Abstract
Background/Aims
Thrombin Activatable Fibrinolysis Inhibitor (TAFI), in addition to suppressing fibrinolysis, can be involved as a natural anti-inflammatory molecule in the inflammatory process in acute pancreatitis (AP). The goal of this study was to discover the significance of early determination of the values of TAFI in the assessment of the severity of AP.
Materials and Methods
The prospective study included 92 patients with AP. In accordance with the revised Atlanta classification, we divided all patients into 3 groups (I-mild AP, II- moderate AP and III-severe AP). All patients were further classified into group A (mild AP) and group B (moderate and severe AP) with the aim of separating the patients with complicated and potentially bad prognosis. Biochemical markers, inflammatory biomarkers, coagulation parameters and TAFI were analyzed in all patients.
Results
The level of TAFI were significantly higher among the patients with the complicated form (group B) of AP (p=0.002). The analysis of the ROC curve in regard to the inflammatory biomarkers (fibronectin and CRP) has shown that TAFI possesses the best discriminatory ability for complicated forms of AP (AUC=0.724, p=0.013), with the sensitivity of 83.30% and the specificity of 56.00%.
Conclusion
The level of TAFI in plasma is higher in patients with moderate or severe AP. Determining the level of TAFI as a single parameter has a greater significance in the early estimation of the severity of AP than inflammatory biomarkers that we have analyzed.
Keywords: Pancreatitis, C-reactive protein, thrombin-activatable fibrinolysis inhibitor
INTRODUCTION
Acute pancreatitis (AP) is an inflammatory disease of the pancreas that can spread to surrounding structures as well as to distant organs (1). Regardless of etiology, AP begins as an inflammatory process that is set off by the early release and activation of pancreatic enzymes in the acinar cells of the pancreas (2,3). Key factors for the progression of the process and for the development of pancreatic and extrapancreatic complications include the apoptosis of the acinar cells, release of cytokines, activation of the coagulation system, tissue ischemia, and tissue necrosis (4).
The inflammatory response in AP is a result of the interactions between proinflammatory and anti-inflammatory mediators. In AP, the inflammatory process is connected to the process of coagulation. This connection is provided by the special cell receptors [protease-activated receptors (PARs)] present in inflammatory and endothelial cells. The coagulation proteases are bind to PARs, which are then activated to participate in the alteration of the inflammatory response (5,6). Animal studies have shown that the activation of hemostasis contributes to the progression of AP; thus, the inhibition of hemostasis would have a beneficial effect on the severity of the disease (7). Studies on the clinical significance of various parameters of the coagulation system in predicting complications caused by AP are limited.
Recent studies have shown that thrombin activatable fibrinolysis inhibitor (TAFI), in addition to suppressing fibrinolysis, can be involved in the inflammatory process as a natural anti-inflammatory molecule. This role of TAFIs depends on its ability to deactivate activated factors, such as complements C3a and C5a, as well as proinflammatory mediators, such as bradykinin and thrombin-cleaved osteopontin (6,8–10). Owing to this unique biochemical activity, TAFI can be assumed to significantly affect the inflammatory process during AP; therefore, the role of TAFI in the early estimation of the severity of AP can also be considered. To our knowledge, only one study in the literature has evaluated plasma TAFI levels in patients with AP (11).
The early determination of the severity of AP is important for the early recognition of pancreatic complications, triage of patients to higher levels of care such as an intensive care unit, therapeutic decisions, and prognostication. Timely therapy prevents the progression of processes that lead to multiple organ dysfunction syndrome, which is the most common cause of mortality in patients with AP.
Identifying prognostic parameters both individually or in combination that could provide a precise prognosis of AP outcomes is imperative. In the clinical setting, different clinical parameters, scoring systems, laboratory markers, and imaging methods are used to determine the severity of AP (12–14).
A great deal of effort has been made so far in the research of laboratory parameters that would be helpful in the timely determination of patients with AP and their stratification into groups with differential risks of complications and lethal outcomes. Unfortunately, no efficient, simple, and inexpensive parameter or system with high specificity and sensitivity for estimating the severity and prognosis of AP has been established. Taking into account the connection between inflammation and TAFI in AP as well as the absence of a superior system of predictable results, the aim of this study is to analyze the role of TAFI in estimating the severity and prognosis of AP.
MATERIALS AND METHODS
A prospective research, in the period from January 2012 to August 2016, examined 92 patients with AP. The present study only examined patients whose symptoms had appeared at 24–48 h before hospitalization. The study did not evaluate pregnant women, patients undergoing anticoagulant therapy, or patients with chronic liver disease and/or malignancy. The diagnosis of AP was established on the basis of characteristic signs and symptoms (periumbilical or epigastric pain, vomiting, and jaundice); increase levels of amylase and lipase in the serum at least three times greater than the upper limit of normal; and ultrasonographic evidence of pancreatic inflammation. Patients were classified according to the criteria of the Revised Atlanta Classification (15). Patients (N=52) with mild AP showed neither local nor systemic complications. Patients (N=22) with moderate AP showed local or systemic complications and/or transient organ failure, corrected within 48 h. Patients (N=18) with severe AP showed persistent organ failure for more than >48 h. According to the Modified Marshall Scoring System, organ failure included cardiovascular, pulmonary, and nephrological complications (15). These patients were hospitalized in the intensive care unit. Patients with an infection of pancreatic necrosis (44.44% of patients with severe AP) were transferred to the clinic for surgery. A lethal outcome was noted in 5 (5.4%) patients. In order to better predict a complicated AP, patients were divided into the following groups: group A with mild AP and group B with moderate or severe AP (complicated AP with potentially bad prognosis).
The whole blood count (hemoglobin, white blood cell, and platelet counts); biochemical markers [amylase, lipase, aspartat aminotranspherasis (AST), alanin aminotranspherasis (ALT), albumin, glucose, lactate dehydrogenase (LDH), urea, creatinine, and calcium]; inflammatory biomarkers [C-reactive protein (CRP), procalcitonin, and fibronectin]; erythrocyte sedimentation rate; coagulation parameters [prothrombin time (PT), activated partial thromboplastin time (APTT), fibrinogen, and D-dimer]; and TAFI levels were tested for all patients. The concentration of circulating TAFI in the plasma of patients was measured using a commercial enzyme-linked immunosorbent assay (ELISA) test (sandwich ELISA), which is based on the competitive binding of polyclonal antibodies with a specificity for TAFI, according to the manufacturer’s guidelines (Wuhan USCN Business Co., Ltd., USA). The concentration was determined by a standard curve and expressed in ng/mL. According to the manufacturer’s guidelines, there is no significant cross-reactivity or interference with other proteins.
All the examined patients provided their personal consent related to the use of their blood samples for this research. All patients provided a written consent to participate in the study. The study protocol was approved by the Local Ethics Committee (No: 12-1720/3).
Statistical analysis
Data were expressed as mean and standard deviation or frequencies and percentages. Categorical variables between different groups were compared using the chi-squared test or Fisher’s exact test. Continuous variables were assessed for normality using Kolmogorov-Smirnov test. Normally distributed data were tested with unpaired t-test. Non-normally distributed values between the two groups were compared using Mann–Whitney U-test. The discriminative ability of TAFI, fibronectin, and CRP was tested by ROC curve analysis. A p-value <0.05 was considered statistically significant. Statistical analysis of data was performed using the R version 2.15.3 software (R Foundation for Statistical Computing, Vienna, Austria) (16).
RESULTS
After the analysis of demographic data, groups A and B were determined to be matched for sex (p=0.694). Statistically, patients in the group A were significantly older than those in the group B (p=0.031). The biliary etiology of AP among patients in the group A was significantly higher than that among patients in the group B (73.10% vs. 57.50%, p=0.046) (Table 1).
Table 1.
Patient demographics according to AP severity
| Characteristics | Group A | Group B | p |
|---|---|---|---|
| Sex (M/F) | 24/28 | 21/19 | 0.6941 |
| Etiology, N (%) | |||
| Biliary | 38 (73.1) | 23 (57.50) | 0.046 |
| Alcoholic | 6 (11.5) | 13 (32.50) | |
| Idiopathic | 8 (15.4) | 4 (10.00) | |
Chi squared test
The statistical analysis of the whole blood count as well as of some biochemical and inflammatory markers revealed that the number of leukocytes was significantly higher among patients in the group B, whereas the remaining parameters were not significantly different between the groups. In the present study, CRP at admission was determined to be significantly higher among patients in the group B. However, the difference was not statistically significant (p=0.052) (Table 2).
Table 2.
Blood test results and biochemical and biohumoral markers according to AP severity
| Characteristics | Group A | Group B | p |
|---|---|---|---|
| ESR (mm/h) | 37.22±22.75 | 40.63±30.78 | 0.907 |
| HCT | 40.21±5.77 | 42.26±5,40 | 0.085 |
| WBC (×103/mm3) | 11.22±5.35 | 13.99±5.30 | 0.002* |
| PLT (×103/mm3) | 211.52±76.33 | 251.40±120.63 | 0.084 |
| Serum amylase (U/L) | 1283.57±1103.15 | 1353.22±886.81 | 0.361 |
| Urinary amylase (U/L) | 8887.29±15158.67 | 8977.42±1103.38 | 0.097 |
| Serum lipase (U/L) | 2550.10±2399.59 | 2060.57±2086.01 | 0.547 |
| Calcium (mmol/L) | 2.42±0.19 | 2.37±0.23 | 0.470 |
| LDH (U/L) | 561.64±316.67 | 576.77±313.09 | 0.885 |
| CRP (mg/L) | 43.48±54.47 | 86.89±89.99 | 0.052 |
| PCT (ng/mL) | 3.63±10.44 | 1.66±4.29 | 0.896 |
| Fibronectin | 262.66±36.59 | 253.15±30.77 | 0.465 |
ESR: erythrocyte sedimentation rate; HCT: hematocrit; WBC: white blood cell; PLT: platelet; LDH: lactate dehydrogenase; CRP: C-reactive protein; PCT: procalcitonin;
p<0.05
The statistical analysis of coagulation parameters revealed that APTT concentration was significantly higher among patients in the group A (p=0.042). TAFI levels were significantly higher among patients with complicated forms (moderate or severe) of AP (p=0.002) (Figure 1).
Figure 1.
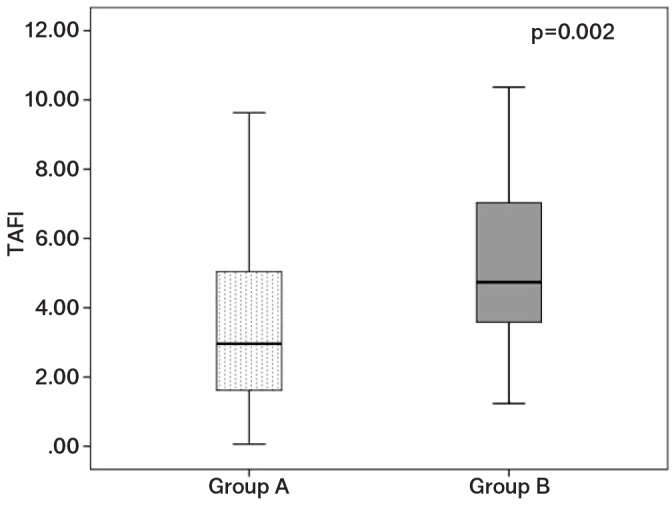
TAFI levels of patients with mild (group A) and moderate or severe AP (group B)
Group A: mild; Group B: moderate or severe pancreatitis
The analysis of the ROC curve for inflammatory biomarkers (CRP and fibronectin) revealed that TAFI showed the best discriminatory ability for complicated forms (moderate and severe) of AP (AUC=0.724, p=0.013), with a sensitivity of 83.30% and a specificity of 56.00% (Figure 2). The remaining tested parameters were not statistically significant (Table 3).
Figure 2.
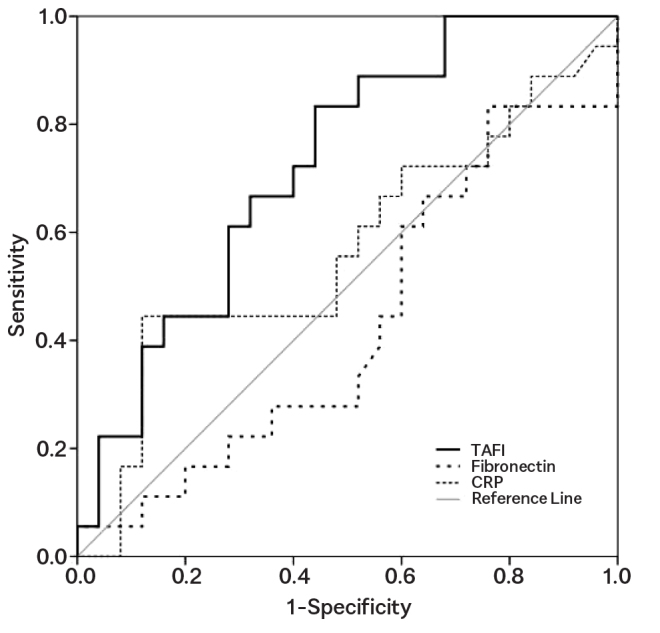
ROC curve of TAFI values, fibronectin, and CRP for the evaluation of acute pancreatitis
Table 3.
Coagulation parameters according to AP severity
| Characteristics | Group A | Group B | p |
|---|---|---|---|
| PT (s) | 79.11±24.74 | 79.57±15.05 | 0.676 |
| APTT (s) | 26.82±.21 | 24.88±3.82 | 0.042* |
| INR | 1.30±0.59 | 1.20±0.17 | 0.631 |
| Fibrinogen (g/L) | 6.99±2.08 | 7.75±2.16 | 0.191 |
| D-dimer (ng/mL) | 1034.17±1256.07 | 1263.79±1269.82 | 0.127 |
| TAFI (ng/mL) | 3.49±2.41 | 5.17±2.40 | 0.002* |
PT: prothrombin time; APTT: activated partial thromboplastin time; INR: international normalized ratio; TAFI: thrombin activator fibrinolysis inhibitor;
p<0.05
DISCUSSION
The timely recognition of patients with AP who have a risk of developing complications or have already developed complications is highly important.
Different multifactorial scoring systems use parameters at the same time that need to be determined at different times, and these systems are often very complicated to use (12–17). The most sensitive parameter or scoring system has not been verified to date (18).
The first result of our study is the finding that CRP at admission was higher among patients with complicated AP (group B). However, the difference was not statistically significant probably owing to the small sample or the premature determination of CRP.
CRP is a non-specific inflammatory mediator that is often used to evaluate the severity of AP. Analysis of CRP is widely available in laboratories, and various researchers have suggested using CRP as a prognostic marker for severe AP (18–20). However, the main problem is that the specificity of CRP in the prediction of the severity of AP is only 80% (15). Analysis of CRP has a sensitivity of 73% and specificity of 71% in the prediction of the severity of AP (14). CRP does not reflect the severity of AP when these levels are measured in the early phase after onset. CRP has to be measured at >48 h after the onset of clinical symptoms because CRP levels peak at 24–48 h after the onset of pancreatitis (21–22). Stirling et al. (23) have suggested that a rise of >90 mg/dL from admission or an absolute value of >190 mg/dL at 48 h predicts severe disease with the greatest accuracy.
The analysis of blood test results in this study revealed that the number of leukocytes at admission was significantly higher among patients in the group B. However, leukocytosis is not sufficiently specific to assess the severity of AP.
The second result of our study is the finding that significantly higher levels of TAFI were detected in the groups of patients with complicated forms of AP (group B) than in those with mild AP (group A). TAFI values were determined at the admission of patients. ROC analysis revealed that TAFI is a useful prognostic parameter that allows the differentiation of complicated forms of AP from mild AP.
The analysis of ROC curve demonstrated that TAFI shows a better discriminatory ability for mild and complicated forms of AP than inflammatory biomarkers (fibronectin and CRP), with a sensitivity of 83.30% and a specificity of 56.00%.
Despite the fact that there is substantial experimental evidence suggesting an important role of the coagulation system in AP, studies on the clinical significance of coagulation parameters in assessing the severity and predicting the course of AP remain scarce. TAFI is one of the coagulation parameters that following the start of the inflammatory process can influence the severity and outcome of AP. TAFI is a 58 kDa carboxypeptidase that is synthesized in the liver and circulates in the plasma as a zymogen and is activated mostly by thrombin/thrombomodulin complex and converted to the active enzyme (TAFI) (24–25). TAFI plays the role of a regulatory protein in the coagulation/fibrinolysis balance, inflammation, and active thrombin/thrombomodulin from the surface of endothelial cells (26–27). Taking into account its unique biochemical activity, one can assume that TAFI, in addition to suppressing fibrinolysis, significantly affects inflammation during AP. TAFI is now recognized as another physiological substrate for the thrombin/thrombomodulin complex. Through the activation of PAR on monocytes, smooth muscle cells, and endothelial cells, thrombin can act as an anti-inflammatory molecule, providing a direct link between coagulation and inflammation (28). This dual function in the regulation of the coagulation and inflammatory processes makes TAFI an interesting protein in the pathophysiology of AP.
Existing clinical studies have investigated the value and effect of TAFI in patients with ulcerative colitis and Crohn’s disease, type 2 diabetes, rheumatoid arthritis, and cardiovascular diseases; studies regarding the role of TAFI in AP are limited to date (9,27–29). A study by Sayilir et al. (11) has aimed to examine TAFI as an indicator of inflammation as well as its possible association with clinical and radiological parameters in AP. In the present study, which included 21 patients with AP, elevated TAFI levels were detected at the initial phases of AP with a significant decline in these values following treatment. TAFI-Ag was found to have high sensitivity, specificity, and predictive value in AP. The analysis of the overall accuracy of TAFI in determining the severity of AP was found to be 83.3%, with a sensitivity of 80.9% and a specificity of 85.7%.The limitation of our study is primarily in the relatively small sample size. Accomplishing such studies in a larger cohort would be interesting.
In conclusion, we found that plasma TAFI levels are higher in patients with complicated forms of AP. Determining TAFI levels as a single parameter has a greater significance in the early estimation of the severity of AP than determining other inflammatory biomarkers. In addition, TAFI may play a key role in regulating the crosstalk between coagulation, fibrinolysis, and inflammation. Further research is certainly imperative. We believe that TAFI can be an effective indicator of disease activity. For the wider use of this parameter, the standardization of test kits for its detection in the serum is warranted.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Local Ethics Committee (Decision No: 12-1720/3).
Informed Consent: Written informed consent was obtained from all the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - B.R.D.; Design - B.RD., S.T.R.; Supervision - B.RD., S.G.; Resources - B.RD.; Materials - B.RD., S.T.R.; Data Collection and/or Processingn - B.RD., A.I.; Analysis and/or Interpretation - B.RD., A.I.; Literature Search - B.RD., S.TR., A.I.; Writing - B.R.D., S.T.R.; Critical Reviews - S.G., A.I.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet. 2008;12:143–52. doi: 10.1016/S0140-6736(08)60107-5. [DOI] [PubMed] [Google Scholar]
- 2.Ince AT, Baysal B. Pathophysiology, classification and available guidelines of acute pancreatitis. Turk J Gastroenterol. 2014;25:351–7. doi: 10.5152/tjg.2014.13005. [DOI] [PubMed] [Google Scholar]
- 3.Stevenson K, Carter RS. Acute pancreatitis. Surgery. 2013;31:295–303. doi: 10.1016/j.mpsur.2013.04.004. [DOI] [Google Scholar]
- 4.Do JH. Mechanism of severe acute pancreatitis: focusing on development and progression. Korean J Pancreas Biliary Tract. 2015;20:115–23. doi: 10.15279/kpba.2015.20.3.115. [DOI] [Google Scholar]
- 5.Gould T, Mai S, Liaw P. Coagulation Abnormalities in Acute Pancreatitis. In: Rodrigo L, editor. Pancreatitis-Treatment and Complications. InTech Publishers; Canada: [DOI] [Google Scholar]
- 6.Pawlinski R, Pedersen B, Schabbauer G, et al. Role of tissue factor and protease-activated receptors in a mouse model of endotoxemia. Blood. 2004;103:1342–7. doi: 10.1182/blood-2003-09-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lisman T, Porte RJ. Activation and regulation of hemostasis in acute liver failure and acute pancreatitis. Semin Thromb Hemost. 2010;36:437–43. doi: 10.1055/s-0030-1254052. [DOI] [PubMed] [Google Scholar]
- 8.Ottesen LH, Bladbjerg EM, Osman M, et al. Protein C activation during the initial phase of experimental acute pancreatitis in the rabbit. Dig Surg. 1999;16:486–95. doi: 10.1159/000018774. [DOI] [PubMed] [Google Scholar]
- 9.Owczarek D, Undas A, Foley JH, Nesheim ME, Jabłonski K, Mach T. Activated thrombin-activatable fibrinolysis inhibitor (TAFIa) is associated with inflammatory markers in inflammatory bowel diseases: TAFIa level in patients with IBD. J Crohns Colitis. 2012;6:13–20. doi: 10.1016/j.crohns.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Leung LL, Nishimura T, Myles T. Regulation of tissue inflammation by thrombin-activatable carboxypeptidase B (or TAFI) Adv Exp Med Biol. 2008;632:61–9. doi: 10.1007/978-0-387-78952-1_5. [DOI] [PubMed] [Google Scholar]
- 11.Sayilir A, Beyazit Y, Yesil Y, et al. Plasma thrombin-activatable fibrinolysis inhibitor as an indicator of inflammation and disease severity in acute pancreatitis. Clin Res Hepatol Gastroenterol. 2012;36:498–504. doi: 10.1016/j.clinre.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Meher S, Mishra TS, Sasmal PK, et al. Role of Biomarkers in Diagnosis and Prognostic Evaluation of Acute Pancreatitis. Journal of Biomarkers. 2015;2015 doi: 10.1155/2015/519534. 519534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khanna AK, Meher S, Prakash S, et al. Comparison of Ranson, Glasgow, MOSS, SIRS, BISAP, APACHE-II, CTSI Scores, IL- 6, CRP, and Procalcitonin in Predicting Severity, Organ Failure, Pancreatic Necrosis, and Mortality in Acute Pancreatitis. HPB Surgery. 2013;367581:1–10. doi: 10.1155/2013/367581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang Jeffrey CF. Acute Pancreatitis Workup. Medscape. Available at https://emedicine.medscape.com/article/181364-overview. Updated: Feb 13, 2017.
- 15.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis- 2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–11. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 16.Core Team R. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2014. [Accessed March 3, 2015]. Available at: http://www.R-project.org/ [Google Scholar]
- 17.Tonsi AF, Bacchion M, Crippa S, Malleo G, Bassi C. Acute pancreatitis at the beginning of the 21st century: the state of the art. World J Gastroenterol. 2009;15:2945–59. doi: 10.3748/wjg.15.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Başak F, Hasbahçeci M, Şişik A, et al. Can C-reactive protein levels increase the accuracy of the Ranson score in predicting the severity and prognosis of acute pancreatitis? A prospective cohort study. Turk J Gastroenterol. 2017;28:207–13. doi: 10.5152/tjg.2017.16686. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg JA, Hsu J, Bawazeer M, et al. Clinical practice guideline: management of acute pancreatitis. Can J Surg. 2016;59:128–40. doi: 10.1503/cjs.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pezzilli R, Melzi d‘Eril GV, Morselli-Labate AM, Merlini G, Barakat B, Bosoni T. Serum amyloid A, procalcitonin, and C-reactive protein in early assessment of severity of acute pancreatitis. Dig Dis Sci. 2000;45:1072–8. doi: 10.1023/A:1005525329939. [DOI] [PubMed] [Google Scholar]
- 21.Al-Bahrani AZ, Ammori BJ. Clinical laboratory assessment of acute pancreatitis. Clin Chim Acta. 2005;362:26–48. doi: 10.1016/j.cccn.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Imamura T, Tanaka S, Yoshida H, et al. Significance of measurement of high-sensitivity C-reactive protein in acute pancreatitis. J Gastroenterol. 2002;37:935–8. doi: 10.1007/s005350200157. [DOI] [PubMed] [Google Scholar]
- 23.Stirling AD, Moran NR, Kelly ME, Ridgway PF, Conlon KC. The predictive value of C-reactive protein (CRP) in acute pancreatitis-is interval change in CRP an additional indicator of severity? HPB. 2017;19:874–80. doi: 10.1016/j.hpb.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Bouma BN, Meijers JC. Thrombin-activatable fibrinolysis inhibitor (TAFI, plasma procarboxypeptidase B, procarboxypeptidase R, procarboxypeptidase U) J Thromb Haemost. 2003;1:1566–74. doi: 10.1046/j.1538-7836.2003.00329.x. [DOI] [PubMed] [Google Scholar]
- 25.Boffa MB, Nesheim ME, Koschinsky ML. Thrombin activable fibrinolysis inhibitor (TAFI): molecular genetics of an emerging potential risk factor for thrombotic disorders. Curr Drug Targets Cardiovasc Haematol Disord. 2001;1:59–74. doi: 10.2174/1568006013337999. [DOI] [PubMed] [Google Scholar]
- 26.Lempinen M, Puolakkainen P, Kemppainen E. Clinical value of severity markers in acute pancreatitis. Scand J Surg. 2005;94:118–23. doi: 10.1177/145749690509400207. [DOI] [PubMed] [Google Scholar]
- 27.Myles T, Nishimura T, Yun TH, et al. Thrombin-activatable fibrinolysis inhibitor, a potential regulator of vascular inflammation. J Biol Chem. 2003;19:51059–67. doi: 10.1074/jbc.M306977200. [DOI] [PubMed] [Google Scholar]
- 28.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–64. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 29.Erdogan M, Solmaz S, Canataroglu A, et al. Plasma thrombin-activatable fibrinolysis inhibitor (TAFI) antigen levels in diabetic foot ulcers. Endocrine. 2010;37:449–54. doi: 10.1007/s12020-010-9329-1. [DOI] [PubMed] [Google Scholar]


