Abstract
Background/Aims
Recent studies have shown that transforming growth factor-β1 (TGF-β1) is prominently associated with acute rejection. This study aimed to explore the role of mesenchymal stem cells (MSCs) in the maintenance of the long-term survival of orthotopic liver transplants (OLTs) via the regulation of TGF-β1 in an experimental rat model.
Materials and Methods
We used Lewis rats as donors and ACI rats as recipients. Hematoxylin and eosin staining was performed to evaluate histomorphological changes, and Western blot was performed to measure protein expression.
Results
The expression of TGF-β1 in the liver allografts and spleen and protein levels of forkhead box P3 (FoxP3), interleukin-10 (IL-10), and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) were measured using Western blot. The suppressive capacity of CD4+CD25+ regulatory T cells was evaluated using the MTT assay. Cell-mediated immunotoxicity was evaluated using the mixed lymphocyte reaction of CD4+ T cells and cytotoxic T lymphocyte (CTL) assay of CD8+ T cells. The results showed that MSCs prolonged the survival of the OLT mice by regulating the expression of TGF-β1 at different time points. The administration of MSCs promoted a prolonged survival in the ACI recipients (105±6.6 d) compared with the MSC-untreated recipients (16.2±4.0 d). On the postoperative day (POD) 7, the MSC-treated recipients showed a significantly higher expression of TGF-β1, FoxP3, IL-10, and CTLA-4 than the MSC-untreated recipients. However, on POD 100, the MSC-treated recipients showed a lower expression of TGF-β1 and FOxP3 than that on POD 7. Moreover, on POD 7, CD4+CD25+ regulatory T cells extracted from the MSC-treated recipients showed a higher expression of FoxP3, IL-10, CTLA-4, and suppressive capacity. On POD 7, CD4+ T cells from the MSC-treated recipients showed more significantly diminished proliferative functions than the MSC-untreated recipients; further, a reduced allospecific CTL activity of CD8+ T cells was observed in the MSC-treated recipients.
Conclusion
MSCs may represent a promising cell therapeutic approach for inducing immunosuppression or transplant tolerance.
Keywords: TGF-β1, mesenchymal stem cell, liver transplantation, CD4+CD25+ regulatory T cell
INTRODUCTION
For end-stage liver diseases, orthotopic liver transplantation (OLT) is an effective treatment. Nevertheless, recipients must avoid rejection by taking immunosuppressive agents. The mechanism of these agents is mainly the suppression of the proliferation of T cells and expression of interleukin-2 (IL-2). Although improved therapeutic methods are associated with survival rates of transplanted patients, the side effects triggered by these immunosuppressive agents and risk assessment on the long-term administration of nonspecific immunosuppressive drugs have presented a series of challenges (1). Consequently, to minimize or completely eliminate immunosuppressants, the induction of a sustained state of donor-specific transplant tolerance should be the ultimate goal. Mesenchymal stem cells (MSCs) are being explored in the treatment of a series of diseases that currently have limited or no therapeutic options (2,3). Recently, some researchers have reported that MSCs can differentiate into organ-specific cells in vivo when they were successfully engrafted in the injured organ (4,5). Some far-reaching immunomodulatory effects have also been observed in MSCs (6), and they can be used in allotansplantation without rejection (7,8). Therefore, MSCs may have beneficial therapeutic effects for liver transplantation (9). The transforming growth factor-β1 (TGF-β1), as a multifunctional and pleiotropic cytokine, can induce the development of acute and chronic rejection. TGF-β1 inhibited organ transplant rejection injury during the early postoperative period. Additionally, TGF-β1 not only increases the levels of collagen but also decreases collagen decomposing to induce fibroblast migration and promoted matrix deposition, resulting in transplant organ fibrosis and arteriosclerosis (10). Recently, some research has shown that the mechanism of TGF-β1 for the induction or maintenance of immune tolerance is associated with CD4+CD25+ regulatory T cells. TGF-β1 is indispensable for the expansion, survival, and functional properties of these CD4+CD25+ regulatory T cells (11,12). However, whether MSCs regulate CD4+CD25+ regulatory T cells via TGF-β1 in long survival recipients following liver transplantation is unclear. In this study, for the first time, we discovered the mechanism of MSC-induced liver transplant tolerance.
In our study, the effects of MSCs on survival in the model of orthotopic liver transplant (OLT) rats have been investigated, as well as the underlying mechanism through which MSCs modulate the function of CD4+CD25+ regulatory T cells via the TGF-β1 signal pathway.
MATERIALS AND METHODS
Cell culture
Mesenchymal stem cells are adult stem cells with self-replication ability and multi-directional differentiation potential, and in this study, all MSCs were extracted from the corresponding donors and frozen in liquid nitrogen (13). The acquired cells were cultured in DMEM (Gibco BRL, NY, USA) supplemented with heat-inactivated 10% (v/v) fetal bovine serum (Gibco, USA), 50.0 μg/mL gentamycin, 2.0 mM L-glutamine, 100.0 μM non-essential amino acids, 10.0 mM HEPES, and 55.0 μM 2-mercaptoethanol under an atmosphere of 95% air and 5% CO2 at 37°C. MSCs between passages 8 and 11 were used in all in vivo experiments. Meanwhile, the functions of MSCs were not evaluated.
Animals and experimental design
Inbred male Lewis (RT1n) rats were used as donors and ACI (RT1l) rats were used as recipients, and these weighed between 250 and 300 g. Experimental rats were all raised under specific pathogen-free conditions in the light of requirements of the Institute of Laboratory Animal Resources of Xuzhou Medical College. All animal experiments were approved by the Institutional Animal Care and Use Committee. OLT was performed under ether anesthesia (14). In our study, we used an improved experimental model of OLT in rats. In conclusion, the hepatic portal vein of the donors perfused 4°C University of Xuzhou preservation solution. Vascular reconstruction was then performed. The superior hepatic vena cava, portal vein, and hepatic inferior vena cava were anastomosed. Arterial reconstruction was not performed. None of the recipients received any immunosuppressive agent, and they were randomly assigned to four groups (14 per group) as mentioned below.
In Group A, we injected saline to the recipients via the right jugular vein at 30 min after OLT.
In Group B, we injected MSCs (2.5×105 cells in 100.0 μL total volume) to the recipients via the right jugular vein at 30 min after OLT.
In Group C, the B6 mice were used as donors and the C3H mice as recipients. These were injected with MSCs (2.5×105 cells in 100.0 μL total volume) extracted from the B6 mice.
In Group D, the ACI rats were used as donors and were injected with MSCs (2.5×105 cells in 100.0 μL total volume) extracted from itself.
To assess the morphology of the liver and determine the concentration of cytokines, the spleen and liver samples were acquired from a half of the experimental subjects in each group at different time points (days 7 and 100 after transplantation). The remaining seven mice in each group were raised to assess the survival rates.
The Banff classification of the pathology of liver allograft
For hematoxylin and eosin (H&E) staining and histological analysis, liver tissues were fixed with 10% neutral-buffered formalin and embedded in paraffin wax. The tissues were then cut into 5.0-μm thick sections and affixed to slides. The paraffin was removed, and slides were stained with H&E to assess the morphological changes in the liver. The resultant H&E stained liver sections were classified using the Banff classification schema (15).
Isolation of T cells from liver and spleen
The liver and spleen cells were dissociated by mashing the tissues and putting them through a 100.0-μm mesh steel sieve. Leukocytes were isolated via centrifugation for 15 min at 840×g, with the cells suspended in 1.05 g/mL Percoll solution (Pharmacia Biotech, Sweden). The remaining cell fractions containing leukocytes were washed and resuspended in a red blood cell lysis buffer (155.0 mM NH4Cl, 10.0 mM KHCO3, 0.1 mM Na2EDTA). Further, isothiocyanate-conjugated anti-CD3 antibody (CD3-FITC, Serotec) was used with fluorescein to stain these cells. T cells were collected using activated cell sorting with a FACS Vantage cell sorter (Becton Dickinson, Australia) after removing the dead cells, which were labeled by propidium iodide (1.0 μg/mL).
Sorting of CD4+CD25+ and CD4+CD25− T cells
The spleens were harvested at specific time points. Splenocytes were stained with anti-mouse CD4 mAb and anti-mouse CD25 mAb (7D4) after incubation with the 2.4G2 antibody. Next, CD4+CD25+ and CD4+CD25− T cells were sorted using a fluorescence-activated cell sorter (FACS Calibur, Becton Dickinson, CA, USA). The purity of these cells was ≥98%.
Western blot analysis
To analyze the expression of TGF-β1, T cells of the spleens and grafts were harvested from the MSC-untreated and MSC-treated mice on POD 7.
To evaluate the expression levels of IL-10, FoxP3, and CTLA-4, CD4+CD25+ regulatory T cells from spleens were sorted by FACS as mentioned above.
Briefly, protein lysates were mixed with loading buffer and boiled for 5 min, and the same amounts of protein were loaded in and separated by sodium dodecyl sulfate-polyacrylamide gels at 200 V for 60 min. This was followed by the transfer to nitrocellulose membranes (Amersham Biosciences) at 100 V for 30 min at room temperature and incubation with primary antibodies, including anti-TGF-β1, anti-FoxP3, anti-IL-10, and anti-CTLA-4 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). The β-actin antibody was purchased from Cell Signaling Technology (Danvers, MA, USA). The secondary antibodies, anti-goat/rabbit/mouse immunoglobulin G (IgG)-HRP, were purchased from the Zhongshan Company (Beijing, China). The expression levels of these proteins were evaluated using chemiluminescence (ECL system, Amersham, UK) and quantified using Quantity One software (Bio-Rad). Protein expression levels were standardized to β-actin.
Mixed lymphocyte reaction (MLR) and CTL assay
Splenocytes were obtained from the MSC-treated and MSC-untreated mice on POD 7 and 100. CD4+ and CD8+ T cells were purified using a magnetic-activated cell sorter (Miltenyi Biotec, Bergisch-Gladbach, Germany) based on the manufacturer’s protocol. The resulting purity in all experiments was ≥98%. All CD4+ T cells and 35 Gy-irradiated splenocytes from Lewis rats were co-cultured under 37°C and 5% CO2 for 3, 5, or 7 d, and the proliferation rates were measured using the MTT assay (Cell Titer, 96, Aqueous One Solution Assay; Promega). The MTT solution (20.0 μl, 5.0 mg/mL in PBS) was added into the cells in each well, and the cells were then incubated at 37°C for 4 h. A microtitre plate reader (Titertek Multiskan MCC) was used to measure the absorbance of samples at 490 nm. All three experiments were independently performed, and the mean values were used to construct the survival curves.
CD8+ T cells and 35 Gy-irradiated splenocytes from TGF-β1+/− KO (B6 background) mice were co-cultivated with IL-2 (50.0 U/mL) at a ratio of 1:2. After 3, 5, or 7 d, an LDH assay (G1780-CytoTox 96 Non-Radioactive Cytotoxicity Assay, Promega) was performed to analyze the cytotoxic lymphocyte (CTL) activity. As targets, B16 cells were added to the effector cells. All cells were cultured under 5% CO2 at 37°C for 24 h, and the cell-free supernatant was then retrieved after centrifugation at 503.1×g for 5 min to analyze LDH. In the end, 50.0 μL supernatant was drawn and 50.0 μL mix-substrate was added into each well of the 96-well plate. The plates were incubated under dark conditions at room temperature for 30 min. To calculate the released amount of LDH, the absorbance in each well was measured. The CTL killing activity was expressed using the following formula (16).
Inhibitory capability of CD4+CD25+ regulatory T cells
CD4+CD25− T cells isolated from the C3H mice were co-cultured with 35 Gy-irradiated splenocytes from TGF-β1+/− KO (B6 background) mice (as the control). Next, the CD4+CD25+ T cells from Group A7d or Group B7d were added. The proliferation of CD4+CD25− T cells was measured after 3, 5, or 7 d using the MTT assay. The percentage of suppressive capacity was calculated by defining the absorption of the control as 100%. Three independent experiments were performed, and the survival curves were constructed.
Statistical analysis
All data are presented as mean±standard deviation. Analysis of variance was used to compare among more than two groups, and the Kaplan-Meier method was used to compare the survival rates among the groups. p≤0.05 shows a statistical significance. All experiments were repeated no less than three times.
RESULTS
Survival rate of transplant recipients
TGF-β1 is believed to have a positive impact on liver allograft rejection and on the survival time of recipients in the models of OLT rats. To demonstrate this hypothesis, we evaluated the survival rates of the MSC-untreated mice (Group A), MSC-treated mice (Group B), and two control groups (Groups C and D). As shown in Figure 1a, the MSC-untreated mice rejected allografts within 14 d, and graft survival time was 6.4±0.5 d. The graft survival times were longer in the two control groups than in the TGF-β1+/− KO mice, indicating that TGF-β1 is essential for allogeneic transplantation tolerance.
Figure 1. a, b .
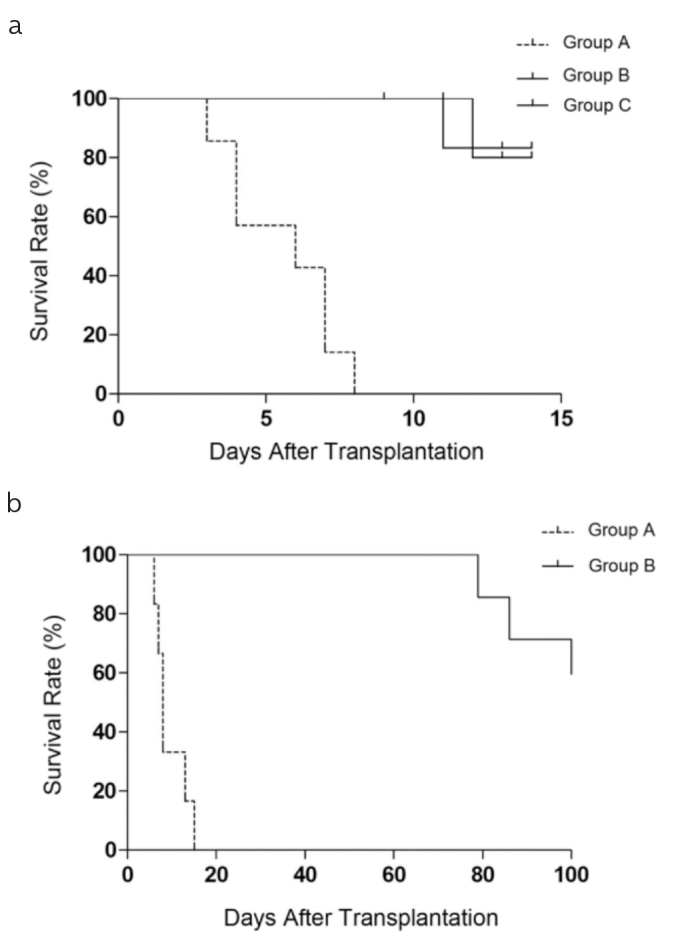
The Kaplan-Meier survival curve of recipients. (a) Survival of the MSC-untreated mice (Group A) compared with that of the two control groups. (b) Survival rates were significantly increased in the MSC-treated mice (Group B) than in the MSC-untreated mice (Group A), and the number of animals at risk in this figure was 14.
Given that TGF-β1 mice presented longer survival (Figure 1a), we sought to address whether MSCs in the allogeneic long survivors were associated with the expression of TGF-β1. In the MSC-treated mice, graft survival time ranged from 78 to 116 d (98±6.6 d) (Figure 1b). These findings revealed that the mean survival time was notably prolonged in the MSC-treated mice and that TGF-β1 was essential in this process.
Morphological alterations in the liver
As demonstrated in Figure 2, allografts from the MSC-untreated and MSC-treated mice have obvious histological differences. Severe acute rejection was found in the MSC-untreated mice. In addition, hepatocytes showed severe degeneration and vacuolation, and hepatic sinusoids presented serious expansion and were filled with several erythrocytes. Some inflammatory cells infiltrated into the portal areas, and sinusoidal endothelial cells presented a severe swelling. The MSC-untreated mice had a progressing acute rejection. The rejection activity index (RAI) scores of the MSC-treated mice significantly decreased compared with those of the MSC-untreated mice (p<0.05). The two control groups (Groups C and D) had negligible amounts of noticeable acute rejection in the liver tissues, and the scores significantly decreased than those of the MSC-untreated and MSC-treated mice, in line with the morphological changes (p<0.05).
Figure 2. a, b.
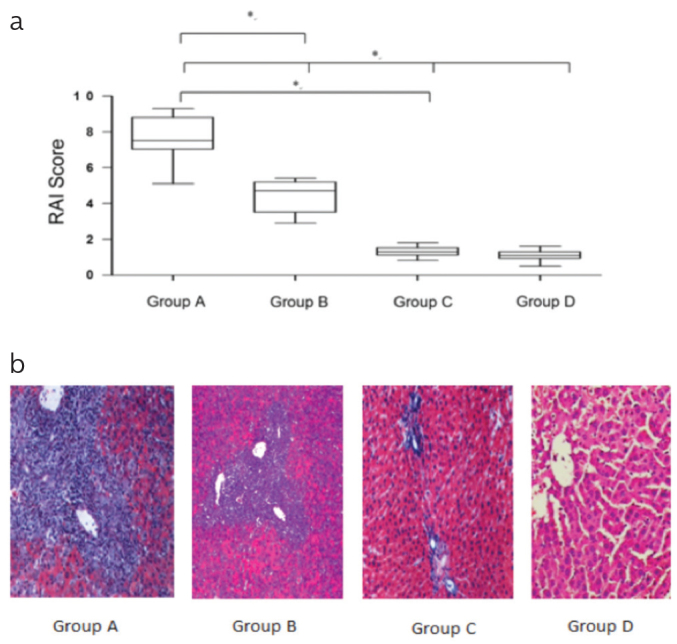
(a) Histological grading of liver allografts using the Banff scheme revealed significant differences in the rejection activity index (RAI) scores among the MSC-untreated, MSC-treated, and control mice (*p<0.05). The MSC-untreated mice (Group A) were associated with a higher score (*p<0.05, Group A versus Group B, Group C, and Group D). (b) The MSC-untreated mice showed parenchymal necrosis, severe endotheliitis, and destruction of the bile ducts. The MSC-treated and control mice had virtually normal histology. The MSC-untreated mice were characterized by a prominent portal infiltrate with varying degrees of endotheliitis and bile duct inflammation.
Long survival of liver transplants induced by MSCs is dependent on regulation of expression of TGF-β1 at different time points
Using Western blot, the MSC-dependent expression of TGF-β1 in liver allografts was evaluated at different time points. On POD 7, the MSC-treated mice showed a significantly higher expression of TGF-β1 than the MSC-untreated mice (p<0.05). Surprisingly, the MSC-treated mice showed a significantly lower expression of TGF-β1 on POD 100 than on POD 7 (p<0.05). On POD 100, the expression was not different between the MSC-treated mice and the two groups of control mice. The expression of TGF-β1 in the spleen samples was similar to that in the liver allografts (Figure 3).
Figure 3. a–c.
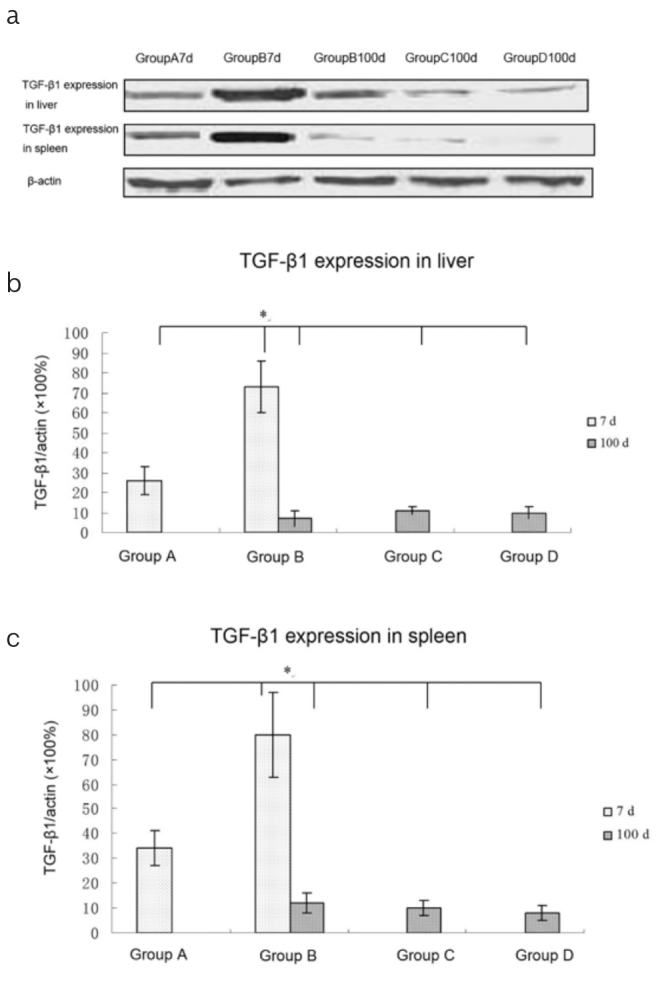
The expression of TGF-β1 in the liver grafts and spleens: (a) At the indicated times, the grafts and spleens were harvested and the expression of TGF-β1 was examined using Western blot. (b, c) Quantitative analysis of TGF-β1 in the liver (b) and spleen (c); On POD 7, the MSC-treated mice (Group B) showed significantly higher expression of TGF-β1 than the MSC-untreated mice (Group A; p<0.05). On POD 100, the MSC-treated mice showed significantly lower expression of TGF-β1 than that on POD 7 (p<0.05). On POD 100, the expression of TGF-β1 was not different among the MSC-treated mice and the two groups of control mice (*p<0.05 compared with MSC-treated mice on POD 7).
Impact of TGF-β1 on the functions of CD4+ and CD8+ T cells
Ample evidence has revealed that TGF-β1 may inhibit the function of T cells (17–19). To evaluate the effect of TGF-β1+/− T cells on allogeneic stimulation, the MLR of CD4+T cells and the CTL activity of CD8+ T cells were evaluated. Figure 4a shows that CD4+ T cells from the MSC-untreated mice had markedly increased proliferative functions (higher OD value) on POD 7 than those from the MSC-treated mice and Group C and D mice on POD 100. Further, the MSC-untreated mice showed a significant increase in the allospecific CTL activity on POD 7 (Figure 4b).
Figure 4. a, b.
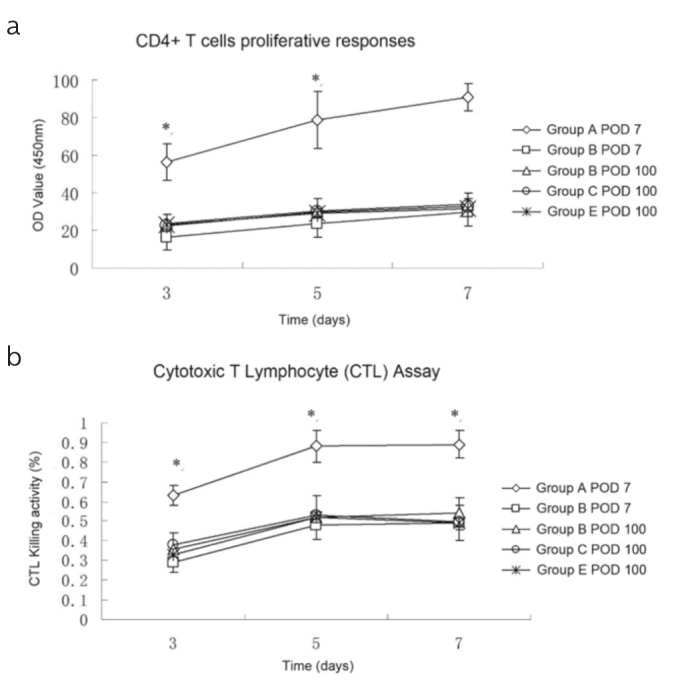
(a) CD4+ T cells were co-cultured with irradiated TGF-β1+/− KO mouse splenocytes for 3, 5, or 7 d, and proliferative responses were measured. The CD4+ T cell proliferation increased when harvested from the MSC-untreated mice on POD 7 (p<0.001 vs. all the other grafts grouped together). (b) CD8+ T cells were co-cultured with irradiated TGF-β1+/− KO mouse splenocytes in the presence of IL-2 after 3, 5, or 7 d. Live cells were collected, and the cytotoxic activity against B12 cells was assessed. There was an increased cytotoxic T lymphocyte activity of T cells from the MSC-untreated mice on POD 7 (p<0.001 vs. all of the other grafts grouped together).
Effect of TGF-β1 on the functions of CD4+CD25+ T cells
Our data revealed that TGF-β1 might regulate the functions of CD4+CD25+ T cells in liver transplants. Therefore, we explored the effect of TGF-β1 on the expression of (20,21), IL-10, CTLA-4, and FoxP3 in CD4+CD25+ T cells. CD4+CD25+ T cells from the spleens of the MSC-untreated, MSC-treated, and control mice were purified over 95% (Figure 5a). CD4+CD25+ T cells from the MSC-untreated mice presented a higher expression of IL-10, CTLA4, and FoxP3 than those from the MSC-treated mice on POD 7 and 100. However, there were no differences in the expression between the MSC-treated mice and the two control groups on POD 100 (Figure 5b). Next, the suppressive capacity of CD4+CD25+ regulatory T cells was measured. Purified CD4+CD25− T cells from the TGF-β1+/− KO mice were co-cultured with irradiated splenocytes from the C3H mice. Then, CD4+CD25+ T cells from the MSC-untreated and MSC-treated mice were added on POD 7, and the proliferation rate of CD4+CD25− T cells was evaluated at the indicated times. As shown in Figure 5c, the CD4+CD25+ T cells from the MSC-treated mice strongly suppressed the proliferation rate of CD4+CD25− T cells than those from the MSC-untreated mice on POD 7, suggesting that CD4+CD25+ T cells from the MSC-treated mice presented markedly increased inhibitory activities.
Figure 5. a, b.
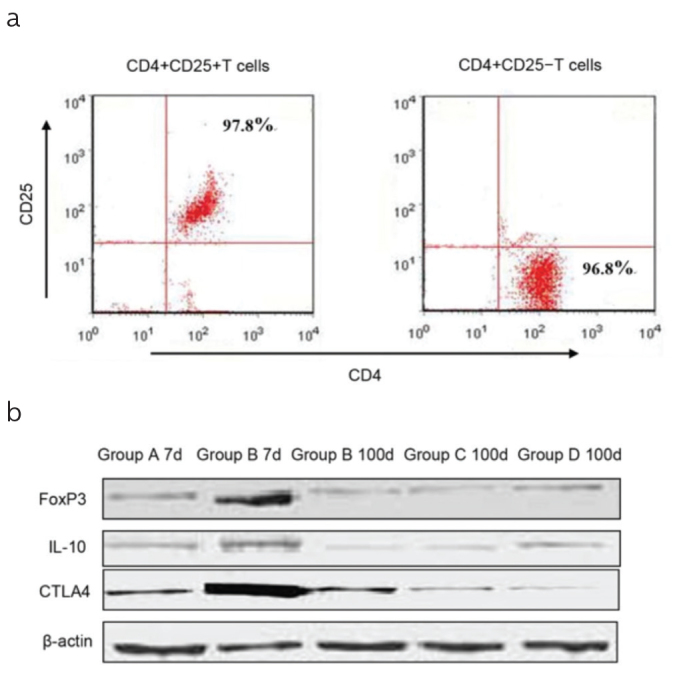
(a) CD4+CD25+ T cells were sorted from the spleens by flow cytometry. The purity of these cells was >95%. The plots shown are representative results. (b) The expression of FoxP3, IL-10, and CTLA-4 in CD4+CD25+ T cells. At the indicated times, FoxP3, IL-10, and CTLA-4 of CD4+CD25+ T cells were measured using Western blot. On POD 7, the MSC-treated mice (Group B) showed significantly higher expression of FoxP3, IL-10, and CTLA-4, and this expression did not differ between the MSC-treated mice and the two groups of control mice on POD 100. Data are presented as mean±SD (*p<0.001; MSC-treated mice vs. all the other grafts grouped together).
DISCUSSION
Several lines of evidence has shown that BMSCs protect against liver injury, inhibit the fibrosis of hepatic stellate cells, and compromise virus control during acute hepatitis B virus infection by activating the TGF-β1 signal pathway (22–25), which revealed that TGF-β1 has a strong immunosuppressive effect in vivo and can inhibit acute organ transplant rejection. The immunosuppressive effects of TGF-β1 are 10,000 times greater than those of cyclosporine A, and TGF-β1 was previously used as an immunosuppressive agent in animal experiments, such as in a rat model of allogeneic heart transplantation (26). Also, some studies have revealed that TGF-β1 has a synergistic effect with CD44 during the epithelial-mesenchymal transition in the liver cancer cells and induces the transition of the characteristic variation of hepatic progenitors. Mesenchymal stem cells can promote the invasion of pancreatic adenocarcinoma cells and drive tumor progression in advanced primary liver cancer via the TGF-β1 signal pathway (27–30).
In theory, a high expression level of TGF-β1 receptor should reduce the rate of acute rejection. Consistent with previous studies, our data showed that TGF-β1 was beneficial for tolerance during the early stages of liver transplantation (Figure 1). We also noted that TGF-β1 is essential for the MSC-induced transplant tolerance. TGF-β1 may inhibit the functions of T cells that contribute to the acceleration of the process of acute rejection (31). TGF-β1 affects the proliferation, differentiation, and apoptosis of T cells and exhibits multiple effects on B cells, macrophages, natural killer cells, and dendritic cells (32,33). However, recent reports have indicated that the mechanisms of TGF-β1 in immune response are somewhat paradoxical because it is generally known as an inhibitory cytokine. More evidence has revealed that it has unexpected properties such as of a master regulator. For instance, it regulates immune responses in several models of autoimmunity (34,35).
Similarly, in most studies, TGF-β1 has been shown to prevent graft versus host disease (36,37). In solid organ transplantation, recent studies have shown that TGF-β1 could depress allograft rejection during the early stages (38,39), but a high expression of TGF-β1 is harmful for long-term allograft survival (39). Based on these theories, our results established that TGF-β1 was essential for inhibiting the acute rejection (Figure 1a). We studied whether MSCs promote long-term graft survival by decreasing the expression of TGF-β1. Consistent with previous findings, we demonstrated that the MSC-treated mice survived longer than the MSC-untreated mice (Figure 1b), indicating that allograft acceptance induced by MSCs relies on the presence of TGF-β1. Histological changes were consistent with the survival curves (Figure 2).
Independent studies from other groups have shown contrasting results. In some studies, TGF-β1 has been shown to have protective effects on graft rejection, whereas in other studies, it has been shown to have damaging effects (40–42). TGF-β1 can inhibit acute immune responses caused by transplant rejection as well as the CD8+ T cell-mediated rejection, and it was unnecessary by the graft to maintain a long-term survival rate (43,44). In the present study, we found that MSCs could regulate the expression of TGF-β1 in the liver allografts and spleen at different time points following transplantation. In the early stage, MSCs induced the expression of TGF-β1, but in long-surviving transplants, the expression was much lower (Figure 3).
In the past, the function of MSCs has been evaluated in vivo and in vitro immunomodulatory and “tissue reconstruction” properties, making them interesting in various clinical settings and particularly in organ transplantation (45). Previous studies have shown that umbilical-cord-derived mesenchymal stromal cells (UC-MSCs) have a therapeutic effect on the ischemic-type biliary lesions following liver transplantation (46), and clinical experimental research has also shown that MSCs infused following liver transplantation have beneficial effects on patients (47); however, the mechanisms of the protective effect of MSCs on TGF-β1 for a long-term transplant survival are unclear. The allospecific CTL and MLR activities showed a noticeable growth when the MSC-untreated mice were used as the source of effector cells than when the MSC-treated mice and the two control groups were used (Figure 4), indicating that endogenously produced TGF-β1 can limit the function of the activation of T cells. The finding represents a potential mechanism, whereby MSCs facilitate long-term acceptance through TGF-β1. Additionally, several studies have shown that TGF-β1 may impact regulatory T cells on their suppressive capacity (40–42). Thus, another explanation could be the effect of TGF-β1 on CD4+CD25+ regulatory T cells. We found that CD4+CD25+ T cells from the MSC-treated mice on POD 7 had an increased expression of FoxP3, CTLA-4, and IL-10 (Figure 5b). Additionally, data from our experiments support the role of TGF-β1 in regulating CD4+CD25+ T cells because the inhibitory activities of CD4+CD25+ regulatory T cells were obviously diminished in the MSC-untreated mice on POD 7 (Figure 5). The detailed mechanisms on the effects of TGF-β1 on the CD4+CD25+ regulatory T cells and how MSCs adjust the expression of TGF-β1 in organ transplantation remain to be explored.
In summary, our results showed that MSCs could protect against graft failure by inhibiting the liver transplant rejection via the regulation of the expression of TGF-β1 in the mouse model. Thus, the MSC-dependent expression of TGF-β1 promotes long-term allograft survival by attenuating the activity of CD4+ and CD8+ T cells and by improving the suppressive capacity of CD4+CD25+ regulatory T cells. Our study also suggested that MSCs enhance the function of CD4+CD25+ regulatory T cells through TGF-β1 in long-term survival rates of transplants. Overall, the functions of TGF-β1 may be different between the injury-promoting and injury-protecting responses. Deeper understanding of TGF-β1 regulated by MSCs in allogeneic rejection may contribute to the development of optimized regimens in the prevention of clinical organ rejection following transplantation.
Footnotes
Ethics Committee Approval: All animal experiments were approved by the Xuzhou Medical College Institutional Animal Care and Use Committee.
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - J.N.; Design - J.N.; Supervision - J.N.; Resources - J.N.; Materials - Y.W.; Data Collection and/or Processing - Y.W.; Analysis and/or Interpretation - B.L.; Literature Search - B.L.; Writing Manuscript - J.N.; Critical Review - B.L., Y.Y.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study was supported by grants from the National Natural Science Foundation of China (NSFC) (30801125).
REFERENCES
- 1.Arasaratnam RJ. Evaluating sirolimus-based immunosuppression for the prevention of cytomegalovirus replication after liver transplantation. Clin Transplant. 2015;29:723. doi: 10.1111/ctr.12597. https://doi.org/10.1111/ctr.12597 [DOI] [PubMed] [Google Scholar]
- 2.de Oliveira LF, Almeida TR, Ribeiro Machado MP, et al. Priming Mesenchymal Stem Cells with Endothelial Growth Medium Boosts Stem Cell Therapy for Systemic Arterial Hypertension. Stem Cells Int. 2015;2015:685383. doi: 10.1155/2015/685383. https://doi.org/10.1155/2015/685383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bitterman PB. Rebuilding the injured lung. Ann Am Thorac Soc. 2015;12:S64–9. doi: 10.1513/AnnalsATS.201411-529MG. https://doi.org/10.1513/AnnalsATS.201411-529MG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldenberg-Cohen N, Avraham-Lubin BC, Sadikov T, Askenasy N. Effect of coadministration of neuronal growth factors on neuroglial differentiation of bone marrow-derived stem cells in the ischemic retina. Invest Ophthalmol Vis Sci. 2014;55:502–12. doi: 10.1167/iovs.13-12223. https://doi.org/10.1167/iovs.13-12223 [DOI] [PubMed] [Google Scholar]
- 5.Mora AL, Rojas M. Adult stem cells for chronic lung diseases. Respirology. 2013;18:1041–6. doi: 10.1111/resp.12112. https://doi.org/10.1111/resp.12112 [DOI] [PubMed] [Google Scholar]
- 6.Akbulut S, Yılmaz M, Arabacı E, Kutlu R, Yılmaz S. Metastasis of carcinoid tumor to the transplanted liver graft: A rare case report. Turk J Gastroenterol. 2014;25:106–9. doi: 10.5152/tjg.2014.4851. https://doi.org/10.5152/tjg.2014.4851 [DOI] [PubMed] [Google Scholar]
- 7.Girdlestone J, Pido-Lopez J, Srivastava S, et al. Enhancement of the immunoregulatory potency of mesenchymal stromal cells by treatment with immunosuppressive drugs. Cytotherapy. 2015;17:1188–99. doi: 10.1016/j.jcyt.2015.05.009. https://doi.org/10.1016/j.jcyt.2015.05.009 [DOI] [PubMed] [Google Scholar]
- 8.Cortinovis M, Casiraghi F, Remuzzi G, Perico N. Mesenchymal stromal cells to control donor-specific memory T cells in solid organ transplantation. Curr Opin Organ Transplant. 2015;20:79–85. doi: 10.1097/MOT.0000000000000145. https://doi.org/10.1097/MOT.0000000000000145 [DOI] [PubMed] [Google Scholar]
- 9.Bar Z, Özçay F, Yılmaz Özbek Ö, Haberal N, Sarialioglu F, Haberal M. A single-center experience of post-transplant lymphoproliferative disorder (ptld) cases after pediatric liver transplantation: Incidence, outcomes, and association with food allergy. Turk J Gastroenterol. 2018;29:354–60. doi: 10.5152/tjg.2018.17731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eurich D, Neumann UP, Boas-Knoop S, et al. Transforming growth factor-β1-gene polymorphism in the development of kidney disease after liver transplantation. Transplantation. 2012;93:555–60. doi: 10.1097/TP.0b013e318242be0b. https://doi.org/10.1097/TP.0b013e318242be0b [DOI] [PubMed] [Google Scholar]
- 11.Jin LP, Chen QY, Zhang T, Guo PF, Li DJ. The CD4+CD25 bright regulatory T cells and CTLA-4 expression in peripheral and decidual lymphocytes are down-regulated in human miscarriage. Clin Immunol. 2009;133:402–10. doi: 10.1016/j.clim.2009.08.009. https://doi.org/10.1016/j.clim.2009.08.009 [DOI] [PubMed] [Google Scholar]
- 12.Ishigame H, Zenewicz LA, Sanjabi S, et al. Excessive Th1 responses due to the absence of TGF-β signaling cause autoimmune diabetes and dysregulated Treg cell homeostasis. Proc Natl Acad Sci USA. 2013;110:6961–6. doi: 10.1073/pnas.1304498110. https://doi.org/10.1073/pnas.1304498110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang FG, Yao Y, Feng Y, Hua CG, Tang XF. Mesenchymal stem cells transduced by stromal cell-derived factor-1α augment ischemic free flaps’ survival. Ann Plast Surg. 2011;66:92–7. doi: 10.1097/SAP.0b013e3181f3e3b3. https://doi.org/10.1097/SAP.0b013e3181f3e3b3 [DOI] [PubMed] [Google Scholar]
- 14.Kamada N, Calne RY. A surgical experience with five hundred thirty liver transplants in the rat. Surgery. 1983;93:64–9. [PubMed] [Google Scholar]
- 15.Demetris AJ, Batts KP, Dhillon AP. Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25:658–63. doi: 10.1002/hep.510250328. https://doi.org/10.1002/hep.510250328 [DOI] [PubMed] [Google Scholar]
- 16.Dierksheide JE, Baiocchi RA, Ferketich AK, et al. IFN-γ gene polymorphisms associate with development of EBV+ lymphoproliferative disease in PBL-SCID mice. Blood. 2005;105:1558–65. doi: 10.1182/blood-2003-07-2476. https://doi.org/10.1182/blood-2003-07-2476 [DOI] [PubMed] [Google Scholar]
- 17.Huber S, Stahl FR, Schrader J, Lüth S, et al. Activin a promotes the TGF-beta-induced conversion of CD4+CD25- T cells into Fox3+ induced regulatory T cells. J Immunol. 2009;182:4633–40. doi: 10.4049/jimmunol.0803143. https://doi.org/10.4049/jimmunol.0803143 [DOI] [PubMed] [Google Scholar]
- 18.Yamada A, Ushio A, Arakaki R, et al. Impaired Expansion of Regulatory T Cells in a Neonatal Thymectomy-Induced Autoimmune Mouse Model. Am J Pathol. 2015;185:2886–97. doi: 10.1016/j.ajpath.2015.07.007. https://doi.org/10.1016/j.ajpath.2015.07.007 [DOI] [PubMed] [Google Scholar]
- 19.Sledzińska A, Hemmers S, Mair F, et al. TGF-β signalling is required for CD4+ T cell homeostasis but dispensable for regulatory T cell function. PLoS Biol. 2013;11:e1001674. doi: 10.1371/journal.pbio.1001674. https://doi.org/10.1371/journal.pbio.1001674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devaud C, Darcy PK, Kershaw MH. Foxp3 expression in T regulatory cells and other cell lineages. Cancer Immunol Immunother. 2014;63:869–76. doi: 10.1007/s00262-014-1581-4. https://doi.org/10.1007/s00262-014-1581-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Liang Y, LeBlanc M, Benner C, Zheng Y. Function of a Foxp3 cis-element in protecting regulatory T cell identity. Cell. 2014;158:734–48. doi: 10.1016/j.cell.2014.07.030. https://doi.org/10.1016/j.cell.2014.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu TB, Li L, Luo XD, Lin H. Bmscs protect against liver injury via suppressing hepatocyte apoptosis and activating TGF-β1/bax singling pathway. Biomed Pharmacother. 2017;96:1395–402. doi: 10.1016/j.biopha.2017.11.023. https://doi.org/10.1016/j.biopha.2017.11.023 [DOI] [PubMed] [Google Scholar]
- 23.Wu SP, Yang Z, Li FR, Liu XD, Chen HT, Su DN. Smad7-overexpressing rat bmscs inhibit the fibrosis of hepatic stellate cells by regulating the tgf-β1/smad signaling pathway. Exp Ther Med. 2017;14:2568–76. doi: 10.3892/etm.2017.4836. https://doi.org/10.3892/etm.2017.4836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qu M, Yuan X, Liu D, et al. Bone marrow-derived mesenchymal stem cells attenuate immune-mediated liver injury and compromise virus control during acute hepatitis b virus infection in mice. Stem Cells Dev. 2017;26:818–27. doi: 10.1089/scd.2016.0348. https://doi.org/10.1089/scd.2016.0348 [DOI] [PubMed] [Google Scholar]
- 25.Xuan J, Feng W, An Zt, et al. Anti-tgfβ-1 receptor inhibitor mediates the efficacy of the human umbilical cord mesenchymal stem cells against liver fibrosis through tgfβ-1/smad pathway. Mol Cell Biochem. 2017;429:113–22. doi: 10.1007/s11010-017-2940-1. https://doi.org/10.1007/s11010-017-2940-1 [DOI] [PubMed] [Google Scholar]
- 26.Cohen AH, Nast CC. TGF-beta in renal allograft rejection. Miner Electrolyte Metab. 1998;24:197–201. doi: 10.1159/000057370. https://doi.org/10.1159/000057370 [DOI] [PubMed] [Google Scholar]
- 27.Park NR, Cha JH, Jang JW, et al. Synergistic effects of CD44 and TGF-β1 through AKT/GSK-3β/β-catenin signaling during epithelial-mesenchymal transition in liver cancer cells. Biochem Biophys Res Commun. 2016;477:568–74. doi: 10.1016/j.bbrc.2016.06.077. https://doi.org/10.1016/j.bbrc.2016.06.077 [DOI] [PubMed] [Google Scholar]
- 28.Wang P, Cong M, Liu T, et al. The characteristics variation of hepatic progenitors after tgf-β1-induced transition and egf-induced reversion. Stem Cells Int. 2016;2016:6304385. doi: 10.1155/2016/6304385. https://doi.org/10.1155/2016/6304385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou HS, Su XF, Fu XL, et al. Mesenchymal stem cells promote pancreatic adenocarcinoma cells invasion by transforming growth factor-β1 induced epithelial-mesenchymal transition. Oncotarget. 2016;7:41294–305. doi: 10.18632/oncotarget.9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vitalone MJ, Sigdel TK, Salomonis N, Sarwal RD, Hsieh SC, Sarwal MM. Transcriptional Perturbations in Graft Rejection. Transplantation. 2015;99:1882–93. doi: 10.1097/TP.0000000000000809. https://doi.org/10.1097/TP.0000000000000809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rojas A, Zhang P, Wang Y, et al. A positive tgf-β/c-kit feedback loop drives tumor progression in advanced primary liver cancer. Neoplasia. 2016;18:371–86. doi: 10.1016/j.neo.2016.04.002. https://doi.org/10.1016/j.neo.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oh SA, Li MO. TGF-β: guardian of T cell function. J Immunol. 2013;191:3973–9. doi: 10.4049/jimmunol.1301843. https://doi.org/10.4049/jimmunol.1301843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwashima M, Love R. Potential of targeting TGF-β for organ transplant patients. Future Med Chem. 2013;5:281–9. doi: 10.4155/fmc.12.215. https://doi.org/10.4155/fmc.12.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schon HT, Weiskirchen R. Immunomodulatory effects of transforming growth factor-β in the liver. Hepatobiliary Surg Nutr. 2014;3:386–406. doi: 10.3978/j.issn.2304-3881.2014.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tu E, Chia PZ, Chen W. TGFβ in T cell biology and tumor immunity: Angel or devil. Cytokine Growth Factor Rev. 2014;25:423–35. doi: 10.1016/j.cytogfr.2014.07.014. https://doi.org/10.1016/j.cytogfr.2014.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo H, Hao Y, Tang B, Zeng D, Shi Y, Yu P. Mouse forestomach carcinoma cells immunosuppress macrophages through TGF-β1. Turk J Gastroenterol. 2012;23:658–65. doi: 10.4318/tjg.2012.0563. https://doi.org/10.4318/tjg.2012.0563 [DOI] [PubMed] [Google Scholar]
- 37.Kohrt HE, Tian L, Li L, Alizadeh AA, et al. Identification of gene microarray expression profiles in patients with chronic graft-versus-host disease following allogeneic hematopoietic cell transplantation. Clin Immunol. 2013;148:124–35. doi: 10.1016/j.clim.2013.04.013. https://doi.org/10.1016/j.clim.2013.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karimi MH, Ebadi P, Pourfathollah AA. Association of cytokine/costimulatory molecule polymorphism and allograft rejection: a comparative review. Expert Rev Clin Immunol. 2013;9:1099–112. doi: 10.1586/1744666X.2013.844462. https://doi.org/10.1586/1744666X.2013.844462 [DOI] [PubMed] [Google Scholar]
- 39.Hegner B, Schaub T, Dragun D. Editorial: Triple-agent TGF-β. J Leukoc Biol. 2013;93:459–62. doi: 10.1189/jlb.1212652. https://doi.org/10.1189/jlb.1212652 [DOI] [PubMed] [Google Scholar]
- 40.Assadiasl S, Ahmadpoor P, Nafar M, et al. Regulatory T cell subtypes and TGF-β1 gene expression in chronic allograft dysfunction. Iran J Immunol. 2014;11:139–52. [PubMed] [Google Scholar]
- 41.Sumitomo S, Fujio K, Okamura T, et al. Transcription factor early growth response 3 is associated with the TGF-β1 expression and the regulatory activity of CD4-positive T cells in vivo. J Immunol. 2013;191:2351–9. doi: 10.4049/jimmunol.1202106. https://doi.org/10.4049/jimmunol.1202106 [DOI] [PubMed] [Google Scholar]
- 42.Pandiyan P, Zhu J. Origin and functions of pro-inflammatory cytokine producing Foxp3(+) regulatory T cells. Cytokine. 2015;76:13–24. doi: 10.1016/j.cyto.2015.07.005. https://doi.org/10.1016/j.cyto.2015.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar S, Naqvi RA, Ali R, Rani R, Khanna N, Rao DN. CD4+CD25+ T regs with acetylated FoxP3 are associated with immune suppression in human leprosy. Mol Immunol. 2013;56:513–20. doi: 10.1016/j.molimm.2013.04.015. https://doi.org/10.1016/j.molimm.2013.04.015 [DOI] [PubMed] [Google Scholar]
- 44.Vandermeulen M, Gregoire C, Briguet A, Lechanteur C, Beguin Y, Detry O. Rationale for the potential use of mesenchymal stromal cells in liver transplantation. World J Gastroenterol. 2014;20:16418–32. doi: 10.3748/wjg.v20.i44.16418. https://doi.org/10.3748/wjg.v20.i44.16418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y-C, Liu W, Fu B-S, et al. Therapeutic potentials of umbilical cord–derived mesenchymal stromal cells for ischemic-type biliary lesions following liver transplantation. Cytotherapy. 2017;19:194–9. doi: 10.1016/j.jcyt.2016.11.005. https://doi.org/10.1016/j.jcyt.2016.11.005 [DOI] [PubMed] [Google Scholar]
- 46.Detry O, Vandermeulen M, Delbouille M-H, et al. Infusion of mesenchymal stromal cells after deceased liver transplantation: A phase I–II, open-label, clinical study. J Hepatol. 2017;67:47–55. doi: 10.1016/j.jhep.2017.03.001. https://doi.org/10.1016/j.jhep.2017.03.001 [DOI] [PubMed] [Google Scholar]
- 47.Zare H, Jamshidi S, Dehghan MM, Saheli M, Piryaei A. Bone marrow or adipose tissue mesenchymal stem cells: Comparison of the therapeutic potentials in mice model of acute liver failure. J Cell Biochem. 2018;119:5834–42. doi: 10.1002/jcb.26772. https://doi.org/10.1002/jcb.26772 [DOI] [PubMed] [Google Scholar]


