Abstract
Background/Aims
Recently, mucosal inflammation has been proposed to be one of the mechanisms underlying the pathophysiology of irritable bowel syndrome (IBS); however, there are controversial results regarding this hypotheses. Our aim was to evaluate immune cell infiltration in rectal and ileal biopsy specimens of patients with IBS and to compare it with those of healthy controls.
Materials and Methods
In total, 36 patients with IBS (15 with diarrhea and 21 with constipation) and 16 healthy volunteers were enrolled. Ileocolonoscopy and ileal/rectal biopsies were performed. Rectal and terminal ileal biopsy specimens were evaluated for mucosal immune cell infiltration using immunohistochemical analysis. Serotonin positivity as well as counts of intraepithelial lymphocytes (IEL) and CD4+, CD8+, CD20+, and CD3+ cells were determined by a single pathologist who is an expert in the gastrointestinal system.
Results
CD3+ and CD4+ cell counts in rectal and terminal ileal biopsy specimens were lower in the IBS group than in the controls. Conversely, there was no statistically significant difference between the IBS and control groups in terms of serotonin positivity as well as counts of IEL and CD20+ and CD8+ cells. Comparison between the IBS subgroups revealed a higher number of IEL in rectal biopsy specimens of the diarrhea dominant group. In the IBS subgroups, immune cell counts in terminal ileal and rectal biopsy specimens showed a positive correlation.
Conclusion
IBS and its subgroups showed lower immune cell counts than the controls in our study. These results indicate that there is no significant mucosal inflammation in homogeneous groups of patients with IBS. Rectal biopsies may be sufficient for the evaluation of inflammation in IBS.
Keywords: Irritable bowel syndrome, inflammation, constipation, diarrhea
INTRODUCTION
Irritable bowel syndrome (IBS) is a disease characterized by chronic recurrent abdominal pain or discomfort and altered bowel habits. Its prevalence is 10%–15% worldwide (1). IBS is a functional disorder that is diagnosed according to symptom-based criteria known as the Rome criteria (2).
The etiology of IBS remains unclear. The potential mechanisms underlying symptoms are multiple. One of the mechanisms gaining traction in the pathophysiology of IBS is immunomodulation in the brain and bowel motor system, which is triggered by low-grade inflammation. This theory has been supported by numerous recent studies (3). Recently, some of these studies have endorsed that low-grade mucosal inflammation could lead to alteration in colon motility and visceral perception. It has been reported that colon biopsy specimens of patients with IBS showed higher concentrations of plasma cells, goblet cells, and mast cells (4). Moreover, it has been reported that the expression of T lymphocytes, enterochromaffin cells, and interleukin-1β was increased in the lamina propria of the colons of patients with IBS (5–7). Furthermore, patients with post-infectious IBS and diarrhea without infection history have been reported to show more intraepithelial lymphocytes (IEL), enterochromaffin cells, and mast cells in their colon biopsies than patients who presented with constipation-dominant IBS (IBS-C) and healthy volunteers (8). Quantitative histology, cytokines, and mediators of mucosal biopsy specimens of patients with IBS have been examined (9,10). However, these studies have reported conflicting results. Some studies have shown increased mucosal immune cell infiltration in colonic biopsy specimens of patients with IBS, but these studies have also reported no difference between patients with IBS and healthy controls according to mucosal inflammation (9). In a recent review that evaluated 66 studies, the authors could not make any certain conclusions from their results due to the lack of standardization in the study design (10).
In the present study, we aimed to investigate the presence of mucosal immune cell infiltration in mucosal biopsy specimens of patients with IBS and to compare this result with results in healthy volunteers.
MATERIALS AND METHODS
Study design and subjects
In total, 36 patients with IBS that was diagnosed according to the Rome III criteria were enrolled in the study. All patients were questioned regarding IBS symptoms (constipation, diarrhea, and abdominal discomfort), and they reported the presence of IBS symptoms for at least 1 year. We included patients with diarrhea or constipation-dominant IBS (IBS-D or IBS-C) and excluded those with alternating subtypes. Moreover, we excluded patients with a history of acute gastroenteritis before diagnosis from the study. The inclusion and exclusion criteria are briefly mentioned below:
Inclusion criteria
Patients aged 18–70 years
Patients diagnosed with IBS-D and IBS-C according to the Rome III criteria
Patients with normal serum inflammatory markers (C-reactive protein and erythrocyte sedimentation rate)
Patients with normal ileocolonoscopy
Exclusion criteria
Patients using any immunosuppressive medication
Patients who fulfill the criteria of microscopic colitis
Patients with post-infectious IBS
Patients with co-morbid disease (Patients with metabolic disorders, such as hypertension and diabetes mellitus; malignancy; and inflammatory disorders were excluded, but those with psychiatric disorders, such as depression and anxiety disorders, were not excluded.)
In total, 16 patients were enrolled as the healthy control group. The inclusion and exclusion criteria of the control group were as follows.
Inclusion criteria for the control group
Patients Aged 18 to 70 years,
Patients with normal ileocolonoscopy
Exclusion criteria for the control group
Patients using any medication
Patients with co-morbid disease (Patients with metabolic disorders, such as hypertension and diabetes mellitus; malignancy; and inflammatory disorders were excluded, but those with psychiatric disorders, such as depression and anxiety disorders, were not excluded)
Patients with any disease in their past medical history
Patients with any of the gastrointestinal symptoms (e.g., abdominal pain, diarrhea, constipation, or dyspepsia)
Written informed consent was obtained from each patient before performing the study procedures.
Endoscopy and pathology
For endoscopic investigation, a Fujinon 450 system EC450WL5 (Fujifilm, Tokyo, Japan) colonoscope was used. During colonoscopy procedures, rectal and terminal ileal mucosal biopsy specimens were obtained from all patients. Paraffin blocks were prepared from the biopsy materials; from these blocks, six 2–3-μm-thick serial sections were obtained. One section was used for routine hematoxylin-eosin staining, and the rest were used for immunohistochemical analyses. To provide dryness and to secure the sections, slides prepared for immunohistochemistry were placed in an incubator at 56°C for a night. On the following day, the slides were placed in a Benchmark XT/ IHC Staining module (Ventana Medical System, Tucson, Arizona, USA), and deparaffinization, antigen retrieval, primary antibody incubation, amplification, and counterstaining with hematoxylin processes were performed. (The properties of primary antibodies are summarized in Table 1.) Immune complexes were visualized by diaminobenzidine precipitation. After these automatic steps, the slides were removed and washed using water with detergent and placed into absolute alcohol and xylene for 3 min each three times. Finally, the slides were sealed using DPX-‘Mountant for microscopy’® (VWR International Ltd., Leics, England).
Table 1.
Properties of antibodies
| Antibody and clone | Producer | Dilution ratio | Incubation duration |
|---|---|---|---|
| CD3 | Thermo Fisher Scientific | 1/30 | 56 minutes |
| Clone: PS1 Ref:MS-401-S1 | Anatomical Pathology | ||
| Lot: 401S904H (Mouse) | Cheshire WA7 1PR, UK | ||
| CD20 | Thermo Fisher Scientific | 1/250 | 28 minutes |
| Clone: L26 Ref: MS-340-S | Anatomical Pathology | ||
| Lot: 340S1005M (Mouse) | Cheshire WA7 1PR, UK | ||
| CD4 | Thermo Fisher Scientific | 1/10 | 92 minutes |
| Clone: 4B12 Ref:MS-1528-S | Anatomical Pathology | ||
| Lot: 1528S1007I (Mouse) | Cheshire WA7 1PR, UK | ||
| CD8 | VECTOR Laboratories | 1/20 | 32 minutes |
| Clone:1A5 | Burlingame, CA 94010, USA | ||
| Lot:6004864 (Mouse) | |||
| Serotonin | Thermo Fisher Scientific | 1/20 | 52 minutes |
| Clone: 5HT-H209 | Anatomical Pathology | ||
| Ref: MS-1431-S1 | Cheshire WA7 1PR, UK | ||
| Lot: 1431S1011C |
CD: cluster of differentiation
Immunohistochemical examinations were conducted by a single pathologist (GY) who was blinded to the clinical status of the patients. Cytoplasmic staining was accepted as positive for serotonin, and positive-stained cell counts were determined per 100 enterocytes in rectal and terminal ileal biopsy specimens of each patient. The cluster of differentiation CD3+, CD4+, CD8+, and CD20+ positive cells in the lamina propria and in rectal and terminal ileal biopsy specimens were counted within five consecutive non-overlapping high-power fields (HPF; final magnification, 40′; FN, 22). IELs per 100 enterocytes were counted in CD3-stained sections (Figures 1–3). Fields containing parts of lymphoid aggregates were avoided.
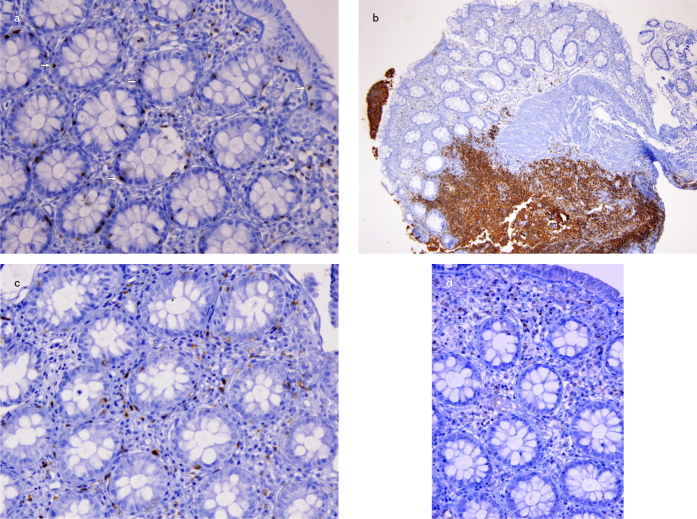
Figure 1. a–d.
CD3+ intraepithelial lymphocytes were counted per 100 enterocytes in rectal mucosa (20X) (a); CD20+ (4x) (b); CD8+ (20x) (c); CD4+ (20x) (d) lymphocytes are shown
Figure 2. a–d.
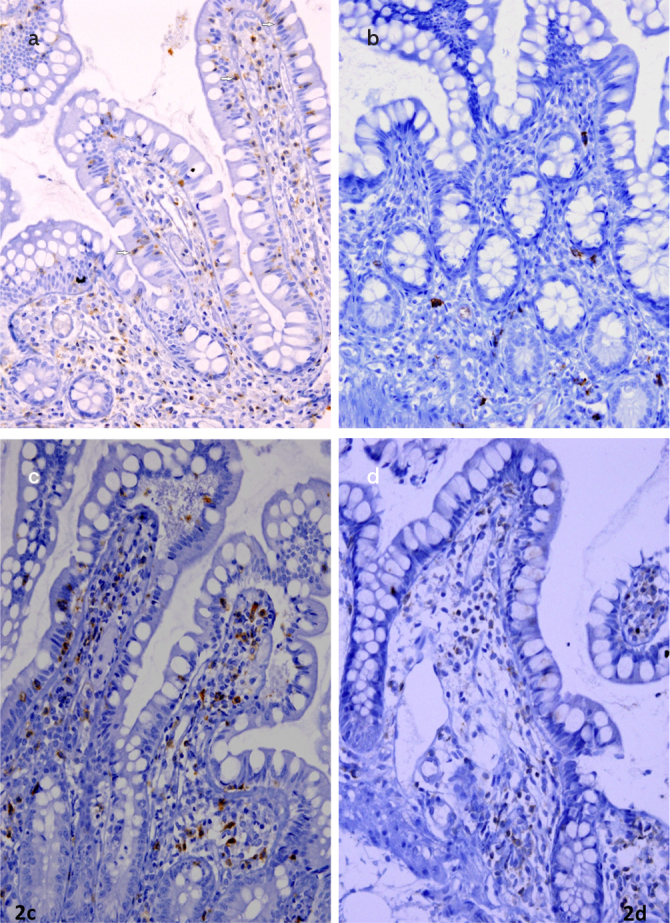
Images from the terminal ileum mucosa, immunohistochemically positive CD3+ (20×) (a); CD20+ (4×) (b); CD8+ (20×) (c); CD4+ (10×) (d); positive T lymphocytes in lamina propria are shown; intraepithelial lymphocytes were counted in CD3+ stained preparations (arrow)
Figure 3. a, b.
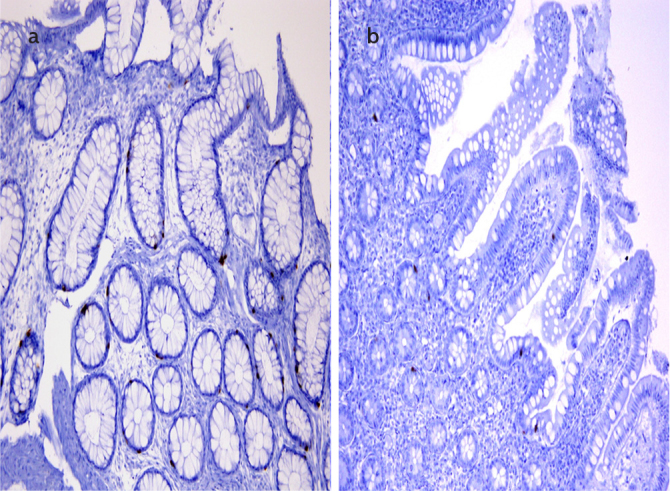
Serotonin-positive enterochromophilic cells (10×) in the rectal mucosa (a) and terminal ileum (b) are shown immunohistochemically
Statistical analysis
The Statistical Package for Social Sciences (SPSS) version 22.0 for Windows (IBM Corp.; Armonk, NY, USA) was used for the statistical analysis of data. Descriptive data are given as the number of patients and frequency. Categorical variables are expressed as the number of patients and percentage value. The comparison of categorical variables was performed using chi-squared and Fisher’s exact tests. Continuous variables are expressed as mean and standard deviation. Shapiro-Wilk test was used to determine whether the continuous variables were normally distributed. For continuous variables, Student’s t-test and Mann-Whitney U test were used relative to the normality of the distribution of the variables. Spearman’s correlation test was used to analyze the association between the variables. A p value of <0.05 was considered statistically significant.
This study was conducted in accordance with the Declaration of Helsinki. The Ethics Committee of İstanbul University İstanbul School of Medicine approved the protocol of the study (2006/503).
RESULTS
In total, 52 patients were included in the study; 16 patients were controls, and the remaining 36 were patients with IBS. In the IBS group, 15 patients presented with IBS-D and 21 patients presented with IBS-C. The mean age of the patients with IBS was 46.4±14.3 years, and 52.8% patients were females. The mean age of the control group was 47±14.8 years, and 43.8% patients were females. Two groups were similar in terms of age and sex (p>0.05) distribution. The IBS-D and IBS-C groups were also similar in terms of age (44.6±15.7 vs. 47.7±13.5 years, respectively) and sex distribution (53.3% vs. 52.4% were female, respectively) (p>0.05). The number of inflammatory cells in mucosal biopsy specimens of the groups is shown in Table 2.
Table 2.
Distribution of inflammatory cells in mucosal biopsy specimens
| Mean cell count | Control* | IBS | Control vs IBS (p) | IBS-D | IBS-C | IBS-D vs IBS-C (p) |
|---|---|---|---|---|---|---|
| Serotonin rectum | 10.1±7 | 11.4±6.6 | 0.373 | 12.2±6.5 | 10.9±6.8 | 0.458 |
| Serotonin ileum | 4.5±4.2 | 4.4±4.1 | 0.887 | 4.6±4.9 | 4.2±3.5 | 0.862 |
| IEL rectum | 15±8.2 | 15.1±12.7 | 0.578 | 19.2±13.4 | 12.1±11.5 | 0.024 |
| IEL ileum | 35.1±16.2 | 36.6±19.7 | 0.921 | 38.1±23.3 | 35.5±17.5 | 0.923 |
| CD3 rectuma, d | 134.4±60.7 | 92±43.1 | 0.013 | 89.5±48.4 | 93.7±40 | 0.360 |
| CD3 ileume | 159.4±40.2 | 127.1±60.1 | 0.031 | 144.1±77.1 | 114.8±42.2 | 0.336 |
| CD20 rectum | 6.4±5.6 | 6.3±4.2 | 0.713 | 6.5±3.9 | 6.1±4.5 | 0.606 |
| CD20 ileum | 11.9±9.8 | 16.3±12.8 | 0.156 | 13.6±6.9 | 18.1±15.6 | 0.962 |
| CD4 rectumb, f | 65±34.3 | 35.7±24.8 | 0.004 | 40.5±29.5 | 32.2±20.9 | 0.441 |
| CD4 ileumg | 90.3±29.2 | 51.9±35.8 | 0.001 | 61.5±47 | 45±24 | 0.460 |
| CD8 rectumc | 64±25.1 | 54.5±29.4 | 0.104 | 45.2±25 | 61.1±31 | 0.112 |
| CD8 ileum | 83.6±22.9 | 79.7±34.3 | 0.984 | 79.7±40.2 | 79.8±30.4 | 0.797 |
Control vs IBS-D:
p=0.044;
p=0.028;
p=0.046
Control vs IBS-C:
p=0.023;
p=0.004;
p=0.006;
p<0.001
IEL: intraepithelial lymphocytes; CD: cluster of differentiation; IBS: irritable bowel syndrome; IBS-D: irritable bowel syndrome diarrhea dominant; IBS-C: irritable bowel syndrome constipation dominant
IBS vs. control group
Mean CD3+ cell counts in rectal (134.4±60.7 vs. 92±43.1) (p=0.013) and terminal ileal (159.4±40.2 vs. 127.1±60.1) (p=0.031) biopsy specimens were significantly lower in the IBS group than in the control group. Mean CD4+ cell counts in rectal (65±34.3 vs. 35.7±24.8) (p=0.004) and terminal ileal (90.3±29.2 vs. 51.9±35.8) (p=0.001) biopsy specimens were significantly lower in the IBS group than in the control group.
When rectal and terminal ileal biopsy specimens of the IBS and control groups were compared, the number of serotonin-positive cells, IELs, CD20+ cells, and CD8+ cells showed no statistically significant difference (p>0.05) (Table 2).
IBS subtypes vs. controls
When rectal biopsy specimens of the IBS-D and control groups were compared, mean CD3+ (89.5±48.4 vs. 134.4±60.7) (p=0.044) and CD4+ (40.5±29.5 vs. 65±34.3) (p=0.028) cell counts in the IBS-D group were lower. Further, regarding CD8+ cells, the IBS-D group had a lower number of cells in the rectum compared with the controls (45.2±25 vs. 64±25.1) (p=0.046).
In the comparison of the IBS-D and control groups, serotonin-positive cells; IELs; CD20+ cell counts in terminal ileal and rectal biopsy specimens; and CD3+, CD4+, and CD8+ cell counts in terminal ileal biopsy specimens showed no statistically significant difference (p>0.05) (Table 2).
The comparison of the control and IBS-C groups showed lower numbers of CD3+ cells in rectal (134.4±60.7 vs. 93.7±40) (p=0.023) and terminal ileal biopsy specimens (159.4±40.2 vs. 114.8±42.2) (p=0.004) of the IBS-C group. Further, rectal (65±34.3 vs. 32.2±20.9) (p=0.006) and terminal ileal (90.3±29.2 vs. 45±24) (p<0.001) biopsy specimens of the IBS-C group showed significantly lower CD4+ cell counts than those of the control group.
When rectal and terminal ileal biopsy specimens of the IBS-C and control groups were compared, the counts of serotonin-positive cells, IELs, and CD20+ and CD8+ cells were found to be similar (p>0.05) (Table 2).
IBS-C vs. IBS-D comparison
When the IBS-D and IBS-C groups were compared, IEL count in rectal biopsy specimens of the IBS-D group was higher than in the IBS-C group (19.2±13.4 vs. 12.1±11.5, p=0.024). The difference was statistically significant.
Cell counts in terminal ileal and rectal biopsy specimens of the IBS groups positively correlated in term of counts of IELs (r=0.615, p<0.001), CD3+ cells (r=0.701, p<0.001), CD4+ cells (r=0.800, p<0.001), and CD8+ cells (r=0.602, p<0.001).
In the evaluation of IBS subgroups according to cell counts, rectal and terminal ileal biopsy specimens of the IBS-D subgroup positively correlated with one another in terms of the counts of serotonin-positive cells (r=0.619 and p=0.03), IELs (r=0.820 and p<0.001), CD3+ cells (r=0.766 and p=0.01), CD4+ cells (r=0.885 and p<0.001), and CD8+ cells (r=0.720 and p=0.02). In contrast, terminal ileal and rectal biopsy specimens of the IBS-C group positively correlated with one another in terms of the counts of CD3+ cells (r=0.728 and p<0.001), CD4+ cells (r=0.645 and p=0.002), and CD8+ cells (r=0.587 and p=0.005).
DISCUSSION
Irritable bowel syndrome is a chronic disease associated with a decreased quality of life. The pathophysiology of IBS remains unknown. Recently, investigators have focused on the effect of mucosal inflammation in IBS (1–3). Although recent studies investigating mucosal inflammation in IBS have shown increased cellular infiltration in the mucosa, their results appear to be controversial. In addition, there are data supporting a lack of significant difference between mucosal cell infiltration in patients with IBS and control groups. A study conducted by Cremon et al. in colonic biopsies of patients with IBS has shown that counts of CD3+ cells, CD8+ cells, and T lymphocytes were increased (9). In contrast, studies showing have also shown no change in the number of CD3+ and CD8+ cells in patients with IBS (11,12). In a study conducted by Braak et al. (13), the authors have shown that CD8+ T lymphocyte count was lower in patients with IBS than in healthy controls in descending colon biopsy specimen. In the same study, no difference was reported between the descending colon biopsy specimens of the IBS and control groups in terms of CD3+ T cells. In our study, the number of CD3+ T lymphocytes was lower in rectal biopsies of patients with IBS (and both IBS-D/ IBS-C subgroups) than in that of the controls. Furthermore, the number of CD3+ cells in terminal ileal biopsy specimens was lower in the IBS group than in the control group. According to our findings, CD8+ T lymphocyte counts in terminal ileal and rectal biopsy specimens were similar in the IBS and control groups. However, in rectal biopsy specimens of the IBS-D subgroup, there was a statistically significant reduction in the number of CD8+ cell type compared with those of the controls. Similarly, Chang et al. (14) have demonstrated a reduced expression of proinflammatory cytokines in the sigmoid colon of patients with IBS. There are also studies reporting colonic mucosal gene expression indicating compromised mucosal immunity in IBS and the downregulation of genes related to T cells and macrophages (15,16). Taken together, our data indicate the possibility of mucosal immune dysregulation rather than immunoactivation. A study by Verma-Gandhu et al. (17) has reported that T lymphocytes played a critical role in visceral nociception, and the authors have proposed that these cells provided anti-nociceptive influence in the gut. Our results support this theory, and the results of Verma-Gandhu et al. (17), which suggested that reduced rather than increased colonic mucosal T lymphocytes infiltration might be one of the factors that can explain the symptoms of patients with IBS. A study by Hughes et al. has supported the claim that immune function is altered in patients with IBS. This alteration, particularly in monocytes/macrophages but not in lymphocytes, had functional consequences on viscerosensory afferent nerves in IBS (18).
A study by Forshammar et al. (19) has shown that CD20+ cells in colon biopsy specimens of patients with IBS, diagnosed according to the Rome II criteria, were lower than in a control group of healthy volunteers. In a study by Cremon et al. (9), B lymphocyte counts in colon biopsy specimens of patients with IBS, again, diagnosed according to the Rome II criteria, and in healthy volunteers did not differ. In our study, consistent with the previously reported data, the number of CD20+ B lymphocytes in rectal and terminal ileal biopsy specimens showed no significant differences between the control and IBS groups. Comparisons of the IBS subgroups with the control group revealed no significantly differences in terms of CD 20+ cell counts.
Spiller et al. (6) have compared post-dysenteric patients with IBS with a control group. The initial rectal biopsies revealed significantly increased serotonin-positive and CD4+ cell counts in IBS compared with the controls. In repeated rectal biopsies, CD4+ cell count declined over time, whereas serotonin-positive cell count remained high. In our study, CD4+ T lymphocytes in rectal and terminal ileal biopsy specimens were significantly lower in the IBS group than in the controls. The assessment of serotonin-positive cells in two biopsy regions revealed no significant difference compared with the control group. These different results might be due to the fact that the patients with IBS in the Spiller et al.’s study were post-dysenteric, whereas the patients in our study had no history of acute infectious diarrhea. The contradictory results in the literature regarding mucosal immune cell infiltration might be due to heterogeneous patient groups.
Colonic mucosal biopsies have generally been used in studies that aimed to evaluate mucosal inflammation in patients with IBS. There are only three studies that have evaluated immune cell infiltration in the small intestine. In one of these studies, the duodenum was the evaluation site of inflammation in patients IBS, whereas the jejunum was the evaluation site in the remaining other two studies (20–22). There are no data regarding immune cell infiltration in the ileum of patients with IBS. In our study, we investigated the terminal ileum for mucosal inflammation in IBS and its subtypes and showed that CD3+ and CD4+ T lymphocyte counts were lower in the ileum of the IBS group than in the control group.
Rectal and terminal ileal biopsy specimens of the IBS and subgroups in our study were positively correlated in terms of the number of inflammatory cells. These data suggest that rectal biopsy may be sufficient for evaluating inflammation in patients diagnosed with IBS according to the Rome criteria. However, the terminal ileum should be macroscopically examined in each colonoscopy performed with the clinical suspicion of inflammatory bowel disease. This is even more important in patients with a predominant symptom of diarrhea. If the terminal ileal mucosa appears normal on macroscopic examination, rectal biopsy may be sufficient.
In this study, we showed that there is no increase in inflammation in patients with IBS compared with control patients in contrast to the current data. Our result should be validated in larger cohorts. Our sample size may be one of our study’s limitations. As another limitation, serum cytokine levels might also be evaluated as inflammation markers, but the aim of our study was to evaluate mucosal inflammatory markers, and serum cytokine levels might be the subject of another study.
Although the recent studies on the pathophysiology of IBS have demonstrated controversial results regarding mucosal inflammation, IBS and its subgroups were found to have lower immune cell counts than the healthy controls in our study. Therefore, our results question the role of mucosal inflammation in the pathophysiology of non-post-infectious IBS.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of İstanbul University İstanbul School of Medicine (2006/503).
Informed Consent: Written informed consent was obtained from all the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - F.A., S.K.; Design - F.A., Ü.A.; Supervision - F.A.; Resources - S.G., F.A., Ü.A., M.G.; Materials - Ü.A., B.B.; Data Collection and/or Processing - A.Ö., S.E., F.A.; Analysis and/or Interpretation - R.İ., F.A.; Literature Review - R.İ, F.A.; Writer - R.İ, F.A.; Critical Review - S.K., M.G., K.D., Ç.K., F.B.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: Provision of materials of our study was afforded by İstanbul University Scientific Research Projects Unit (Project No: 7361).
REFERENCES
- 1.Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108–31. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 2.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–91. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 3.Collins SM. Is the irritable gut an inflamed gut? Scand J Gastroenterol. 1992;192( suppl):102–5. doi: 10.3109/00365529209095988. [DOI] [PubMed] [Google Scholar]
- 4.Barbara G, De Giorgio R, Stanghellini V, Cremon C, Corinaldesi R. A role for inflammation in irritable bowel syndrome? Gut. 2002;51:41–4. doi: 10.1136/gut.51.suppl_1.i41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gwee KA, Leong YL, Graham C, et al. The role of psychological and biological factors in postinfective gut dysfunction. Gut. 1999;44:400–6. doi: 10.1136/gut.44.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spiller RC, Jenkins D, Thornley JP, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–11. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gwee KA, Collins SM, Read NW, et al. Increased rectal mucosal expression of interleukin 1beta in recently acquired post-infectious irritable bowel syndrome. Gut. 2003;52:523–6. doi: 10.1136/gut.52.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunlop S, Jenkins D, Spiller R. Distinctive histological patterns of chronic inflammatory cells in rectal biopsies of patients with different clinical subtypes of IBS. Gastroenterology. 2002;122:A60. [Google Scholar]
- 9.Cremon C, Gargano L, Morselli-Labate AM, et al. Mucosal immune activation in irritable bowel syndrome: gender-dependence and association with digestive symptoms. Am J Gastroenterol. 2009;104:392–400. doi: 10.1038/ajg.2008.94. [DOI] [PubMed] [Google Scholar]
- 10.Martin-Viñas JJ, Quigley EM. Immune response in irritable bowel syndrome: A systematic review of systemic and mucosal inflammatory mediators. J Dig Dis. 2016;17:572–81. doi: 10.1111/1751-2980.12379. [DOI] [PubMed] [Google Scholar]
- 11.Chadwick VS, Chen W, Shu D, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778–83. doi: 10.1053/gast.2002.33579. [DOI] [PubMed] [Google Scholar]
- 12.Lee KJ, Kim YB, Kim JH, Kwon HC, Kim DK, Cho SW. The alteration of enterochromaffin cell, mast cell, and lamina propria T lymphocyte numbers in irritable bowel syndrome and its relationship with psychological factors. J Gastroenterol Hepatol. 2008;23:1689–94. doi: 10.1111/j.1440-1746.2008.05574.x. [DOI] [PubMed] [Google Scholar]
- 13.Braak B, Klooker TK, Wouters MM, et al. Mucosal Immune Cell Numbers and Visceral Sensitivity in Patients with Irritable Bowel Syndrome: Is There Any Relationship? Am J Gastroenterol. 2012;107:715–726. doi: 10.1038/ajg.2012.54. [DOI] [PubMed] [Google Scholar]
- 14.Chang L, Sundaresh S, Elliott J, et al. Dysregulation of the hypothalamicpituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterol Motil. 2009;21:149–59. doi: 10.1111/j.1365-2982.2008.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aerssens J, Camilleri M, Talloen W, et al. Alterations in mucosal immunity identified in the colon of patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2008;6:194–205. doi: 10.1016/j.cgh.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macsharry J, O’Mahony L, Fanning A, et al. Mucosal cytokine imbalance in irritable bowel syndrome. Scand J Gastroenterol. 2008;43:1467–76. doi: 10.1080/00365520802276127. [DOI] [PubMed] [Google Scholar]
- 17.Verma-Gandhu M, Bercik P, Motomura Y, et al. CD4+ T-cell modulation of visceral nociception in mice. Gastroenterology. 2006;130:1721–8. doi: 10.1053/j.gastro.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 18.Hughes PA, Moretta M, Lim A, Grasby DJ, et al. Immune derived opioidergic inhibition of viscerosensory afferents is decreased in Irritable Bowel Syndrome patients. Brain Behav Immu. 42:191–203. doi: 10.1016/j.bbi.2014.07.001. 20140. [DOI] [PubMed] [Google Scholar]
- 19.Forshammar J, Isaksson S, Strid H, et al. A pilot study of colonic B cell pattern in irritable bowel syndrome. Scand J Gastroenterol. 2008;43:1461–6. doi: 10.1080/00365520802272126. [DOI] [PubMed] [Google Scholar]
- 20.Walker MM, Talley NJ, Prabhakar M, et al. Duodenal mastocytosis, eosinophilia, and intraepithelial lymphocytosis as possible disease markers in the irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther. 2009;29:765–73. doi: 10.1111/j.1365-2036.2009.03937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guilarte M, Santos J, de Torres I, et al. Diarrhoea-predominant IBS patients show mast cell activation and hyperplasia in the jejunum. Gut. 2007;56:203–9. doi: 10.1136/gut.2006.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tornblom H, Lindberg G, Nyberg B, Veress B. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. 2002;123:1972–9. doi: 10.1053/gast.2002.37059. [DOI] [PubMed] [Google Scholar]