Abstract
Background/Aims
Helicobacter pylori infection is a risk factor for gastric cancer and colorectal cancer (CRC). MDM2 SNP309 G/G homozygosity is known to be the genetic background that influences the severity of inflammation in the gastric mucosa, and it corresponds to CRC development. We examined the role of screening colonoscopy in H. pylori-related chronic gastritis and the association of patients who have MDM2 SNP309 G/G homozygosity and advanced colorectal neoplasia (CRN) susceptibility.
Materials and Methods
A prospective cross-sectional study was used to investigate H. pylori-related gastritis in 331 consecutive asymptomatic patients who had MDM2 SNP309 G/G homozygosity and who were enrolled from November 2014 to July 2017. The MDM2 SNP309 polymorphism was genotyped by real-time PCR hybridization probe assay.
Results
Totally, there were 331 patients with H. pylori-related gastritis, of whom 39 (8.76%) had advanced CRN. The H. pylori-positive group comprised 180 patients (54.36%). H. pylori infection was associated with advanced CRN (OR: 2.09, 95% CI: 1.56–2.80; p=0.01) and had an increased risk of advanced CRN (OR: 4.24, 95% CI: 1.76–5.21; p=0.01) after adjusting for confounding factors. Patients with H. pylori infection had a significantly increased risk of high-grade dysplasia or invasive adenocarcinoma (OR: 2.96, 95% CI: 1.48–4.17; p=0.03).
Conclusion
Chronic gastritis patients infected with H. pylori and who had MDM2 SNP309 G/G homozygosity had an increased risk of advanced CRN, particularly high-grade dysplasia including invasive adenocarcinoma. Screening colonoscopy in these patients might benefit colorectal polyp diagnosis and prevention and early CRC treatment in the Thai population.
Keywords: Helicobacter pylori, chronic gastritis, atrophy, colorectal neoplasm, colorectal cancer, screening colonoscopy
INTRODUCTION
Colorectal cancer (CRC) is the third and fifth most common type of cancer in males and females in Thailand, respectively (1). Many studies have suggested that alcohol consumption, smoking, obesity, dietary habits, and family history of CRC are risk factors for the development of CRC (2–5). The association of Helicobacter pylori-related gastritis and CRC is inconclusive (6,7). Several studies have demonstrated that there is an association of H. pylori infection with CRC, whereas other studies have not shown such a positive association (8,9). Discrepancies in results between these studies may be attributed to differences in the strain of H. pylori causes the infection. Infection with the cytotoxin-associated gene A-positive (CagA+) strain is associated with a higher risk of colorectal adenocarcinoma than infection with the CagA- strain (10). Screening colonoscopy can be performed to reduce the incidence of and mortality due to advanced colorectal neoplasia (CRN). In a large single-center study in Thailand, the incidence of colorectal polyps was 30.6%, as determined by screening colonoscopy; however, there is no report on screening colonoscopy in patients with chronic gastritis (11). Clinical practice guidelines for CRC screening in 2016 do not recommend performing screening colonoscopy for CRC in patients with chronic gastritis (12). A recent study in Thailand, “Thailand Consensus on H. pylori Treatment 2015.” does not recommend performing screening colonoscopy for CRC in H. pylori-infected patients with chronic gastritis or premalignant gastric mucosa (13). Mouse double minute 2 (MDM2) is an oncoprotein that acts as a negative regulator inhibiting p53 tumor suppressor activity (14). Studies have shown that the MDM2 gene is involved in the inflammatory process, with p53-independent activation of nuclear factor-kappa beta. Altered expression of this gene is likely to contribute to the modulation of inflammation and carcinogenesis. It has been proposed that MDM2 overexpression results in a weakened p53 tumor suppressor pathway, resulting in a higher mutation rate, poorer DNA repair processes, and reduced apoptosis. Thus, the ultimate result is faster and more frequent tumor formation (15). The levels of cell lines and tissues with the SNP309 G/G homozygous genotype shown at the RNA and the protein levels were elevated in cells with SNP309. Cells with SNP309 G/G homozygosity and a higher level of MDM2 protein had a lower apoptotic response than cells with T/T at the SNP309 locus (16,17). In our previous studies, genetic polymorphism of the MDM2 gene (SNP309) showed that G/G homozygosity is correlated with type 4 and type 5 gastric mucosal patterns, suggesting that the G/G genotype of MDM2 SNP309 contributes to histopathological severity (18–20). The association of the MDM2 SNP309 polymorphism with CRC risk has also been reported (21–24). Terng et al. found that CRC risk is related to the upregulation of MDM2. They showed that patients with MDM2 upregulation are increased risk of CRC (25).
The role of screening colonoscopy in H. pylori-related chronic gastritis has been inconclusive, even in Thailand. Therefore, the present study was aimed to determine the role of screening colonoscopy in patients with H. pylori-related chronic gastritis who carried the homozygous G/G genotype of MDM2 SNP309 and to investigate the association of MDM2 SNP309 G/G homozygosity with advanced CRN susceptibility in the Thai population.
MATERIALS AND METHODS
Patients
Esophagogastroduodenoscopy (EGD) was performed in 1,012 subjects with chronic abdominal pain who were enrolled between November 2014 and July 2017. In total, 331 patients with chronic gastritis were included; they carried the MDM2 SNP309 G/G genotype and were without a history of the following: H. pylori eradication therapy in the past 2 months; previous gastric surgery; use of gastrointestinal medications including PPIs; use of antimicrobials, H2-blockers, or bismuth compounds in the past 2 months; and CRC or incomplete colonoscopy preparation (Figure 1). Good clinical practice recommendations following the guidelines of the Declaration of Helsinki were followed. All patients provided informed consent. The study protocol was accepted by the Ethics Committee for Research Involving Human Subjects (EC-57–34).
Figure 1.
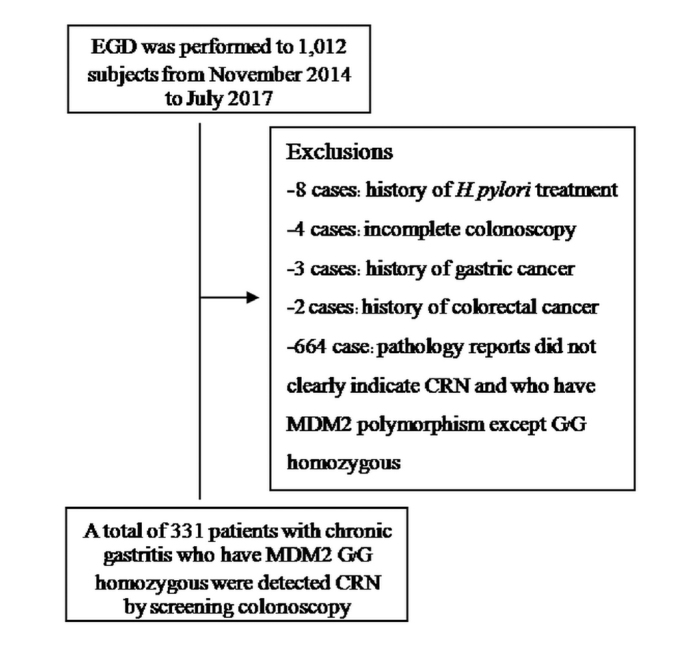
Flow chart of the study population
Diagnosis of H. pylori infection, chronic gastritis, and advanced CRN
H. pylori infection was diagnosed while performing a histopathological examination. Biopsy samples were taken from the observation area, and H. pylori was detected using the rapid urease test on site (ProntodyleR, GASTREX, France). H. pylori infection was demonstrated by PCR. Chronic gastritis was diagnosed by five pathologists from a laboratory in Bangkok. The histopathology of advanced CRN was defined as adenomas that were >1 cm in diameter, high-grade dysplasia or invasive adenocarcinomas, villous adenomas, and >3 adenomas, according to the World Health Organization classification (26).
MDM2 SNP309 polymorphisms analysis
MDM2 SNP309 genotypes were evaluated by real-time PCR using the LightCycler® 480 system (Roche Diagnostics, Neuilly-sur-Seine, France). The identification of PCR products was completed by melting curve analyses. Target PCR products were generated using primers as previously reported. The use of 3 μL of DNA templates in 20 μL of a PCR reaction mixture consisted of forward and reverse primers (20 M each), sensor and anchor probes (20 M each), 2 μL of Fast Start DNA Master Hybridization Probes, and 25 mM of MgCl2. PCR amplification included initial denaturation at 95°C for 10 min, annealing at 60°C for 10 s, and extension at 72°C for 17 s. G/G homozygous, T/T homozygous, and G/T heterozygous genotypes were analyzed using LightCycler® 480 Software 1.5 (Roche Diagnostics). The success rate of genotyping for each SNP was over 94%.
Endoscopic procedure
Esophagogastroduodenoscopy and colonoscopy procedures were conducted using a gastrointestinal video endoscope (Olympus EVIS EXERA III, CV-190, Japan). The whole stomach was investigated by performing conventional endoscopy. After mucosa of the whole stomach was noted, specific gastric mucosa was collected from the site using “site-specific biopsy” (27). Colonoscopy was performed in patients with chronic gastritis with MDM2 SNP309 G/G homozygosity.
Statistical analysis
The association of the status of H. pylori infection with colonoscopy findings in patients with chronic gastritis and MDM2 SNP309 G/G homozygosity was analyzed using univariate analysis and logistic regression model analysis by adjusting for confounding factors. p values of <0.05 were considered statistically significant. All statistical analyses were performed using Statistical Package for Social Sciences Version 20.0 (IBM Corp.; Armonk, NY, USA).
RESULTS
Baseline characteristics of the study population
A prospective study of 1,012 subjects who underwent EGD for screening was performed from November 2014 to July 2017. Totally, 331 patients participated and were analyzed, while 664 patients were excluded because of their pathology reports, which did not clearly indicate advanced CRN. Patients who had the MDM2 polymorphism except for G/G homozygosity included 8 with a history of H. pylori treatment, 4 with incomplete colonoscopy, 3 with a history of GC, and 2 with a history of CRC (Figure 1). The mean age of the patients was 49.1±8.2 years in the H. pylori-negative group and 44.2±6.1 years in the H. pylori-positive group. However, there were statistically significant differences in the distribution of gender between patients in the two groups. The baseline characteristics of the patients are shown in Table 1.
Table 1.
Baseline characteristics and the H. pylori status of patients with chronic gastritis who had MDM2 SNP309 G/G homozygosity and who underwent screening colonoscopy
| Characteristics | H. pylori (−) (n=151) | H. pylori (+) (n=180) | p |
|---|---|---|---|
| Age, years | 49.1±8.2 | 44.2±6.1 | 0.16 |
| Male sex | 77 (50.9%) | 68 (37.7%) | 0.01 |
| Body mass index <25 kg/m2 | 22.8±5.0 | 23.1±5.3 | 0.13 |
| Smoking | 22 (14.5%) | 24 (13.3%) | 0.07 |
| Alcohol | 18 (11.9%) | 20 (11.1%) | 0.14 |
| Diabetes | 22 (14.5%) | 24 (13.3%) | 0.42 |
| Hypertension | 14 (9.2%) | 17 (9.4%) | 0.54 |
| Dyslipidemia | 19 (12.5%) | 21 (11.6%) | 0.72 |
| Family history of colorectal cancer | 12 (7.9%) | 15 (8.3%) | 0.27 |
The chi-square test was used for categorical variables, and the t-test was used for continuous variables
Association of H. pylori-related gastritis with MDM2 SNP309 G/G homozygosity and CRN
Among the 331 patients, the H. pylori-positive rate was 54.36%. H. pylori-positive patients with chronic gastritis who carried the MDM2 SNP309 G/G homozygous genotype had a high prevalence of overall CRN (OR: 1.98, 95% CI: 1.24–2.16; p=0.01). Chronic gastritis patients who had H. pylori-infection and who had MDM2 SNP309 G/G homozygosity had a high prevalence of advanced CRN (OR: 2.09, 95% CI: 1.56–2.80; p=0.01), particularly high-grade dysplasia or invasive adenocarcinoma (OR: 5.16; 95% CI: 1.94–8.71; p=0.01), as determined by univariate analysis (Table 2). In logistic regression and multivariate analyses, H. pylori infection demonstrated an increased risk of overall and advanced CRN (OR: 3.89, 95% CI: 1.64–1.72; p=0.02 and OR: 4.24, 95% CI: 1.76–5.21; p=0.01, respectively) (Table 3).
Table 2.
Association of H. pylori infection status with colonoscopy findings in patients with chronic atrophic gastritis who had MDM2 SNP309 G/G homozygosity and who underwent surveillance colonoscopy (univariate analysis)
| Characteristics | H. pylori (−) (n=151) | (+) (n=180) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| No. (%) | OR (95% CI) | p | No. (%) | OR (95% CI) | p | |
| Colonoscopy findings | ||||||
| Overall CRN | 13 (8.6%) | 1.42 (0.86–1.83) | 0.03 | 39 (21.6%) | 1.98 (1.24–2.16) | 0.001 |
| Advanced CRN | 8 (5.2%) | 1.12 (0.26–1.37) | 0.06 | 31 (17.2%) | 2.09 (1.56–2.80) | 0.001 |
| Villous adenomatous polyp | 1 (0.6%) | 1.06 (0.81–1.67) | 0.10 | 5 (2.7%) | 2.13 (1.28–3.56) | 0.041 |
| Size of polyp <1 cm | 2 (1.3%) | 1.27 (0.38–2.78) | 0.45 | 9 (5.0%) | 1.18 (0.96–2.58) | 0.034 |
| High-grade dysplasia or invasive adenocarcinoma | 4 (2.6%) | 2.04 (1.18–4.87) | 0.02 | 14 (7.7%) | 5.16 (1.94–8.71) | 0.001 |
| No. of adenomas<3 | 1 (0.6%) | 1.02 (0.41–1.38) | 0.28 | 3 (1.6%) | 1.15 (0.69–2.21) | 0.072 |
| Other CRN | 5 (3.3%) | 1.43 (0.56–2.24) | 0.12 | 8 (4.4%) | 1.24 (0.98–2.32) | 0.024 |
The chi-square test was used for categorical variables, and the t-test was used for continuous variables
Table 2.
Association of H. pylori infection status with colonoscopy findings in patients with chronic atrophic gastritis who had MDM2 SNP309 G/G homozygosity and who underwent surveillance colonoscopy (univariate analysis)
| Characteristics | H. pylori (−) (n=151) | H. pylori (+) (n=180) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| No. (%) | OR (95% CI) | p | No. (%) | OR (95% CI) | p | |
| Colonoscopy findings | ||||||
| Overall CRN | 13 (8.6%) | 0.95 (0.75–1.21) | 0.18 | 39 (21.6%) | 3.89 (1.64–8.25) | 0.02 |
| Advanced CRN | 8 (5.2%) | 1.49 (0.57–1.48) | 0.24 | 31 (17.2%) | 4.24 (1.76–5.21) | 0.01 |
| Villous adenomatous polyp | 1 (0.6%) | 0.76 (0.52–1.47) | 0.52 | 5 (2.7%) | 1.03 (0.78–1.38) | 0.36 |
| Size of polyp≥1 cm | 2 (1.3%) | 1.14 (0.68–2.17) | 0.61 | 9 (5.0%) | 1.02 (0.86–2.13) | 0.64 |
| High-grade dysplasia or invasive adenocarcinoma | 4 (2.6%) | 1.24 (0.97–3.76) | 0.14 | 14 (7.7%) | 2.96 (1.48–4.71) | 0.03 |
| No. of adenoma≥3 | 1 (0.6%) | 0.92 (0.71–1.48) | 0.37 | 3 (1.6%) | 1.05 (0.79–1.22) | 0.47 |
| Other CRN | 5 (3.3%) | 0.89 (0.56–1.24) | 0.12 | 8 (4.4%) | 1.14 (0.72–2.13) | 0.09 |
OR: odds ratio; CI: confidence interval; CRN: colorectal neoplasm
Logistic regression was used to analyze the data
Adjustments were made by incorporating all relevant factors, such as age, gender, family history of colorectal cancer, body mass index, diabetes, hypertension, dyslipidemia, smoking, and alcohol consumption, into the analysis
DISCUSSION
H. pylori infection is associated with many gastroenterologic diseases as well as hematologic diseases. It is a risk factor for colorectal polyps and CRC. Several studies have demonstrated the correlation between H. pylori infection and CRN development. In 1995, the incidence of hypergastrinemia was reported to high in H. pylori- positive patients (5.2 fold), while gastrin or its processing intermediates were present in a high proportion of patients with CRC (28). In a prospective study, 8.6% of patients with CRC had high serum gastrin levels. Hypergastrinemia is associated with an increased risk of CRC (OR: 3.9, 95% CI: 1.5–9.8) (29). A case-control study showed that hypergastrinemia is a risk factor for colon cancer and distal colon (OR: 3.2, 95% CI: 1.4–7.5) (30). The intestinal flora-changing hypothesis has been proposed. The hypochlorhydric status promoted the fermentation of bacteria in the large intestine, which may be an etiology of colonic malignancy resulting in the malabsorption of proteins. The inflammation-mediated hypothesis, in which IL-8 is an autocrine growth factor in colon carcinoma cell lines, has been proposed (31). The seropositivity of H. pylori CagA+ is associated with an increased risk of GC and colonic cancer (OR: 10.6, 95% CI: 2.7–41.3; p=0.001) (32). It has been proposed that H. pylori infection can increase the risk of CRC through mechanisms related to chronic infection and/or alteration of bacterial flora that comprise the gastrointestinal microenvironment. Other evidence has shown that colorectal and gastric cancers may share aspects of a common etiology. Specifically, CRC has consistently been found to be the most common synchronous cancer among patients with gastric cancer, where primary gastric cancers are increased following CRC diagnosis and, correspondingly, second primary CRCs are increased following gastric cancer diagnosis (33). Thus, our study revealed a significant association of H. pylori infection with the prevalence of colorectal polyps, particularly adenomas with high-grade dysplasia or invasive adenocarcinoma in the Thai population (OR: 2.96, 95% CI: 1.48–4.17; p=0.003). With MDM2 SNP309 G/G homozygosity, there is an increased risk of H. pylori infection. Therefore, MDM2 SNP309 G/G homozygosity increased the risk of advanced CRN. However, multicenter studies with a large population are needed for the high number of H. pylori-positive patients who exhibit a high prevalence of H. pylori infection. Further investigations of the association of H. pylori infection with CRC risk may take into account H. pylori strain types to test the hypothesis.
In conclusion, patients with chronic gastritis infected with H. pylori and who carried the MDM2 SNP309 G/G homozygous genotype were at an increased risk of advanced CRN, particularly high-grade dysplasia including invasive adenocarcinoma. Screening colonoscopy in these patients might benefit colorectal polyp diagnosis and prevention and early CRC treatment in the Thai population. Further evaluations of other H. pylori factors associated with gastritis can provide an understanding of underlying factors influencing the clinical consequences of colorectal polyp or CRN development.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Suranaree University of Technology (Decision Date: December 1, 2015; Decision No: EC-57-34).
Informed Consent: Written informed consent was obtained from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - T.T.; Design - T.T.; Supervision - T.T.; Resources - T.T.; Materials - T.T.; Data Collection and/or Processing - T.T., T.S., W.W.; Analysis and/or Interpretation - T.T., T.S., W.W.; Literature Search - T.T., T.S.; Writing Manuscript - T.T., T.S.; Critical Review - T.T., T.S.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: This study was supported by a grant from Suranaree University of Technology (SUT) and by the office of the higher education commission under NRU project of Thailand.
REFERENCES
- 1.Imsamran W, Chaiwerawuttana A, Waingnon S, et al. National Cancer Institute. Cancer in Bangkok Thailand. 2012;6:2004–6. [Google Scholar]
- 2.Ganesh B, Talole SD, Dikshit R. A case-control study on diet and colorectal cancer from Mumbai, India. Cancer Epidemiol. 2009;33:189–93. doi: 10.1016/j.canep.2009.07.009. https://doi.org/10.1016/j.canep.2009.09.002 [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Zhao Y, Jiang J, et al. Polymorphisms in DNA repair genes XRCC1, XRCC3 and XPD, and colorectal cancer risk: a case-control study in an Indian population. J Cancer Res Clin Oncol. 2010;136:1517–25. doi: 10.1007/s00432-010-0809-8. https://doi.org/10.1007/s00432-010-0809-8 [DOI] [PubMed] [Google Scholar]
- 4.De Stefani E, Ronco AL, Boffetta P, et al. Nutrient-derived dietary patterns and risk of colorectal cancer: a factor analysis in Uruguay. Asian Pac J Cancer Prev. 2012;13:231–5. doi: 10.7314/apjcp.2012.13.1.231. https://doi.org/10.7314/APJCP.2012.13.1.231 [DOI] [PubMed] [Google Scholar]
- 5.Durko L, Malecka-Panas E. Lifestyle Modifications and Colorectal Cancer. Curr Colorectal Cancer Rep. 2014;10:45–54. doi: 10.1007/s11888-013-0203-4. https://doi.org/10.1007/s11888-013-0203-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong SN, Lee SM, Kim JH, et al. Helicobacter pylori infection increases the risk of colorectal adenomas: cross-sectional study and meta-analysis. Dig Dis Sci. 2012;57:2184–94. doi: 10.1007/s10620-012-2245-x. https://doi.org/10.1007/s10620-012-2245-x [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Hoffmeister M, Weck MN, Chang-Claude J, Brenner H. Helicobacter pylori infection and colorectal cancer risk: evidence from a large population-based case-control study in Germany. Am J Epidemiol. 2012;175:441–50. doi: 10.1093/aje/kwr331. https://doi.org/10.1093/aje/kwr331 [DOI] [PubMed] [Google Scholar]
- 8.Machida-Montani A, Sasazuki S, Inoue M, et al. Atrophic gastritis, Helicobacter pylori, and colorectal cancer risk: a case-control study. Helicobacter. 2007;12:328–32. doi: 10.1111/j.1523-5378.2007.00513.x. https://doi.org/10.1111/j.1523-5378.2007.00513.x [DOI] [PubMed] [Google Scholar]
- 9.Chen XZ, Schottker B, Castro FA, et al. Association of helicobacter pylori infection and chronic atrophic gastritis with risk of colonic, pancreatic and gastric cancer: A ten-year follow-up of the ESTHER cohort study. Oncotarget. 2016;7:17182–93. doi: 10.18632/oncotarget.7946. https://doi.org/10.18632/oncotarget.7946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haim S, Doug P, Aryeh F, et al. Relationship between Helicobacter pylori CagA status and colorectal cancer. American Journal of Gastroenterology. 2001;96:3406–10. doi: 10.1111/j.1572-0241.2001.05342.x. https://doi.org/10.1111/j.1572-0241.2001.05342.x [DOI] [PubMed] [Google Scholar]
- 11.Aswakul P, Prachayakul V, Lohsiriwat V, Bunyaarunnate T, Kachintorn U. Screening colonoscopy from a large single center of Thailand - something needs to be changed? Asian Pac J Cancer Prev. 2012;13:1361–4. doi: 10.7314/apjcp.2012.13.4.1361. https://doi.org/10.7314/APJCP.2012.13.4.1361 [DOI] [PubMed] [Google Scholar]
- 12.Ransohoff DF, Sox HC. Clinical Practice Guidelines for Colorectal Cancer Screening: New Recommendations and New Challenges. JAMA. 2016;315:2529–31. doi: 10.1001/jama.2016.7990. https://doi.org/10.1001/jama.2016.7990 [DOI] [PubMed] [Google Scholar]
- 13.Mahachai V, Vilaichone RK, Pittayanon R, et al. Thailand Consensus on Helicobacter pylori Treatment 2015. Asian Pac J Cancer Prev. 2016;17:2351–60. [PubMed] [Google Scholar]
- 14.Bond GL, Hu W, Levine A. A single nucleotide polymorphism in the MDM2 gene: from a molecular and cellular explanation to clinical effect. Cancer Res. 2005;65:5481–4. doi: 10.1158/0008-5472.CAN-05-0825. https://doi.org/10.1158/0008-5472.CAN-05-0825 [DOI] [PubMed] [Google Scholar]
- 15.Bond GL, Levine AJ. A single nucleotide polymorphism in the p53 pathway interacts with gender, environmental stresses and tumor genetics to influence cancer in humans. Oncogene. 2007;26:1317–23. doi: 10.1038/sj.onc.1210199. https://doi.org/10.1038/sj.onc.1210199 [DOI] [PubMed] [Google Scholar]
- 16.Bond GL, Hu W, Bond EE, et al. A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell. 2004;119:591–602. doi: 10.1016/j.cell.2004.11.022. https://doi.org/10.1016/j.cell.2004.11.022 [DOI] [PubMed] [Google Scholar]
- 17.Hong Y, Miao X, Zhang X, et al. The role of P53 and MDM2 polymorphisms in the risk of esophageal squamous cell carcinoma. Cancer Res. 2005;65:9582–7. doi: 10.1158/0008-5472.CAN-05-1460. https://doi.org/10.1158/0008-5472.CAN-05-1460 [DOI] [PubMed] [Google Scholar]
- 18.Tongtawee T, Dechsukhum C, Leeanansaksiri W, et al. Role of the Mdm2 SNIP 309 Polymorphism in Gastric Mucosal Morphologic Patterns of Patients with Helicobacter pylori Associated Gastritis. Asian Pac J Cancer Prev. 2016;17:1057–60. doi: 10.7314/apjcp.2016.17.3.1057. https://doi.org/10.7314/APJCP.2016.17.3.1057 [DOI] [PubMed] [Google Scholar]
- 19.Tongtawee T, Dechsukhum C, Leeanansaksiri W, et al. Genetic Polymorphism of MDM2 SNP309 in Patients with Helicobacter Pylori-Associated Gastritis. Asian Pac J Cancer Prev. 2015;16:7049–52. doi: 10.7314/apjcp.2015.16.16.7049. https://doi.org/10.7314/APJCP.2015.16.16.7049 [DOI] [PubMed] [Google Scholar]
- 20.Tongtawee T, Dechsukhum C, Talabnin K, et al. Correlation between Patterns of Mdm2 SNIP 309 and Histopathological Severity of Helicobacter pylori Associated Gastritis in Thailand. Asian Pac J Cancer Prev. 2015;16:7781–4. doi: 10.7314/apjcp.2015.16.17.7781. https://doi.org/10.7314/APJCP.2015.16.17.7781 [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Pageon L, Post SM. Impact of the Mdm2 (SNP309-G) allele on a murine model of colorectal cancer. Oncogene. 2015;34:4412–20. doi: 10.1038/onc.2014.377. https://doi.org/10.1038/onc.2014.377 [DOI] [PubMed] [Google Scholar]
- 22.Wang W, Du M, Gu D, et al. MDM2 SNP309 polymorphism is associated with colorectal cancer risk. Sci Rep. 2014;4:4851. doi: 10.1038/srep04851. https://doi.org/10.1038/srep04851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu Q, Zhang G, Chen H, Zheng Y, Cheng J. Current evidence on the relationship between SNP309 polymorphism in the MDM2 gene and colorectal cancer risk. Tumour Biol. 2013;34:3721–9. doi: 10.1007/s13277-013-0956-z. https://doi.org/10.1007/s13277-013-0956-z [DOI] [PubMed] [Google Scholar]
- 24.Qin X, Peng Q, Tang W, et al. An updated meta-analysis on the association of MDM2 SNP309 polymorphism with colorectal cancer risk. PLoS One. 2013;8:e76031. doi: 10.1371/journal.pone.0076031. https://doi.org/10.1371/journal.pone.0076031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quyun C, Ye Z, Lin SC, Lin B. Recent patents and advances in genomic biomarker discovery for colorectal cancers. Recent Pat DNA Gene Seq. 2010;4:86–93. doi: 10.2174/187221510793205764. https://doi.org/10.2174/187221510793205764 [DOI] [PubMed] [Google Scholar]
- 26.Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumors of the digestive system. Vol. 3. WHO Press; 2010. pp. 103–35. [Google Scholar]
- 27.Tongtawee T, Dechsukhum C, Leeanansaksiri W, et al. Improved Detection of Helicobacter pylori Infection and Premalignant Gastric Mucosa Using “Site Specific Biopsy”: A Randomized Control Clinical Trial. Asian Pac J Cancer Prev. 2015;16:8487–90. doi: 10.7314/apjcp.2015.16.18.8487. https://doi.org/10.7314/APJCP.2015.16.18.8487 [DOI] [PubMed] [Google Scholar]
- 28.Ciccotosto GD, McLeish A, Hardy KJ, Shulkes A. Expression, processing, and secretion of gastrin in patients with colorectal carcinoma. Gastroenterology. 1995;109:1142–53. doi: 10.1016/0016-5085(95)90572-3. https://doi.org/10.1016/0016-5085(95)90572-3 [DOI] [PubMed] [Google Scholar]
- 29.Thorburn CM, Friedman GD, Dickinson CJ, Vogelman JH, Orentreich N, Parsonnet J. Gastrin and colorectal cancer: a prospective study. Gastroenterology. 1998;115:275–80. doi: 10.1016/s0016-5085(98)70193-3. https://doi.org/10.1016/S0016-5085(98)70193-3 [DOI] [PubMed] [Google Scholar]
- 30.Georgopoulos SD, Polymeros D, Triantafyllou K, et al. Hypergastrinemia is associated with increased risk of distal colon adenomas. Digestion. 2006;74:42–6. doi: 10.1159/000096593. https://doi.org/10.1159/000096593 [DOI] [PubMed] [Google Scholar]
- 31.Brew R, Erikson JS, West DC, Kinsella AR, Slavin J, Christmas SE. Interleukin-8 as an autocrine growth factor for human colon carcinoma cells in vitro. Cytokine. 2000;12:78–85. doi: 10.1006/cyto.1999.0518. https://doi.org/10.1006/cyto.1999.0518 [DOI] [PubMed] [Google Scholar]
- 32.Shmuely H, Passaro D, Figer A, et al. Relationship between Helicobacter pylori CagA status and colorectal cancer. Am J Gastroenterol. 2001;96:3401–10. doi: 10.1111/j.1572-0241.2001.05342.x. https://doi.org/10.1111/j.1572-0241.2001.05342.x [DOI] [PubMed] [Google Scholar]
- 33.Epplein M, Pawlita M, Michel A, Peek RM, Jr, Cai Q, Blot WJ. Helicobacter pylori protein-specific antibodies and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2013;22:1964–74. doi: 10.1158/1055-9965.EPI-13-0702. https://doi.org/10.1158/1055-9965.EPI-13-0702 [DOI] [PMC free article] [PubMed] [Google Scholar]


