Abstract
Background/Aims
The hepatitis C virus (HCV) infection is important cause of chronic hepatitis. Liver biopsy is considered the gold standard for assessment of fibrosis but this procedure is an invasive procedure. We aimed to evaluate the diagnostic efficiency of non-invasive serum biomarkers, separately and in combinations, on liver fibrosis in treatment-naive chronic hepatitis C (CHC) patients.
Materials and Methods
Two hundred and sixteen treatment-naive CHC patients were enrolled from 32 locations across Turkey in this open-labelled, non-interventional prospective observational study. FibroTest®, aspartate aminotransferase-to-platelet ratio index(APRI), aspartate aminotransferase and alanine aminotransferase ratio (AAR), fibrosis index based on four factors (FIB-4), Age-platelet(AP) index and Forns index were measured and compared with Metavir scores got from liver biopsies.
Results
Data from 182 patients with baseline liver biopsy were suitable for analysis. One hundred and twenty patients (65.9%) had F0–F1 fibrosis and 62 patients (34.1%) had F2–F4 fibrosis. APRI 0.732 area under the curve(AUC) indicated advanced fibrosis with 69% sensitivity and 77% specificity. FIB-4 0.732 AUC and FibroTest 0.715 AUC indicated advanced fibrosis with 69% and 78.4% sensitivity, and 75% and 71.4% specificity, respectively. The combined use of tests also led to an increase in AUC and specificity. Combinations of FibroTest with APRI and/or FIB-4, and FIB-4 with APRI were optimal for the evaluation of liver fibrosis.
Conclusion
Fibrotest, FIB-4, APRI, AP index and Forns index exhibit good diagnostic performance for determining liver fibrosis in CHC patients, and the use of at least two tests together will increase their diagnostic value still further.
Keywords: Chronic hepatitis C infection, liver fibrosis, FibroTest, APRI, Forns index, Fib-4, non-invasive serum biomarkers
INTRODUCTION
Hepatitis C virus (HCV) infection is a disease that affects approximately 2.8% of the world population and 1% of the Turkish population (1,2). The determination of hepatic fibrosis plays a significant role in treatment decisions and follow-up of chronic hepatitis C (CHC) patients (3). Liver biopsy is the gold standard in the determination of hepatic fibrosis; however, it is an invasive procedure, can result in serious complications, cannot be performed on every patient, and is difficult to repeat. Sampling errors, as well as differences of interpretation among pathologists, may occur (4–6). Currently, there are several noninvasive methods, such as serum biomarkers and imaging methods, for the assessment of liver fibrosis in CHC infection (7). Ultrasonography, magnetic resonance imaging, computed tomography, and transient elastography (TE-FibroScan) are examples of imaging methods. TE is the most recommended diagnostic imaging method that measures liver stiffness; however, it is too expensive and is used only in a few hospitals in Turkey. Even more, the reimbursement agency does not pay the TE fee in Turkey. However, serum biomarkers have numerous advantages, including the absence of risks, objective interpretation, patient acceptance, cost effectiveness, and the fact that they are easily applicable and repeatable tests (8). These biomarkers can be direct (collagen, hyaluronic acid, laminin, and YKL-40) and indirect [FibroTest (FT), ActiTest (AT), aspartate aminotransferase and alanine aminotransferase ratio (AAR), aspartate aminotransferase-to-platelet ratio index (APRI), four factors (FIB-4), age-platelet (AP) index, and Forns index]. Direct biomarkers are liver matrix components, the routine clinical use of which is limited. In contrast, indirect biomarkers are easy to perform serum-based tests, which include biochemical parameters routinely measured in the peripheral blood of CHC patients (9–12). These serum biomarkers have successfully identified hepatic fibrosis in CHC patients, but noninvasive serum biomarkers have some limitations, such as low accuracy of discrimination between intermediate stages of fibrosis. The diagnostic performance of serum biomarkers can be increased when these are used in combination in the determination of hepatic fibrosis (3).
In this study, the diagnostic performances of serum biomarkers such as FT, APRI, AAR, FIB-4, the AP index, and the Forns index were analyzed separately and in combination in treatment-naïve CHC patients. The results were compared with those from liver biopsy in terms of predicting fibrosis.
MATERIALS AND METHODS
In this noninterventional, observational study, 216 treatment-naïve CHC patients aged between 18 and 65 years from 32 locations in Turkey were included. The study patients were anti-HCV positive for at least 6 months and had detectable levels of HCV-RNA by PCR for CHC. The patient recruitment began on November 1, 2008, and it continued for 24 months after approval of the ethics committee. Informed consent was obtained from all patients before starting any procedure.
Patients co-infected with HIV or hepatitis B virus, patients with suspected hepatocellular carcinoma or acquired/inherited liver disease, patients with a serious concomitant disease (renal failure, hypertension, DM, etc.), patients using immunosuppressive drugs during the previous 6 months, patients with abnormal hematological parameters that contraindicated liver biopsy (prolonged prothrombin time, low platelet count, etc.), and patients who used alcohol regardless of duration and who abused drugs were excluded from the study. Demographic, clinical, histological, and laboratory data were recorded.
Percutaneous liver biopsy was performed using a disposable Hepafix suction needle (B. Braun, Melsungen, Germany). The same pathologist examined all liver biopsy samples. The stage of fibrosis was determined using the METAVIR scoring system (13). Two groups were established based on fibrosis scores: Group 1, F0–F1 (no fibrosis) and Group 2, F2–F4 (significant/advanced fibrosis). Blood samples for serum biomarkers were collected simultaneously with the liver biopsy procedure and stored at −80°C until analysis.
Noninvasive serum biomarkers, FT, APRI, AAR, FIB-4, the AP index, and the Forns index were calculated for all patients using laboratory data based on a single center with the formulas shown in Table 1 (8,9,11,14–20).
Table 1.
Noninvasive serum biomarker methods
| Serum Biomarkers | Methods |
|---|---|
| Fibrotest® (FT) | (Biopredictive, Paris, France) Patented formula combining α-2-macroglobulin, γGT, apolipoprotein A1, haptoglobin, total bilirubin, age, and gender |
|
|
|
| Forns Index | 7.811–3.131×(platelet count)+0.781 × (GGT)+3.467×(age)−0.014× (cholesterol) |
|
|
|
| AST to Platelet Ratio (APRI) | AST (Upper Limit of Normal) (IU/L)/platelet (109/L)×100 |
|
|
|
| FIB-4 | [Age (year)×AST (IU/L)]/[platelet (109/L)×√ALT (IU/L)] |
|
|
|
| Age-platelet (AP) index | Age (year) (<30=0; 30–39=1; 40–49=2; 50–59=3; 60–69=4; ≥70=5) + platelet (109/L) (≥225=0; 200–224=1; 175–199=2; 150–174=3; 125–149=4; <125=5) |
|
|
|
| AST/ALT ratio (AAR) | AST (IU/L)/ALT (IU/L) |
APRI: aspartate aminotransferase-to-platelet ratio index; FIB-4: fibrosis index based on four factors; AST: aspartate aminotransferase; ALT: alanine aminotransferase
The following combinations were calculated simultaneously: FibroTest+APRI, FibroTest+APRI+FIB-4, FibroTest+FIB-4, FibroTest+AP index, FibroTest+Forns index, FibroTest+AAR, APRI+FIB-4, APRI+AP index, APRI+AAR, APRI+Forns index, FIB-4+AP index, FIB-4+AAR, FIB-4+ Forns index, AP index+AAR, AP index+Forns index, and AAR+Forns index.
Statistical analysis
Descriptive statistical analysis was conducted using the Statistical Package for Social Sciences (SPSS) 13.0 software (SPSS Inc.; Chicago, IL, USA). Data were expressed as mean±standard deviation (SD), percentage (%), or median (min–max), as appropriate. p-values of <0.05 were considered statistically significant. Categorical data were analyzed using the Chi-squared and Fisher’s tests. Data obtained in measurements of normal distribution were analyzed using the Kolmogorov-Smirnov test. Data conforming to normal distribution were analyzed using the Student’s-t test, and non-conforming data were analyzed using the Mann-Whitney U test. Correlation analysis was performed using the Spearman’s correlation test. The receiver operating characteristic (ROC) analysis was performed to calculate the sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) of the noninvasive serum biomarkers for the prediction of hepatic fibrosis.
RESULTS
Of the 216 patients recorded in this study, 182 (84.3%) were eligible for assessment. Thirty-four patients were excluded from the analysis due to insufficient liver biopsy samples, absence of one or more laboratory parameters, or missing data entry.
The mean (SD) age was 49.9±11.4 years, and 59.9% of the 182 patients were female. One hundred and twenty (65.9%) patients were classified as having METAVIR fibrosis stage F0–F1 (no fibrosis; Group 1) and 62 (34.1%) patients as having F2–F4 (significant/advanced fibrosis; Group 2). Patients with higher fibrosis scores were observed to have a higher average age, indicating that advanced age was correlated with higher fibrosis (p=0.003). No statistical significance was observed between gender and fibrosis. The patients with advanced fibrosis had higher mean ALT and AST values (p<0.005). Although platelet counts were lower in patients with advanced fibrosis, no statistically significant difference was observed compared with patients with no fibrosis (p=0.053). There was a significant difference in terms of APRI, FIB-4, the AP index, and the Forns index between the two groups (p<0.05). This finding shows that APRI, FIB-4, the AP index, and the Forns index are important serum biomarkers in the determination of liver fibrosis (Table 2). AAR was not significant in the determination of the stage of fibrosis. The correlation between the METAVIR fibrosis scores and FibroTest results (r=0.321; p<0.001) increased with the stage of fibrosis.
Table 2.
Demographic features and laboratory parameters of the 182 patients
| Serum Biomarkers Methods | ||||
|---|---|---|---|---|
| Mean age | 48.4±12.2 | 53.1±9.0 | 0.003 | |
| Gender, n (M/F) | 45/75 | 30/32 | 0.193 | |
| Mean AST, U/L | 43.2±32.5 | 65.7±48.2 | 0.000 | |
| Mean ALT, U/L | 60.2±55.6 | 77.5±62.6 | 0.004 | |
| Mean platelet count (×103/microL) | 235.4±57.7 | 215.9±71.0 | 0.053 | |
| Protein | 7.6±0.5 | 7.5±0.5 | 0.327 | |
| Albumin | 4.3±0.6 | 4.3±0.5 | 0.654 | |
| APRI | 0.51±0.5 | 0.95±1.2 | 0.000 | |
| AAR | 0.85±0.3 | 1.03±0.6 | 0.060 | |
| FIB-4 | 1.30±0.9 | 2.35±2.1 | 0.000 | |
| AP index | 4.59±1.7 | 5.41±1.7 | 0.002 | |
| Forns index | 4.37±1.6 | 5.28±2.0 | 0.034 | |
| F0 | 31 | 9 | ||
| FibroTest | F1–F2 | 40 | 20 | 0.003 |
| F3 | 15 | 9 | ||
| F4 | 5 | 13 | ||
AST: aspartate aminotransferase; ALT: alanine aminotransferase; APRI: aspartate aminotransferase-to-platelet ratio index; AAR: AST/ALT ratio; FIB-4: fibrosis index based on four factors; AP index: age-platelet index
METAVIR fibrosis scores and FibroTest (r=0.321; p<0.001), APRI (r=0.302; p<0.001), the Forns index (r=0.339; p=0.001), and the AP index (r=0.254; p=0.001) results were also positively correlated.
The ROC analysis of noninvasive tests revealed that the area under the curve (AUC) exceeded the reference line in the majority of tests (Figure 1). The APRI 0.732 AUC indicated advanced fibrosis with 69% sensitivity and 77% specificity. The FIB-4 0.732 AUC and FibroTest 0.715 AUC indicated advanced fibrosis with 69% and 78.4% sensitivity and 75% and 71.4% specificity, respectively. The combined use of tests also led to an increase in AUC and specificity. A combination of FibroTest with APRI and/or FIB-4 and of APRI with FIB-4 was identified to be optimal for the assessment of fibrosis (Table 3). The error bar graph analysis of noninvasive tests showed that FibroTest, APRI, and FIB-4 could be used to differentiate patients with advanced fibrosis from patients with minimal fibrosis (Figure 2–7).
Figure 1.
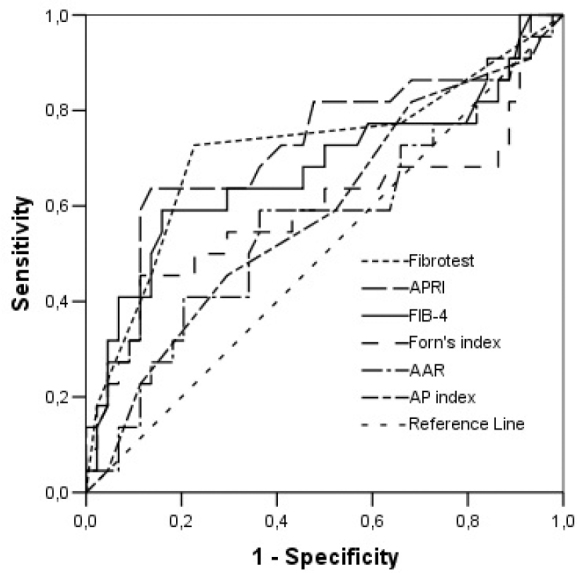
ROC curve analysis of noninvasive tests for diagnosis of liver fibrosis
Table 3.
Sensitivity, specificity, and predictive values of serum biomarkers either alone or combined
| Cutoff | p | AUC | Sensitivity (%) | Specificity (%) | NPV (%) | PPV (%) | |
|---|---|---|---|---|---|---|---|
| APRI | >0.5 | 0.0001 | 0.732 | 69.0 | 77.0 | 81.1 | 63.5 |
| AAR | >1.0 | 0.059 | 0.589 | 37.3 | 79.8 | 69.2 | 51.2 |
| FIB-4 | >1.5 | 0.0001 | 0.732 | 69.0 | 75.0 | 80.6 | 61.5 |
| AP index | ≥6 | 0.002 | 0.639 | 54.2 | 70.7 | 75.2 | 48.5 |
| Forns index | >5 | 0.045 | 0.629 | 56.7 | 68.3 | 75.9 | 47.2 |
| Fibrotest | ≥F2 | 0.0001 | 0.715 | 78.4 | 71.4 | 85.5 | 60.6 |
| Fibrotest + APRI | ≥F2 | 0.0001 | 0.729 | 56.4 | 89.5 | 81.0 | 72.1 |
| >0.5 | |||||||
| Fibrotest + APRI + FIB-4 | ≥F3 | 0.0001 | 0.724 | 54.9 | 89.9 | 79.5 | 73.7 |
| >0.5 | |||||||
| >1.5 | |||||||
| Fibrotest + FIB-4 | ≥F2 | 0.0001 | 0.719 | 62.5 | 81.3 | 81.2 | 62.5 |
| >1.5 | |||||||
| Fibrotest + AP index | ≥F2 | 0.018 | 0.616 | 43.1 | 80.0 | 74.3 | 51.2 |
| >6 | |||||||
| Fibrotest + Forns index | ≥F2 | 0.132 | 0.597 | 37.9 | 81.4 | 76.0 | 45.8 |
| >5 | |||||||
| Fibrotest + AAR | ≥F2 | 0.148 | 0.571 | 26.4 | 87.9 | 69.0 | 53.8 |
| >1.0 | |||||||
| APRI + FIB-4 | >0.5 | 0.0001 | 0.740 | 62.1 | 86.0 | 79.6 | 72.0 |
| >1.5 | |||||||
| APRI + AP index | >0.5 | 0.0001 | 0.689 | 47.5 | 90.3 | 76.7 | 71.8 |
| >6 | |||||||
| APRI + AAR | >0.5 | 0.0094 | 0.621 | 27.1 | 97.1 | 69.9 | 84.2 |
| >1.0 | |||||||
| APRI + Forns index | >0.5 | 0.0364 | 0.599 | 25.9 | 94.0 | 68.6 | 71.4 |
| >5 | |||||||
| FIB-4 + AP index | >1.5 | 0.0001 | 0.687 | 54.2 | 83.2 | 77.7 | 62.7 |
| >6 | |||||||
| FIB-4 + AAR | >1.5 | 0.0119 | 0.617 | 32.2 | 91.3 | 70.1 | 67.9 |
| >1.0 | |||||||
| FIB-4 + Forns index | >1.5 | 0.0055 | 0.658 | 44.4 | 87.2 | 77.3 | 61.5 |
| >5 | |||||||
| AP index + AAR | >6 | 0.0317 | 0.599 | 28.3 | 91.4 | 71.1 | 63.0 |
| >1.0 | |||||||
| AP index + Forns index | >6 | 0.0446 | 0.606 | 34.9 | 86.3 | 75.9 | 51.7 |
| >5 | |||||||
| AAR + Forns index | >1.0 | 0.238 | 0.570 | 22.2 | 91.8 | 70.5 | 57.1 |
| >5 |
NPV: negative predictive value; PPV: positive predictive value; APRI: aspartate aminotransferase-to-platelet ratio index; AAR: AST/ALT ratio; FIB-4: fibrosis index based on four factors; AP index: age-platelet index
Figure 2.
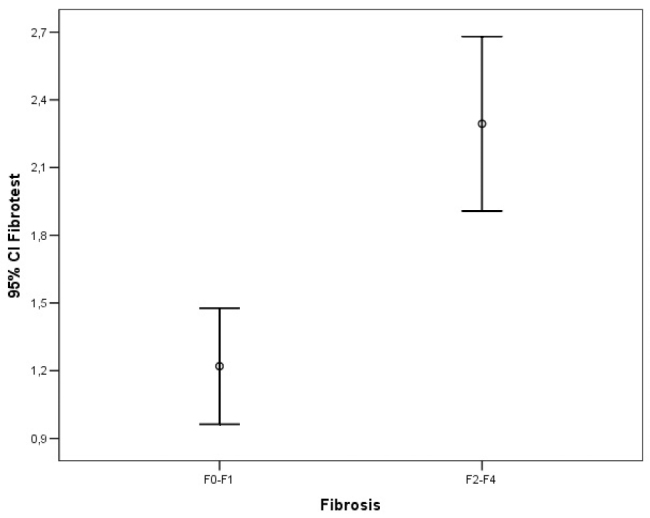
Error bar graphic images of FibroTest for diagnosis of liver fibrosis
Figure 3.
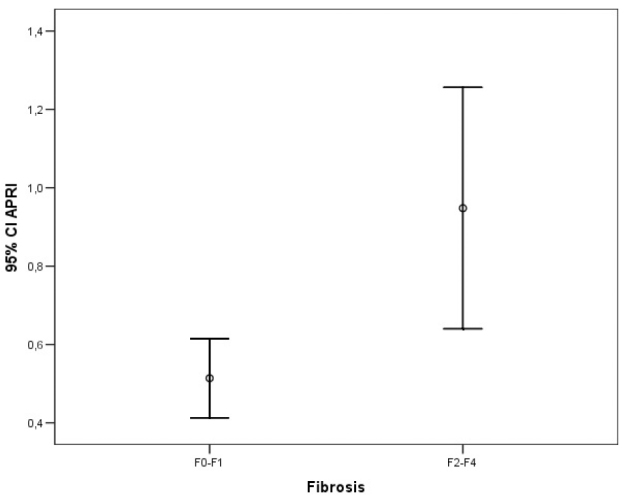
Error bar graphic images of APRI for diagnosis of liver fibrosis
Figure 4.
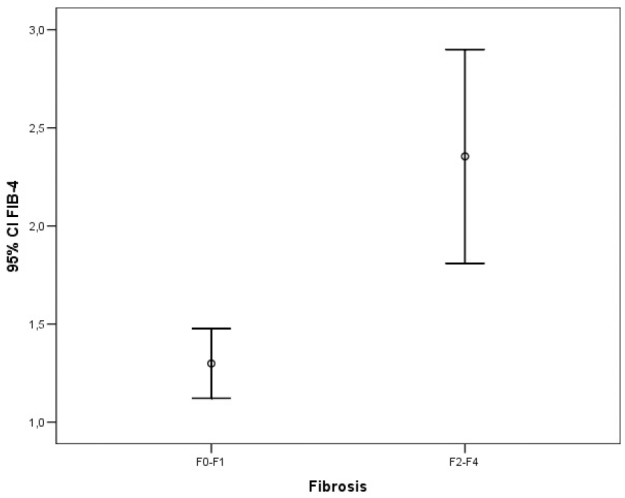
Error bar graphic images of FIB-4 for diagnosis of liver fibrosis
Figure 5.
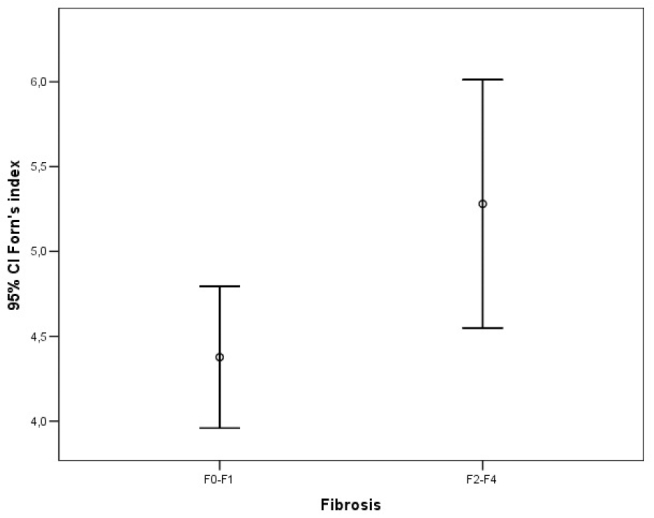
Error bar graphic images of the Forns index for diagnosis of liver fibrosis
Figure 6.
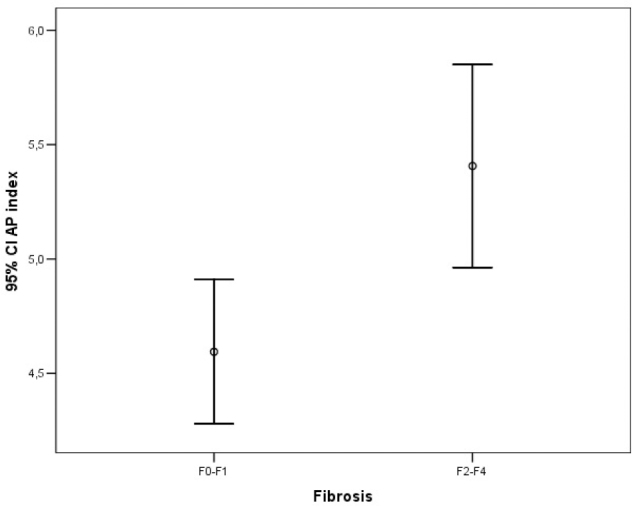
Error bar graphic images of the AP index for diagnosis of liver fibrosis
Figure 7.
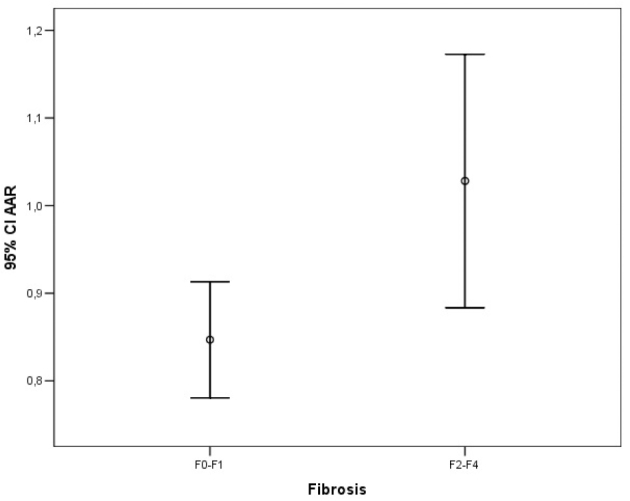
Error bar graphic images of AAR for diagnosis of liver fibrosis
DISCUSSION
Liver biopsy is the gold standard method for the determination of hepatic fibrosis, but it has many disadvantages, such as side effects, patient reluctance, inadequate sample, evaluation errors, and high cost (21,22). These limitations have led to the development of noninvasive alternative methods for the evaluation of liver fibrosis. These methods can be summarized as serum biomarkers and imaging methods for the assessment of liver fibrosis in CHC infection (7). TE is the most recommended diagnostic imaging method that measures liver stiffness, as mentioned in the introductory section. In many studies comparing the performance of TE and serum biomarkers mostly in viral hepatitis, it has been found that TE and serum biomarkers have equal performance for detecting significant fibrosis (20). Although the combination of TE and serum biomarkers is more effective than the combination of two serum biomarkers for a significant fibrosis finding, it is more expensive when compared with serum biomarkers (20). However, serum biomarkers have emerged as a leading methodology, as these tests are repeatable, noninvasive, and cost-effective. The costs of these tests vary, FibroTest being the most expensive. The others can be easily calculated using equations involving the patient’s routine biochemical tests. Serum biomarkers are recommended by the American Association for the Study of Liver Diseases, the European Association for the Study of the Liver, and the World Health Organization for determining stage of fibrosis in patients with HCV infection in the light of these advantages (23–25). However, these tests may exhibit low accuracy in differentiating intermediate or low stages of fibrosis from increased stages of fibrosis. In addition, interpreting the results may be difficult since various stages of fibrosis have different cutoff values. The validation of serum biomarkers for evaluating fibrosis is of critical importance for the routine clinical use of these tests. When applied individually in cases of significant fibrosis, the diagnostic value of these tests does not exceed 80%. For this reason, considering more than one serum biomarker can increase the susceptibility of the tests.
The data collected from the 182 enrolled patients clearly showed that fibrosis scores increase with age (p=0.003). This may be attributed to infection with HCV at an early age that remained untreated for many years due to a lack of patient awareness. The advanced age may thus have affected the efficacy of noninvasive tests, particularly FIB-4, the AP index, and the Forns index, in the determination of fibrosis (Table 2). Significant differences were observed in the APRI, FIB-4, AP index, and Forns index values between the fibrosis groups (p<0.05), indicating that APRI, FIB-4, the AP index, and the Forns index are important serum biomarkers for the determination of significant fibrosis. A positive correlation was observed between APRI, FIB-4, the AP index, and the Forns index and the METAVIR fibrosis scores. There was also a positive correlation between FibroTest and fibrosis. Sensitivity analysis showed that the FibroTest demonstrated the highest sensitivity, 78.4%, followed by APRI, FIB-4, the Forns index, and the AP index. AAR exhibited the lowest sensitivity, although no statistically significant difference was observed between the groups. The specificity values of all tests were close to one another. NPV was higher than PPV in all tests in this study. The AUC values were similar, the highest values being observed in the APRI, FIB-4, and FibroTest tests. The AUC, sensitivity, specificity, NPV, and PPV from noninvasive tests were similar to or lower than those reported in the original publications for those tests (9,17,26–31). Wai et al. (9) reported that APRI PPV for the determination of significant fibrosis was higher than NPV. AUC was reported at 0.80. However, the opposite was observed in the present study, in which NPV was higher than PPV. This difference may be attributable to the use of the Ishak score by Wai et al. (9) for fibrosis scoring, as well as the greater number of patients with cirrhosis and advanced fibrosis in that study. In the meta-analysis by Lin et al. (11), for the prediction of significant fibrosis, the estimated PPV and NPV of the 0.5 cutoffs were 55% and 69%, respectively. At the 1.5 cutoff, the estimated PPV and NPV were 82% and 63%, respectively. At the 0.7 cutoff, the estimated PPV and NPV were 70% and 79%, respectively. PPV and NPV of APRI for the prediction of significant fibrosis differ at various thresholds. In this meta-analysis, the best cutoff for diagnosing significant fibrosis was 0.7, whereas for diagnosing cirrhosis it was 1. In our study, at the 0.5 cutoff, the estimated PPV and NPV of APRI were 63.5% and 81.1%, respectively. In other studies, for investigating the diagnostic performance of APRI, different results have been reported for the estimated significant fibrosis and cirrhosis (28–30). In the study of Leroy et al. (32), when >0.5 cutoff was used for APRI in the diagnosis of significant fibrosis (F2–F4), the specificity was found to be only 26.8%; however, when >1.5 cutoff was used, the specificity was found to be 87.8%. At the same time, the concomitant presence of FibroTest>0.59 and APRI>2 improved PPV for significant fibrosis to 96.7% and for extensive fibrosis to 92.2%. The fact that our results are different from study results of Leroy et al. (32) may be due to the low number of patients with advanced fibrosis. Our study concluded that APRI had better performance for mild fibrosis.
While the FIB-4 AUC and sensitivity values in this study were parallel to those reported by Sterling et al. (27), the NPV value was higher. However, it should be noted that Sterling et al. (27) investigated patients co-infected with HCV/HIV and used the Ishak score for fibrosis scoring. In a study by Vallet-Pichard et al. (26), the FIB-4 index enabled the correct identification of patients with severe fibrosis (METAVIR F3–F4) with a 0.85 AUC. An FIB-4 index lower than 1.45 had an NPV of 94.7% to exclude any extensive fibrosis (F3–F4) with a sensitivity of 74.3% and a specificity of 80.1%. A higher FIB-4 index (>3.25) was recommended to confirm the existence of significant fibrosis. In our study, an FIB-4 index higher than 1.5 had PPV and NPV of 61.5% and 80.6%, respectively, with a sensitivity of 69% and a specificity of 80.1%.
Noninvasive tests exhibited improved diagnostic performance in patients with advanced fibrosis (17,29,31). The poor performance of noninvasive tests in this research compared with that of the original studies may be attributed to the fact that only 34% of patients had serious fibrosis and that the number of patients with cirrhosis was low. Recently, several studies have shown that the diagnostic performance of serum biomarker tests increases significantly when these are used in combination (33–36). Mummadi et al. (10) showed that there was a significant correlation between change in the stage of fibrosis and change in APRI and FIB-4. In this study, APRI had a positive predictive value (PPV) of 67% and a negative predictive value of 67% (NPV) for progression of fibrosis. As for FIB-4, PPV and NPV were 75%. The results of our study are similar, with a specificity of >80% being observed when the tests were used in combination. The combination of FibroTest with APRI and/or FIB-4 and APRI with FIB-4 exhibited good diagnostic performance for determining significant fibrosis. The FibroTest is more expensive than other serum biomarkers, and the use of APRI or FIB-4 is therefore recommended for the detection of hepatic fibrosis, particularly when resources are limited (25). However, none of these tests are of equal value in the assessment of both fibrosis and cirrhosis. For instance, APRI has been successfully validated for the diagnosis of both significant fibrosis and cirrhosis, whereas FIB-4 is useful only for the diagnosis of significant fibrosis (METAVIR stage≥F2) (25). One of the greatest advantages of combining tests is the resulting decrease in diagnostic differences. This decrease in diagnostic differences with the use of serum biomarker tests is clearly demonstrated in the present study. Our results show that the specificity of tests increases when these are used together.
In conclusion, there is a consistent and growing need for easily reproducible tests for the effective diagnosis of advanced hepatic fibrosis. Studies comparing the efficacies of liver biopsy and noninvasive tests provide guidance for the development and introduction of new techniques. This study concludes that APRI, FIB-4, AP index, Forns index, and FibroTest, as well as the combined use of these tests, can help identify CHC patients with significant/advanced fibrosis. However, none of the parameters according to cutoff points have a good diagnostic performance. The concurrent use of at least two of these tests will be useful for the evaluation and follow-up of CHC patients with fibrosis. In particular, the use of FIB-4 and APRI together may be indicative of significant/advanced fibrosis.
Acknowledgements
The authors would like to thank the Chronic Hepatitis C Patient Group for their contribution to this study.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of Karadeniz Technical University School of Medicine.
Informed Consent: Written informed consent was obtained from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - İ.K.; Design - İ.K., S.E., F.Ö.; Supervision - İ.K., S.E., F.Ö.; Resource - S.E., F.Ö.; Data Collection and/or Processing - İ.K., G.Y., M.P., T.D., S.K., M.C., A.K., S.A., Ö.A., H.T., Y.G., Ö.D., A.A.G., R.G., Z.K., H.T., N.B., S.E., F.Ö.; Analysis and/or Interpretation - İ.K., G.Y.; Literature Search - İ.K., G.Y., M.P., T.D., S.K., M.C., A.K., S.A., Ö.A., H.T., Y.G., Ö.D., A.A.G., R.G., Z.K., H.T., N.B., S.E., F.Ö.; Writing - İ.K., G.Y., S.E., F.Ö.; Critical Reviews - İ.K., G.Y., M.P., T.D., S.K., M.C., A.K., S.A., Ö.A., H.T., Y.G., Ö.D., A.A.G., R.G., Z.K., H.T., N.B., S.E., F.Ö.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: This study was financially supported by Roche Pharmaceuticals (Protocol No: ML21920).
REFERENCES
- 1.Messina JP, Humphreys I, Flaxman A, et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61:77–87. doi: 10.1002/hep.27259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tozun N, Ozdogan O, Cakaloglu Y, et al. Seroprevalence of hepatitis B and C virus infections and risk factors in Turkey: a fieldwork TURHEP study. Clin Microbiol Infect. 2015;21:1020–6. doi: 10.1016/j.cmi.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 3.de Schiavon LL, Narciso-Schiavon JL, de Carvalho-Filho RJ. Non-invasive diagnosis of liver fibrosis in chronic hepatitis C. World J Gastroenterol. 2014;20:2854–66. doi: 10.3748/wjg.v20.i11.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gebo KA, Herlong HF, Torbenson MS, et al. Role of liver biopsy in management of chronic hepatitis C: a systematic review. Hepatology. 2002;36:S161–72. doi: 10.1002/hep.1840360721. [DOI] [PubMed] [Google Scholar]
- 5.Saadeh S, Cammell G, Carey WD, Younossi Z, Barnes D, Easley K. The role of liver biopsy in chronic hepatitis C. Hepatology. 2001;33:196–200. doi: 10.1053/jhep.2001.20534. [DOI] [PubMed] [Google Scholar]
- 6.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 7.Gressner OA, Beer N, Jodlowski A, Gressner AM. Impact of quality control accepted inter-laboratory variations on calculated Fibrotest/Actitest scores for the non-invasive biochemical assessment of liver fibrosis. Clin Chim Acta. 2009;409:90–5. doi: 10.1016/j.cca.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Halfon P, Munteanu M, Poynard T. Fibrotest-Actitest as a non-invasive marker of liver fibrosis seven years ago: a 2008 updated meta-analyses of FT diagnostic and prognostic values in chronic liver diseases. Gastroenterol Clin Biol. 2008;32:22–39. doi: 10.1016/S0399-8320(08)73991-5. [DOI] [PubMed] [Google Scholar]
- 9.Wai CT, Greenson JK, Fontana RJ, et al. A simple non-invasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–26. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 10.Mummadi RR. Role of simple biomarkers in predicting fibrosis progression in HCV infection. World J Gastroenterol. 2010;16:5710–5. doi: 10.3748/wjg.v16.i45.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin ZH, Xin YN, Dong QJ, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726–36. doi: 10.1002/hep.24105. [DOI] [PubMed] [Google Scholar]
- 12.Sebastiani G, Alberti A. How far is non-invasive assessment of liver fibrosis from replacing liver biopsy in hepatitis C? Journal of Viral Hepatitis. 2012;19( Suppl 1):18–32. doi: 10.1111/j.1365-2893.2011.01518.x. [DOI] [PubMed] [Google Scholar]
- 13.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–93. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 14.Chinnaratha MA, Jeffrey GP, MacQuillan G, et al. Prediction of morbidity and mortality in patients with chronic hepatitis C by non-invasive liver fibrosis models. Liver International. 2014;34:720–7. doi: 10.1111/liv.12306. [DOI] [PubMed] [Google Scholar]
- 15.Sène D, Limal N, Messous D, et al. Biological markers of liver fibrosis and activity as non-invasive alternatives to liver biopsy in patients with chronic hepatitis C and associated mixed cryoglobulinemia vasculitis. Clin Biochem. 2006;39:715–21. doi: 10.1016/j.clinbiochem.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 16.Myers RP, Tainturier MH, Ratziu V, et al. Prediction of liver histological lesions with biochemical markers in patients with chronic hepatitis B. J Hepatol. 2003;39:222–30. doi: 10.1016/S0168-8278(03)00171-5. [DOI] [PubMed] [Google Scholar]
- 17.Forns X, Ampurdanès S, Llovet JM, et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36:986–92. doi: 10.1016/S0270-9139(02)00107-6. [DOI] [PubMed] [Google Scholar]
- 18.Stauber RE, Lackner C. Noninvasive diagnosis of hepatic fibrosis in chronic hepatitis C. World J Gastroenterol. 2007;13:4287–94. doi: 10.3748/wjg.v13.i32.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poynard T, Bedossa P. Age and platelet count: a simple index for predicting the pres- ence of histological lesions in patients with antibodies to hepatitis C virus. METAVIR and CLINIVIR Cooperative Study Groups. J Viral Hepatol. 1997;4:199–208. doi: 10.1046/j.1365-2893.1997.00141.x. [DOI] [PubMed] [Google Scholar]
- 20.EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237–64. doi: 10.1016/j.jhep.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Kleiner DE. The liver biopsy in chronic hepatitis C: a view from the other side of the microscope. Semin Liver Dis. 2005;25:52–64. doi: 10.1055/s-2005-864781. [DOI] [PubMed] [Google Scholar]
- 22.Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614–8. doi: 10.1111/j.1572-0241.2002.06038.x. [DOI] [PubMed] [Google Scholar]
- 23.Chung RT, Davis GL, Jensen DM, Masur H, Saag MS, Thomas DL, Aronsohn AI, et al. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. AASLD/IDSA HCV Guidance Panel. Hepatology. 2015;62:932–54. doi: 10.1002/hep.27950. [DOI] [PubMed] [Google Scholar]
- 24.European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2014. J Hepatol. 2014;61:373–95. doi: 10.1016/j.jhep.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Guidelines for the Screening, Care and Treatment of Persons with Hepatitis C Infection. Geneva: World Health Organization; 2014. Apr, WHO Guidelines Approved by the Guidelines Review Committee. [PubMed] [Google Scholar]
- 26.Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and Fibrotest. Hepatology. 2007;46:32–6. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 27.Sterling RK, Lissen E, Clumeck N, et al. APRICOT Clinical Investigators. Development of a simple non-invasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–25. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 28.Shaheen AA, Myers RP. Diagnostic accuracy of the aspartate aminotransferase-platelet ratio index for the prediction of hepatitis C-related fibrosis: a systematic review. Hepatology. 2007;46:912–21. doi: 10.1002/hep.21835. [DOI] [PubMed] [Google Scholar]
- 29.Silva RG, Jr, Fakhouri R, Nascimento TV, Santos IM, Barbosa LM. Aspartate aminotransferase-to-platelet ratio index for fibrosis and cirrhosis prediction in chronic hepatitis C patients. Braz J Infect Dis. 2008;12:15–9. [PubMed] [Google Scholar]
- 30.Güzelbulut F, Çetinkaya ZA, Sezikli M, Yaşar B, Ozkara S, Övünç AO. AST-platelet ratio index, Forns index and FIB-4 in the prediction of significant fibrosis and cirrhosis in patients with chronic hepatitis C. Turk J Gastroenterol. 2011;22:279–85. doi: 10.4318/tjg.2011.0213. [DOI] [PubMed] [Google Scholar]
- 31.Halfon P, Bacq Y, De Murret A, et al. Comparison of test performance profile for blood tests of liver fibrosis in chronic hepatitis C. J Hepatol. 2007;46:395–402. doi: 10.1016/j.jhep.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 32.Leroy V, Hilleret MN, Sturm N, et al. Prospective comparison of six non-invasive scores for the diagnosis of liver fibrosis in chronic hepatitis C. J Hepatol. 2007;46:775–82. doi: 10.1016/j.jhep.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Sebastiani G. Serum biomarkers for the non-invasive diagnosis of liver fibrosis: the importance of being validated. Clin Chem Lab Med. 2012;50:595–7. doi: 10.1515/cclm-2011-0850. [DOI] [PubMed] [Google Scholar]
- 34.Castera L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–50. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 35.Martinez SM, Crespo G, Navasa M, Forns X. Non-invasive assessment of liver fibrosis. Hepatology. 2011;53:325–35. doi: 10.1002/hep.24013. [DOI] [PubMed] [Google Scholar]
- 36.Boursier J, de Ledinghen V, Zarski JP, et al. Comparison of eight diagnostic algorithms for liver fibrosis in hepatitis C: new algorithms are more precise and entirely non-invasive. Hepatology. 2012;55:58–67. doi: 10.1002/hep.24654. [DOI] [PubMed] [Google Scholar]


