Abstract
Background/Aims
Celiac disease (CD) is an autoimmune enteropathy that develops in individuals with genetic susceptibility as a result of a permanent sensitivity to gluten found in grains. The prevalence of CD in Turkey is between 0.3% and 1%. However, the prevalence of CD in Çorum, a city in middle Anatolia in Turkey, is unknown. The purpose of this study was to identify the prevalence of childhood CD in Çorum and to detect patients with silent and atypical CD.
Materials and Methods
The sample size was calculated using a stratified sampling method, to provide the sample number that would best represent this population. Screenings were conducted using rapid tissue transglutaminase IgA test kits.
Results
A total of 1730 students were included in the study; 877 (50.6%) were female. Of students in the city center, 301 (34%) were in primary school, 299 (34%) were in secondary school, and 283 (32%) were in high school. As for towns, 847 (49%) students from 92 schools were included in the study. Eight children had positive screening results; 4 (50%) were female, and the average age was 11.6±3.4 (9–17) years. According to the celiac serology results and endoscopic duodenum biopsies, all children with positive screening results were diagnosed with CD. The prevalence of CD was found to be 0.46% in schoolchildren.
Conclusion
Various studies in Turkey have reported a prevalence of CD between 0.6% and 0.9%, with 0.47% reported in a multicenter study. The present study identified CD prevalence as 0.46% (1 in 216) among children in Çorum, Turkey.
Keywords: Celiac disease, child, school screening, Turkey
INTRODUCTION
Celiac disease (CD) is an autoimmune, systemic disease with intestinal and nonintestinal findings, which develops as a result of permanent sensitivity to gluten in the diet of genetically susceptible individuals (1). Gluten is a term used for proteins rich in proline and glutamine (prolamins) that are found in wheat, barley, and rye; these include gliadin in wheat, hordein in barley, and secalin in rye (2). CD is the only autoimmune disease with a trigger and that enters remission with removal of the trigger (3). CD can be symptomatic, with different clinical presentations. Although malabsorption findings are predominant in classical CD, currently about half of patients with CD have extraintestinal disorders (4–6). The prevalence of CD is influenced by racial and environmental factors. Until 1990, CD was considered to exist primarily among white people in Europe; it was considered rare in other areas. Its prevalence in Europe was reported as 1/1000–6500 (7,8). For example, the prevalence of CD in the United Kingdom was 1/8000 in 1950, and it is currently approximately 1/100. The CD prevalence in the United States was 1/5000 in 1950, and it is 1/133 today. The reason for this change in prevalence is owing, to a great extent, to recognition of atypical forms of the disease, increased medical awareness of CD, and significant developments in sensitive screening tests (9).
Çorum is a city located between the Central Black Sea and Central Anatolia in Turkey; the city is 244 km from Ankara, the capital of the country. The population of Çorum is 527,863. The city has a very extensive history, as it was the capital city of the Hittite Empire (10).
According to recent studies, the worldwide prevalence of CD has been reported as 0.5%–1% (5,6). In Turkey, the prevalence of CD is between 0.3% and 1%, according to data of the Ministry of Health (11). However, the actual number of people with CD in the province of Çorum is unclear, as a large number of patients have not been diagnosed.
The objective of our study was to determine the frequency of childhood CD in the province of Çorum, to identify silent celiac and atypical celiac patients, and to increase information and sensitivity about the disease.
MATERIALS AND METHODS
There are 82,124 primary, secondary, and high school students in the city center and towns of the province of Çorum. Considering the different socioeconomic characteristics of the city center and towns, we used a stratified random sampling method for sample selection. Four large towns, including the city center (Osmancık, Sungurlu, Alaca, and İskilip), were considered one stratum each, and the sample number was determined according to the number of students (Figure 1). In addition, the number of all primary, secondary, and high school students was determined, and care was taken to reflect the towns according to the weight of strata. After the number of samples was determined, which students to include in the sample was determined according to a simple random sampling method. To determine the research sample size, the minimum number needed to reflect the universe statistically was calculated using the formula of “sample size with a known number of individuals in the universe;” p=0.01, q=0.09, the sampling error was accepted as d=0.05, and the t-table statistical value was accepted as t=1.96. Students with a known diagnosis of CD were not included in the study. Celiac screening was conducted using rapid celiac test kits (BiocardTM celiac test, Labsystems Diagnostics Oy Vantaa, Finland) with a sensitivity of 97.8% and specificity of 96.3%, which can qualitatively measure tissue transglutaminase (tTg) IgA with an immunochromatographic method. The reaction obtained by mixing the reagent solution with a fingerstick blood sample (10 μL) can be assessed within the first 5 minutes. In the kit, one line in the control area is a negative result, lines on both the control and test area (two lines) are positive, and no line is considered IgA deficiency.
Figure 1.
Sample selection areas in Çorum, Turkey
The study was funded by the Scientific Research Projects Unit, Hitit University. Approval of the study was obtained from the Local Clinical Research Ethics Board, and signed volunteer consent forms were obtained from parents for study participants. A survey including information about each child’s delivery method, birth weight, breastfeeding characteristics, and other sociodemographic data, was administered.
IgA and tTg IgA were checked in serum samples from children who were positive for CD and IgA deficiency. In addition, the serum tTg IgG and endomysial antibody IgG levels were measured in children with selective IgA deficiency. Tissue transglutaminase IgA, IgG, and endomysial antibody IgG (Euroimmun, Germany) were determined with micro enzyme-linked immunosorbent assay. An endoscopic duodenum biopsy was performed on patients with positive antibodies by using a pediatric gastroscope (Fujifilm EG 530 FP Japan). Multiple biopsy samples were obtained from the second part of the duodenum. The diagnoses for children with positive results were made according to the ESPGHAN 2012 criteria (12), and duodenum biopsies were assessed according to the Marsh-Oberhuber criteria (13). The Statistical Package for Social Sciences (SPSS) Version 22.0 (IBM Corp.; Armonk, NY, USA) was used for data analysis in this study. Descriptive statistics are presented as numbers and percentage (%) for qualitative variables and as average±standard deviation for quantitative variables.
RESULTS
This study was conducted between May 1, 2016 and May 1, 2017. The screening was conducted by a team of 15 doctors and nurses. There were a total 1730 students from 216 schools included in the study: 883 from the province of Çorum, 213 from Osmancık, 214 from Sungurlu, 220 from Alaca, and 200 from İskilip. A total of 877 (50.6%) students were female, 584 (33.7%) were primary school students, 585 (33.8%) were secondary school students, and 561 (32.5%) were high school students.
Of the 883 (51%) students screened in the province of Çorum, 455 (51.5%) were female, 301 (34%) were primary school students, 299 (34%) were secondary school students, and 283 (32%) were high school students. In 92 schools in the towns, 847 (49%) students were included in the study; 422 (49.8%) were female, 283 (33.4%) were primary school students, 286 (33.8%) were secondary school students, and 278 (32.8%) were high school students (Table 1).
Table 1.
Distribution of students participating in the survey according to regions and schools
| Screening Regions | Number of Students | Schools | Total Number of Students | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Female | Male | Primary Schools | Secondary Schools | High Schools | |||
| City Center | 455 (51.5%) | 428 (48.5%) | 301 (34%) | 299 (34%) | 283 (32%) | 883 (%51) | |
| Towns | Osmancık | 112 (52.5%) | 101 (47.5%) | 73 (34%) | 76 (36%) | 64 (30%) | 213 (12.3%) |
| Sungurlu | 107 (50%) | 107 (50%) | 76 (36%) | 65 (30%) | 73 (34%) | 214 (12.4%) | |
| Alaca | 105 (48%) | 115 (52%) | 74 (33.5%) | 74 (33.5%) | 72 (33%) | 220 (12.8%) | |
| İskilip | 98 (49%) | 102 (51%) | 60 (30%) | 71 (35.5%) | 69 (34.5%) | 200 (11.5%) | |
| Total Number of Students | 877 (50.6%) | 853 (49.4%) | 584 (33.7%) | 585 (33.8%) | 561 (32.5%) | 1730 (100%) | |
According to rapid celiac screening, the IgA deficiency was found in six children (Figure 2). Serum IgA levels of these children were also below 6 mg/dL, and other immunoglobulin levels were normal. These children were diagnosed with selective IgA deficiency. The frequency of selective IgA deficiency was found to be 3.4/1000 (1:288). The results of screening showed a total of 8 students had CD: 5 in the city center of Çorum, 1 in Osmancık, 1 in Alaca, and 1 in Sungurlu (Figure 2). Four (50%) of these students were female, and the average age was 11.6±3.4 (9–17) years. Four (50%) were primary school students, 2 (25%) were secondary school students, and 2 (25%) were high school students. Three of the children had atypical CD, and 5 had silent CD. Two children had chronic abdominal pain, and 1 had a complaint of growth retardation. Physical examination revealed short stature in 2 children. In laboratory assessments, 3 children were detected with iron deficiency, 3 with vitamin B12 deficiency, 3 with vitamin D deficiency, and 1 with folate deficiency. One child had Hashimoto thyroiditis. Two bulbus biopsy samples, and 4 duodenum biopsy samples were taken from these patients. A Marsh 3c lesion was found in 2 patients, Marsh 3b lesion was found in 1 patient, and Marsh 3a lesion in 5 patients (Table 2). With these findings, all 8 children were diagnosed with CD, and they were started on a gluten-free diet. In line with these results, we concluded that the frequency of CD was 4.6/1000 in Çorum, and 1 out of every 216 children in Çorum had CD. Upon screening the families of these children, 2 siblings of 2 patients were found to have CD.
Figure 2.
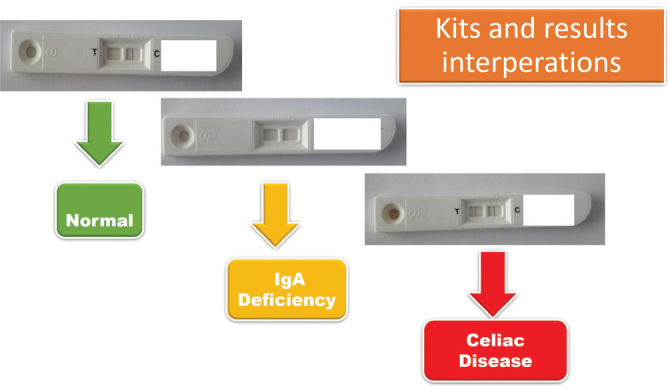
Kits and results interpretations
Table 2.
Clinical and laboratory characteristics of patients with celiac disease detected by screening
| Age (years) | Gender | Complaint | Physical Examination | Pathological Laboratory Findings | IgA mg/gL | tTg IgA U/mL | EMA IgA U/mL | Pathology* | Celiac Type | Family Screening |
|---|---|---|---|---|---|---|---|---|---|---|
| 16 | Male | None | Normal | Iron/B12/D vitamin deficiency | 75 | 74 | 88 | 3a | Silent | − |
| 8 | Female | None | Normal | Hashimoto thyroiditis D vitamin deficiency | 115 | 81 | 72 | 3a | Silent | + (her sister) |
| 14 | Female | Stomach ache | Normal | Anemia/Iron/B12/Folate deficiency | 588 | 89 | 88 | 3a | Atypical | − |
| 16 | Female | Growth retardation | Short stature | Iron, B12 deficiency | 86 | 98 | 86 | 3c | Atypical | − |
| 10 | Male | None | Normal | Normal | 142 | 88 | 92 | 3b | Silent | + (his sister) |
| 8 | Female | None | Normal | Normal | 230 | 198 | 184 | 3a | Silent | − |
| 9 | Male | Stomach ache | Short stature | D vitamin deficiency | 135 | 82 | 88 | 3c | Atypical | − |
| 8 | Male | None | Normal | Normal | 137 | 35 | 32 | 3a | Silent | − |
Marsh Oberhuber
tTg: tissue transglutaminase; EMA: endomysial antibody
DISCUSSION
Celiac disease is a systemic disease that occurs as a result of the interaction of genetic, immunologic, and environmental factors (7). The two most important factors that determine the frequency of the disease in any society are the frequency of major histocompatibility complex class II alleles that encode for human leukocyte antigen (HLA)-DQ and the person’s exposure to gluten-containing dietary products (9). The most important genetic predisposition for the disease is DQ2 and DQ8 loci, which have the HLA class 2 genes. HLA DQ2 is positive in 90%–95% of patients with CD, and HLA DQ8 is positive in 5%–10% of patients with CD (1). The contribution of HLA types to genetic risk for CD is estimated to be approximately 30%–50% (9). Thus, individuals with a history of CD in their family have a higher risk compared with the general population (14). CD can occur with typical (classical) and atypical findings. The frequency of typical (classical) CD is about 1/1000 (15). Classical CD includes findings of chronic diarrhea, abdominal distension, and weight loss in the 6–24 months after gluten is included in the diet (5,7). However, almost 50% of patients are currently diagnosed with non-GIS symptoms; thus, CD is accepted as a systemic disease. With the increase in diagnostic means and recognition of asymptomatic and atypical cases, CD incidence has increased significantly in the last 20 years, and the age at diagnosis has increased. Recently, it is thought that there may be 5–7 undiagnosed patients for each diagnosed patient (5). Due to these atypical and silent findings, the disease is likened to the “iceberg model,” with symptomatic patients making up only 10% of the iceberg (16).
Current recommendations emphasize the need to screen risk groups (such as people with type 1 diabetes, autoimmune thyroid disease, Down syndrome, and selective IgA deficiency) and first-degree relatives for CD. In a current cohort study, no obvious recommendation was made about the use of screening for asymptomatic (silent) patients with CD; it was concluded that further studies are needed (17). Population screening studies have shown that 50% to 70% of the patients diagnosed with CD are in fact asymptomatic (15). It is impossible to identify asymptomatic children without screening. Screening not only makes early diagnosis possible, but it can also be used to improve life quality for patients by introducing a gluten-free diet, even if patients are asymptomatic (18). Despite increased awareness and screening programs, delays of about 7–10 years often occur in the diagnosis of CD (19). If the diagnosis is delayed and treatment does not begin early, there is greater risk of developing serious conditions such as malignant diseases, metabolic bone diseases, and autoimmune diseases (20). A cohort study showed that the mortality risk of undiagnosed patients with CD was nearly 4 times higher than that of the normal population (21).
In current screening studies, the frequency of CD in children was found to be 0.9% in Germany, 0.2%–0.6% in Russia, 0.99% in the United Kingdom, and 0.71% in the United States (15,22–24). In a screening study conducted in Turkey by Dalgıç et al. (25) among 20,190 school children in 62 cities between 2006 and 2008, the frequency of a biopsy-confirmed CD diagnosis was 0.47%; however, when including participants who did not undergo biopsy, it was estimated that the prevalence could be as high as 1/58. The first local prevalence study in Turkey was conducted by Demirçeken et al. (26) between 2002 and 2003 among 1000 children in Ankara. In that study, the frequency of biopsy-confirmed CD was 0.9%. Screening was later conducted by Ertekin et al. (27) in Erzurum, a city in east Anatolia in Turkey, among 1263 children, and celiac autoimmunity was found to be 1/115; the frequency of biopsy-confirmed CD was found to be 1/158. In a screening study conducted in 502 Turkish university students between the ages 17 and 24 years, the frequency of the disease was found to be 0.8% (16). In a study conducted in 1554 children, Sezgin et al. (28) found that celiac autoimmunity was 0.77%, and the frequency of diagnosis of biopsy-confirmed CD was 0.39%. In our study, screening was conducted among 1730 children, with 883 in the city center and 847 in surrounding towns. To the best of our knowledge, ours is the first celiac screening study in the province of Çorum and the Black Sea region of Turkey. A total of 8 children had positive results upon celiac screening. In all these children, a diagnosis of CD was confirmed by serology and duodenum biopsies. Three of the children had atypical CD, and 5 had silent (asymptomatic) CD. Although the disease is more frequent in adult women, its frequency is equal for both sexes during childhood (29). Accordingly, the prevalence in males and females was similar in our study (0.45% vs 0.47%). Among first-degree relatives of patients with CD, the CD risk was higher compared with the normal population (6); in this group, the biopsy-confirmed CD prevalence rate is between 2.8% and 12%. This rate reaches 30% in HLA-identical siblings and 30%–70% in monozygotic twins (30). Two siblings of children in our study, aged 5 and 8 years, were found to have CD.
Limitation of this study is that the screening was performed with a qualitative test although the test kits is highly sensitive and specific. For this reason, the titers in sera tTg IgA antibodies of the screened children could not be quantified. Therefore, children with low sera tTg IgA antibodies levels may have been found to have false negative in their kits. In this study, we could not comment on the specificity of the test kits, but the sensitivity of kits was very high because all children who were found positive in their kit had CD.
In conclusion, the frequency of CD was found to be 0.46% (1:216) in Çorum, Turkey. These results show that the frequency of CD is high in Çorum, as has been reported in other studies. Although screening is not routinely recommended for patients with silent CD, screening programs should be more frequent in Turkey as well as in other countries worldwide. To identify patients with atypical CD, it is important to increase information and awareness about the disease and to periodically screen individuals in high-risk groups, even if they are asymptomatic.
Acknowledgements
We thank Hitit University Rectorship and the Çorum Provincial Directorate of National Education for supporting this project at every step in a good cooperation. We thank Şahin Özcan, Hayati Özdemir and İsmail Serdar Yakar from the Provincial Directorate of National Education Research and Development Unit for participating actively and making a great effort. We also thank Ayşe Koyun, Büşra İlhan, Eda Nur Eroğlu, Esra Yüksel, Hüsne Bozdemir, Kübra Bayraktar, Kübra Böcekçi, Meral Küçükkale, Merve Bilaloğlu, Pamuk Gözübüyük, Sena Ankara and Yasemin Yılmaz from Health School for working voluntarily during the survey process of the study.
Footnotes
“See Editorial Comment 530–1”
Ethics Committee Approval: Ethics committee approval was received for this study from the Hitit University Clinical Research Ethics Board.
Informed Consent: Written informed consent was obtained from parents of the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - A.C., N.B.E., E.D.; Design - A.C., N.B.E., E.D.; Supervision - A.C., N.B.E., E.D.; Resources - N.B.E.; Materials - A.C.; Analysis and/or Interpretation - A.C., E.D.; Literature Search - A.C.; Writing Manuscript - A.C., N.B.E., E.D.; Critical Review - A.C., N.B.E., E.D.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Maki M. Celiac Disease. In: Kleinman RE, Goulet OJ, Mielli-Vergani G, Sanderson IR, Sherman PM, Shneider BL, editors. Walker’s Pediatric Gastrointestinal Disesase. Shelton, CT: People’s Medikal Publishing House; 2008. pp. 319–28. [Google Scholar]
- 2.Kagnoff MF. Celiac disease: patogenesis of a model immunogenetic disease. J Clin Invest. 2007;117:41–9. doi: 10.1172/JCI30253. https://doi.org/10.1172/JCI30253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill ID, Klish WJ. Management of celiac disease in children. Available form: URL http://www.uptodate.com/contents/celiac-disease-in-children-beyond-the-basics.
- 4.Ravikumara M, Tuthill DP, Jenkins HR. The changing clinical presentation of coeliac disease. Arch Dis Child. 2006;91:969–97. doi: 10.1136/adc.2006.094045. https://doi.org/10.1136/adc.2006.094045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lionetti E, Catassi C. New clues in celiac disease epidemiology, pathogenesis, clinical manifestations, and treatment. Int Rev Immunol. 2011;30:219–31. doi: 10.3109/08830185.2011.602443. https://doi.org/10.3109/08830185.2011.602443 [DOI] [PubMed] [Google Scholar]
- 6.Uenishi TH, Gandolfi L, Almeida LM, et al. Screening for celiac disease in 1st degree relatives: a 10-year follow-up study. BMC Gastroenterol. 2014;14:36. doi: 10.1186/1471-230X-14-36. https://doi.org/10.1186/1471-230X-14-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guandalini S. Celiac disease. In: Guandalini S, editor. Essential Pediatric Gastroenterology Hepatology Nutrition. Chicago: McGraw-Hill; 2004. pp. 221–30. [Google Scholar]
- 8.Kang Jy, Kang AH, Gren A, Gwee KA, Ho Ky. Systematic review: world wide variation in the frequency of coeliac disease and changes over time. Aliment Pharmacol Ther. 2013;38:226–45. doi: 10.1111/apt.12373. https://doi.org/10.1111/apt.12373 [DOI] [PubMed] [Google Scholar]
- 9.Cummins AG, Roberts-Thomson IC. Prevalence of celiac disease in the Asia-Pacific region. J Gastroenterol Hepatol. 2009;24:1347–51. doi: 10.1111/j.1440-1746.2009.05932.x. https://doi.org/10.1111/j.1440-1746.2009.05932.x [DOI] [PubMed] [Google Scholar]
- 10.Çorum guide-city information. Available Form: URL http://www.dimpletravel.com/info/turkey/corum/
- 11.Çölyak hastalığı görülme sıklığı ve illere dağılımı. Available Form: Url http://Beslenme.Gov.Tr/İndex.Php?Page=519.
- 12.Husby S, Koletzko S, Korponay-Szabó IR, et al. ESPGHAN Working Group on Coeliac Disease Diagnosis; ESPGHAN Gastroenterology Committee; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–60. doi: 10.1097/MPG.0b013e31821a23d0. https://doi.org/10.1097/MPG.0b013e31821a23d0 [DOI] [PubMed] [Google Scholar]
- 13.Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185–94. doi: 10.1097/00042737-199910000-00019. https://doi.org/10.1097/00042737-199910000-00019 [DOI] [PubMed] [Google Scholar]
- 14.Karakoyun M, Deviren R, Öztürk O, Genç RE, Aydoğdu S. Celiac disease screening on students of the department of nutritions and dietetics and medical school at Ege University. J Ped Research. 2015;2:66–9. https://doi.org/10.4274/jpr.52824 [Google Scholar]
- 15.Laass MW, Schmitz R, Uhlig HH, Zimmer KP, Thamm M, Koletzko S. The prevalence of celiac disease in children and adolescents in Germany. Dtsch Arztebl Int. 2015;112:553–60. doi: 10.3238/arztebl.2015.0553. https://doi.org/10.3238/arztebl.2015.0553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodrigues AF, Jenkins HR. Coeliac disease in children. Curr Paediatr. 2006;16:317–32. https://doi.org/10.1016/j.cupe.2006.07.010 [Google Scholar]
- 17.Chou R, Bougatsos C, Blazina I, Mackey K, Grusing S, Selph S. Screening for Celiac Disease: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2017;317:1258–68. doi: 10.1001/jama.2016.10395. https://doi.org/10.1001/jama.2016.10395 [DOI] [PubMed] [Google Scholar]
- 18.Corrao G, Corazza GR, Bagnardi V, et al. Mortality in patients with coeliac disease and their relatives: a cohort study. Lancet. 2001;358:356–61. doi: 10.1016/s0140-6736(01)05554-4. https://doi.org/10.1016/S0140-6736(01)05554-4 [DOI] [PubMed] [Google Scholar]
- 19.Fuchs V, Kuppa K, Huhtala H, et al. Factors associated with long diagnostic delay in celiac disease. Scand J Gastroenterol. 2014;49:1304–10. doi: 10.3109/00365521.2014.923502. https://doi.org/10.3109/00365521.2014.923502 [DOI] [PubMed] [Google Scholar]
- 20.Gursoy S, Guven K, Simsek T, et al. The prevalence of unrecognized adult celiac disease in Central Anatolia. J Clin Gastroenterol. 2005;39:508–11. doi: 10.1097/01.mcg.0000165664.87153.e1. https://doi.org/10.1097/01.mcg.0000165664.87153.e1 [DOI] [PubMed] [Google Scholar]
- 21.Rubio-Tapia A, Kyle RA, Kaplan EL, et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology. 2009;137:88–93. doi: 10.1053/j.gastro.2009.03.059. https://doi.org/10.1053/j.gastro.2009.03.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mustalahti K, Catassi C, Reunanen A, et al. The prevalence of celiac disease in Europa: results of centralized, international mass screening project. Ann Med. 2010;42:587–95. doi: 10.3109/07853890.2010.505931. https://doi.org/10.3109/07853890.2010.505931 [DOI] [PubMed] [Google Scholar]
- 23.Savvateeva LV, Erdes SI, Antishin AS, Zamyatnin AA., Jr Overview of Celiac Disease in Russia: Regional Data and Estimated Prevalence. J Immunol Res. 2017;2017:2314813. doi: 10.1155/2017/2314813. https://doi.org/10.1155/2017/2314813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubio-Tapia A, Ludvigsson JF, Brantner TL, Murray JA, Everhart JE. The prevalence of celiac disease in the United States. Am J Gastroenterol. 2012;107:1538–44. doi: 10.1038/ajg.2012.219. https://doi.org/10.1038/ajg.2012.219 [DOI] [PubMed] [Google Scholar]
- 25.Dalgic B, Sari S, Basturk B, et al. Prevalence of celiac disease in healthy Turkish school children. Am J Gastroenterol. 2011;106:1512–7. doi: 10.1038/ajg.2011.183. https://doi.org/10.1038/ajg.2011.183 [DOI] [PubMed] [Google Scholar]
- 26.Demirçeken FG, Kansu A, Kuloğlu Z, Girgin N, Güriz H, Ensari A. Human tissue transglutaminase antibody screening by immunochromatographic line immunoassay for early diagnosis of celiac disease in Turkish children. Turk J Gastroenterol. 2008;19:14–21. [PubMed] [Google Scholar]
- 27.Ertekin V, Selimoğlu MA, Kardaş F, Aktaş E. Prevalence of celiac disease in Turkish children. J Clin Gastroenterol. 2005;39:689–91. doi: 10.1097/01.mcg.0000174026.26838.56. https://doi.org/10.1097/01.mcg.0000174026.26838.56 [DOI] [PubMed] [Google Scholar]
- 28.Sezgin O, Sarıtaş B, Aydın İ, Şaşmaz T, Lınke ES. Celiac disease prevalence in Turkey: A Population based cross-sectional study. Acta Medica Mediterranea. 2016;32:719–27. [Google Scholar]
- 29.Lebenthal E, Shteyer E, Branski D. The changing clinical presentation of celiac disease. Pediatr Adolesc Med. 2008;12:18–22. https://doi.org/10.1159/000128609 [Google Scholar]
- 30.Doğan Y, Yıldırmaz S, Özercan İB. Prevalence of celiac disease among first-degree relatives of patients with celiac disease. JPGN. 2012;55:205–8. doi: 10.1097/MPG.0b013e318249378c. https://doi.org/10.1097/MPG.0b013e318249378c [DOI] [PubMed] [Google Scholar]


