ABSTRACT
A wide variety of factors are required for the conversion of pre-tRNA molecules into the mature tRNAs that function in translation. To identify factors influencing tRNA biogenesis, we previously performed a screen for strains carrying mutations that induce lethality when combined with a sup61-T47:2C allele, encoding a mutant form of . Analyzes of two complementation groups led to the identification of Tan1 as a protein involved in formation of the modified nucleoside N4-acetylcytidine (ac4C) in tRNA and Bud13 as a factor controlling the levels of ac4C by promoting TAN1 pre-mRNA splicing. Here, we describe the remaining complementation groups and show that they include strains with mutations in genes for known tRNA biogenesis factors that modify (DUS2, MOD5 and TRM1), transport (LOS1), or aminoacylate (SES1) . Other strains carried mutations in genes for factors involved in rRNA/mRNA synthesis (RPA49, RRN3 and MOT1) or magnesium uptake (ALR1). We show that mutations in not only DUS2, LOS1 and SES1 but also in RPA49, RRN3 and MOT1 cause a reduction in the levels of the altered . These results indicate that Rpa49, Rrn3 and Mot1 directly or indirectly influence biogenesis.
KEYWORDS: sup61, tRNASer, tRNA maturation, tRNA modification, modified nucleosides
Introduction
Eukaryotic transfer RNA genes are transcribed by RNA polymerase III generating precursor tRNAs (pre-tRNAs) that undergo a series of processing steps to generate mature functional tRNAs [1–3]. These steps include the removal of 5´ leader and 3´ trailer sequences, posttranscriptional addition of CCA to the 3´ termini, and the removal of intervening sequences in pre-tRNAs transcribed from intron-containing genes. All pre-tRNAs also undergo post-transcriptional modification of a subset of their nucleosides [1–3]. Some modified nucleosides are found in essentially all tRNA species while others are present in a specific or a subclass of tRNAs. Many pre-tRNA processing steps occur in the nucleus, including end-maturation and numerous nucleoside modifications [1–3]. However, some nucleoside modifications only occur after nuclear export. Moreover, the subcellular location of pre-tRNA splicing varies between organisms and in Saccharomyces cerevisiae splicing occurs after the pre-tRNA has been exported to the cytoplasm.
The essential and intron-containing sup61+ (tS(CGA)C) gene encodes the only serine isoacceptor, , that efficiently decodes UCG codons in S. cerevisiae [4]. We previously identified four mutant sup61 alleles in a screen for strains that require the TRM2 gene, encoding the tRNA (m5U54) methyltransferase, for growth [5]. The sup61 trm2 double mutants are viable at 25°C but inviable or very slow growing at 30°C. The m5U54 residue is present in and the lack of Trm2 causes decreased abundance of the altered species. In addition to the requirement for TRM2, direct tests showed that the growth of the sup61 mutants is dependent on other enzymes that catalyze nucleoside modifications in [5]. These enzymes (Pus4, Trm1, and Trm3) are required for the formation of pseudouridine (Ψ) at position 55, N2,N2-dimethylguanosine () at position 26 and 2′-O-methylguanosine (Gm) at position 18 in tRNAs [6–8]. Similar to the absence of Trm2, the lack of Pus4, Trm1, or Trm3 causes a reduction in the levels of the altered . Moreover, introduction of a null allele of LHP1 into the sup61 mutants was found to inhibit growth and reduce the levels of the altered [5]. The Lhp1 protein promotes tRNA folding and protects the 3ʹ end of pre-tRNAs from exonucleolytic digestion [9,10]. Collectively, these findings suggest that the species in the sup61 mutants is sensitized to perturbations of the tRNA maturation pathway.
To identify novel factors promoting tRNA maturation, we performed a screen for mutations lethal in combination with one of the four sup61 alleles [11]. The screen identified mutants representing 12 different complementation groups and detailed analyzes of one group led to the identification of TAN1 as a gene required for the formation of N4-acetylcytidine (ac4C) in tRNA [11]. The ac4C nucleoside is present at position 12 in leucine and serine isoacceptors, and the inactivation of TAN1 causes a lack of ac4C in tRNA and a reduction in the abundance of the altered [11]. Another complementation group consisted of a strain with a mutation in the BUD13 gene [12]. BUD13 encodes a subunit of the heterotrimeric pre-mRNA retention and splicing (RES) complex, which promotes splicing and nuclear retention of a subset of intron-containing pre-mRNAs [13–18]. The requirement for Bud13 in the sup61 mutant is caused by an important role of the RES complex in splicing and nuclear retention of TAN1 pre-mRNA and consequently in the formation of ac4C [12]. In this study, we describe the remaining mutants identified in the screen and show that they do not only define strains with mutations in genes for known tRNA biogenesis factors but also those involved in RNA polymerase I and II transcription or Mg2+ uptake.
Results
Several different factors that promote modification of are required for growth of sup61-T47:2C cells
The screen for mutations lethal in combination with the sup61-T47:2C allele, encoding a species with an alteration in the variable arm (Figure 1(a)), identified mutants representing 12 complementation groups [11]. One group consisted of strains in which the mutation was genetically linked to the sup61 locus and this group was excluded from further analysis. The mutant gene in each of the 11 remaining groups (Table 1) was identified by complementing the phenotype with a yeast genomic library and subsequent confirmation that the original mutation was genetically linked to the locus for the complementing gene. As the sup61-T47:2C allele generates a requirement for several different tRNA modifying enzymes [5,11], we expected that the screen would identify factors involved in the modification of . In addition to trm1, tan1 and bud13 mutants, the screen identified strains with mutations in the DUS2 and MOD5 genes (Table 1) [11,12,19]. These genes encode the tRNA modifying enzymes that catalyze the formation of dihydrouridine (D) at position 20 and N6-isopentenyladenosine (i6A) at position 37, respectively (Figure 1(a)) [20–23]. To demonstrate unambiguously that DUS2 and MOD5 are required for growth of cells with the altered , we independently combined, in the presence of a rescuing sup61+-containing plasmid, the sup61-T47:2C mutation with dus2Δ and mod5Δ alleles. The resulting sup61-T47:2C dus2Δ and sup61-T47:2C mod5Δ strains were dependent on the plasmid for growth at both 30°C and 25°C (Figures 1(b) and S1), confirming that the alteration in causes a requirement for Dus2 and Mod5.
Figure 1.
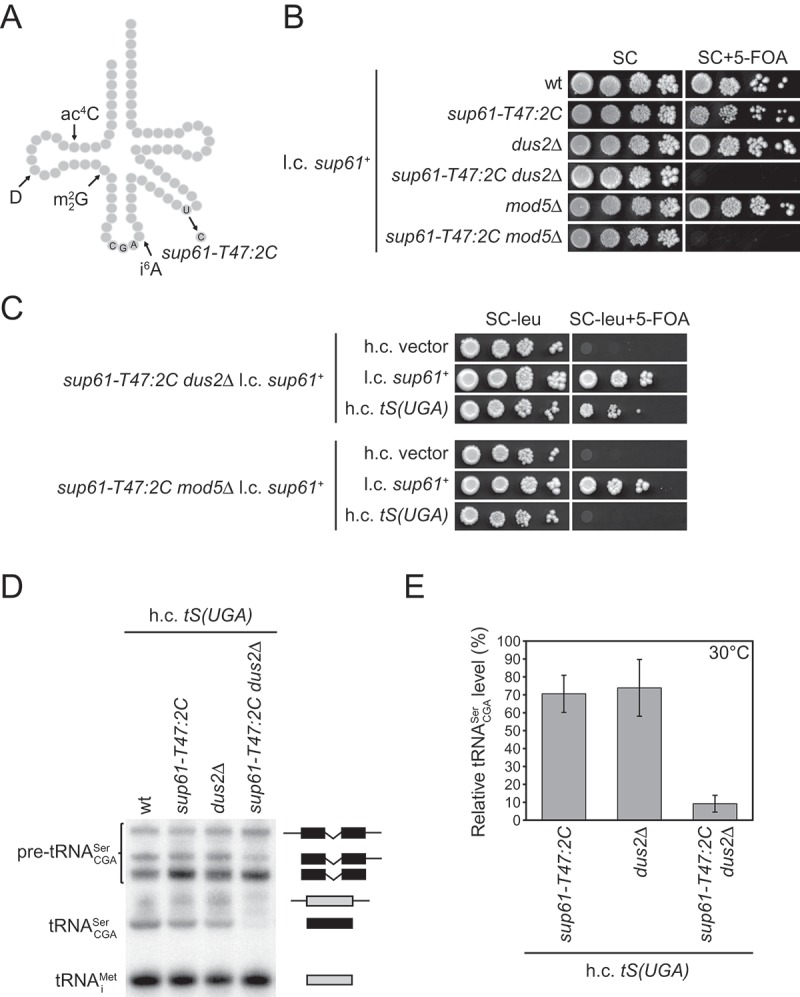
The DUS2 and MOD5 genes are required for growth of sup61-T47:2C cells. (a) Schematic secondary structure of . The alteration caused by the sup61-T47:2C allele and the positions of ac4C12, D20, 26 and i6A37 are indicated. (b) Effects of dus2Δ and mod5Δ alleles on growth of sup61-T47:2C cells. The wild-type (UMY2220), sup61-T47:2C (MJY926), dus2Δ (UMY2872), sup61-T47:2C dus2Δ (UMY4286), mod5Δ (UMY2565), and sup61-T47:2C mod5Δ (UMY4285) strains carrying the low-copy (l.c.) URA3 plasmid pRS316-sup61+ were grown over-night at 30°C in liquid synthetic complete medium (SC). The cells were serially diluted, spotted onto SC plates and SC plates containing 5-fluoroorotic acid (SC+ 5-FOA), and incubated at 30°C for 3 days. Only cells that have lost the URA3 plasmid are able to grow on 5-FOA-containing medium [64]. (c) Effects of increased tS(UGA)P dosage on growth of sup61-T47:2C dus2Δ and sup61-T47:2C mod5Δ cells. The sup61-T47:2C dus2Δ and sup61-T47:2C mod5Δ strains from (b) were transformed with the empty high copy (h.c) LEU2 plasmid pRS425 [65], the l.c. LEU2 plasmid pRS315-sup61+, and the h.c. LEU2 plasmid pRS425-tS(UGA)P. After purifications by single cell streaks on SC plates lacking uracil and leucine (SC-ura-leu), the transformants were grown over-night at 30°C in liquid SC medium lacking leucine (SC-leu). Cells were serially diluted, spotted onto SC-leu and SC-leu+ 5-FOA plates, and incubated at 30°C for 3 days. (d) Northern analysis of total RNA isolated from the wild-type, sup61-T47:2C, dus2Δ, and sup61-T47:2C dus2Δ strains carrying the h.c. LEU2 plasmid pRS425-tS(UGA)P. The cells were grown in SC-leu medium at 30°C. The blot was probed for pre-, , and using radiolabeled oligonucleotides. serves as the loading control. The position and identity of the (black) and (gray) species are indicated on the right. (e) Influence of a dus2Δ allele on the abundance of the mature . The mature signal in (d) and two additional independent experiments was normalized to the corresponding signal and the value for each strain expressed relative to that for the wild-type, which was set to 100%. The standard deviation is indicated.
Table 1.
The mutants define twelve complementation groups.
| Complementation group | Number of mutants | Gene |
|---|---|---|
| I | 3 | sup61-T47:2C |
| II | 2 | TRM1 |
| III | 3 | TAN1 |
| IV | 1 | BUD13 |
| V | 2 | DUS2 |
| VI | 2 | MOD5 |
| VII | 2 | SES1 |
| VIII | 1 | LOS1 |
| IX | 1 | MOT1 |
| X | 2 | RPA49 |
| XI | 1 | RRN3 |
| XII | 1 | ALR1 |
As the inactivation of any of several tRNA modifying factors in sup61-T47:2C cells causes a decreased abundance of the altered [5,11], it seemed likely that mutations in DUS2 or MOD5 would induce a similar defect. Even though the sup61+ gene is essential, we previously showed that the inviability of sup61Δ cells is suppressed by increased dosage of a gene (tS(UGA)P) coding for [24]. This finding suggested that the introduction of a high-copy tS(UGA)P plasmid into the double mutants may suppress the dependence on the sup61+ plasmid and allow analyzes of levels. Increased dosage of the tS(UGA)P gene counteracted the requirement for the sup61+ plasmid in the sup61-T47:2C dus2Δ strain both at 30°C and 25°C (Figures 1(c) and S1). However, the requirement for the sup61+ plasmid was not suppressed by increased tS(UGA)P dosage in sup61-T47:2C mod5Δ cells, preventing further analyzes of the strain (Figures 1(c) and S1). An i6A37 residue is also found in [25] and the inability of the high-copy tS(UGA)P plasmid to suppress the sup61-T47:2C mod5Δ strain suggests that the isopentenyl group at A37 enhances the ability of to read the near-cognate UCG codon.
To investigate the influence of Dus2 on abundance, we used northern blotting to analyze transcripts in wild-type, sup61-T47:2C, dus2Δ, and sup61-T47:2C dus2Δ strains carrying the high-copy tS(UGA)P plasmid. The blots were also probed for , which served as the loading control. The analyzes revealed a decreased abundance of mature in the dus2Δ sup61-T47:2C strain compared to the dus2Δ and sup61-T47:2C single mutants (Figure 1(d,e)). Thus, the effect of the dus2Δ allele on the abundance of the altered is similar to that observed for the inactivation of other tRNA modifying factors [5,11].
The abundance of the sup61-T47:2C-encoded is reduced in cells with mutant ses1 or los1 alleles
In addition to tRNA modification mutants, we expected that the screen would define strains with reduced function in other steps of tRNA biogenesis. Accordingly, two of the remaining complementation groups consisted of strains carrying mutations in the SES1 or LOS1 gene (Table 1). The essential SES1 gene codes for the seryl-tRNA synthetase that aminoacylates serine tRNA isoacceptors [26] whereas LOS1 encodes a nonessential factor that mediates nuclear export of pre-tRNAs [3,27–29]. One of the two isolated sup61-T47:2C ses1 double mutants (ses1-40) was able to lose the rescuing sup61+ vector at 25°C, generating a strain that is slow-growing at 25°C and inviable at 30°C (Figure 2(a)). Similarly, a sup61-T47:2C los1Δ double mutant is slow-growing at 25°C and inviable at 30°C (Figure 2(a)). Northern blot analyzes of wild-type, sup61-T47:2C, ses1-40, sup61-T47:2C ses1-40, los1Δ, and sup61-T47:2C los1Δ cells grown at 25°C revealed that the abundance of the mature is reduced in the double mutants compared to the respective single mutants (Figure 2(b,c)). Further, the ses1-40 sup61-T47:2C mutant shows reduced levels of partially processed pre- (Figure 2(b) and Table S1), indicating that the stability and/or processing of the mutant pre- is affected by the ses1-40 allele. Similarly, the accumulation of unspliced pre- in los1Δ cells [27,30] is less pronounced in the sup61-T47:2C los1Δ mutant (Figure 2(b) and Table S1).
Figure 2.
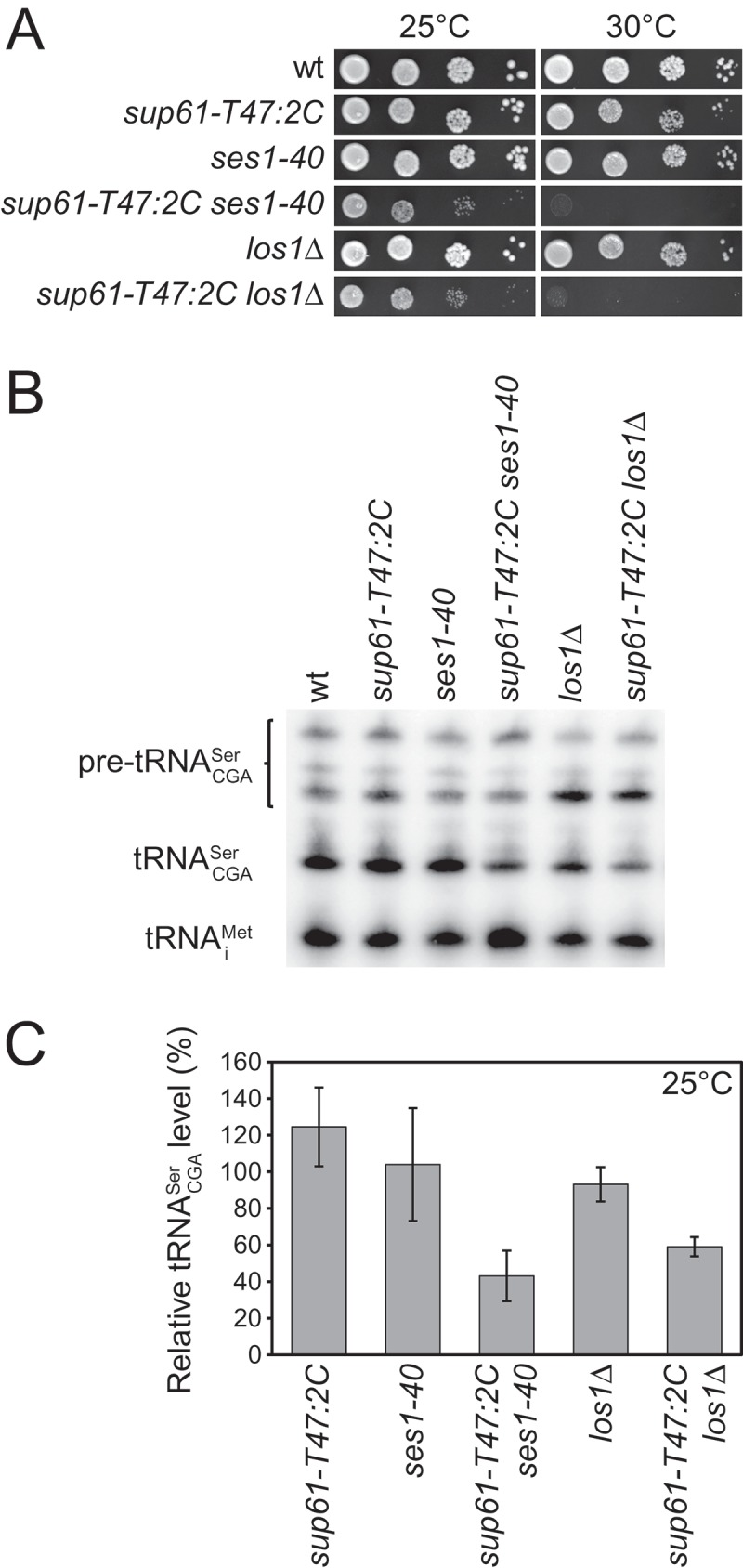
Influence of the ses1-40 and los1Δ alleles on growth and abundance. (a) Effects of the ses1-40 and los1Δ alleles on growth of sup61-T47:2C cells. The wild-type (UMY2220), sup61-T47:2C (MJY926), ses1-40 (MJY924), sup61-T47:2C ses1-40 (MJY925), los1Δ (UMY2441), and sup61-T47:2C los1Δ (UMY2861) strains were grown over-night at 25°C in liquid SC medium. The cells were serially diluted, spotted onto SC plates, and incubated at 25°C for 3 or at 30°C for 2 days. (b) Northern analysis of total RNA isolated from the strains described in (a) grown in SC medium at 25°C. The blot was probed for pre-, , and using radiolabeled oligonucleotides. serves as the loading control. (c) Influence of ses1-40 and los1Δ alleles on the levels of mature . The signal in (b) and two additional independent experiments was normalized to the corresponding signal and the value for each strain expressed relative to that for the wild-type, which was set to 100%. The standard deviation is indicated.
In addition to its essential role in aminoacylation, Ses1 was recently shown to stimulate the formation of 3-methylcytidine (m3C) at position 32 in and [31]. The methylation of C32 is catalyzed by the Trm140 protein and the modification is known to be present in three out of four tRNASer and all three tRNAThr species [32,33]. Ses1 was found to co-purify with Trm140 and its stimulatory role in the formation of m3C was restricted to the serine isoacceptors [31]. To investigate the possibility that the ses1-40 allele influences the viability of sup61-T47:2C cells by inducing a tRNA modification defect, we used HPLC to analyze the nucleoside composition of total tRNA from wild-type and ses1-40 cells grown at 30°C. We also analyzed the nucleoside composition of total tRNA from the los1Δ mutant grown at 30°C. The analyzes showed that the levels of the modified nucleosides normally found in , including m3C, are unaffected in total tRNA from the ses1-40 and los1Δ mutants (Table S2 and Figure S2). However, any effect of the ses1-40 allele on the m3C levels in the serine isoacceptors may be masked by the m3C residues in the tRNAThr species. To directly test if the absence of m3C affects growth of sup61-T47:2C cells, we combined a trm140Δ allele with the sup61-T47:2C mutation. The sup61-T47:2C trm140Δ strain was viable with only a minor growth defect compared to the sup61-T47:2C single mutant (Figure S2). This finding suggests that any effect of the ses1-40 allele on the formation of m3C in is not the cause of the growth defect of sup61-T47:2C ses1-40 cells. Collectively, our results show that Ses1 and Los1 are important to maintain the levels of the altered .
Factors involved in RNA polymerase I and II transcription influence the growth of sup61-T47:2C cells
Three complementation groups defined strains with mutations in genes, RPA49, RRN3 or MOT1, encoding factors involved in RNA polymerase I and/or II transcription (Table 1). RPA49 encodes a nonessential subunit of RNA polymerase I (Pol I) [34] whereas RRN3 codes for an essential Pol I transcription factor [35]. The MOT1 gene codes for an essential protein that has global effects on gene expression by controlling the distribution and activity of the TATA-binding protein (TBP) at promoters of RNA polymerase II (Pol II) transcribed genes [36–40]. In addition to its role in Pol II transcription, Mot1 has also been suggested to regulate Pol I transcription [41].
Cells deleted for the RPA49 gene are viable, but they show a slow-growth phenotype that is more pronounced at 25°C than at 30°C (Figure S3) [34,42]. However, the growth defect caused by one of the two rpa49 alleles (rpa49-27) identified from the screen was less severe than that induced by the deletion (Figure S3). The sup61-T47:2C rpa49-27 strain was able to lose the rescuing sup61+ plasmid at 25°C generating a strain that is viable but very slow growing at 30°C (Figure 3(a)). Similarly, the sup61-T47:2C rrn3-32 and sup61-T47:2C mot1-190 strains were able to lose the rescuing sup61+ plasmid at 25°C. The sup61-T47:2C rrn3-32 strain is viable but slow-growing at 30°C whereas the sup61-T47:2C mot1-190 strain is inviable at 30°C and very slow-growing at 25°C (Figure 3(a)).
Figure 3.
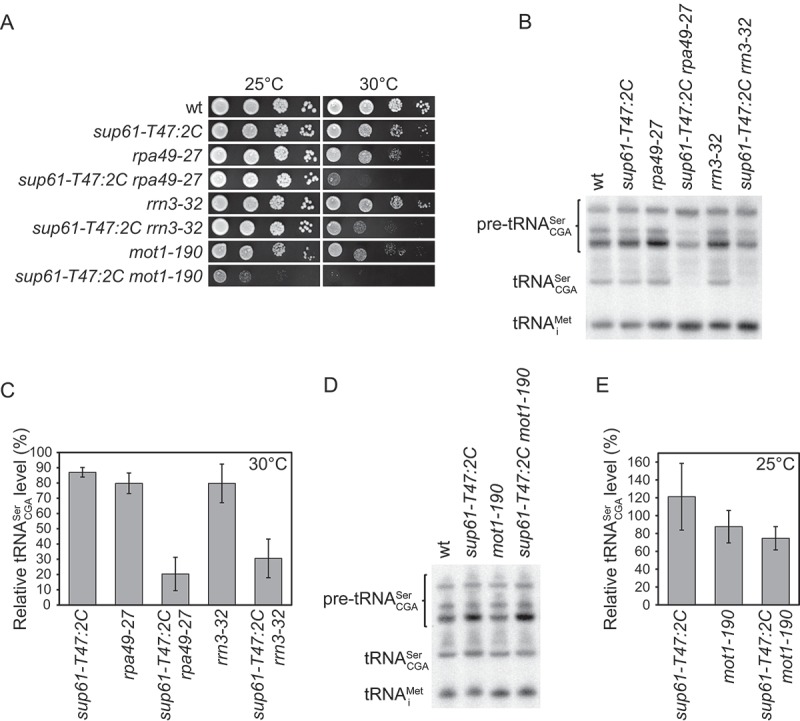
Mutations in the MOT1, RPA49 and RRN3 genes are detrimental to cells with a sup61-T47:2C allele. (a) Effects of the mot1-190, rpa49-27 and rrn3-32 alleles on growth of sup61-T47:2C cells. The wild-type (UMY2220), sup61-T47:2C (MJY926), rpa49-27 (MJY890), sup61-T47:2C rpa49-27 (MJY891), rrn3-32 (MJY894), sup61-T47:2C rrn3-32 (MJY895), mot1-190 (MJY892), and sup61-T47:2C mot1-190 (MJY893) strains were grown over-night at 25°C in liquid SC medium. Cells were serially diluted, spotted onto SC plates, and incubated at 25°C for 3 or at 30°C for 2 days. (b) Northern blot analysis of total RNA isolated from wild-type, sup61-T47:2C, rpa49-27, sup61-T47:2C rpa49-27, rrn3-32 and sup61-T47:2C rrn3-32 strains grown in SC medium at 30°C. The blot was probed for pre-, , and using radiolabeled oligonucleotides. serves as the loading control. (c) Influence of rpa49-27 and rrn3-32 alleles on the levels of mature . The signal in (b) and two additional independent experiments was normalized to the corresponding signal and the value for each strain expressed relative to that for the wild-type, which was set to 100%. The standard deviation is indicated. (d) Northern blot analysis of total RNA isolated from wild-type, sup61-T47:2C, mot1-190, and sup61-T47:2C mot1-190 strains grown in SC medium at 25°C. The blot was probed as described in (b). (e) Influence of the mot1-190 allele on abundance. The signal in (d) and three additional independent experiments was normalized to the corresponding signal and the value for each strain expressed relative to that for the wild-type, which was set to 100%. The standard deviation is indicated.
The effect of the rpa49-27, rrn3-32 and mot1-190 alleles on abundance was examined in cells grown at either 30°C (rrn3-32 and rpa49-27) or 25°C (mot1-190). These analyzes revealed that the level of the mature is reduced in the sup61-T47:2C rrn3-32 and sup61-T47:2C rpa49-27 strains compared to the respective single mutants (Figure 3(b,c)). Moreover, the sup61-T47:2C rrn3-32 and sup61-T47:2C rpa49-27 strains show reduced levels of partially processed pre- (Figure 3(b) and Table S1). Only a slight decrease in levels was observed in the sup61-T47:2C mot1-190 strain (Figure 3(d,e)), suggesting that the effect on abundance may not, at least by itself, explain the severe growth defect of the double mutant. Analyzes of the nucleoside composition of total tRNA from the rpa49-27, rrn3-32 and mot1-190 single mutants revealed no apparent tRNA modification defect (Table S2), indicating that Rpa49, Rrn3 and Mot1 influences levels by another mechanism.
Mutations in the ALR1 gene are detrimental for growth of sup61-T47:2C cells
The final complementation group consisted of a strain with a mutation in the ALR1 gene, which encodes the main Mg2+ importer of S. cerevisiae [43,44]. In addition to the importance of Mg2+ as a cofactor/counterion, it plays an important role in establishing and maintaining structures of nucleic acids, proteins and membranes. Strains with mutations in ALR1 show reduced intracellular Mg2+ levels and an alr1Δ mutant is, depending on genetic background, either inviable or very slow growing on standard media [43–45]. These phenotypes are partially suppressed by supplementation of the media with Mg2+ [43,44]. We found that supplementation of the medium with Mg2+ does not suppress the sup61+-plasmid dependence of sup61-T47:2C alr1-11 cells at either 30°C or 25°C (Figures 4(a, b) and S4). Moreover, analyzes of the effects of the alr1 allele on levels was prevented by the finding that increased expression does not suppress the plasmid dependence of the sup61-T47:2C alr1-11 strain even on Mg2+-supplemented medium (Figures 4(c) and S4). As no tRNA modification defect is apparent in alr1-11 cells (Table S2), the cause of the synergistic interaction remains unclear. Nevertheless, our findings suggest that cells with the altered are sensitive to defects in Mg2+ uptake.
Figure 4.
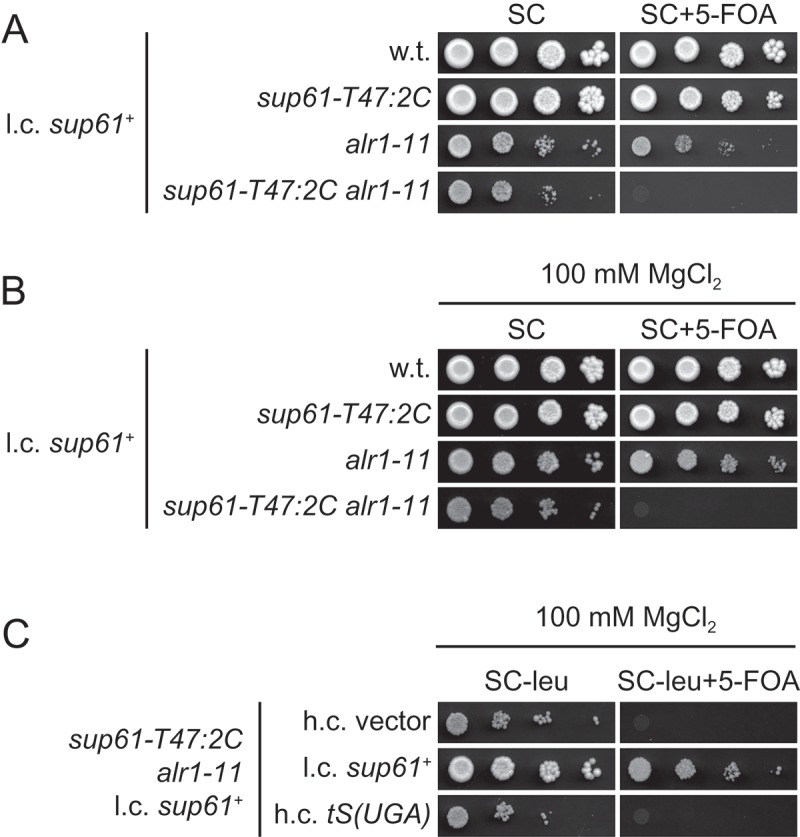
Genetic interactions between the alr1-11 and sup61-T47:2C alleles. (a and b) Growth phenotypes of strains with an alr1-11 and/or sup61-T47:2C allele. The wild-type (UMY2220), sup61-T47:2C (MJY926), alr1-11 (UMY3002), sup61-T47:2C alr1-11 (UMY2967) strains carrying the l.c. URA3 plasmid pMJ1421 (sup61+) were grown overnight at 30°C in liquid SC medium (A) or in liquid SC medium supplemented with 100 mM MgCl2 (b). Cells were serially diluted, spotted onto SC and SC+ 5-FOA (A) or SC and SC+ 5-FOA plates containing 100 mM MgCl2 (b), and incubated at 30°C for 3 days. (c) Effect of increased tS(UGA)P dosage on growth of sup61-T47:2C alr1-11 cells. The sup61-T47:2C alr1-11 strain described in (a) was transformed with the indicated h.c. or l.c. LEU2 plasmids. Transformants were grown at 30°C in liquid SC-leu medium supplemented with 100 mM MgCl2. Cells were serially diluted, spotted onto SC-leu and SC-leu+ 5-FOA plates containing 100 mM MgCl2, and incubated at 30°C for 3 days.
Discussion
We previously showed that the viability of sup61-T47:2C cells is dependent on several different factors promoting maturation of [5,11,12]. In this study, we describe additional factors required for growth of strains with the altered form of . We show that mutations in genes required for modification (DUS2 and MOD5), transport (LOS1), and aminoacylation (SES1) of are deleterious to cells with the sup61-T47:2C allele. Similar to the inactivation of other factors influencing tRNA biogenesis, the dus2, los1, and ses1 alleles cause a reduced abundance of the altered . These findings provide further support for the notion that sup61-T47:2C cells are sensitized for perturbations in tRNA biogenesis.
In addition to mutations in genes for known tRNA biogenesis factors, we found that mutations in the MOT1, RPA49, or RRN3 gene inhibit growth of sup61-T47:2C cells and cause a reduction in the levels of the altered . The Mot1 protein is thought to influence both Pol I and Pol II transcription [36–41,46] whereas the function of Rpa49 and Rrn3 appears to be restricted to Pol I transcription [34,35,42]. The mechanisms by which Mot1, Rpa49 and Rrn3 influence tRNA biogenesis is unclear, but it is possible that they indirectly influence the process through their involvement in Pol I transcription. Transcription of tRNA genes as well as 5´-end processing of pre-tRNAs has been reported to localize to the nucleolus [47–49]. Moreover, dissociation of pre-tRNA from the nucleolus and defects in 5´ end processing have been observed in cells defective in Pol I transcription or rRNA processing [50,51]. As mutations in MOT1, RPA49, and RRN3 affect nucleolar morphology [52,53], the mot1-190, rpa49-27, and rrn3-32 alleles may influence biogenesis by disrupting the balance and/or order of pre-tRNA processing. The rpa49-27 and rrn3-32 alleles induce reduced levels of not only the mature but also the partially processed forms of the altered (Figure 3(b) and Table S1), indicating that pre-tRNA processing and/or stability is affected in the mutants. The abundance of the primary unprocessed pre- is, however, largely unaffected by the mot1-190, rpa49-27 and rrn3-32 alleles (Figure 3(b,d) and Table S1), suggesting that the mutations do not cause a defect in 5´-end processing.
The finding that a mutation in the ALR1 gene is lethal in combination with the sup61-T47:2C allele indicates that a reduction in intracellular Mg2+ levels may cause inefficient folding and/or decreased stability of the altered . Alternatively, the genetic interaction could be a consequence of the effect of reduced Mg2+ on translational fidelity [54–56], i.e. decreased intracellular Mg2+ levels may reduce the ability of the altered to read the UCG codon. It should, however, be noted that Mg2+ is important for a vast number of cellular processes and it cannot be excluded that the genetic interaction is caused by a more indirect mechanism.
Cells with mutations in the single-copy and essential sup61+ are sensitized for defects in tRNA biogenesis. The screen for mutations lethal when combined with a sup61-T47:2C allele is an unbiased genetic approach to identify factors promoting tRNA biogenesis. As our screen was far from being saturated, additional factors would likely be identified in an expanded screen.
Materials and methods
Yeast strains, media, and genetic procedures
Strains used in this study are listed in Table S3. Yeast media were prepared essentially as described [57] using a slightly different composition of the drop-out mix [58]. Difco yeast nitrogen base without amino acids was purchased from Becton Dickinson (291940).
The screen for mutations lethal in combination with the sup61-T47:2C mutation and the subsequent identification of plasmids that complement the phenotype has been described [11]. To investigate linkage between the complementing gene and the original mutation, we cloned the gene or a DNA fragment close to the gene into an integrative vector and targeted the plasmid marker (TRP1 or URA3) to the corresponding locus in strain UMY2256. This strain was crossed to the relevant mutant(s), carrying pMJ1421 (URA3, ADE3, sup61+) or pMJ1422 (TRP1, ADE3, sup61+) [11], and tetrad analysis revealed that the ability to lose the sup61+ plasmid always co-segregated with the integrated TRP1/URA3 marker, i.e., the mutation was in all cases linked to the locus for the complementing gene.
One copy of the DUS2, MOD5, RPA49, and TRM140 genes was independently deleted in the diploid strain UMY2366 by a PCR-mediated strategy [59,60]. The generated heterozygous diploids were allowed to sporulate and the dus2Δ (UMY2872), mod5Δ (UMY2565), rpa49Δ (MJY1111) and trm140Δ (MJY1110) strains obtained from tetrads. The dus2Δ and mod5Δ strains were crossed to a sup61-T47:2C strain, carrying a sup61+ gene on a plasmid, and rescued double mutants obtained from tetrads. The sup61-T47:2C trm140Δ double mutant was obtained from a cross between UMY2256 and MJY1110.
The sup61-T47:2C alr1-11, sup61-T47:2C ses1-40, sup61-T47:2C rrn3-32, sup61-T47:2C mot1-190 and sup61-T47:2C rpa49-27 mutants identified in the screen, all carrying a rescuing sup61+-containing plasmid, were backcrossed at least three times to UMY2256. The backcrossed strains were crossed to UMY2219 and alr1-11, ses1-40, rrn3-32, mot1-190, and rpa49-27 single mutants obtained from tetrads.
Plasmid constructions
A low copy LEU2 plasmid harboring the sup61+ gene (pRS315-sup61+) was constructed by cloning a BamHI/HindIII DNA fragment from pRS316-sup61+ [5] into the corresponding sites of pRS315 [61]. Plasmids pMJ1421, pMJ1422, and pRS425-tS(UGA)P have been described [5,24].
RNA methods
Total RNA for northern blotting was isolated [58] from exponentially growing cells at an optical density at 600 nm (OD600) of ≈ 0.5. An aliquot of slow-growing double mutants was, during harvesting, streaked on a SC plate and incubated at the appropriate temperature. Only cell pellets from cultures that contained no or negligible amounts of faster growing revertants were processed further. The procedures for northern blotting have been described [5,11]. The oligonucleotides used to detect precursor and mature were 5´- AGCCGAACTTTTTATTCCATTCG-3´ and 5´-GCCCAAGAGATTTCGAGTCTCT-3´, respectively. To detect , the oligonucleotide 5´-GGACATCAGGGTTATGAGCC-3´ was used.
For HPLC analyzes of modified nucleosides, the strains were grown in SC medium at 30°C to OD600 ≈ 0.8. Cells were harvested and total tRNA isolated as previously described [62]. Typically, 50µg of total tRNA was digested to nucleosides [63] using nuclease P1 (Sigma-Aldrich, N8630) and bacterial alkaline phosphatase (Sigma-Aldrich, P4252). The hydrolysate was analyzed by HPLC [63] using a Develosil C30 reversed phase column (Phenomenex, CH0-5690) and buffer A (0.01M NH4H2PO4, 2.5% methanol (v/v) at pH 5.3), buffer B (0.01M NH4H2PO4, 20% methanol (v/v) at pH 5.1), and buffer C (0.01M NH4H2PO4, 35% acetonitrile (v/v)) as the elution buffers. To be able to separate m3C from U, the methanol concentration in buffer A was changed to 6% (v/v).
Funding Statement
This work was supported by Magnus Bergvalls Foundation (2017-02098 to MJ); Åke Wibergs Foundation (M14-0207 to MJ); Insamlingsstiftelsen Umeå universitet (FS 2.1.6-1888-15 to MJ); Swedish Research Council (621-2016-03949 to AB); and Karin and Harald Silvanders Foundation/Insamlingsstiftelsen Umeå universitet (FS 2.1.6-1870-16 to AB).
Acknowledgments
We are grateful to Anders Esberg for providing the los1Δ strain.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplementary data for this article can be accessed here.
References
- [1].Phizicky EM, Hopper AK.. tRNA biology charges to the front. Genes Dev. 2010;24(17):1832–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hopper AK. Transfer RNA post-transcriptional processing, turnover, and subcellular dynamics in the yeast Saccharomyces cerevisiae. Genetics. 2013;194(1):43–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chatterjee K, Nostramo RT, Wan Y, et al. tRNA dynamics between the nucleus, cytoplasm and mitochondrial surface: location, location, location. Biochim Biophys Acta. 2018;1861(4):373–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Etcheverry T, Salvato M, Guthrie C. Recessive lethality of yeast strains carrying the SUP61 suppressor results from loss of a transfer RNA with a unique decoding function. J Mol Biol. 1982;158(4):599–618. [DOI] [PubMed] [Google Scholar]
- [5].Johansson MJO, Byström AS. Dual function of the tRNA(m(5)U54)methyltransferase in tRNA maturation. RNA. 2002;8(3):324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Becker HF, Motorin Y, Planta RJ, et al. The yeast gene YNL292w encodes a pseudouridine synthase (Pus4) catalyzing the formation of psi55 in both mitochondrial and cytoplasmic tRNAs. Nucleic Acids Res. 1997;25(22):4493–4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ellis SR, Morales MJ, Li JM, et al. Isolation and characterization of the TRM1 locus, a gene essential for the N2,N2-dimethylguanosine modification of both mitochondrial and cytoplasmic tRNA in Saccharomyces cerevisiae. J Biol Chem. 1986;261(21):9703–9709. [PubMed] [Google Scholar]
- [8].Cavaille J, Chetouani F, Bachellerie JP. The yeast Saccharomyces cerevisiae YDL112w ORF encodes the putative 2ʹ- O-ribose methyltransferase catalyzing the formation of Gm18 in tRNAs. RNA. 1999;5(1):66–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yoo CJ, Wolin SL. The yeast la protein is required for the 3ʹ endonucleolytic cleavage that matures tRNA precursors. Cell. 1997;89(3):393–402. [DOI] [PubMed] [Google Scholar]
- [10].Chakshusmathi G, Kim SD, Rubinson DA, et al. A La protein requirement for efficient pre-tRNA folding. Embo J. 2003;22(24):6562–6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Johansson MJO, Byström AS. The Saccharomyces cerevisiae TAN1 gene is required for N4-acetylcytidine formation in tRNA. RNA. 2004;10(4):712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhou Y, Chen C, Johansson MJO. The pre-mRNA retention and splicing complex controls tRNA maturation by promoting TAN1 expression. Nucleic Acids Res. 2013;41(11):5669–5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dziembowski A, Ventura AP, Rutz B, et al. Proteomic analysis identifies a new complex required for nuclear pre-mRNA retention and splicing. Embo J. 2004;23(24):4847–4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhou Y, Johansson MJO. The pre-mRNA retention and splicing complex controls expression of the Mediator subunit Med20. RNA Biol. 2017;14(10):1411–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tuo S, Nakashima K, Pringle JR. Apparent defect in yeast bud-site selection due to a specific failure to splice the pre-mRNA of a regulator of cell-type-specific transcription. PLoS One. 2012;7(10):e47621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Spingola M, Armisen J, Ares M Jr.. Mer1p is a modular splicing factor whose function depends on the conserved U2 snRNP protein Snu17p. Nucleic Acids Res. 2004;32(3):1242–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schmidlin T, Kaeberlein M, Kudlow BA, et al. Single-gene deletions that restore mating competence to diploid yeast. FEMS Yeast Res. 2008;8(2):276–286. [DOI] [PubMed] [Google Scholar]
- [18].Scherrer FW Jr., Spingola M. A subset of Mer1p-dependent introns requires Bud13p for splicing activation and nuclear retention. RNA. 2006;12(7):1361–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Johansson MJO, Byström AS. Transfer RNA modifications and modifying enzymes in Saccharomyces cerevisiae In: editor, Grosjean H. Fine-tuning of RNA functions by modification and editing. Topics in Curr Genetics. Vol. 12 Berlin (Heidelberg): Springer-Verlag; 2005. p. 87–120. [Google Scholar]
- [20].Xing F, Martzen MR, Phizicky EM. A conserved family of Saccharomyces cerevisiae synthases effects dihydrouridine modification of tRNA. RNA. 2002;8(3):370–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Xing F, Hiley SL, Hughes TR, et al. The specificities of four yeast dihydrouridine synthases for cytoplasmic tRNAs. J Biol Chem. 2004;279(17):17850–17860. [DOI] [PubMed] [Google Scholar]
- [22].Dihanich ME, Najarian D, Clark R, et al. Isolation and characterization of MOD5, a gene required for isopentenylation of cytoplasmic and mitochondrial tRNAs of Saccharomyces cerevisiae. Mol Cell Biol. 1987;7(1):177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Laten H, Gorman J, Bock RM. Isopentenyladenosine deficient tRNA from an antisuppressor mutant of Saccharomyces cerevisiae. Nucleic Acids Res. 1978;5(11):4329–4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Johansson MJO, Esberg A, Huang B, et al. Eukaryotic wobble uridine modifications promote a functionally redundant decoding system. Mol Cell Biol. 2008;28(10):3301–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Piper PW. A correlation between a recessive lethal amber suppressor mutation in Saccharomyces cerevisiae and an anticodon change in a minor serine transfer RNA. J Mol Biol. 1978;122(2):217–235. [DOI] [PubMed] [Google Scholar]
- [26].Weygand-Durasevic I, Johnson-Burke D, Soll D. Cloning and characterization of the gene coding for cytoplasmic seryl- tRNA synthetase from Saccharomyces cerevisiae. Nucleic Acids Res. 1987;15(5):1887–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hopper AK, Schultz LD, Shapiro RA. Processing of intervening sequences: a new yeast mutant which fails to excise intervening sequences from precursor tRNAs. Cell. 1980;19(3):741–751. [DOI] [PubMed] [Google Scholar]
- [28].Hellmuth K, Lau DM, Bischoff FR, et al. Yeast Los1p has properties of an exportin-like nucleocytoplasmic transport factor for tRNA. Mol Cell Biol. 1998;18(11):6374–6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sarkar S, Hopper AK. tRNA nuclear export in Saccharomyces cerevisiae: in situ hybridization analysis. Mol Biol Cell. 1998;9(11):3041–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hurt DJ, Wang SS, Lin YH, et al. Cloning and characterization of LOS1, a Saccharomyces cerevisiae gene that affects tRNA splicing. Mol Cell Biol. 1987;7(3):1208–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Han L, Marcus E, D’Silva S, et al. S. cerevisiae Trm140 has two recognition modes for 3-methylcytidine modification of the anticodon loop of tRNA substrates. RNA. 2017;23(3):406–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].D’Silva S, Haider SJ, Phizicky EM. A domain of the actin binding protein Abp140 is the yeast methyltransferase responsible for 3-methylcytidine modification in the tRNA anti-codon loop. RNA. 2011;17(6):1100–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Noma A, Yi S, Katoh T, et al. Actin-binding protein ABP140 is a methyltransferase for 3-methylcytidine at position 32 of tRNAs in Saccharomyces cerevisiae. RNA. 2011;17(6):1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Liljelund P, Mariotte S, Buhler JM, et al. Characterization and mutagenesis of the gene encoding the A49 subunit of RNA polymerase A in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1992;89(19):9302–9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yamamoto RT, Nogi Y, Dodd JA, et al. RRN3 gene of Saccharomyces cerevisiae encodes an essential RNA polymerase I transcription factor which interacts with the polymerase independently of DNA template. Embo J. 1996;15(15):3964–3973. [PMC free article] [PubMed] [Google Scholar]
- [36].Davis JL, Kunisawa R, Thorner J, et al. (Mot1 gene-product) Affects gene-expression and is required for viability in the yeast saccharomyces-cerevisiae. Mol Cell Biol. 1992;12(4):1879–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Auble DT, Hansen KE, Mueller CG, et al. Mot1, a global repressor of RNA polymerase II transcription, inhibits TBP binding to DNA by an ATP-dependent mechanism. Genes Dev. 1994;8(16):1920–1934. [DOI] [PubMed] [Google Scholar]
- [38].Geisberg JV, Moqtaderi Z, Kuras L, et al. Mot1 associates with transcriptionally active promoters and inhibits association of NC2 in Saccharomyces cerevisiae. Mol Cell Biol. 2002;22(23):8122–8134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Andrau JC, Van Oevelen CJC, Van Teeffelen HAAM, et al. Mot1p is essential for TBP recruitment to selected promoters during in vivo gene activation. Embo J. 2002;21(19):5173–5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Viswanathan R, Auble DT. One small step for Mot1; one giant leap for other Swi2/Snf2 enzymes? Biochim Biophys Acta. 2011;1809(9):488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dasgupta A, Sprouse RO, French S, et al. Regulation of rRNA synthesis by TATA-binding protein-associated factor Mot1. Mol Cell Biol. 2007;27(8):2886–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gadal O, Mariotte-Labarre S, Chedin S, et al. A34.5, a nonessential component of yeast RNA polymerase I, cooperates with subunit A14 and DNA topoisomerase I to produce a functional rRNA synthesis machine. Mol Cell Biol. 1997;17(4):1787–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Graschopf A, Stadler JA, Hoellerer MK, et al. The yeast plasma membrane protein Alr1 controls Mg2+ homeostasis and is subject to Mg2+-dependent control of its synthesis and degradation. J Biol Chem. 2001;276(19):16216–16222. [DOI] [PubMed] [Google Scholar]
- [44].MacDiarmid CW, Gardner RC. Overexpression of the Saccharomyces cerevisiae magnesium transport system confers resistance to aluminum ion. J Biol Chem. 1998;273(3):1727–1732. [DOI] [PubMed] [Google Scholar]
- [45].Tran HG, Steger DJ, Iyer VR, et al. The chromo domain protein chd1p from budding yeast is an ATP-dependent chromatin-modifying factor. Embo J. 2000;19(10):2323–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sikorski TW, Buratowski S. The basal initiation machinery: beyond the general transcription factors. Curr Opin Cell Biol. 2009;21(3):344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Thompson M, Haeusler RA, Good PD, et al. Nucleolar clustering of dispersed tRNA genes. Science. 2003;302(5649):1399–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Duan Z, Andronescu M, Schutz K, et al. A three-dimensional model of the yeast genome. Nature. 2010;465(7296):363–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bertrand E, Houser-Scott F, Kendall A, et al. Nucleolar localization of early tRNA processing. Genes Dev. 1998;12(16):2463–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Briand JF, Navarro F, Gadal O, et al. Cross Talk between tRNA and rRNA synthesis in Saccharomyces cerevisiae. Mol Cell Biol. 2001;21(1):189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kendall A, Hull MW, Bertrand E, et al. A CBF5 mutation that disrupts nucleolar localization of early tRNA biosynthesis in yeast also suppresses tRNA gene-mediated transcriptional silencing. Proc Natl Acad Sci USA. 2000;97(24):13108–13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Albert B, Leger-Silvestre I, Normand C, et al. RNA polymerase I-specific subunits promote polymerase clustering to enhance the rRNA gene transcription cycle. J Cell Biol. 2011;192(2):277–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Neumuller RA, Gross T, Samsonova AA, et al. Conserved regulators of nucleolar size revealed by global phenotypic analyses. Sci Signal. 2013;6(289):ra70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Szer W, Ochoa S. Complexing ability and coding properties of synthetic polynucleotides. J Mol Biol. 1964;8:823–834. [DOI] [PubMed] [Google Scholar]
- [55].Schlanger G, Friedman SM. Ambiguity in a polypeptide-synthesizing extract from Saccharomyces cerevisiae. J Bacteriol. 1973;115(1):129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Johansson MJO, Jacobson A. Nonsense-mediated mRNA decay maintains translational fidelity by limiting magnesium uptake. Genes Dev. 2010;24(14):1491–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Amberg DC, Burke DJ, Strathern JN. Methods in yeast genetics. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2005. [Google Scholar]
- [58].Johansson MJO. Determining if an mRNA is a substrate of nonsense-mediated mRNA decay in Saccharomyces cerevisiae In: Wajapeyee N, Gupta R, editors. Eukaryotic transcriptional and post-transcriptional gene expression regulation. Methods Mol Biol. Vol. 1507 New York (NY): Humana Press; 2017. p. 169–177. [DOI] [PubMed] [Google Scholar]
- [59].Brachmann CB, Davies A, Cost GJ, et al. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast. 1998;14(2):115–132. [DOI] [PubMed] [Google Scholar]
- [60].Longtine MS, McKenzie A 3rd, Demarini DJ, et al. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14(10):953–961. [DOI] [PubMed] [Google Scholar]
- [61].Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122(1):19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Chen C, Huang B, Eliasson M, et al. Elongator complex influences telomeric gene silencing and DNA damage response by its role in wobble uridine tRNA modification. PLoS Genet. 2011;7(9):e1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Gehrke CW, Kuo KC. Ribonucleoside analysis by reversed-phase high performance liquid chromatography In: Gehrke CW, Kuo KC, editors. Journal of chromatography library. Vol. Chromatography and modification of nucleosides analytical methods for major and modified nucleosides: HPLC, GC, MS, NMR, UV and FT-IR. Amsterdam: Elsevier; 1990. p. A3–A71. [Google Scholar]
- [64].Boeke JD, LaCroute F, Fink GR. A positive selection for mutants lacking orotidine-5ʹ-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197(2):345–346. [DOI] [PubMed] [Google Scholar]
- [65].Christianson TW, Sikorski RS, Dante M, et al. Multifunctional yeast high-copy-number shuttle vectors. Gene. 1992;110(1):119–122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


