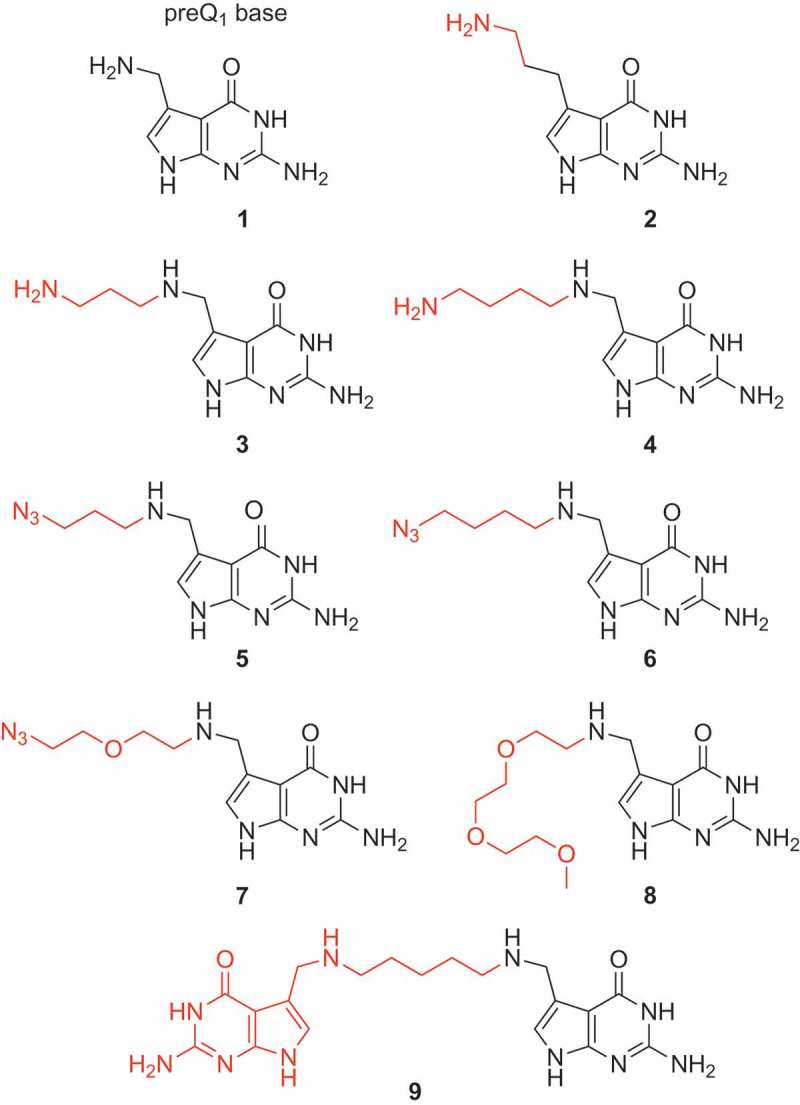
Chemical structures of the preQ1 ligand derivatives synthesized for this study (for synthetic details see the Supporting Information).
Second, azidoalkyl tethers as shown for derivative 5, 6 and 7 (Figure 1E, Figure 2) have been envisaged because of their potential for further straightforward functionalization with labeling compounds, e.g. fluorophores or biotin, using bioorthogonal Click or Staudinger reactions.
Moreover, we set out to analyze the impact of ethylene glycol moieties as shown for compound 8 (Figure 1E, Figure 2). In a different context, earlier studies on oligoribonucleotide duplexes carrying this functionalization demonstrated a Mg2+-chelating effect that can be utilized for enthalpic stabilization of RNA double helices [32]. We therefore speculated that this effect might be advantageous to stabilize small molecule–RNA interactions as well. Finally, we wanted to analyze the binding properties of a ligand dimer 9 (Figure 2) that bridges two preQ1 units via a short pentane linker to both 7-aminomethyl groups.
Synthesis of tethered preQ1 ligands
To get access to the preQ1 derivatives displayed in Figure 2, we have developed a robust protocol for reductive amination of 7-(aminomethyl)-7-deazaguanine 1 [33] and the corresponding phthalimido-protected aminoalkylaldehydes and azidoalkylaldehydes, respectively, using tetramethylammonium triacetoxyborohydride in dimethylformamide and acetic acid. The phthalimido group was then cleaved with aqueous hydrazine solution. All tethered preQ1 derivatives were purified by reversed-phase chromatography applying an acetonitrile gradient to aqueous eluents containing one percent of trifluoroacetic acid. The products were thus obtained as salts of trifluoroacetic acid in excellent purity. Details of preparation are given in the Supporting Information. The developed routes provide significantly higher yields compared to direct alkylation of preQ1 using bromoalkyl substrates [34].
Binding thermodynamics and kinetics of tethered preQ1 ligands to class-I and -II riboswitches
Ligand affinities (KD) as well as on-rates (kon) for ligand binding were measured based on a fluorescence spectroscopic approach (Fig. S1, Figure 3) that utilizes site-specifically 2-aminopurine (Ap) labeled RNA [35]. For preQ1 class-I riboswitches, we focused on the specific aptamer sequence from Thermoanaerobacter tengcongensis (Tte); for preQ1 class-II riboswitches, we used the aptamer sequence from Streptococcus pneumoniae (Spn) (Figure 4A). For both, suitable positions for Ap substitutions (U22Ap class I; A11Ap class II) have been identified [36–38].
Figure 3.
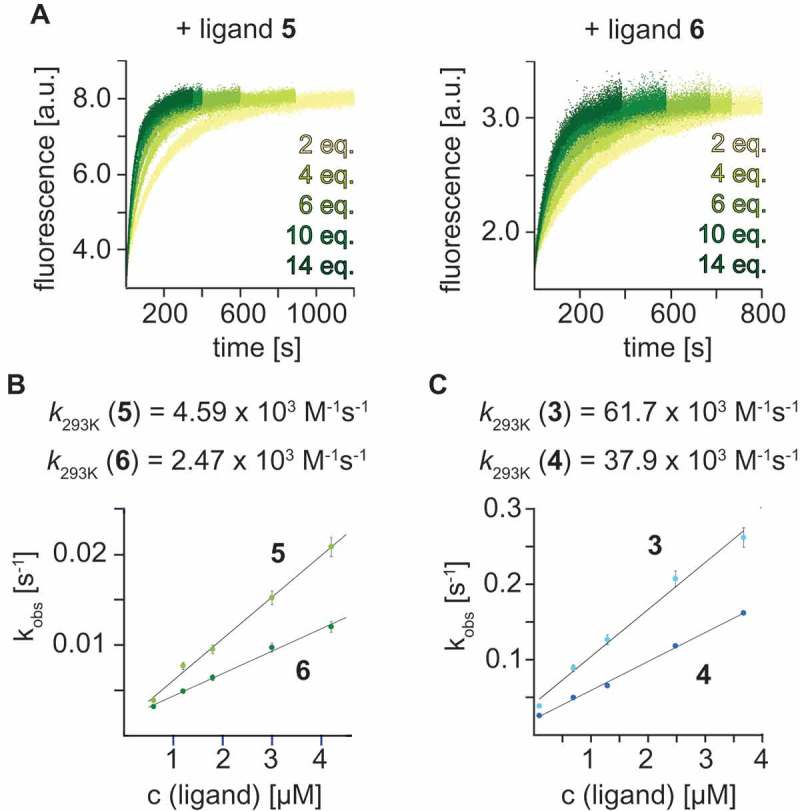
Stopped-flow fluorescence spectroscopy was used to monitor the kinetics of ligand preQ1 binding to the Tte preQ1 class-I riboswitch. A) Real time aminopurine (Ap) fluorescence time traces of the Tte U22Ap variant at different concentrations of 3-azidopropyl- and 4-azidobutyl-modified preQ1 5 and 6; B) Rate constants k293 from plots of observed rate kobs versus ligand concentration. C) Same as (B) but for the 3-aminopropyl and 4-aminobutyl-modified preQ1 3 and 4. c(RNA) = 0.3 µM, c(MgCl2) = 2 mM, 100 mM KCl, 50 mM MOPS, pH 7.5, 293 K. Ligand concentration c(ligand) as indicated.
Figure 4.
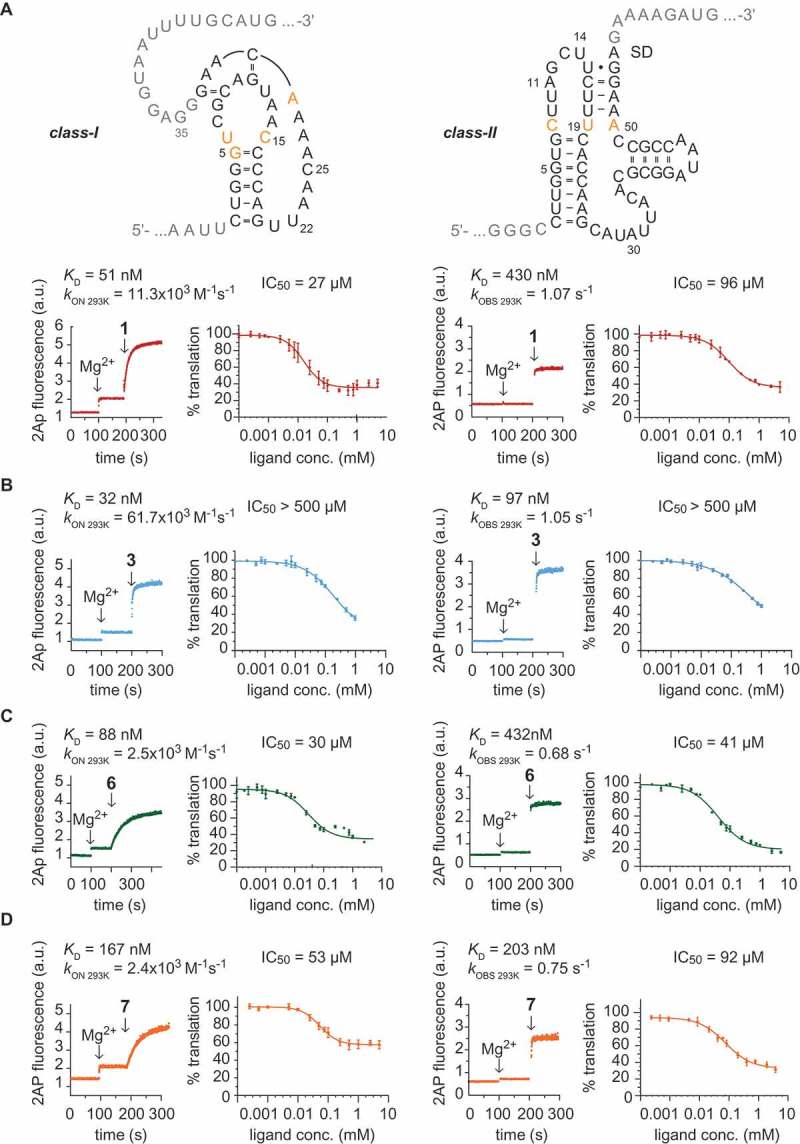
Comparison of in vitro and in vivo performance of preQ1 class-I and -II riboswitches with preQ1 and selected derivatives. A) Sequences of the Tte class-I (top left) and Streptococcus pneumoniae (Spn) class-II (top right) riboswitches used in this study. Nucleobase letters in black indicate the synthetic RNA aptamer sequences for KD and kon determinations (U22Ap and A11Ap substitution, respectively). Nucleobase letters in grey indicate the integration of the aptamers into the reporter mRNA. Letters in orange indicate nucleobases of the binding pocket that directly interact with the ligand via H-bonding. Exemplary fluorescence time traces of Ap-labeled preQ1 RNAs in response to Mg2+and preQ1 ligand 1 (conditions: 0.5 μM RNA, 100 mM KCl, 50 mM MOPS, pH 7.5, 293 K. Ligands: 2 mM MgCl2, 5 μM preQ1 1); affinities KD were obtained from plots of normalized AP fluorescence intensities plotted as a function of ligand concentrations (for details see Fig. S1); rate constants kon(293) of the Tte preQ1 class-I riboswitch were obtained from plots of observed rates kobs vs ligand concentrations (for details see Figure 3 and Fig. S3); binding to the Spn preQ1-II riboswitch was independent of ligand concentration; for details of kobs determination see Fig. S4. Dose-dependent repression of gene expression: B105 E. coli cells transformed with constructs expressing eGFP under translational control of preQ1 class-I and -II riboswitches were assayed for eGFP fluorescence in the presence of different preQ1 1 concentrations (0.10 μM to 5 mM). Data represent the mean of three biological replicates, with error bars indicating standard deviation. The data were fit with a four-parameter logistic function to derive the IC50 values as indicated. B) Same as (A), but for ligand derivative 3. C) Same as (A), but for ligand derivative 6. D) Same as (A), but for ligand derivative 7.
Of note, the availability of the 7-aminomethyl group of native ligand 1 is particularly important for class-II riboswitches. Affinity was 7-fold reduced for ligand analog 2 (7-(3-aminopropyl)-7-deazaguanine; Fig. S2A) that comprised an alkyl spacer placing the amino group at greater distance from the ligand core (Figure 2); likely, the longer chain hinders positioning of the amino group to generate contacts to the phosphate of A70–A71 and to U31 in the RNA binding pocket.
Concerning the class-I preQ1 riboswitch aptamer, the KD values of amino- and azidopropyl and -butyl tethered ligands (3 to 6) measured in aqueous buffer at pH 7.5 (50 mM MOPS, 100 mM KCl, 293 K) in the presence of 2 mM MgCl2 were comparable to native ligand 1, varying only by a factor of 1.7 (Table 1, Figure 4B-D, Fig. S1 and S2). Ligands carrying longer chains, such as azidoethoxyethyl-preQ1 7 and triethylene glycol-linked preQ1 8, experienced a 4-fold and 18-fold decrease in affinity, respectively (Table 1, Fig. S2). We speculate that the conformational changes of the tethered group that are required for a chelating Mg2+ interaction (as indicated in Figure 1E) might lead to an entropic destabilization that compensates the enthalpic stabilization. Also, for the preQ1 dimer 9, a loss in affinity (11-fold) was found (Table 1).
Table 1.
Class-I preQ1 riboswitches – Thermodynamic and kinetic parameters of ligand binding and cellular activity.
| Ligand No. | KD [nM] | kon [M−1s−1 x10−3 | koff [s−1] * | IC50 [µM] ** |
|---|---|---|---|---|
| 1 | 51 | 11.3 | 0.000576 | 27 (75%) |
| 2 | 71 | 4.3 | 0.000305 | n.d. |
| 3 | 32 | 61.7 | 0.001974 | > 500 |
| 4 | 41 | 37.9 | 0.001554 | > 1000 |
| 5 | 30 | 4.6 | 0.000138 | 52 (65%) |
| 6 | 88 | 2.5 | 0.000220 | 30 (70%) |
| 7 | 167 | 2.4 | 0.000401 | 53 (42%) |
| 8 | 922 | 3.4 | 0.003135 | 152 (56%) |
| 9 | 590 | 3.5 | 0.002065 | 308 (38%) |
* off-rates were calculated from koff = KD x kon (ref. [51]);
** number in brackets represents percentage of translational repression at saturating ligand concentration;
n.d. not detectable
Interestingly, for the preQ1 class-II riboswitch aptamer, the affinities measured for aminopropyl and -butyl tethered ligands (3 and 4) were increased by 3- to 4-fold compared to the native ligand, consistent with stabilizing interactions between the additional ammonium group and the RNA phosphate backbone at the entrance of the binding pocket (Table 2, Figure 4B, Fig. S1B and S2A). In contrast to 3 and 4, the KD values of the corresponding azidopropyl and -butyl tethered ligands (5 and 6) were comparable to the native ligand, varying only by a factor of 1.7 (Table 2, Figure 4C, Fig. S1B and S2C). Also, for azidoethoxyethyl-preQ1 7 the affinity remained comparable, however, the triethylene glycol-linked preQ1 8 and the preQ1 dimer 9, experienced a 10-fold and 4-fold loss in affinity, respectively (Table 2). From this in vitro analysis, it becomes obvious that varying affinities of a particular ligand derivative towards class-I and class-II aptamers likely originate from their distinct structural features leading to differential accomodation and interaction with the tether.
Table 2.
Class-II preQ1 riboswitches – Thermodynamic and kinetic parameters of ligand binding and cellular activity.
| Ligand No. | KD [nM] | kobs [s−1] | koff [s−[1]] * | IC50 [µM] ** |
|---|---|---|---|---|
| 1 | 430 | 1.07 | 0.084733 | 96 (65%) |
| 2 | 2830 | > 10*** | n.d. | n.d. |
| 3 | 97 | 1.05 | 0.019982 | > 500 |
| 4 | 148 | 0.80 | 0.022999 | > 1000 |
| 5 | 254 | 0.70 | 0.033841 | 71 (80%) |
| 6 | 432 | 0.68 | 0.054080 | 40 (80%) |
| 7 | 203 | 0.75 | 0.029262 | 92 (68%) |
| 8 | 4580 | 0.72 | 0.031532 | 116 (65%) |
| 9 | 1030 | 1.06 | 0.181061 | 328 (53%) |
* off-rates were calculated from koff = kobs/(1+([L]/KD)) (ref. [51]);
** number in brackets represents percentage of repression at saturating ligand concentration;
*** estimated value;
n.d. not determined
With regard to ligand binding kinetics, we note that previous studies have demonstrated that for the Tte class-I aptamer, preQ1 binding kinetics are strongly dependent on preQ1 concentrations [36]. Employing the U22Ap riboswitch variant for the 2ApFold fluorescence approach here, we determined an on-rate kon of 11.3 × 103 M−1s−1 for preQ1 1 (Table 1). For the Spn class-II preQ1 counterpart, however, we found that binding kinetics of preQ1 1 (based on the corresponding A11Ap variant) were independent of ligand concentration, with kobs of 1.07 ± 0.30 s−1 (over the same range of 2 to 14-fold excess of ligand over RNA as applied to the class-I counterpart). This suggests that a conformational change or conformational adaption of the class-II preQ1 RNA is possibly rate-limiting for the ligand binding process.
For the aminoalkyl ligand derivatives 3 and 4 we found three to five-fold faster on-rates kon for binding to the class-I aptamer compared to native preQ1 1 (Table 1, Figure 3C, Fig. S2B and S3). By contrast, the corresponding azidoalkyl ligands 5 and 6 with the same tether lengths were observed to have two and five-fold slower on-rates kon than preQ1 1 for binding to the class-I aptamer (Table 1, Figure 3B, Fig. S2C). The faster on-rates for ligands 3 and 4 are consistent with the possibility for specific electrostatic interactions between the additional ammonium moieties and the phosphate backbone, and more generally, with an increase in local concentration due to improved electrostatic interactions with the negatively charged RNA. They are also consistent with an earlier proposed induced-fit binding mode of the preQ1 class-I riboswitch [39,40].
Not unexpectedly, for the class-II riboswitch where ligand binding is not the rate limiting step but likely a conformational change that occurs in the RNA pocket, all preQ1 ligand derivatives 3 to 9 exhibited rates kobs that were comparable to that of the native ligand (Table 2, Figure 4, Fig. S2 and S4). Only a slight rate difference among the ligand derivatives was observed, with azidobutyl modified preQ1 6 being slowest (kobs 0.68 ± 0.30 s−1) and aminopropyl modified preQ1 3 together with dimer 9 being fastest (kobs 1.05 ± 0.16 s−1 and kobs 1.06 ± 0.11 s−1) (Table 2, Fig. S4). The here observed concentration-independence of class-II riboswitches with respect to binding rates is consistent with the conformational capture model that was deduced from NMR spectroscopic investigations [41]. This model proposed that stem P4 is poised to act as a ‘screw cap’ on preQ1 recognition to block ligand exit and stabilize the binding pocket.
Cellular activity of functionalized preQ1 derivatives in a preQ1 deficient E. coli strain
Previously, preQ1 riboswitches have attracted attention as platforms for the engineering of orthogonal riboswitches to control gene expression. Micklefield and coworkers used a rational targeted approach in the evaluation of synthetic compounds with riboswitch mutants and identified an orthogonal riboswitch−ligand pair that effectively repressed the transcription of selected genes in B. subtilis [42]. More recently, rationally engineered preQ1 riboswitches have been applied for inducible gene regulation in mycobacteria [43].
In this study, we investigated how the affinity and kinetic parameters obtained in vitro for functionalized preQ1 derivatives (2 to 9) translate into cellular activity (Figure 4). We therefore engineered a preQ1 class I or class II riboswitch- controlled reporter gene (green fluorescence protein, GFP) and monitored its production in response to the different ligands in vivo in E. coli. To avoid potential interference of endogenous preQ1 with the assay, we used an E. coli strain bearing an inactivating mutation of the queC gene, which encodes a protein involved in the early steps of queuosine synthesis [44]. Tte and Spn preQ1 riboswitches act at the level of translation by sequestering the Shine-Dalgarno sequence via ligand-triggered alternative RNA folding [21]. Successful binding of the preQ1 ligand therefore results in a decrease of GFP production, which can be measured directly in the bacterial culture by determining GFP fluorescence. We used an inducible reporter system (pQE70 bacterial expression system) to repress GFP transcription in the absence of the inducer (IPTG) and added the different ligands concomitantly with the IPTG. Fluorescence measurements at 6 h after induction revealed that the native preQ1 ligand 1 was capable of dose-dependent regulation of class I–controlled GFP expression with an IC50 value of 27 μM and 75% repression observed at preQ1 concentrations of 1 mM or higher (Figure 4A) while for the class II riboswitch, the IC50 value amounted to 96 μM with 65% repression at 1 mM or higher preQ1 concentrations (Figure 4A).
We then evaluated the importance for in vivo activity of the native 7-aminomethyl group as a structural subunit in analogs of preQ1 by measuring IC50 values of compound 2. Although this compound comprises the 7-deazaguanine core, the replacement of the 7-aminomethyl by a 7-(3-aminopropyl) substituent renders it practically inactive in vivo regardless of the riboswitch tested (Fig. S2A). This was especially surprising in the case of the class I riboswitch because in vitro, compound 2 displayed nearly the same affinity to class I aptamers as the native ligand.
Unexpectedly, the aminoalkylated ligands 3 and 4 that showed up to 4-fold higher affinities (class-II) and up to 5-fold increased on-rates (class-I) in vitro exhibited poor regulation ability in vivo. IC50 values for both riboswitch classes were at least 10-fold higher than those for native preQ1 1 (Table 1, Figure 4B, Fig. S2B). However, when azido instead of amino groups were present at tethers of the same lengths, as in preQ1 derivatives 5 and 6, the in vivo activity was restored (for class I) or even improved (for class II) compared to the native preQ1 1 ligand (Table 1, Figure 4C, Fig. S2C). Intriguingly, those ligands had shown significantly slower on-rates in in vitro binding studies (for class-I) (Figure 4C). Together, these findings demonstrate that azidoalkylated ligands exhibit excellent bioavailability and are potent triggers of riboswitch conformation changes resulting in the repression of translation in vivo. On the other hand, it appears that despite superior in vitro affinity of aminoalkylated ligands, they are less suitable for in vivo applications. Potential reasons for that could be reduced cellular uptake [45] or interference with polyamine metabolism in the cell [46].
We also tested the in vivo activity of azidoethoxyethyl preQ1 derivative 7 and found that it was comparable to that of the native preQ1 ligand for the class II riboswitch and slightly higher for the class I type (Figure 4D). The triethylene glycol modification of the ligand (derivative 8) resulted in strongly decreased in vitro affinities of this ligand for both riboswitch classes. Interestingly, however, the in vivo activity of derivative 8 towards the class-II riboswitch was essentially equal to the native ligand (Fig. S2D). For the class-I riboswitch, a similar trend was observed in that the decrease in IC50 value was less pronounced than expected considering its low affinity in vitro (Fig. S1D). Finally, the ligand dimer 9 showed clearly inferior in vivo activity towards both class-I and -II riboswitches compared to the native ligand 1 (Tables 1 and 2).
Conclusions
For several reasons riboswitches have been considered attractive targets for antimicrobial drug development [1–5]: They are well structured and allow stable binding of low–molecular weight compounds to RNA with affinities as found for interactions between established antibiotics and ribosomal RNA. Furthermore, riboswitches have not been identified in mammals which should reduce the risk of undesired side effects. Finally, they are often located upstream of genes encoding enzymes that are involved in the synthesis of the metabolite that triggers the very riboswitch. By designing suitable metabolite analogs that outcompete the natural ligand for interaction with the riboswitch, the production of the metabolite will be inhibited by preventing the expression of the synthesis genes. If the respective metabolite is essential for life, this will lead to a growth stop and/or death of the bacterial cell. Several studies have demonstrated that riboswitches are indeed druggable [6–8,16,47–49]. The most prominent investigation employed a phenotypic screen and identified ribocil that acts as a structurally distinct mimic of the natural ligand flavin mononucleotide to repress ribB gene expression and inhibit cell growth [7].
Modest success, however, derived from ligand design that relied on the modulation of the nature and/or position of heteroatoms of the ligand core and/or the decoration of accessible positions with substituents that are typically used in medicinal chemistry as e.g. reported recently for guanine-sensing riboswitches in the bacterial pathogen Clostridioides difficile [6]. To the best of our knowledge, such studies have not yet included the evaluation of azide functionalization of ligands. Our study now demonstrates that the attachment of short azido-tethers to the native ligand of preQ1 riboswitches leads to improved efficacy (> 2-fold decreased IC50) and significantly increased repression (from 65% to 80%) of a GFP reporter in E. coli. These findings were unexpected because the thermodynamic and kinetic parameters kon and KD determined in vitro were clearly inferior to amino-modified derivatives and rather similar to the native ligand. It was furthermore unexpected that the amino-modified derivatives that gave the highest affinities in vitro, exhibited the lowest cellular activities of all preQ1 derivatives investigated.
For future prospects, azido-modified preQ1 ligands due to their excellent bioavailability and in vivo activity may constitute highly promising platforms for in vivo labeling approaches. The here presented azido-tethered preQ1 derivatives are amenable for bioorthogonal labeling reactions with diverse reporter groups such as fluorophores. Recently, the structure-guided design of fluorescent S-adenosyl-L-methionine (SAM) analogs has been successfully introduced for a high throughput screen to target SAM-I riboswitch RNAs [50]. Such screens are likely expandable to preQ1 riboswitches based on the derivatives presented. Finally, inspiration for live cell imaging applications of preQ1-fluorophor conjugates can be drawn from a recent RNA imaging assay using the cobalamin riboswitch as an RNA tag and a series of probes containing the cobalamin ligand as a fluorescence quencher to elicit fluorescence turn-on upon binding RNA [17].
Funding Statement
This work was supported by the Austrian Science Fund FWF [I1040, P27947, P27024]; Austrian Research Promotion Agency FFG [West Austrian BioNMR 858017].
Acknowledgments
We thank Roland Rokita for synthetic contributions to preQ1 and Daniel Fellner for reagent synthesis for RNA solid-phase synthesis. We thank Christoph Kreutz for continuous NMR spectroscopic support.
Disclosure statement
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental data for this article can be accessed here.
References
- [1].Blount KF, Breaker RR.. Riboswitches as antibacterial drug targets. Nat Biotechnol. 2006;24:1558–1564. [DOI] [PubMed] [Google Scholar]
- [2].Rekand IH, Brenk R. Ligand design for riboswitches, an emerging target class for novel antibiotics. Future Med Chem. 2017;9:1649–1662. [DOI] [PubMed] [Google Scholar]
- [3].Lünse CE, Schüller A, Mayer G. The promise of riboswitches as potential antibacterial drug targets. Int J Med Microbiol. 2014;304:79–92. [DOI] [PubMed] [Google Scholar]
- [4].Deigan KE, Ferré-D’Amaré AR. Riboswitches: discovery of drugs that target bacterial gene-regulatory RNAs. Acc Chem Res. 2011;44:1329–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Groher F, Suess B. In vitro selection of antibiotic-binding aptamers. Methods. 2016;106:42–50. [DOI] [PubMed] [Google Scholar]
- [6].Yan L-H, Le Roux A, Boyapelly K, et al. Purine analogs targeting the guanine riboswitch as potential antibiotics against Clostridioides difficile. Eur J Med Chem. 2018;143:755–768. [DOI] [PubMed] [Google Scholar]
- [7].Howe JA, Wang H, Fischmann TO, et al. Selective small-molecule inhibition of an RNA structural element. Nature. 2015;526:672–677. [DOI] [PubMed] [Google Scholar]
- [8].Dar D, Shamir M, Mellin JR, et al. Term-seq reveals abundant ribo-regulation of antibiotics resistance in bacteria. Science. 2016;352:aad9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Breaker RR. Riboswitches and translation control. Cold Spring Harb Perspec Biol. 2018. DOI: 10.1101/cshperspect.a032797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Serganov A, Nudler E. A decade of riboswitches. Cell. 2013;152:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Batey RT. Riboswitches: still a lot of undiscovered country. Rna. 2015;21:560–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Haller A, Soulière MF, Micura R. The dynamic nature of RNA as key to understanding riboswitch mechanisms. Acc Chem Res. 2011;44:1339–1348. [DOI] [PubMed] [Google Scholar]
- [13].Lang K, Rieder R, Micura R. Ligand-induced folding of the thiM TPP riboswitch investigated by a structure-based fluorescence spectroscopic approach. Nucleic Acids Res. 2007;35:5370–5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Uhm H, Kang W, Ha KS, et al. Single-molecule FRET studies on the cotranscriptional folding of a thiamine pyrophosphate riboswitch. Proc Natl Acad Sci USA. 2018;115:331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Garst AD, Batey RT. A switch in time: detailing the life of a riboswitch. BBA Gene Regul Mech. 2009;1789:584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wang H, Mann PA, Xiao L, et al. Dual-targeting small-molecule inhibitors of the staphylococcus aureus FMN riboswitch disrupt riboflavin homeostasis in an infectious setting. Cell Chem Biol. 2017;24:576–588. [DOI] [PubMed] [Google Scholar]
- [17].Braselmann E, Wierzba AJ, Polaski JT, et al. A multicolor riboswitch-based platform for imaging of RNA in live mammalian cells. Nat Chem Biol. 2018;14:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kellenberger CA, Chen C, Whiteley AT, et al. RNA-based fluorescent biosensors for live cell imaging of second messenger cyclic di-AMP. J Am Chem Soc. 2015;137:6432–6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sunbul M, Jäschke A. SRB-2: a promiscuous rainbow aptamer for live-cell RNA imaging. Nucleic Acids Res. 2018;6:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sinha J, Reyes SJ, Gallivan JP. Reprogramming bacteria to seek and destroy an herbicide. Nat Chem Biol. 2010;6:464–470. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [21].McCown PJ, Liang JJ, Weinberg Z, et al. Structural, functional, and taxonomic diversity of three PreQ1 riboswitch classes. Chem Biol. 2014;21:880–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hutinet G, Swarjo MA, de Crécy-Lagard V. Deazaguanine derivatives, examples of crosstalk between RNA and DNA modification pathways. RNA Biol. 2017;14:1175–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vinayak M, Pathak C. Queuosine modification of tRNA: its divergent role in cellular machinery. Biosci Rep. 2009;30:135–148. [DOI] [PubMed] [Google Scholar]
- [24].Bienz M, Kubli E. Wild-type tRNATyrG reads the TMV RNA stop codon, but Q base-modified tRNATyrQ does not. Nature. 1981;294:188–190. [DOI] [PubMed] [Google Scholar]
- [25].Yokoyama S, Miyazawa T, Iitaka Y, et al. Three-dimensional structure of hyper-modified nucleoside Q located in the wobbling position of tRNA. Nature. 1979;282:107–109. [DOI] [PubMed] [Google Scholar]
- [26].Noguchi S, Nishimura Y, Hirota Y, et al. Isolation and characterization of an Escherichia coli mutant lacking tRNA-guanine transglycosylase. Function and biosynthesis of queuosine in tRNA. J Biol Chem. 1982;257:6544–6550. [PubMed] [Google Scholar]
- [27].Durand JM, Okada N, Tobe T, et al. vacC, a virulence-associated chromosomal locus of Shigella flexneri, is homologous to tgt, a gene encoding tRNA-guanine transglycosylase (Tgt) of Escherichia coli K-12. J Bacteriol. 1994;176:4627–4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Roth A, Winkler WC, Regulski EE, et al. A riboswitch selective for the queuosine precursor preQ1 contains an unusually small aptamer domain. Nat Struct Mol Biol. 2007;14:308–317. [DOI] [PubMed] [Google Scholar]
- [29].Meyer MM, Roth A, Chervin SM, et al. Confirmation of a second natural preQ1 aptamer class in Streptococcaceae bacteria. Rna. 2008;14:685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jenkins JL, Krucinska J, McCarty RM, et al. Comparison of a preQ1 riboswitch aptamer in metabolite-bound and free states with implications for gene regulation. J Biol Chem. 2011;286:24626–24637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liberman JA, Salim M, Krucinska J, et al. Structure of a class II preQ1 riboswitch reveals ligand recognition by a new fold. Nat Chem Biol. 2013;9:353–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wu X, Pitsch S. Synthesis and pairing properties of oligoribonucleotide analogues containing a metal-binding site attached to beta-D-allofuranosyl cytosine. Nucleic Acids Res. 1998;26:4315–4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Levic J, Micura R. Syntheses of 15N-labeled pre-queuosine nucleobase derivatives. Beilstein J Org Chem. 2014;10:1914–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ehret F, Zhou CY, Alexander SC, et al. Site-specific covalent conjugation of modified mRNA by tRNA guanine transglycosylase. Mol Pharm. 2018;15:737–742. [DOI] [PubMed] [Google Scholar]
- [35].Haller A, Altman RB, Soulière MF, et al. Folding and ligand recognition of the TPP riboswitch aptamer at single-molecule resolution. Proc Natl Acad Sci USA. 2013;110:4188–4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Frener M, Micura R. Conformational rearrangements of individual nucleotides during RNA-ligand binding are rate-differentiated. J Am Chem Soc. 2016;138:3627–3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Soulière MF, Haller A, Rieder R, et al. Approach for the selection of 2-Aminopurine substitution sites to investigate RNA folding. J Am Chem Soc. 2011;133:16161–16167. [DOI] [PubMed] [Google Scholar]
- [38].Soulière MF, Altman RB, Schwarz V, et al. Tuning a riboswitch response through structural extension of a pseudoknot. Proc Natl Acad Sci USA. 2013;110:E3256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Suddala KC, Wang J, Hou Q, et al. Mg(2+) shifts ligand-mediated folding of a riboswitch from induced-fit to conformational selection. J Am Chem Soc. 2015;137:14075–14083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Suddala KC, Rinaldi AJ, Feng J, et al. Single transcriptional and translational preQ1 riboswitches adopt similar pre-folded ensembles that follow distinct folding pathways into the same ligand-bound structure. Nucleic Acids Res. 2013;41:10462–10475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kang M, Eichhorn CD, Feigon J. Structural determinants for ligand capture by a class II preQ1 riboswitch. Proc Natl Acad Sci USA. 2014;111:E663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wu M-C, Lowe PT, Robinson CJ, et al. Rational re-engineering of a transcriptional silencing PreQ1 riboswitch. J Am Chem Soc. 2015;137:9015–9021. [DOI] [PubMed] [Google Scholar]
- [43].Van Vlack ER, Topp S, Seeliger JC. Characterization of engineered PreQ1 riboswitches for inducible gene regulation in mycobacteria. J Bacteriol. 2017;199: pii:e00656–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gaur R, Varshney U. Genetic analysis identifies a function for the queC (ybaX) gene product at an initial step in the queuosine biosynthetic pathway in escherichia coli. J Bacteriol. 2005;187:6893–6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Menzi M, Wild B, Pradère U, et al. Towards improved oligonucleotide therapeutics through faster target binding kinetics. Chem Eur J. 2017;23:14221–14230. [DOI] [PubMed] [Google Scholar]
- [46].Bae D-H, Lane DJR, Jansson PJ, et al. The old and new biochemistry of polyamines. Biochim Biophys Acta. 2018;1862:2053–2068. [DOI] [PubMed] [Google Scholar]
- [47].Krajewski SS, Isoz I, Johansson J. Antibacterial and antivirulence effect of 6-N-hydroxylaminopurine in Listeria monocytogenes. Nucleic Acids Res. 2017;45:1914–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Blount KF, Megyola C, Plummer M, et al. Novel riboswitch-binding flavin analog that protects mice against Clostridium difficile infection without inhibiting cecal flora. Antimicrob Agents Chemother. 2015;59:5736–5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mulhbacher J, Brouillette E, Allard M, et al. Novel riboswitch ligand analogs as selective inhibitors of guanine-related metabolic pathways. PLoS Pathog. 2010;6:e1000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hickey SF, Hammond MC. Structure-guided design of fluorescent S-adenosylmethionine analogs for a high-throughput screen to target SAM-I riboswitch RNAs. Chem Biol. 2014;21:345–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hulme EC, Trevethick MA. Ligand binding assays at equilibrium: validation and interpretation. Brit J Pharmacol. 2010;161:1219–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


