ABSTRACT
Few treatment options are available for acute myeloid leukemia (AML) patients. DCLL9718A is an antibody-drug conjugate that targets C-type lectin-like molecule-1 (CLL-1). This receptor is prevalent on monocytes, neutrophils, and AML blast cells, and unlike CD33, is not expressed on hematopoietic stem cells, thus providing possible hematopoietic recovery. DCLL9718A comprises an anti-CLL-1 IgG1 antibody (MCLL0517A) linked to a pyrrolobenzodiazepine (PBD) dimer payload, via a cleavable disulfide-labile linker. Here, we characterize the in vitro and in vivo stability, the pharmacokinetics (PK) and pharmacodynamics (PD) of DCLL9718A and MCLL0517A in rodents and cynomolgus monkeys. Three key PK analytes were measured in these studies: total antibody, antibody-conjugated PBD dimer and unconjugated PBD dimer. In vitro, DCLL9718A, was stable with most (> 80%) of the PBD dimer payload remaining conjugated to the antibody over 96 hours. This was recapitulated in vivo with antibody-conjugated PBD dimer clearance estimates similar to DCLL9718A total antibody clearance. Both DCLL9718A and MCLL0517A showed linear PK in the non-binding rodent species, and non-linear PK in cynomolgus monkeys, a binding species. The PK data indicated minimal impact of conjugation on the disposition of DCLL9718A total antibody. Finally, in cynomolgus monkey, MCLL0517A showed target engagement at all doses tested (0.5 and 20 mg/kg) as measured by receptor occupancy, and DCLL9718A (at doses of 0.05, 0.1 and 0.2 mg/kg) showed strong PD activity as evidenced by notable reduction in monocytes and neutrophils.
Keywords: Antibody-drug conjugate, CLL-1, pharmacokinetics, acute myeloid leukemia, receptor occupancy, PBD dimer
Introduction
Acute myeloid leukemia (AML) continues to be a significant unmet medical need, with greater than 20,000 new cases diagnosed in 2017. 1 Over the past few decades, increased knowledge around AML biology has led to the development of new targeted agents (e.g., mutated FLT3, IDH inhibitors).2 However, these advances have been limited to subpopulations, with the majority of AML patients relying on modifications to doses and schedules of the standard of care cytarabine and anthracycline chemotherapy induction regimens (7 + 3) or improvements in hematopoietic stem cell transplantation methodologies.3 AML has been a target for the therapeutic use of monoclonal antibodies or antibody-drug conjugates (ADCs), partly due the accessibility of the malignant cells and expression of well-defined cell surface antigens. To date, most development efforts with ADCs for AML have focused on targeting CD33, a transmembrane receptor expressed on cells of myeloid lineage, as exemplified by the approval of anti-CD33 Mylotarg® (gemtuzumab ozogamicin) in 2000, which was the first anti-cancer ADC on the market.4 However, as the development of ADC technology continues to mature, development of different and more potent drugs and alternate linker technologies offer potential new treatment modalities.5,6 Among these are AVE9633, an anti-CD33-maytansinoid that demonstrated an acceptable safety profile, but lower than expected efficacy likely due to the DM4 payload, a tubulin inhibitor, which is unlikely to be effective in AML.7 Other ADC modalities for AML include DNA alkylating agents, such as IMGN779, an anti-CD33 ADC with a novel indolinobenzodiazepine conjugated through lysines at 3 dimers per IgG.5 Another novel ADC using a DNA alkylating agent is vadastuximab talirine (SGN-CD33A), an anti-CD33 antibody with engineered cysteines, conjugated to a highly potent pyrrolobenzodiazepine (PBD) dimer via a protease-cleavable linker. While early clinical trials found SGN-CD33A was efficacious, slow recovery of neutrophils and platelets resulted in an unacceptable safety profile, resulting in cessation of clinical trials with SGN-CD33A.8 While these data suggest that DNA damaging agents may be a promising approach for the treatment of AML, targeting CD33, albeit a well-validated target, may have liabilities. Notably, CD33 is also expressed on hematopoietic stem cells (HSCs); therefore, a CD33-targeted ADC with a DNA alkylator, whose activity affects both cycling and non-cycling cells, could potentially reduce bone marrow recovery in AML patients.9,10 A new potential alternative ADC target for the treatment of AML is C-type lectin-like molecule-1 (CLL-1). CLL-1 presents itself as an ideal target for AML given its expression on myeloid cells and the overexpression in most AML patients, while having no expression in HSCs.11-13
DCLL9718A is an ADC being explored for the treatment of AML. It is composed of a humanized anti-CLL-1 antibody (hu6E7.N54A or MCLL0517A) linked to a PBD dimer payload via a cleavable disulfide-labile linker. MCLL0517A is designed to bind with low nanomolar affinity to human and cynomolgus monkey CLL-1.11 Herein, we investigated the pharmacokinetics (PK) and pharmacodynamics (PD) of DCLL9718A in cynomolgus monkeys (binding species), and the PK in mouse and rat (non-binding species). Similar to previously described bioanalytical strategies for ADCs,14 the characterization of DCLL9718A PK included the quantification of three key analytes: DCLL9718A total antibody (measurement of all drug-antibody ratios of the ADC, including fully conjugated, partially deconjugated, and fully deconjugated antibodies), antibody-conjugated PBD dimer (acPBD dimer, measurement of PBD dimer conjugated to the antibody), and unconjugated PDB dimer (measurement of PBD dimer that is not conjugated to the antibody through the linker). In addition, we also characterized the PK of the unconjugated antibody, MCLL0517A, in cynomolgus monkey and mouse to evaluate the effect of conjugation on the PK of DCLL9718A. Furthermore, we characterized the in vitro stability in mouse, rat, cynomolgus monkey, and human plasma, as well as the in vivo stability of DCLL9718A in mouse, rat, and cynomolgus monkey.
Results
Plasma stability of DCLL9718A (ADC) across species
In order to characterize the stability of the ADC, DCLL9718A was incubated in human, cynomolgus monkey, rat, and mouse plasma, and buffer control over 96 hours at 37 ºC. The disulfide linker of DCLL9718A demonstrated good stability in all species, with only a slight decrease in acPBD dimer concentrations over time. The majority of PBD dimer (greater than 80%) remained conjugated to the antibody at 96 hours in all species (Figure 1A). As expected, there was no change in MCLL0517A or DCLL9718A total antibody concentrations across all matrices over 96 hours (Supplemental Figure 1). Overall, the plasma stability profiles of DCLL9718A were comparable across the species tested in. this system. The levels of unconjugated PBD dimer increased over time in all matrices, and by the end of the incubation (96 hours), the concentrations in plasma samples were slightly higher (2 – 3 ng/mL) compared to buffer control (approximately 1 ng/mL) (Figure 1B).
Figure 1.
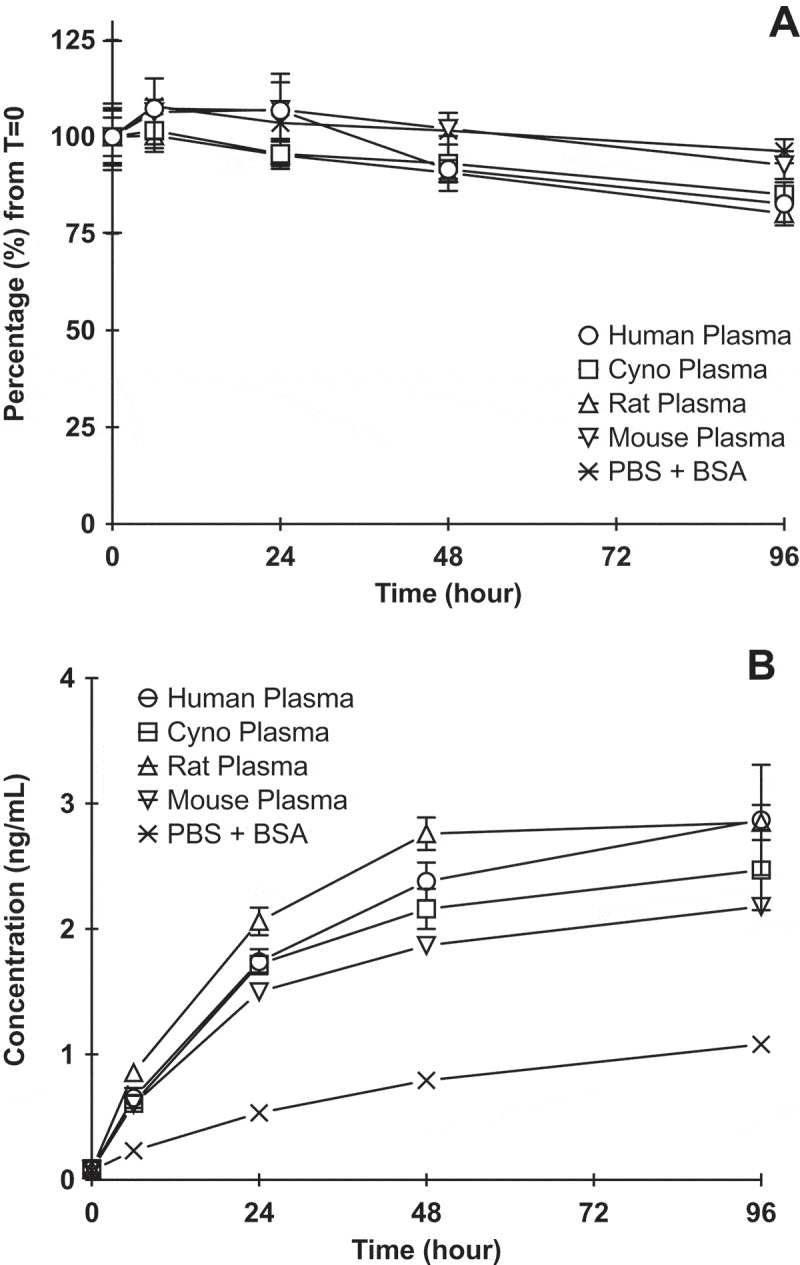
DCLL9718A (ADC) stability in human, cynomolgus monkey, rat, mouse plasma and PBS + 0.5% BSA (control) following incubation of 50 ug/mL DCLL9718A at 37 ºC for 96 hours. (A) acPBD dimer profiles normalized (%) to time zero concentrations, (B) Unconjugated drug release profiles (ng/mL) .
Pharmacokinetics of MCLL0517A (naked antibody) in mouse and cynomolgus monkey
To characterize the overall PK behavior of the antibody component (MCLL0517A) of DCLL9718A, MCLL0517A was examined in both C.B.17 SCID mice (non-binding species) and cynomolgus monkey (binding species). The plasma concentration-time profiles of MCLL0517A following a single IV bolus dose in C.B.17 SCID mice at 5 mg/kg, and in cynomolgus monkeys at doses of 0.5 and 20 mg/kg, as well anti-gD antibody (non-binding control antibody) at 20 mg/kg are shown in Figure 2A and 2B, respectively. The corresponding PK parameters are summarized in Table 1.
Table 1.
Mean (± SD) MCLL0517A NCA PK Parameters.
| Mouse a |
Cynomolgus Monkey |
|||
|---|---|---|---|---|
| PK Parameter | MCLL0517A (5 mg/kg) |
MCLL0517A (0.5 mg/kg, N = 3) |
MCLL0517A (20 mg/kg, N = 2) |
Anti-gD Antibody (20 mg/kg, N = 2) |
| Cmax (µg/mL) | 77.4 ± 5.27 | 10.9 ± 0.520 | 527 | 461 |
| AUC0-inf (day • µg/mL) | 1140 | 24.0 ± 5.03 | 5080 | 5130 |
| CLtotal (mL/day/kg) | 3.75 | 21.5 ± 5.01 | 3.95 | 3.91 |
| Vss (mL/kg) | 128 | 51.4 ± 4.68 | 64.2 | 89.0 |
| T1/2, λz (day) | 24.0 | 1.79 ± 0.448 | 10.4 | 17.3 |
a Represents composite value from naïve pooling of animals. Reported parameter variability in Cmax represents standard error of mean (SEM) and is result of naïve pool approach with NCA.
In the mouse, MCLL0517A plasma concentration-time profile exhibited bi-exponential disposition with a total clearance estimate of 3.75 mL/day/kg and a half-life of 24 days (Table 1). The slow total clearance and long half-life in the mouse are consistent with the lack of binding of MCLL0517A to the murine homologue of the human CLL-1 target.
In the cynomolgus monkey, following a single IV bolus dose of 0.5 or 20 mg/kg, AUC0-inf increased more than dose proportionally. The mean CL estimates in groups administered 0.5 or 20 mg/kg of MCLL0517A were 21.5 and 3.95 mL/day/kg, respectively. The total clearance of MCLL0517A at the high dose (20 mg/kg) was similar to that of non-binding anti-gD antibody (3.91 mL/day/kg) and is consistent with the expected clearance range for a human IgG1 antibody in cynomolgus monkeys.15 These data suggest that the target was saturated at the dose level of 20 mg/kg in cynomolgus monkeys for the duration of the study.
In the cynomolgus monkey, anti-drug antibodies (ADA) were detected in all of the animals administered MCLL0517A, while only one animal given anti-gD antibody had positive post-baseline signals. While all animals that received MCLL0517A had positive post-baseline ADA signals, only 1 animal showed an apparent impact on the PK due to the presence of ADAs (20 mg/kg dose level). Similarly, the one animal given anti-gD antibody with positive post-baseline signal had an apparent impact on the PK due to the presence of ADAs (unpublished data).
Pharmacodynamics of MCLL0517A (naked antibody) in cynomolgus monkeys
Receptor occupancy was also characterized in the cynomolgus monkey following administration of MCLL0517A and anti-gD antibody. In the cynomolgus monkey, MCLL0517A demonstrated a dose dependent reduction in the extent and duration of target occupancy in monocytes (Figure 2C), while the anti-gD antibody, a non-binding control antibody, demonstrated no apparent receptor occupancy. The low dose of MCLL0517A (0.5 mg/kg) demonstrated ~ 80% occupancy immediately following dosing. This observation was transient and quickly returned to baseline (Figure 2C) by 14 days post-dose. Alternatively, the MCLL0517A high dose (20 mg/kg) demonstrated occupancy of 90%, and this occupancy was maintained at greater than 60% until the conclusion of the study (Day 42).
Figure 2.
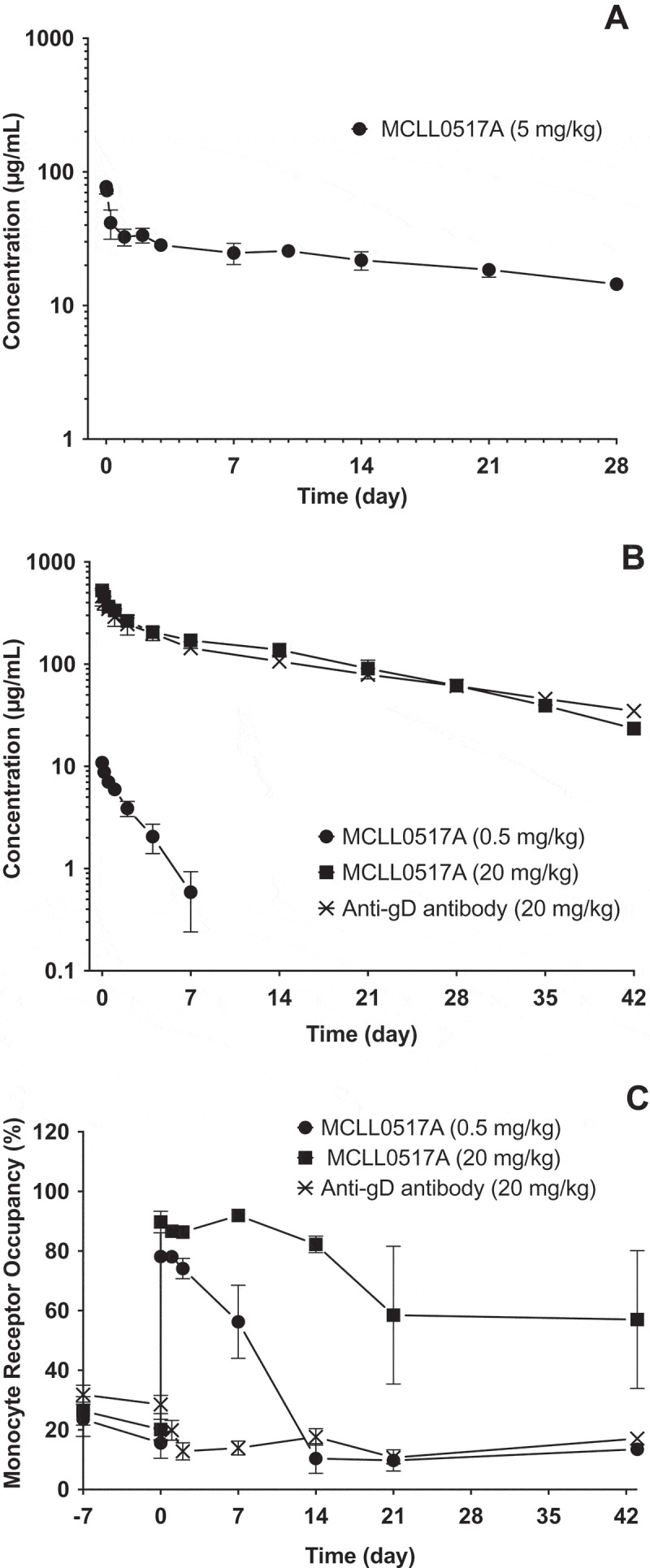
Mean (± SD) concentration-time profiles of MCLL0517A (naked antibody) following IV administration in mouse and cynomolgus monkey. (A) MCLL0517A antibody concentration-time profiles in SCID.bg mice, (B) MCLL0517A and anti-gD (non-binding control antibody) concentration-time profiles in cynomolgus monkey. (C) Monocyte occupancy (%) following IV administration of MCLL0517A or anti-gD antibody in cynomolgus monkey.
Using the receptor occupancy data, in combination with the MCLL0517A PK data in cynomolgus monkeys, a compartmental modeling approach was also used to characterize the serum concentration–time profiles of the MCLL0517A. Precision of the parameter estimations were reported in terms of standard error of the estimate (%SEE) shown in (Table 2). The highest %SEE value relative to estimated parameter values is that of the Michaelis Menten constant (Km = 1.33 ug/mL, %SEE = 0.451) suggesting an increased level of uncertainty with respect to the fitted value. Using this model, the estimated non-specific CL was 3.49 mL/day/kg, which is similar to the non-compartmental total clearance estimates for anti-gD antibody (non-binding antibody) and MCLL0517A at the 20 mg/kg, further suggesting target saturation was achieved at this highest dose of MCLL0517A in this study. The estimated values for the Vmax and Km for the specific CL pathway were 82.7 µg/day/kg and 1.33 µg/mL, respectively, while the maximum value of clearance of MCLL0517A via target engagement (CLINT = Vmax/Km) was calculated to be equal to 62 mL/day/kg.
Table 2.
MCLL0517A Pharmacokinetic Parameters in Cynomolgus Monkeys using a Non-Linear Two-Compartment Model.
| PK Parameter | Estimate | %SEE |
|---|---|---|
| V1 (mL/kg) | 47.9 | 1.51 |
| V2 (mL/kg) | 31.0 | 3.42 |
| CL (mL/day/kg) | 3.49 | 0.242 |
| CLD (mL/day/kg) | 26.8 | 4.34 |
| Vmax (µg/day/kg) | 82.7 | 10.2 |
| Km (µg/mL) | 1.33 | 0.451 |
Pharmacokinetics of DCLL9718A (ADC) in mouse and rat
Following administration of a single intravenous (IV) dose of 5 mg/kg of DCLL9718A in mouse and rat (both non-binding species), DCLL9718A total antibody plasma-concentrations time profiles demonstrated expected bi-phasic disposition, as shown in Figure 4A and 4B. DCLL9718A total antibody total clearance estimates were slow in both mouse and rat, 3.33 mL/day/kg and 4.88 mL/day/kg, respectively, with long half-lives of 16.5 and 28.4 days, respectively. The acPBD dimer plasma-concentration-time profile in mouse also exhibited bi-exponential behavior (Figure 4A), with total clearance estimates about 3-fold faster than DCLL9718A total antibody at 9.35 mL/day/kg and a half-life of 8.72 days (Table 4). For the rat, in lieu of acPBD dimer plasma concentration-data, the average drug-antibody ratio (DAR) was used to characterize the DCLL9718A conjugate behavior, which demonstrated bi-phasic behavior while remaining above 1.4 throughout the study (Figure 4C). The unconjugated PBD dimer concentration-time data in the mouse were also quite low (< 0.5 nM (0.29 ng/mL) at all time-points) and were orders of magnitude lower than DCLL9718A acPBD dimer concentrations (Figure 4A).
Figure 4.
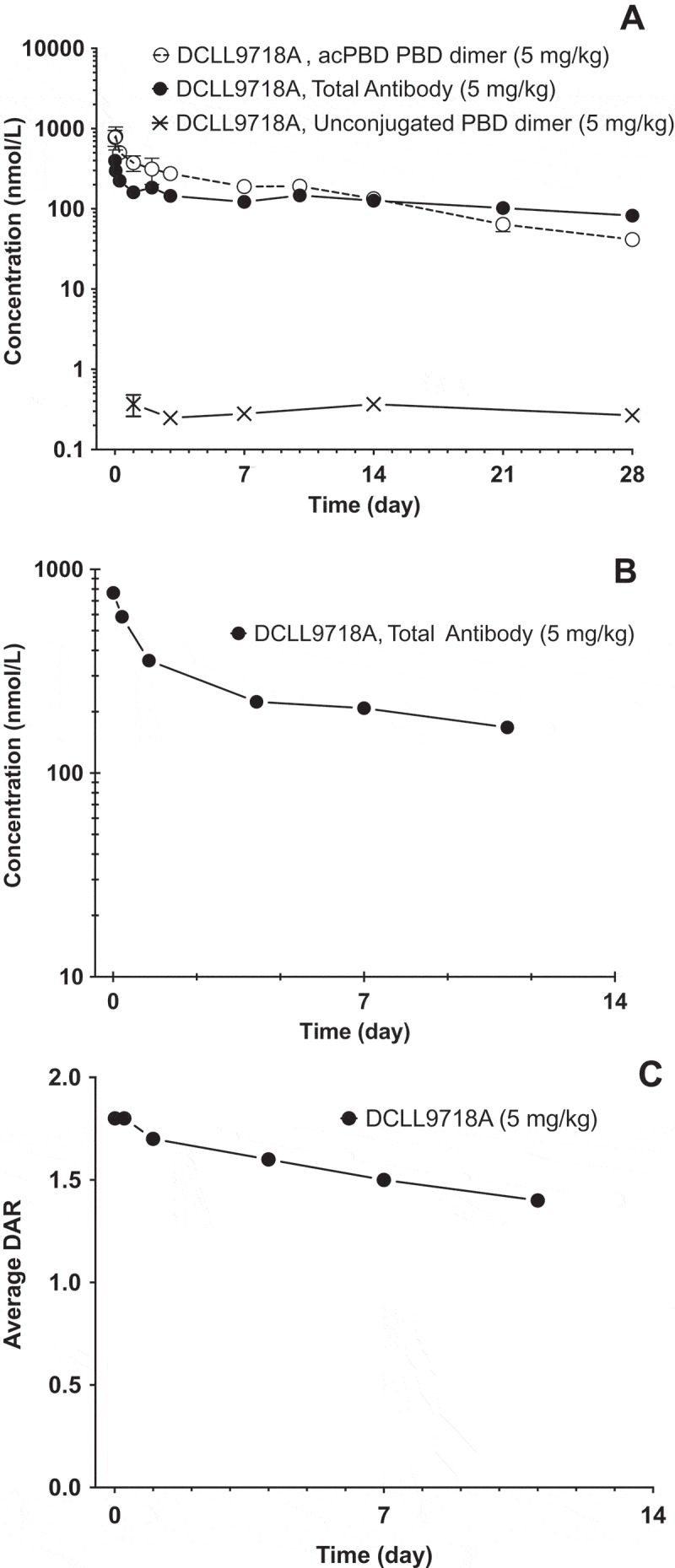
Mean (± SD) plasma concentration-time profiles following IV administration of DCLL9718A in mouse and rat. (A) DCLL9718A total antibody, acPBD PBD dimer and unconjugated PBD dimer plasma concentration-time (nmol/L) profiles following a single IV bolus dose of DCLL9718A in SCID.bg mice. (B) DCLL9718A total antibody, plasma concentration-time (nmol/L) profile following a single IV bolus dose of DCLL9718A in rats. (C) Average drug-antibody ratio (DAR) profile following a single IV bolus dose of 5 mg/kg of DCLL9718A in rats.
Table 4.
Mean (± SD) DCLL9718A acPBD Dimer PK Parameters.
| Mouse a |
Cynomolgus Monkey b |
|||
|---|---|---|---|---|
| PK Parameter | 5 mg/kg | 0.05 mg/kg | 0.1 mg/kg | 0.2 mg/kg |
| Cmax (nM) | 793 ± 146 | 13.9 ± 0.772 | 30.0 ± 2.46 | 65.1 ± 6.77 |
| AUC0-7 (day • nM) | NA | 10.2 ± 0.849 | 24.6 ± 6.02 | 81.7 ± 10.5 |
| AUC0-inf (day • nM) | 4870 | 10.4 ± 0.912 | 25.3 ± 6.58 | 82.0 ± 10.6 |
| CLtotal (mL/day/kg) | 9.35 | 64.0 ± 5.44 | 55.1 ± 11.5 | 31.7 ± 4.76 |
| Vss (mL/kg) | 114 | 76.1 ± 11.3 | 60.5 ± 7.38 | 56.0 ± 7.42 |
| T1/2, λz (day) | 8.72 | 1.94 ± 0.374 | 2.07 ± 1.11 | 1.80 ± 0.236 |
a Represents composite value from naïve pooling of animals. Reported parameter variability in Cmax represents standard error of mean (SEM) and is result of naïve pool approach with NCA.
b Parameters based on first cycle only
Pharmacokinetics and pharmacodynamics of DCLL9718A (ADC) in cynomolgus monkey
The PK profiles of DCLL9718A total antibody following two IV bolus doses of DCLL9718A given 3 weeks apart in cynomolgus monkeys at doses of 0.05, 0.1 and 0.2 mg/kg are shown in Figure 5A, with the corresponding PK parameters, following the first dose, summarized in Table 3. DCLL9718A total antibody Cmax was dose proportional across the dose range tested, while AUC0-inf was dose proportional from 0.05 to 0.1 mg/kg and then greater than dose proportional at 0.2 mg/kg, with mean total clearance estimates of 44.4, 39.4 and 22.4 mL/day/kg at 0.05, 0.1 and 0.2 mg/kg, respectively (Table 3). Similar to the DCLL9718A total antibody concentrations, the acPBD dimer concentrations were proportional at Cmax, while proportionality was observed at 0.05 and 0.1 mg/kg and greater than proportional AUC0-inf was observed at the highest dose. The acPBD dimer concentrations decreased rapidly with mean total clearance estimates of 64.0, 55.1 and 31.7 mL/day/kg (Table 4). For both DCLL9718A total antibody and acPBD dimer, the half-life values were ~ 2 to 3 days.
Figure 5.
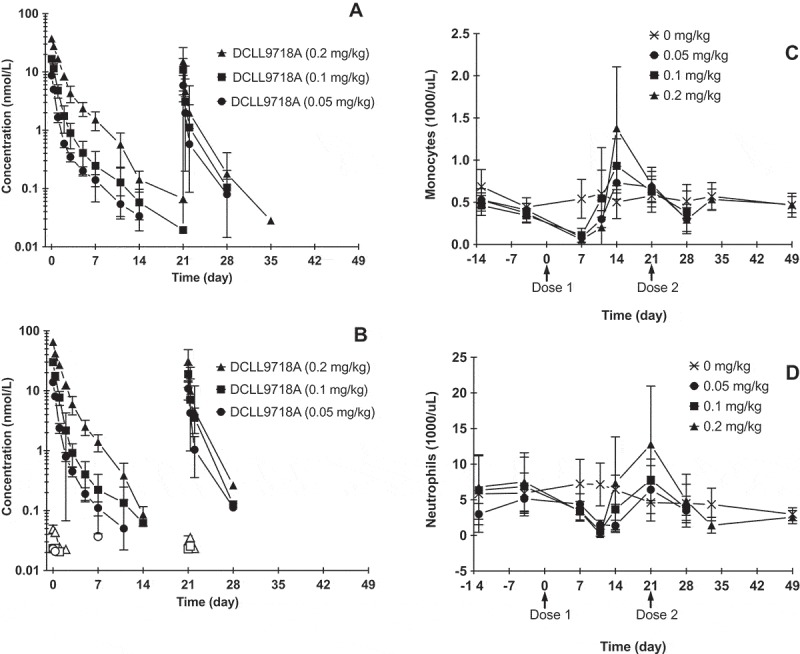
Mean (± SD) DCLL9718A concentration-time profiles and monocyte and neutrophil depletion in cynomolgus monkeys. (A) DCLL9718A total antibody plasma concentration–time profiles, (B) acPBD dimer and unconjugated PBD dimer plasma concentration–time; closed symbols = acPBD dimer, open symbols = unconjugated PBD dimer. Mean (± SD) monocyte (C) and neutrophil (D) depletion over time following administration DCLL9718A.
Table 3.
Mean (± SD) DCLL9718A Total Antibody PK Parameters.
| Mouse a |
Rat |
Cynomolgus Monkey b |
|||
|---|---|---|---|---|---|
| PK Parameter | 5 mg/kg | 5 mg/kg | 0.05 mg/kg | 0.1 mg/kg | 0.2 mg/kg |
| Cmax (nM) | 396 ± 36.5 | 765 ± 46.2 | 8.66 ± 0.631 | 16.7 ± 0.466 | 37.2 ± 2.99 |
| AUC0-7 (day • nM) | NA | NA | 6.93 ± 0.586 | 16.6 ± 4.27 | 55.0 ± 6.06 |
| AUC0-inf (day • nM) | 6780 | 6710 | 7.61 ± 7.08 | 17.8 ± 5.16 | 60.6 ± 8.07 |
| CLtotal (mL/day/kg) | 3.33 | 4.98 | 44.4 ± 4.41 | 39.4 ± 8.15 | 22.4 ± 3.06 |
| Vss (mL/kg) | 133 | 111 | 89.6 ± 7.31 | 70.9 ± 11.7 | 51.9 ± 6.09 |
| T1/2, λz (day) | 28.2 | 16.5 | 3.25 ± 0.326 | 3.46 ± 1.75 | 2.31 ± 0.365 |
a Represents composite value from naïve pooling of animals. Reported parameter variability in Cmax represents standard error of mean (SEM) and is result of naïve pool approach with NCA.
b Parameters based on first cycle only
Following the second dose, there was a notable decrease in DCLL9718A total antibody and acPBD dimer exposure compared to the first dose, which is likely due to the high incidence of ADA (unpublished data). The presence of ADAs also significantly confounded the ability to model or further characterize the DCLL9718A total antibody and acPBD dimer data.
The unconjugated PBD dimer concentrations were low or below the limit of quantification in all animals and never exceeded 0.070 nM (0.041 ng/mL) at any dose level (Figure 5B). Similar to the mouse, at the highest DCLL9718A dose of 0.2 mg/kg, Cmax for the unconjugated PBD dimer was several orders of magnitude lower than peak concentrations of the acPBD dimer.
While the antibody portion, MCLL0517A, notably targets monocytes and neutrophils, depletion of these myeloid cells is only elicited by DCLL9718A. Furthermore, cynomolgus monkeys express CLL-1 on normal monocytes and granulocytes, albeit at lower levels than humans. However, unlike humans, cynomolgus monkeys do express very low level of CLL-1 on hematopoietic stem cells 11. Following the initial dose of DCLL9718A, both monocytes and neutrophils demonstrated a marked decrease at all dose levels (Figure 5C and 5D), and the reduction appeared to be independent of dose. Following the first dose, monocyte reductions reached their nadir at 7 days, and quickly rebounded past baseline by 14 days following clearance of DCLL9718A (Figure 5C). Meanwhile neutrophil reductions reached their nadir at ~ 10 to 14 days post-dose, and quickly rebounded past baseline by 21 days (prior to the second dose) (Figure 5D). Following the second dose of DCLL9718A, there was a small reduction in monocytes, while there was almost no change in neutrophils relative to concurrent controls and to baseline values. This lack of dramatic reduction in monocytes and neutrophils following the second dose was likely due to reduced exposure as a result of the presence of ADAs.
Discussion
The clinical validation of CD33 as an AML target for ADCs has been well established by numerous examples, including gemtuzumab ozogamicin, AVE9633, IMG779, and vadastuximab talirine.16 In the case of vadastuximab talirine, which is conjugated to a highly potent DNA-alkylating PBD dimer, the targeting of CD33 may have been a liability due to its expression on HSCs. Therefore, targeting another exclusive myeloid and AML target such as CLL-1, which is not expressed on HSCs, may reduce these liabilities.11 Herein, we characterized the stability of DCLL9718A, impact of conjugation and disposition of both MCLL0517A and DCLL9718A in the mouse, rat and cynomolgus monkey. Finally, we further evaluated the PD of DCLL9718A in cynomolgus monkeys.
As part of any non-clinical PK characterization of an ADC, it is essential to understand both the stability of the ADC, the overall dispostion of both the antibody component (as measured by the total antibody), and the conjugate (as measured, in this case by acPBD dimer). For DCLL9718A, stability was assessed in vitro, as well as in vivo in the mouse, rat and cynomolgus monkey. In the plasma stability study, DCLL9718A was stable with greater than 80% of the PBD dimer still conjugated to the antibody after 96 hours, with no apparent differences across species (Figure 1). In addition, ~ 2 – 3 ng/mL (~ 3.5 – 5 nM), of the unconjugated PBD dimer was released over the course of the plasma stability study. This small concentration of the unconjugated PBD dimer accounts for ~ 5–20% of the loss of acPBD dimer levels. However, at time zero, this loss of unconjugated PBD dimer constitutes less than 1% of the theoretical total acPBD dimer linked to DCLL9718A. The stability of the linker was also assessed in vivo, in the mouse, rat and cynomolgus monkey. For the mouse and cynomolgus monkey, we measured acPBD dimer concentrations, which for DCLL9718A demonstrated a total clearance that was 2 to 3-fold faster than the DCLL9718A total antibody clearance. This difference in total antibody and conjugate clearance is consistent with other ADCs, including the approved product Kadcyla®, with different linker types (cleavable and non-cleavable).17 The clearance of the acPBD dimer is the sum of several processes, including those governing typical antibody clearance pathways (both target-mediated and non-saturable), but also the de-conjugation of PBD dimer. The overall similarity in the DCLL9718A total antibody and acPBD total clearance estimates suggests that de-conjugation was not the major clearance mechanism for DCLL9718A. In the rat, we measured the DAR, which for the THIOMAB® antibody technology used in our ADC ranges from 0 to 2. Similar to the stability demonstrated in mouse and cynomolgus monkey, the average DAR in the rat remained greater than 1.4 through 11 days, suggesting that most of the PBD dimer remained conjugated to the antibody. Finally, the unconjugated PBD dimer levels were measured in both mouse and cynomolgus monkey, in which very low concentrations, 40 to 500 pM in the monkey and mouse, respectively, were observed. While the low unconjugated concentrations generally do not correlate to a direct measure of stability (see ref. 18), they are consistent with the in vitro stability data, supporting the overall stability of DCLL9718A.
Beyond linker stability, we also characterized the disposition of DCLL9718A and MCLL0517A in the mouse and cynomolgus monkey, which allowed us to evaluate the effect of conjugation on the PK of the ADC. In mouse and rat, DCLL9718A total antibody PK demonstrated expected bi-phasic disposition with a slow clearance and a long half-life, which was as expected given that DCLL9718A does not cross-react to the rodent homolog of CLL-1 (Figure 4A and 4B). Similar disposition and clearance estimates were also observed for MCLL0517A in the mouse, suggesting that the conjugation of PBD-dimer had little or no effect on the overall disposition of DCLL9718A total antibody (Tables 1 and 3).
For the cynomolgus monkeys (binding species), the PK of MCLL0517A demonstrated expected target mediated dispostion, with decreasing total clearance with increasing dose (Table 1). At the high dose of 20 mg/kg, MCLL0517A demonstrated bi-phasic disposition and a total clearance that was within the expected range for an IgG1 antibody.15 It was also in line with a non-binding antibody, anti-gD antibody, suggesting that the target was completely saturated at this dose. The saturation of target at 20 mg/kg was also confirmed by the receptor occupancy data, in which MCLL0517A demonstrated near complete and sustained receptor occupancy. In comparison, the lower dose (0.5 mg/kg) achieved 80% occupancy that quickly declined in parallel with MCLL0517A concentrations. We also evaluated MCLL0517A PK and PD data in a two-compartment model with both target-mediated and non-saturable components, which included incorporation of the receptor occupancy in monocytes (Figure 3). The model well characterized the PK behavior of MCLL0517A and captured the measured receptor occupancy data within variability (Supplemental Figures 2 and 3). However, the estimated value of Km (1.33 µg/mL, 8.9 nM) was slightly higher than the affinity of MCLL0517A of ~ 1 nM. 11 A higher value of Km may suggest that CLL-1 mediated uptake is not limited by affinity. This difference might be explained by the rate of antibody/target internalization, in which a higher concentration is required to reach half of the clearance capacity (i.e., Km) compared to the concentration required to bind half of the receptors (i.e., Kd) in a closed system.
Figure 3.
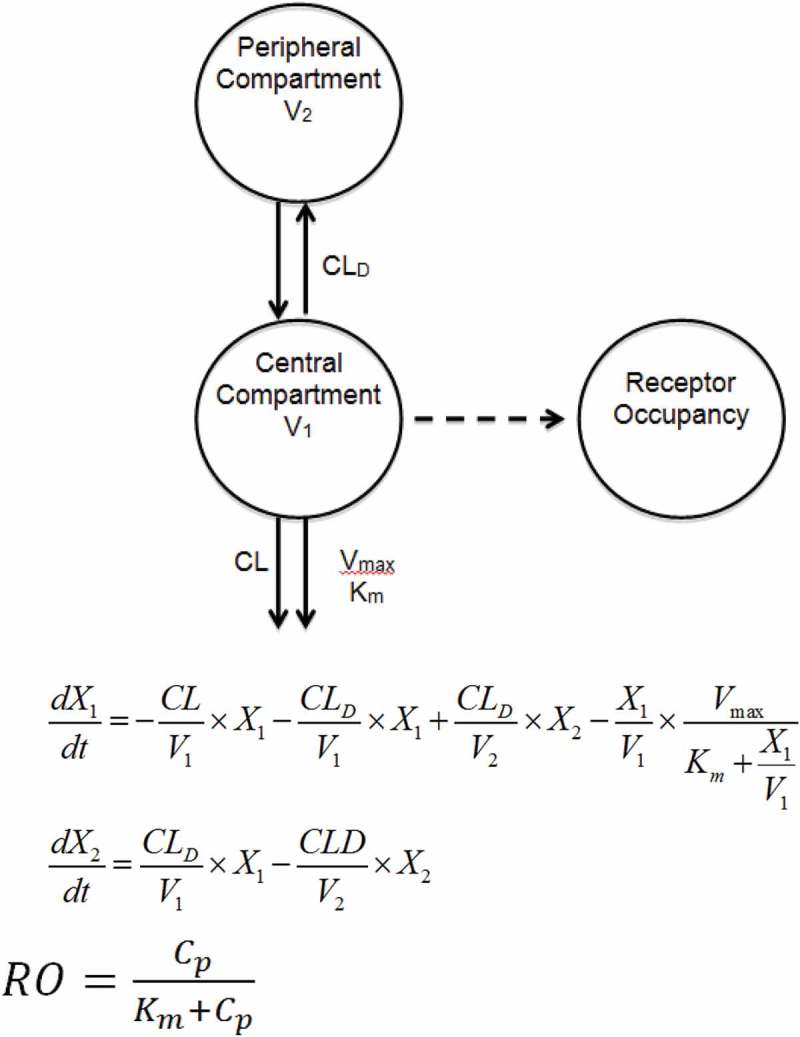
Two-compartment non-linear PK/PD model.
The PK of DCLL9718A in the cynomolgus monkeys was dictated by rapid total clearance of both the DCLL9718A total antibody and acPBD dimer, similar to the low dose of MCLL0517A in the cynomolgus monkey18. At these low doses (0.05 to 0.2 mg/kg), the DCLL9718A target mediated disposition was expected, and marked decreases in monocytes and neutrophils were observed at all dose levels after the first dose. (Figure 5A – 5D). The complete depletion of monocytes and neutrophils at these low doses of DCLL9718A occurred despite receptor occupancy not being saturated or sustained at a similar dose range of 0.5 mg/kg of MCLL0517A (Figure 2C). This suggests, that while some target occupancy/engagement is required for biological activity, the pharmacological activity is based on numerous other factors, such as internalization, cytotoxic drug potency.19 Following the first dose of DCLL9718A, target-mediated disposition is clearly demonstrated, and as a result a rapid decline in monocytes and neutrophils was observed (Figure 5C and 5D). However no ADA sampling was conducted between the first and second dose. Therefore, while there appeared be sufficient exposure to elicit the expected pharmacology after the first dose, the impact of ADAs is unknown. Following the second dose, the impact of ADA was more apparent as notable decreases in exposure of DCLL9718A total antibody and acPBD dimer were observed. As a result of the decreased exposure, there was a reduced pharmacologic effect, and, although the monocytes and neutrophils decreased to some degree, the magnitude was lower compared to the first dose (Figure 5A – 5D). Although the presence of ADAs had an effect on PK and PD following the second dose, and high incidence of ADAs was observed pre-clinically, ADAs in animals are generally not considered predictive in the clinic.20
In summary, DCLL9718A demonstrated good in vitro and in vivo stability in animals, with most of the PBD dimer payload still conjugated to the antibody over the duration tested. Both DCLL9718A and MCLL0517A showed linear PK in the non-binding rodent species, and non-linear PK in cynomolgus monkeys (binding species). The results indicated minimal impact of conjugation on the PK of DCLL9718A total antibody. In addition, in cynomolgus monkey, MCLL0517A showed target engagement within the range of doses tested as measured by receptor occupancy, while DCLL9718A showed strong PD activity as evidenced by substantial reduction in monocytes and neutrophils. The preclinical PK, PD, and stability data for DCLL9718A collectively suppport clinical evaluation of this molecule for the treatment for AML.
Material and methods
THIOMABTM antibody-drug conjugate
The DCLL9718A ADC used for all in vitro and in vivo PK/PD studies was generated at Genentech Inc. (South San Francisco, CA). DCLL9718A is composed of a humanized monoclonal immunoglobulin G1 (IgG1) anti-CLL-1 antibody (MCLL0517A) connected to a PBD dimer payload via a cleavable disulfide-labile linker. DCLL9718A uses THIOMABTM antibody technology resulting in the conjugation of two drug molecules per antibody to engineered cysteine residues.21 MCLL0517A was optimized to obtain approximately one nanomolar binding affinities to both human and cynomolgus monkey CLL-1, as well as efficient internalization and trafficking to lysosomes in HL-60 and 293 cells.11
In Vitro plasma stability study
Human, cynomolgus monkey, rat, and mouse plasma were obtained from Bioreclamation, Inc. (Westbury, NY).22–24 Phosphate-buffered saline + 0.5% bovine serum albumin (Sigma-Aldrich, St. Louis, MO) was used as a buffer control. DCLL9718A or MCLL0517A was spiked into each species matrix to a final concentration of 50 µg/mL and incubated at 37 ºC (in CO2 incubator) with gentle rotation. At the time-points of 0, 6, 24, 48, and 96 hours, samples were transferred to dry ice to stop the incubation, and then stored frozen at −70°C until analysis. The time-zero samples (0 hours) were transferred immediately from wet ice to dry ice before storage.
In vivo PK/TK and PK/PD studies
The in vivo PK studies in mice and rats were approved by the Institutional Animal Care and Use Committee at Genentech, Inc. and were conducted in compliance with the regulations of the Association for Assessment and Accreditation of Laboratory Animal Care. For the mouse study, 30 female naïve C.B.17 SCID mice (6–8 weeks old) were obtained from Charles River Laboratories, Inc. (Hollister, CA). Each animal received a single IV dose of 5 mg/kg of DCLL9718A or MCLL0517A via tail vein injection (n = 15/group). Blood samples were collected from 3 mice in each dosing group at each of the following time points: 10 minutes, 1 and 6 hours, 1, 2, 3, 7, 10, 14, 21, and 28 days. The sample collection was done via retro-orbital bleeds or cardiac puncture. For the retro-orbital bleeds, samples were processed to collect plasma and used to measure DCLL9718A total antibody and antibody-conjugated PBD dimer (acPBD dimer), while the select cardiac puncture samples (1, 3, 7, 14 and 28 days) were processed to collect plasma and used to measure unconjugated PBD dimer concentrations as described in the bioanalysis section. For the rat study, 12 male naïve Sprague-Dawley rats were obtained from Charles River Laboratories, Inc. (Hollister, CA). Each animal received a single IV dose of 5 mg/kg of DCLL9718A via the tail vein injection. Blood samples were collected from 3 rats at each of the following time points: 10 minutes, and 6 hours, 1, 4, 7, and 11 days. Rat blood samples were processed to collect plasma and used to measure DCLL9718A total antibody and the average DAR proportions.
The PK study of unconjugated MCLL0517A in cynomolgus monkeys was approved by the Institutional Animal Care and Use Committee at Charles River Laboratories (Reno, NV). Animals (n = 3 per group) were administered with a single IV injection of 0.5 and 20 mg/kg of MCLL0517A or 20 mg/kg anti-gD antibody (a non-targeting control antibody). The anti-gD antibody was produced and humanized at Genentech, Inc. Blood samples for PK, anti-drug antibodies (ADA) and receptor occupancy analysis were collected from each animal via the femoral vein and processed to collect serum. PK samples were collected at the following time points: pre-dose, 10 min, 4 and 12 hours, 1, 2, 4, 7, 14, 21, 28, 35 and 42 days, while ADA samples were collected at pre-dose, 7, 14, 28 and 42 days. For receptor occupancy, samples were collected at: 1 week prior to dosing, pre-dose, 10 min, 1, 2, 7, 14, 21, 42 days post-dose.
The toxicokinetic study of DCLL9718A in cynomolgus monkeys was approved by the Institutional Animal Care and Use Committee at Charles River Laboratories (Reno, NV). Thirty-two cynomolgus monkeys in each of four dose groups were given two doses, three weeks apart by IV bolus administration at dose levels of 0, 0.05, 0.1, and 0.2 mg/kg. All four groups had six animals (3 male/3 female) in the main study, which were euthanized 7 days following the second dose, while the 0 and 0.2 mg/kg dose levels had four extra animals (2 male/2 female) which were euthanized 28 days following the second dose. For the first dose cycle, PK blood samples were collected at the following time-points: pre-dose, 10 minutes, 8 hours, and 1, 2, 3, 5, 7, 11, 14, and 21 days post-dose, while following the second dose: 10 minutes and 8 hours, 1 7, 14, and 28 days post-dose. PK blood samples were processed to collect plasma and used to measure DCLL9718A total antibody, acPBD dimer and unconjugated PBD dimer. For ADA samples, samples were collected: 2 weeks prior to dosing, pre-dose, and at 21, 28 and 49 days. For hematology parameters (monocyte and neutrophil counts), samples were collected at: 2 weeks prior to dosing, 5 days prior to dosing and 7, 11, 14, 20, 28, 33 and 49 days.
Bioanalysis of plasma samples
DCLL9718A TOTAL ANTIBODY, MCLL0517A and Anti-gD ANTIBODY ASSAY
In Vitro plasma stability; in vivo mouse, rat and cynomolgus monkey samples
During the different stages of development of DCLL9718A, different analytical approaches were applied to quantify the DCLL9718A total antibody and antibody (MCLL0517A or anti-gD antibody) concentrations in mouse, rat and cynomolgus monkey. For determining the DCLL9718A total antibody concentrations in mouse and cynomolgus monkey, as well as the MCLL0517A antibody concentrations in the mouse, a specific peptide-based liquid chromatography (LC)-mass spectrometry (MS)/MS quantitative assay was used. Samples were enriched from mouse plasma via immunoaffinity capture using streptavidin magnetic beads coupled with biotinylated anti-human IgG antibody (clone R10Z, Hoffman-La Roche, Inc., Nutley, NJ) and then subjected to “on-bead” proteolysis with trypsin. A representative signature tryptic peptide selected from the complementarity-determining region of DCLL9718A was identified as the surrogate for quantification of the antibody. The assay lower limit of quantitation (LLOQ) was 2.50 ng/mL in this assay.
For determining MCLL0517A and anti-gD antibody concentrations in cynomolgus monkey and DCLL9718A total antibody concentrations in rats, a bridging enzyme-linked immunosorbent assay (ELISA) technique was employed. DCLL9718A total antibody, MCLL0517A and anti-gD antibodies were captured via sheep anti–human IgG (Cat. # AU003CUS01, The Binding Site, San Diego, CA) followed by detection using a sheep anti-human IgG conjugated to horseradish peroxidase (Cat. # A80-319P-12, Bethyl Labs, Montgomery, TX). The LLOQ of the assay was 20 ng/mL.
ANTIBODY-CONJUGATED PBD DIMER (acPBD Dimer) ASSAY
In Vivo mouse and cynomolgus monkey samples
DCLL9718A conjugate was measured as acPBD dimer. Samples were enriched from mouse plasma via affinity capture using streptavidin magnetic beads coupled with biotinylated anti-human IgG antibody. The DCLL9718A sample was subjected to reduction and alkylation, releasing PBD dimer in its active form and subsequently analyzed using LC-MS/MS. The assay LLOQ for the mouse and cynomolgus monkey samples was 0.0500 nM (29.2 pg/mL PBD dimer).
In Vitro plasma stability
Samples were enriched from mouse, rat, cynomolgus monkey, and human plasma via immunoaffinity capture using Protein A-coated magnetic beads. The DCLL9718A sample was subjected to reduction and alkylation, releasing PBD dimer in its active form and was analyzed using LC-MS/MS. The assay LLOQ for the plasma stability samples was 3.91 nM (2.28 ng/mL PBD dimer).
UNCONJUGATED PBD DIMER ASSAY
In Vitro plasma stability; in vivo mouse and cynomolgus monkey samples
Unconjugated PBD dimer and deuterated PBD dimer (Internal Standard) were extracted from lithium heparin plasma samples on a Water’s Ostro protein precipitation and phospholipid extraction plate (Milford, MA). The supernatant (containing PBD dimer and deuterated PBD dimer) was then analyzed using LC-MS/MS. The assay LLOQ for the plasma stability samples was 0.05 nM (29.2 pg/mL PBD dimer) and for the mouse and cynomolgus monkey samples was 0.02 nM (11.7 pg/mL PBD dimer).
AFFINITY-CAPTURE LC-MS ASSAY
An immunoaffinity capture LC-MS intact antibody assay was used to determine the DAR in rats (see ref. 22). Briefly, biotinylated CLL-1 receptor extracellular domain immobilized on streptavidin-coated magnetic beads was used to specifically capture various DAR species (DAR 0, DAR 1, and DAR 2). The captured ADC was digested by IdeS protease and then eluted from the beads to generate the F(ab′)2 fragments, which were injected onto a reversed phased LC coupled to a Sciex™ triple time-of-flight 5600 mass spectrometer (Redwood City, U.S.A.) operated in the positive ESI mode.
ANTI-DRUG ANTIBODY ASSAY
Cynomolgus monkey serum samples were analyzed by a generic immunocomplex ADA immunoassay (see ref. 23) to detect ADAs against MCLL0517A and anti-gD antibody
or an in-solution bridging ELISA (See ref. 24) to detect ADAs against anti-DCLL9718A antibodies.
Receptor occupancy analysis in cynomolgus monkey
MCLL0517A targets the CLL-1 surface receptor on myeloid cells. To assess the occupancy of the CLL-1 receptor in cynomolgus monkeys administered MCLL0517A and anti-gD antibody, a competing, commercially available anti-CLL-1 monoclonal antibody (R&D systems, Minneapolis, MN; #FAB2946R) was used in conjunction with CD11b labeling to determine the percentage of monocytes expressing unbound CLL-1 receptor. A mouse IgG2b isotype control antibody (BD Biosciences, San Jose, CA; #555745) was utilized as a negative control for CLL-1 labeling and to aid in the gating of receptor occupancy data.
Monocyte and neutrophil analysis in cynomolgus monkey
Blood samples, taken from cynomolgus monkeys before/after administration of DCLL9718A, were analyzed for monocyte and neutrophil counts, which were determined using an Advia® 120/2120 Hematology Analyzer. Whole blood samples were collected and stored on wet ice prior to starting analysis.
Pharmacokinetic Analysis
Non-compartmental analysis
For DCLL9718A total antibody, acPBD dimer or MCLL0517A, the plasma concentration vs. time data was analyzed by non-compartmental approaches (WinNonlin, version 6.3, Pharsight Corp., Mountain View, CA) to provide an estimation of PK parameters. For mice, the plasma concentration vs. time data was naïve pooled together (sparse sampling approach) to provide PK parameter estimations. For rats and cynomolgus monkeys, PK parameters for each individual animal were estimated and summary statistics for each group were calculated. Parameters calculated included the maximum concentration (Cmax); area under the plasma concentration-time curve from time = 0 to infinity (AUC0-inf); area under the plasma concentration-time curve from time = 0 to 7 days post-dose (AUC0-7) for cynomolgus monkeys only; the total clearance (CLtotal); volume of distribution at steady-state (Vss) and terminal half-life (t1/2,λz). Animals that had apparent impact on the PK due to the presence of ADAs were excluded from the PK analysis. Apparent impact was described as an unexpected decrease or lack of quantifiable concentrations not attributable to target-mediated mechanisms
Compartmental analysis
The PK of MCLL0517A in cynomolgus monkeys were also described using a non-linear two-compartment model comprising specific (target-mediated) and non-specific clearance, with structural model and corresponding equations shown in Figure 3, to obtain the following PK parameters: clearance from the central compartment (CL), which is the non-specific clearance pathway; distributional CL (CLD); volumes of distribution of the central compartment (V1) and the peripheral compartment (V2); and parameters of the specific clearance pathway, which are maximum target-mediated elimination rate under conditions of target saturation (Vmax) and the concentration for reaching 50% Vmax (Km). The results were summarized as one estimate for each parameter and the % standard error of the estimate (%SEE). The CLL-1 receptor occupancy pharmacodynamics were modeled using a sigmoidal equation. The inflection point, corresponding to the drug concentration resulting in 50% of target occupation, was set to be equal to Km. PK and RO data were fitted simultaneously using a pooled approach with a proportional weighting function (SAAM v2.3, University of Washington) to estimate PK parameters.
Supplemental Material
Supplemental data for this article can be access on the publisher’s website.
Disclosure of potential conflicts of interest
All authors are or were employees of Genentech, a member of the Roche Group, and hold financial interest in Hoffman-La Roche.
Abbreviations
- acPBD dimer
antibody-conjugated PBD dimer
- ADA
anti-drug antibodies
- ADC
antibody-drug conjugate
- AML
acute myeloid leukemia
- AUC
area under the curve
- CLL-1
C-type lectin-like molecule-1
- Cmax
maximum concentration
- DAR
drug-antibody ratio
- HSC
hematopoietic stem cell
- IgG1
humanized monoclonal immunoglobulin G1
- LLOQ
lower limit of quantitation
- PBD
pyrrolobenzodiazepine
- PD
pharmacodynamics
- PK
pharmacokinetics
- SD
standard deviation
- SEE
standard error of the estimate
References
- 1.Siegel RL, Miller KD, Jemal A.. Cancer Statistics, 2017. 2017;67(1):7–30. doi: 10.3322/caac.21387 PMID: 28055103. [DOI] [PubMed] [Google Scholar]
- 2.Kantarjian H. Acute myeloid leukemia—major progress over four decades and glimpses into the future. Am. J. Hematol. 2016;91:131–145. doi: 10.1002/ajh.24246 PMID: 26598393. [DOI] [PubMed] [Google Scholar]
- 3.Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, Anderson JE, Petersdorf SH. Age and acute myeloid leukemia. Blood. 2006;107(9):3481–3485. doi: 10.1182/blood-2005-09-3724 PMID: 16455952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shor B, Gerber H-P SP. Preclinical and clinical development of inotuzumab-ozogamicin in hematological malignancies. Mol Immunol. 2015;67(2):107–116. doi: 10.1016/j.molimm.2014.09.014 PMID: 25304309. [DOI] [PubMed] [Google Scholar]
- 5.Beck A, Goetsch L, Dumontet C, Corvaia N. Strategies and challenges for the next generation of antibody–drug conjugates. Nat Rev Drug Discov. 2017;16(5):315–337. doi: 10.1038/nrd.2016.268 PMID: 28303026. [DOI] [PubMed] [Google Scholar]
- 6.Jain N, Smith SW, Ghone S, Current TB. ADC Linker Chemistry. Pharm Res. 2015;32(11):3526–3540. doi: 10.1007/s11095-015-1657-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lapusan S, Vidriales MB, Thomas X, De Botton S, Vekhoff A, Tang R, Dumontet C, Morariu-Zamfir R, Lambert JM, Ozoux M-L, et al. Phase I studies of AVE9633, an anti-CD33 antibody-maytansinoid conjugate, in adult patients with relapsed/refractory acute myeloid leukemia. Invest New Drugs. 2012;30:1121–1131. doi: 10.1007/s10637-011-9670-0 PMID: 21519855. [DOI] [PubMed] [Google Scholar]
- 8.US National Library of Medicine. http://clinicaltrials.gov. [DOI] [PubMed]
- 9.Kung Sutherland MS, Walter RB, Jeffrey SC, Burke PJ, Yu C, Kostner H, Stone I, Ryan MC, Sussman D, Lyon RP, et al. SGN-CD33A: a novel CD33-targeting antibody-drug conjugate using a pyrrolobenzodiazepine dimer is active in models of drug-resistant AML. Blood. 2013;122:1455–1463. doi: 10.1182/blood-2013-03-491506 PMID: 23770776. [DOI] [PubMed] [Google Scholar]
- 10.Leong SR, Sukumaran S, Hristopoulos M, Totpal K, Stainton S, Lu E, Wong A, Tam L, Newman R, Vuillemenot B, et al. An anti-CD3/anti–CLL-1 bispecific antibody for the treatment of acute myeloid leukemia. Blood. 2017;129(5):609–618. doi: 10.1182/blood-2016-08-735365 PMID: 27908880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng B, Yu S-F, Del Rosario G, Leong S, Lee GY, Vij R, Chiu C, Liang W-C, Wu Y, Chalouni C, et al. An Anti-CLL-1 Antibody-Drug Conjugates for the Treatment of Acute Myeloid Leukemia. Clin Cancer Res, 2018. June 29 doi: 10.1158/1078-0432.CCR-18-0333 PMID: 29959143. [DOI] [PubMed] [Google Scholar]
- 12.Bakker AB, Van Den Oudenrijn S, Bakker AQ, Feller N, Van Meijer M, Bia JA, Jongeneelen MA, Visser TJ, Bijl N, Geuijen CA, et al. C-type lectin-like molecule-1: a novel myeloid cell surface marker associated with acute myeloid leukemia. Cancer Res. 2004;64(22):8443–8450. doi: 10.1158/0008-5472.CAN-04-1659 PMID: 15548716. [DOI] [PubMed] [Google Scholar]
- 13.Van Rhenen A, Van Dongen GA, Kelder A, Rombouts EJ, Feller N, Moshaver B, Stigter-Van Walsum M, Zweegman S, Ossenkoppele GJ, Schuurhuis J. The novel AML stem cell associated antigen CLL-1 aids in discrimination between normal and leukemic stem cells. Blood. 2007;110(7):2659–2666. doi: 10.1182/blood-2007-03-083048 PMID: 17609428. [DOI] [PubMed] [Google Scholar]
- 14.Kaur S, Xu K, Saad OM, Dere RC, Carrasco-Triguero M. Bioanalytical assay strategies for the development of antibody–drug conjugate biotherapeutics. Bioanalysis. 2013;5(2):201–226. doi: 10.4155/bio.12.299 PMID: 23330562. [DOI] [PubMed] [Google Scholar]
- 15.Deng R, Iyer S, Theil FP, Mortensen DL, Fielder PJ, Prabhu S. Projecting human pharmacokinetics of therapeutic antibodies from nonclinical data: what have we learned?. mAbs. 2011;3(1):61–66. doi: 10.4161/mabs.3.1.13799 PMID: 20962582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antibody Drug PP. Conjugates for Cancer Therapy. Pharmacological Reviews.. 2016;63:1–17. doi: 10.1124/pr.114.009373 PMID: 26589413. [DOI] [PubMed] [Google Scholar]
- 17.Kamath AV, Iyer S. Preclinical Pharmacokinetic Considerations for the Development of Antibody Drug Conjugates. Pharm Res. 2015;32(11):3470–3479. doi: 10.1007/s11095-014-1584-z PMID: 25446773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ross PL, Wolfe JL. Physical and Chemical Stability of Antibody Drug Conjugates: current Status. J Pharm Sci. 2016;105(2):391–397. doi: 10.1016/j.xphs.2015.11.037 PMID: 26869406. [DOI] [PubMed] [Google Scholar]
- 19.Sadekar S, Figueroa I, Tabrizi M. Antibody Drug Conjugates: application of Quantitative Pharmacology in Modality Design and Target Selection. AAPS J. 2015;17(4):828–836. doi: 10.1208/s12248-015-9766-0 PMID: 25933599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ponce R, Abad L, Amaravadi L, Gelzleichter T, Gore E, Green J, Gupta S, Herzyk D, Hurst C, Ivens IA, et al. Immunogenicity of biologically-derived therapeutics: assessment and interpretation of nonclinical safety studies. Regulatory, Toxicol Pharmacol. 2009;54(2):164–182. doi: 10.1016/j.yrtph.2009.03.012 PMID: 19345250. [DOI] [PubMed] [Google Scholar]
- 21.Junutula JR, Raab H, Clark S, Bhakta S, Leipold DD, Weir S, Chen Y, Simpson M, Tsai SP, Dennis MS, et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol. 2008;26(8):925–932. doi: 10.1038/nbt.1480 PMID: 18641636. [DOI] [PubMed] [Google Scholar]
- 22.Su D, Ng C, Khosraviani M, Yu S-F, Cosino E, Kaur S, Xu K. Custom-Designed Affinity Capture LC-MS F(ab′)2 Assay for Biotransformation Assessment of Site-Specific Antibody Drug Conjugates. Anal Chem. 2016;88(23):11340–11346. doi: 10.1021/acs.analchem.6b03410 PMID: 27779866. [DOI] [PubMed] [Google Scholar]
- 23.Carrasco-Triguero M, Davis H, Zhu Y, Coleman D, Nazzal D, Vu P, Kaur S. Application of a Plug-and-Play Immunogenicity Assay in Cynomolgus Monkey Serum for ADCs at Early Stages of Drug Development. J Immunol Res. 2016;2016:.1–14. doi: 10.1155/2016/2618575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carrasco-Triguero M, Yi JH, Dere R, Qiu ZJ, Lei C, Li Y, Mahood C, Wang B, Leipold D, Poon KA. Immunogenicity assays for antibody–drug conjugates: case study with ado-trastuzumab emtansine. Bioanalysis. 2013;5(9):1007–1023. doi: 10.4155/bio.13.64 PMID: 23641693. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


