Abstract
Background/Aims
We aimed to investigate the factors associated with piecemeal resection of colorectal neoplasia (CRN), in spite of endoscopic submucosal dissection (ESD).
Materials and Methods
We analyzed the retrospective data for colorectal ESD cases from January 2005 to April 2014. We also reviewed the piecemeal endoscopic mucosal resection (EMR) for CRNs ≥20 mm, performed over the same period.
Results
En bloc resection was possible in 648 (85.7%) of 756 lesions in 740 patients. Multivariate analysis showed that hybrid ESD (odds ratio (OR), 29.07; 95% confidence interval (CI), 15.46–54.65; p<0.01) and mild or severe submucosal fibrosis (OR, 3.62; 95% CI, 1.94–6.76; p<0.01) were independently associated with piecemeal ESD. The en bloc ESD group showed higher histologic complete resection rate than the piecemeal ESD group (80.4% vs. 56.5%; p<0.01), and the piecemeal ESD group showed higher recurrence rate than in the en bloc ESD group (5.6% [4/72] vs. 0.7% [3/450]; p<0.01). Overall recurrence rate was 1.3% (7/522).
Conclusion
Hybrid ESD and submucosal fibrosis are independently associated with piecemeal ESD. Piecemeal ESD cases recurred more frequently than en bloc ESD cases.
Keywords: Endoscopic submucosal resection, colorectal neoplasia, piecemeal resection
INTRODUCTION
Endoscopic submucosal dissection (ESD) is a highly specialized technique for the en bloc resection of large colorectal neoplasia (CRN) (1). The higher en bloc resection rate of ESD yields lower recurrence rates after treatment in cases of large CRN, as compared to that with conventional endoscopic mucosal resection (EMR) (2,3). However, colorectal ESD is technically more challenging and requires longer procedure times as compared to EMR or piecemeal EMR (4–7). Hence, en bloc resection may not be completed in all ESD cases and the piecemeal or incomplete resection rate of colorectal ESD ranged from 1% to 16% in the previous studies (4, 8–12). Only a few studies have investigated the factors related to incomplete resection or piecemeal resection. One study suggested that longer procedure time, submucosal fibrosis, and paradoxical colonic movement during ESD might be associated with incomplete resection, whereas another reported that en bloc ESD failure was associated with low-volume institution (<30 ESDs for 3 years), usage of a snare, and poor lifting after submucosal injection (13,14).
Recurrence data of piecemeal EMR are widely available in the literature. The local recurrence rates of piecemeal EMR for CRN ranges from 12.2% to 23.5% (2,15–17). In a western retrospective study on the outcomes of EMR (including 46.2% cases of piecemeal resection) for colorectal lesions with a mean size of 23 mm, 30.4% were found to have recurrent or remnant lesions during follow-up (18). However, the recurrence rate of piecemeal ESD has not been extensively investigated relative to piecemeal EMR. A recent Japanese multicenter prospective study reported that the recurrence rate (13.9%) of piecemeal ESD was similar to that (14.9%) of piecemeal EMR (2). However, that study included a small number of piecemeal ESD cases (n=36), as compared to piecemeal EMR cases (n=378); hence, a relatively large number of cases may be needed to evaluate the local recurrence rates of piecemeal ESD.
Here, we aimed to reveal the factors affecting piecemeal resection of large (≥20 mm) colorectal epithelial neoplasia, despite the application of ESD, and to investigate the long-term outcomes of piecemeal ESD cases relative to en bloc ESD by using data from a single center.
MATERIALS AND METHODS
Study population
We reviewed the medical records of patients who underwent ESD for CRNs from January 2005 to April 2014 at our center. Cases with subepithelial lesions such as neuroendocrine tumor, leiomyoma, gastrointestinal stromal cell tumor (GIST), and granular cell tumor were excluded. Furthermore, we excluded cases with a final pathologic diagnosis of hyperplastic or inflammatory polyps. During the study period, 4 endoscopists (D.H.Y., J.S.B., B.D.Y., and K.J.K.) performed ESD and removed 756 lesions in 740 patients. The endoscopists were categorized as high-volume endoscopists (>200 ESDs during the study period) and medium-volume endoscopists (100–200 ESDs during the study period). Regarding the experience before starting colorectal ESD, all endoscopists had performed more than 4,000 colonoscopy procedures including EMR, but none had the experience of gastric ESD. All the ESD cases were also categorized as en bloc or piecemeal resection. Moreover, we analyzed the factors associated with the piecemeal resection of CRNs. After excluding patients who required subsequent surgery and/or chemoradiation or patients who were lost to follow-up, we compared the recurrence rates between en bloc ESD and piecemeal ESD cases (Figure 1). Based on the guidelines of the colorectal ESD Standardization Implementation Working Group (19), colorectal ESD is generally indicated for lesions if en bloc resection was considered challenging via EMR technique due to location or fibrosis.
Figure 1.
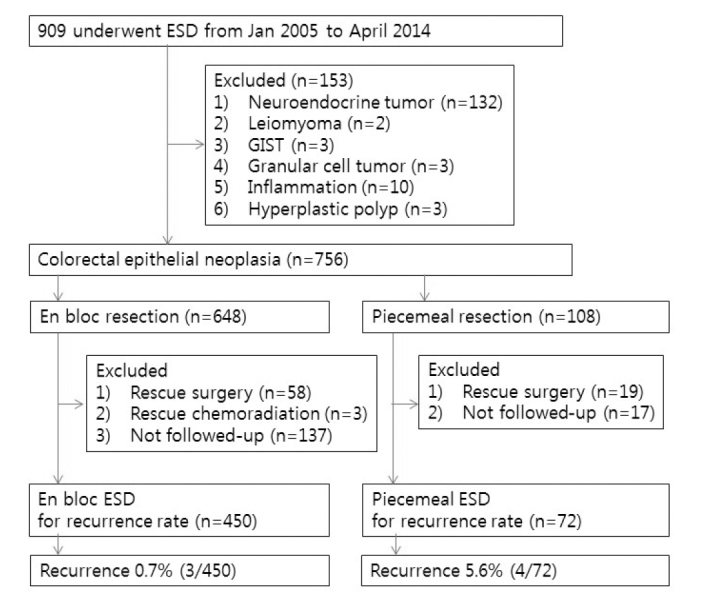
Flow diagram showing the categorization of lesions into the two treatment groups
ESD: endoscopic submucosal dissection; GIST: gastrointestinal stromal tumor
All the data related to the patients, tumors, procedures, and adverse events were collected from the ESD database and medical records. All endoscopic procedures in this study including ESD and surveillance endoscopy were conducted after getting informed consent from the patients. Our institutional review board approved the study protocol (No. 2015-0954).
ESD/hybrid ESD procedure
Details of the ESD technique and devices has been previously published (7,20,21). Transparent cap-attached endoscope was used for ESD. After submucosal injection using sodium hyaluonate solution (Hyal® Shinpoong Co. or Endo-Ease® Unimed Co., Seoul, Korea) mucosal incision and submucosal dissection were performed using a Fixed Flexible knife (Kachu Technology Co., Seoul, Korea) or a Dual knife (Olympus Co., Tokyo, Japan).
Hybrid ESD is a procedure wherein submucosal dissection is performed circumferentially until approximately ≤1 cm of the tissue is remaining, which is then resected with the snaring method (21).
Follow-up endoscopy
All patients underwent periodic follow-up colonoscopies or sigmoidoscopies to assess the local recurrence or remnants. Follow-up endoscopy was performed approximately in 1 year in case of histologic complete resection was achieved. In case of piecemeal resection or a histologically positive lateral resection margin, the first surveillance endoscopy was done within 6 months after endoscopic treatment.
Data collection and study outcomes
The lesion locations were categorized as cecum, ascending colon, transverse colon, descending colon, sigmoid colon, and rectum. The colorectal lesions were classified as laterally spreading tumor granular type (LST-G), laterally spreading tumor non-granular type (LST-NG), and sessile type, based on the morphologic characteristics. The sessile type had 0-Is gross appearance in Paris classification, and did not show a transversal mode of growth on endoscopy (22).
The submucosal fibrosis was graded as none, mild, and severe, according to the endoscopic transparency and appearance of the exposed submucosal layer (23). Intraprocedural bleeding was defined as bleeding during endoscopic resection and interrupted procedure that finally required endoscopic hemostasis with hemostatic forceps. Delayed bleeding was defined as clinical evidence of the occurrence of hematochezia/melena after the procedure that required endoscopic hemostasis. Perforation was identified based on endoscopic and/or radiological findings
All the resected specimens were fixed in 10% formalin and microscopically evaluated. En bloc resection was defined as grossly complete resection of the lesion in a single piece. The greatest dimension of the gross specimen was measured and recorded as the size of the tumor. In cases of piecemeal resection, the greatest dimension of the assembled gross specimen was considered as the size of the lesion if all of resected tumors could be spread and fixed onto a hard Styrofoam. In cases where the pieces of the specimens could not be accurately assembled, the lesion size was endoscopically estimated using open biopsy forceps as a reference.
Histological diagnoses were made according to the Vienna classification (24). The extension of the tumor cells into the resected margin was evaluated. Histologic complete resection was defined as the absence of tumor cells at the lateral and deep resection margins of the specimen. If the pieces of the specimens could not be accurately assembled after the piecemeal resection, it was considered as histologic incomplete resection. Moreover, superficial submucosal cancer was defined as cancer invasion to a depth of <1000 μm from the muscularis mucosa. Curative resection was defined as the clear deep and lateral resection margins, without deep submucosal invasion (>1000 μm from muscularis mucosa) or other unfavorable histologic risk factors (lymphovascular invasion, poor differentiation, or tumor budding) related with lymph node metastasis. Recurrence of CRN after ESD was defined as any histologically identified CRN that recurred at the site of scar of ESD.
Statistical analysis
Continuous parameters were analyzed using Student’s t-test or the Mann-Whitney U test, whereas categorical variables were compared using the χ2 test and Fisher’s exact test, as appropriate. Values of p<0.05 were considered statistically significant. Risk factors for piecemeal resection were analyzed using univariate method. A logistic regression model was used for the multivariate analysis of significant factors detected by univariate analysis, defined as p<0.05, with backward stepwise selection. Cumulative recurrence rates were calculated by the Kaplan-Meier method and compared between each group using the log-rank test. SPSS version 21 for Windows (IBM Corp.; Armonk, NY, USA) was used for the statistical analysis.
RESULTS
Baseline and clinical characteristics of the en bloc and piecemeal ESD groups
Among the 756 lesions in 740 patients who underwent ESD for colorectal epithelial neoplasia, en bloc resection was achieved in 648 (85.7%). The baseline characteristics of the en bloc ESD and piecemeal ESD groups are described in Table 1.
Table 1.
Clinical characteristics, procedure-related variables and histologic outcomes of en bloc and piecemeal endoscopic submucosal dissection for colorectal neoplasia
| En bloc ESD (n=648) | Piecemeal ESD (n=108) | p | |
|---|---|---|---|
| Age, years (mean±SD) | 61.6±9.7 | 62.6±10.4 | 0.329 |
| Sex | 0.082 | ||
| Male, n (%) | 405 (62.5) | 58 (53.7) | |
| Female, n (%) | 243 (37.5) | 50 (46.3) | |
| Tumor size, mm (mean±SD) | 32.5±15.5 | 31.2±11.1 | 0.406 |
| Tumor location, n (%) | 0.422 | ||
| Cecum | 16 (2.5) | 3 (2.8) | |
| Ascending | 95 (14.7) | 21 (19.4) | |
| HF-transverse | 88 (13.6) | 17 (15.7) | |
| SF-descending | 23 (3.5) | 6 (5.6) | |
| Sigmoid | 112 (17.3) | 20 (18.5) | |
| Rectum | 314 (48.5) | 41 (38.0) | |
| Tumor morphology, n (%) | 0.037 | ||
| LST-G | 308 (47.5) | 37 (34.3) | |
| LST-NG | 218 (33.6) | 45 (41.6) | |
| Sessile | 122 (18.8) | 26 (24.1) | |
| Procedure time, min (mean±SD) | 52.7±49.4 | 64.5±47.9 | 0.020 |
| Adverse events, n (%) | |||
| Perforation | 49 (7.6) | 9 (8.3) | 0.780 |
| Intraprocedural bleeding | 69 (10.6) | 25 (23.1) | 0.001 |
| Delayed bleeding | 13 (2.0) | 2 (1.9) | 0.915 |
| Histology, n (%) | 0.115 | ||
| Adenoma | 376 (58.0) | 56 (51.9) | |
| SSA/P | 11 (1.7) | 1 (0.9) | |
| Mucosal cancer | 144 (22.2) | 27 (25.0) | |
| Superficial sm cancer | 67 (10.3) | 8 (7.4) | |
| Deep sm or deeper cancer | 50 (7.7) | 16 (14.8) | |
| Curative resection, n (%) | 473 (73.0) | 54 (50.0) | <0.001 |
| Histological complete resection, n (%) | 521 (80.4) | 61 (56.5) | <0.001 |
| Histological incomplete resection, n (%) | 127 (19.6) | 47 (43.5) | <0.001 |
| Positive or uncheckable lateral margin | 111 (17.1) | 45 (41.7) | <0.001 |
| Positive or uncheckable deep margin | 16 (2.5) | 6 (5.6) | 0.112 |
| Experience of the endoscopist, n (%) | 0.050 | ||
| High-volume endoscopist | 495 (76.4) | 73 (67.6) | |
| Medium-volume endoscopist | 153 (23.6) | 35 (32.4) | |
| ESD type, n (%) | <0.001 | ||
| ESD | 528 (81.5) | 13 (12.0) | |
| Hybrid ESD | 120 (18.5) | 95 (88.0) | |
| Submucosal fibrosis, n (%) | <0.001 | ||
| None | 577 (89.5) | 61 (57.0) | |
| Mild | 55 (8.5) | 35 (32.7) | |
| Severe | 13 (2.0) | 11 (10.3) |
SD: standard deviation; ESD: endoscopic submucosal dissection; HF: hepatic flexure; SF: splenic flexure; LST-G: lateral spreading tumor-granular type; LST-NG: lateral spreading tumor-non-granular type; SSA/P: sessile serrated adenoma or polyp; sm: submucosal
Data are presented as mean±standard deviation or number (%), unless otherwise indicated
Procedure-related and histologic variables of the en bloc and piecemeal ESD groups
With regard to the experience of the endoscopists performing colorectal ESD, high-volume endoscopists tended to have a higher en bloc resection rate as compared to medium-volume endoscopists (76.4% vs. 67.6%, respectively; p=0.050). Hybrid ESD, submucosal fibrosis, and intraprocedural bleeding were more frequent in the piecemeal ESD group than in the en bloc ESD group. Piecemeal ESD group required longer procedure time than en bloc ESD group. Deep submucosal or more advanced invasion was identified histologically in 66 patients; of these patients, 5 refused subsequent surgery, whereas the remaining 61 underwent rescue surgery and/or chemoradiation therapy. Details of the procedure-related variables and histologic outcomes are summarized in Table 1.
The overall curative resection rate and histologic complete resection rate were 69.7% (527/756) and 77.0% (582/756), respectively. Both histologically complete resection rate and curative resection rate were higher in the en bloc ESD group compared with the piecemeal ESD group (p<0.001). Positive or uncheckable lateral resection margin involvement was more frequently noted in the piecemeal ESD group than in the en bloc ESD group (p<0.001).
Factors associated with piecemeal ESD
Univariate analysis indicated that piecemeal ESD was associated with the gross LST-NG type, hybrid ESD, tumors located above the rectum, intraprocedural bleeding, submucosal fibrosis, and procedure time ≥60 min. However, multivariate analysis revealed that only hybrid ESD, submucosal fibrosis, and procedure time ≥60 min were independently associated with piecemeal ESD. Details of the analyses of the factors associated with piecemeal ESD are described in Table 2.
Table 2.
Univariate and multivariate analysis of the related factors of piecemeal endoscopic submucosal dissection
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
|
|
|
|||
| OR (95% CI) | p | OR (95% CI) | p | |
| ESD type | <0.001 | <0.001 | ||
| ESD | Reference | Reference | ||
| Hybrid ESD | 32.15 (17.43–59.33) | 29.07 (15.46–54.65) | ||
| Tumor location | 0.044 | - | ||
| Rectum | Reference | |||
| Above the rectum | 1.54 (1.01–2.33) | |||
| Tumor morphology | - | |||
| LST-G | Reference | |||
| LST-NG | 1.77 (1.03–3.06) | 0.039 | ||
| Sessile | 1.03 (0.61–1.76) | 0.906 | ||
| Intraprocedural bleeding | <0.001 | - | ||
| No | Reference | |||
| Yes | 2.53 (1.52–4.22) | |||
| Submucosal fibrosis | <0.001 | <0.001 | ||
| None | Reference | Reference | ||
| Mild or severe | 6.33 (4.01–10.00) | 3.62 (1.94–6.76) | ||
| Procedure time | <0.001 | 0.049 | ||
| <60 min | Reference | Reference | ||
| ≥60 min | 2.12 (1.39–3.23) | 1.81 (1.00–3.26) | ||
OR: odds ratio; CI: confidence interval; ESD: endoscopic submucosal dissection; LST-NG non-granular-type laterally spreading tumor; LST-G: granular-type laterally spreading tumor
Recurrence in the en bloc and piecemeal ESD groups
A total of 234 cases were eliminated from the analysis of recurrence due to rescue surgery (58 in the en bloc ESD group and 19 in the piecemeal ESD group), rescue chemoradiation (3 rectal cancers in the en bloc ESD group), and absence of follow-up endoscopy (137 in the en bloc ESD group and 17 in the piecemeal ESD group). Accordingly, recurrence was evaluated in 450 cases of en bloc ESD and 72 cases of piecemeal ESD, as shown in Figure 1. The median follow-up period was 15.4 months (inter-quartile rate (IQR), 12.3–29.3 months) in the en bloc ESD group and 13.7 months (IQR, 6.9–31.0 months) in the piecemeal ESD group. There were 7 cases of recurrence during follow-up period and the overall recurrence rate was 1.3% (7/522). Clinicopathologic characteristics of each case are summarized in Table 3. Compared with the en bloc ESD group, the recurrence rate was significantly higher in the piecemeal ESD group (5.6% vs. 0.7%; p=0.008). The cumulative recurrence rate was significantly higher in the piecemeal ESD group (4.7% at 1 year and 7.7% at 3 years) than in the en bloc ESD group (0% at 1 years and 1.9% at 3 years) (p<0.001, Figure 2).
Table 3.
Clinicopathologic characteristics of the cases with recurrence
| No. | Age (years)/Sex | Tumor location | Gross type | Endoscopist | Hybrid ESD | Resection (number of pieces) | Tumor size, mm | Histology | Histological complete resection | Deep resection margin | Lateral resection margin | Number of endoscopy to recur | Time to recurrence, months | Histology of the recurred tumor | Treatment of tumor recurrence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 55/F | R | Sessile | Moderate | Yes | Piecemeal (5) | 50 | Adenoma | No | Negative | Uncheckable | 1 | 6.1 | Adenoma | Endoscopic treatment (APC) |
| 2* | 50/M | R | LST-NG | Moderate | Yes | En bloc | 12 | Superficial sm cancer | No | Positive | Negative | 4 | 17.4 | Adenocarcinoma | Surgery |
| 3 | 67/F | R | LST-G | Moderate | No | En bloc | 56 | M cancer | Yes | Negative | Negative | 2 | 17.8 | Adenocarcinoma | Chemotherapy due to lung metastasis |
| 4 | 78/F | S colon | LST-G | Moderate | Yes | Piecemeal (2) | 35 | M cancer | No | Negative | Positive | 2 | 16.0 | Adenocarcinoma | Endoscopic treatment (EMR) |
| 5 | 66/M | A colon | LST-G | Moderate | Yes | Piecemeal (3) | 49 | Adenoma | No | Negative | Positive | 1 | 3.5 | Adenoma | Endoscopic treatment (EMR) |
| 6 | 58/M | T colon | Sessile | Highly | Yes | Piecemeal (3) | 36 | Adenoma | No | Negative | Positive | 1 | 6.6 | Adenoma | Endoscopic treatment (EMR) |
| 7 | 63/F | A colon | LST-G | Moderate | No | En bloc | 60 | M cancer | No | Negative | Positive | 2 | 32.0 | Adenocarcinoma | Surgery |
ESD: endoscopic submucosal dissection; APC: argon plasma coagulation; EMR: endoscopic mucosal resection; R: rectum; S: sigmoid; A: ascending; T: transverse; LST-NG: non-granular-type laterally spreading tumor; LST-G: granular-type laterally spreading tumor; sm: submucosal; M: intramucosal
Patient 2 refused additional surgery despite a diagnosis of submucosal invasive cancer and positive deep resection margin, and was subsequently diagnosed with local recurrence of invasive cancer at 17.4 months after ESD
Figure 2.
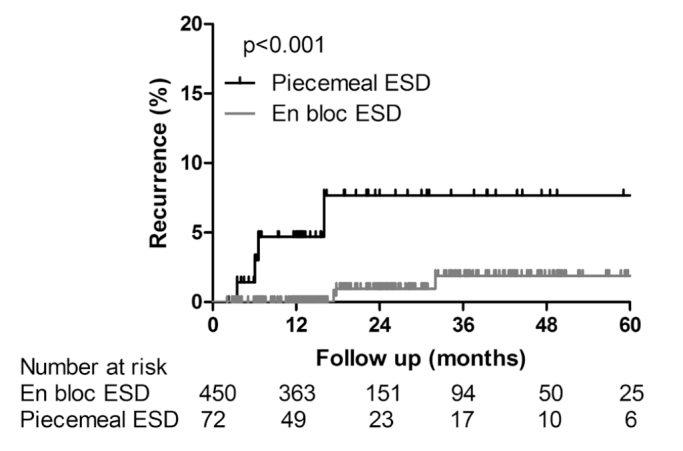
Kaplan-Meier plots in follow-up patients. Comparison of cumulative recurrence rates between en bloc ESD and piecemeal
ESD groups (log rank test, p<0.001)
ESD: endoscopic submucosal dissection
According to the univariate analysis, piecemeal ESD (odds ratio (OR), 8.77; 95% confidence interval (CI), 1.92–40.02; p=0.005), tumor size ≥35 mm (OR, 11.46; 95% CI, 1.37–95.91; p=0.024), histologically incomplete resection (OR, 21.35; 95%, CI 2.54–179.13; p=0.005), and medium-volume endoscopists (OR, 21.35; 95% CI, 2.54–179.13; p=0.005) were associated with recurrence after ESD.
DISCUSSION
In this study, we found that the en bloc resection rate of colorectal ESD was 85.7%, and that the application of hybrid ESD and presence of submucosal fibrosis were independently associated with piecemeal resection. In our clinical practice, hybrid ESD was performed to remove lesions quickly, especially for the safety of patients with intraprocedural problems such as bleeding or higher perforation risk, and to complete the resection of lesions that were hard to remove with standard ESD due to the difficult location or submucosal fibrosis. Hence, the application of hybrid ESD would reflect the cases with difficulties and problems during ESD in our cohort. A previous study suggested that snare use in ESD was an independent factor associated with en bloc resection failure (14). In addition to hybrid ESD or snare use in ESD, previous studies suggested that factors reflecting difficulty in the procedure, such as submucosal fibrosis or poor lifting after submucosal injection, prolonged procedure time, and paradoxical movement, were associated with piecemeal or incomplete ESD (13,14). Given that all these factors are associated with technical difficulty or intraprocedural problems in ESD, the associations of piecemeal ESD with hybrid ESD and submucosal fibrosis observed in the present study appear to be consistent with the findings of previous studies; hence, piecemeal ESD should be considered as an undesirable outcome resulting from technical or situational difficulty during ESD.
As expected, piecemeal resection, despite the application of ESD, led to a significantly higher recurrence rate, as compared to that with en bloc ESD cases (5.6% vs. 0.7%). This is consistent with the results of a recent Japanese prospective study, wherein the recurrence rate was found to be 13.9% in piecemeal ESD cases and 0.7% in en bloc ESD cases (2). They showed that local recurrence was associated with rectal lesions, lesions ≥50 mm in diameter, piecemeal resection, trimming after resection, and a positive horizontal margin. In the present study, we noted that larger lesion size, histologic incomplete resection, and ESD performed by medium-volume endoscopists were associated with recurrence risk, based on univariate analysis. However, we only assessed 7 cases of recurrence in the present study; hence, a further statistical assessment, such as multivariate analysis, to identify the relationships between piecemeal resection and other potential covariates or confounding factors could not be performed in our study. Although piecemeal resection is one of the most important predictive factors for recurrence, additional studies on a larger number of cases should be performed to confirm its impact on local recurrence after ESD of early colorectal epithelial neoplasia.
With regard to the number of pieces in the resection, we found that the recurrence rate was 0.7% (3/450) for 1-piece resection, 2.3% (1/44) for 2-piece resection, and 10.7% (3/28) for ≥3-piece resection. Thus, the local recurrence rate tends to increase with the number of pieces in the resection, consistent with the results of a previous study (2).
Of 4 cases of piecemeal ESD, 3 exhibited local recurrence at the first follow-up endoscopy session after ESD; hence, as mentioned in the current postpolypectomy surveillance guidelines, a follow-up interval of 3–6 months after ESD may be essential in cases of piecemeal resection (25–27). Although the exact mechanism of recurrence could not be identified, a few case reports have described metastasis or recurrence from intramucosal colorectal adenocarcinoma (28,29). In our study, we also noted 2 cases of local recurrence as invasive adenocarcinomas with metastasis in the en bloc ESD group (case 3 and 7 in Table 3), which is extremely rare; the original lesions in these cases were well or moderately differentiated adenocarcinomas confined to the laminar propria. One case (case 3) had a focal least differentiated and the other (case 7) showed severe cautery artifacts at the resection margin. Details of these recurrent cases and the potential mechanism of recurrence have been described in our recent case report (30).
Our study had certain limitations of note. First, as our data were based on a retrospective, single center experience, selection bias related to surveillance and the decision of rescue treatment after piecemeal resection might have influenced the recurrence rate of the piecemeal ESD cases. Second, the surveillance strategy after en bloc and piecemeal ESD was not standardized among the endoscopists, and a considerable proportion of patients were not compliant to surveillance after ESD. Third, because of the relatively short follow-up duration, the recurrence rate might have been underestimated. Despite these limitations, we believe that our study is helpful for understanding the risk of recurrence after piecemeal ESD, relative to en bloc ESD. The establishment of a well-designed, large prospective cohort, and long-term follow-up of that cohort is essential to investigate the risk of recurrence after en bloc and piecemeal ESD, and finally to help develop a surveillance strategy after colorectal ESD.
In conclusion, the hybrid ESD technique and submucosal fibrosis were independent risk factors for piecemeal resection of large colorectal epithelial neoplasia, even in cases where the ESD technique was applied. As piecemeal ESD is associated with a higher risk of recurrence, relative to en bloc ESD, a more meticulous endoscopic surveillance is required.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Institutional Review Board of Ulsan University School of Medicine (No. 2015-0954).
Informed Consent: Written informed consent was obtained from all the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - M.S., D.H.Y.; Design - M.S., D.H.Y.; Supervision - M.S., D.H.Y., J.K., E.M.S., G.U.K., S.W.H., S.H.P., K.J.K., B.D.Y., J.S.B., S.J.M., S.K.Y; Resources - D.H.Y.; Materials - M.S., D.H.Y.; Data Collection and/or Processing - M.S., D.H.Y., J.K., E.M.S., G.U.K., S.W.H., S.H.P., K.J.K., B.D.Y., J.S.B., S.J.M., S.K.Y; Analysis and/or Interpretation - M.S., D.H.Y., J.K., E.M.S., G.U.K., S.W.H., S.H.P., K.J.K., B.D.Y., J.S.B., S.J.M., S.K.Y.; Literature Search - M.S., D.H.Y.; Writing Manuscript - M.S., D.H.Y., J.K., E.M.S., G.U.K., S.W.H., S.H.P., K.J.K., B.D.Y., J.S.B., S.J.M., S.K.Y; Critical Review - M.S., D.H.Y., J.K., E.M.S., G.U.K., S.W.H., S.H.P., K.J.K., B.D.Y., J.S.B., S.J.M., S.K.Y.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: This study was supported by a grant from Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea (No. 2015-653).
REFERENCES
- 1.Tanaka S, Kashida H, Saito Y, et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2015;27:417–34. doi: 10.1111/den.12456. [DOI] [PubMed] [Google Scholar]
- 2.Oka S, Tanaka S, Saito Y, et al. Local recurrence after endoscopic resection for large colorectal neoplasia: a multicenter prospective study in Japan. Am J Gastroenterol. 2015;110:697–707. doi: 10.1038/ajg.2015.96. [DOI] [PubMed] [Google Scholar]
- 3.Fujiya M, Tanaka K, Dokoshi T, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc. 2015;81:583–95. doi: 10.1016/j.gie.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 4.Saito Y, Fukuzawa M, Matsuda T, et al. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc. 2010;24:343–52. doi: 10.1007/s00464-009-0562-8. [DOI] [PubMed] [Google Scholar]
- 5.Lee EJ, Lee JB, Lee SH, Youk EG. Endoscopic treatment of large colorectal tumors: comparison of endoscopic mucosal resection, endoscopic mucosal resection-precutting, and endoscopic submucosal dissection. Surg Endosc. 2012;26:2220–30. doi: 10.1007/s00464-012-2164-0. [DOI] [PubMed] [Google Scholar]
- 6.Tajika M, Niwa Y, Bhatia V, Kondo S, Tanaka T, Mizuno N, et al. Comparison of endoscopic submucosal dissection and endoscopic mucosal resection for large colorectal tumors. Eur J Gastroenterol Hepatol. 2011;23:1042–9. doi: 10.1097/MEG.0b013e32834aa47b. [DOI] [PubMed] [Google Scholar]
- 7.Yang DH, Jeong GH, Song Y, Park SH, Park SK, Kim JW, et al. The feasibility of performing colorectal endoscopic submucosal dissection without previous experience in performing gastric endoscopic submucosal dissection. Dig Dis Sci. 2015;60:3431–41. doi: 10.1007/s10620-015-3755-0. [DOI] [PubMed] [Google Scholar]
- 8.Saito Y, Uraoka T, Matsuda T, et al. Endoscopic treatment of large superficial colorectal tumors: a case series of 200 endoscopic submucosal dissections (with video) Gastrointest Endosc. 2007;66:966–73. doi: 10.1016/j.gie.2007.02.053. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka S, Oka S, Kaneko I, et al. Endoscopic submucosal dissection for colorectal neoplasia: possibility of standardization. Gastrointest Endosc. 2007;66:100–7. doi: 10.1016/j.gie.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 10.Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video) Gastrointest Endosc. 2010;72:1217–25. doi: 10.1016/j.gie.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Fujishiro M, Yahagi N, Kakushima N, et al. Outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms in 200 consecutive cases. Clin Gastroenterol Hepatol. 2007;5:678–83. doi: 10.1016/j.cgh.2007.01.006. quiz 645. [DOI] [PubMed] [Google Scholar]
- 12.Toyonaga T, Man-i M, Fujita T, et al. Retrospective study of technical aspects and complications of endoscopic submucosal dissection for laterally spreading tumors of the colorectum. Endoscopy. 2010;42:714–22. doi: 10.1055/s-0030-1255654. [DOI] [PubMed] [Google Scholar]
- 13.Sato K, Ito S, Kitagawa T, et al. Factors affecting the technical difficulty and clinical outcome of endoscopic submucosal dissection for colorectal tumors. Surgical Endosc. 2014;28:2959–65. doi: 10.1007/s00464-014-3558-y. [DOI] [PubMed] [Google Scholar]
- 14.Takeuchi Y, Iishi H, Tanaka S, et al. Factors associated with technical difficulties and adverse events of colorectal endoscopic submucosal dissection: retrospective exploratory factor analysis of a multicenter prospective cohort. Int J Colorectal Dis. 2014;29:1275–84. doi: 10.1007/s00384-014-1947-2. [DOI] [PubMed] [Google Scholar]
- 15.Seo GJ, Sohn DK, Han KS, et al. Recurrence after endoscopic piecemeal mucosal resection for large sessile colorectal polyps. World J Gastroenterol. 2010;16:2806–11. doi: 10.3748/wjg.v16.i22.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moss A, Williams SJ, Hourigan LF, et al. Long-term adenoma recurrence following wide-field endoscopic mucosal resection (WF-EMR) for advanced colonic mucosal neoplasia is infrequent: results and risk factors in 1000 cases from the Australian Colonic EMR (ACE) study. Gut. 2015;64:57–65. doi: 10.1136/gutjnl-2013-305516. [DOI] [PubMed] [Google Scholar]
- 17.Hotta K, Fujii T, Saito Y, Matsuda T. Local recurrence after endoscopic resection of colorectal tumors. Int J Colorectal Dis. 2009;24:225–30. doi: 10.1007/s00384-008-0596-8. [DOI] [PubMed] [Google Scholar]
- 18.Buchner AM, Guarner-Argente C, Ginsberg GG. Outcomes of EMR of defiant colorectal lesions directed to an endoscopy referral center. Gastrointest Endosc. 2012;76:255–63. doi: 10.1016/j.gie.2012.02.060. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka S, Oka S, Chayama K. Colorectal endoscopic submucosal dissection: present status and future perspective, including its differentiation from endoscopic mucosal resection. J Gastroenterol. 2008;43:641–51. doi: 10.1007/s00535-008-2223-4. [DOI] [PubMed] [Google Scholar]
- 20.Park HW, Byeon JS, Park YS, et al. Endoscopic submucosal dissection for treatment of rectal carcinoid tumors. Gastrointestinal endoscopy. 2010;72:143–9. doi: 10.1016/j.gie.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 21.Byeon JS, Yang DH, Kim KJ, et al. Endoscopic submucosal dissection with or without snaring for colorectal neoplasms. Gastrointest Endosc. 2011;74:1075–83. doi: 10.1016/j.gie.2011.03.1248. [DOI] [PubMed] [Google Scholar]
- 22.The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58(6 Suppl):S3–43. doi: 10.1016/S0016-5107(03)02159-X. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto A, Tanaka S, Oba S, et al. Outcome of endoscopic submucosal dissection for colorectal tumors accompanied by fibrosis. Scandinavian journal of gastroenterology. 2010;45:1329–37. doi: 10.3109/00365521.2010.495416. [DOI] [PubMed] [Google Scholar]
- 24.Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–5. doi: 10.1136/gut.47.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–57. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Arditi C, Gonvers JJ, Burnand B, et al. Appropriateness of colonoscopy in Europe (EPAGE II). Surveillance after polypectomy and after resection of colorectal cancer. Endoscopy. 2009;41:209–17. doi: 10.1055/s-0028-1119646. [DOI] [PubMed] [Google Scholar]
- 27.Yang DH, Hong SN, Kim YH, et al. Korean guidelines for postpolypectomy colonoscopy surveillance. Clin Endosc. 2012;45:44–61. doi: 10.5946/ce.2012.45.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seo HJ, Kim YJ, Cho KB, et al. Nodal metastasis after successful endoscopic submucosal dissection for colorectal mucosal cancer. Endoscopy. 2011;43(Suppl 2 UCTN):E374–5. doi: 10.1055/s-0030-1256705. [DOI] [PubMed] [Google Scholar]
- 29.Lee KH, Kim JS, Cheon KS, Song IS, Kang DY, Kim JY. TisN0M1 Sigmoid colon cancer: a case report. Ann Coloproctol. 2014;30:141–6. doi: 10.3393/ac.2014.30.3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee HJ, Ye BD, Byeon JS, et al. Unusual Local Recurrence with Distant Metastasis after Successful Endoscopic Submucosal Dissection for Colorectal Mucosal Cancer. Clin Endosc. 2017;50:91–5. doi: 10.5946/ce.2016.054. [DOI] [PMC free article] [PubMed] [Google Scholar]


