Abstract
Background/Aims
An organ preservation approach using chemoradiotherapy has been established for anal cancer. This retrospective cohort study aimed to define the clinico-demographic characteristics and outcomes of cases of human immunodeficiency virus (HIV)-negative anal carcinoma during a period of 20 years in a single comprehensive cancer institute.
Materials and Methods
This was a single-center retrospective cohort study of patients who were treated between January 1995 and January 2015. The primary outcome measures that were investigated included overall survival (OS), progression-free survival (PFS), colostomy rates, and colostomy-free survival (CFS).
Results
A total of 28 patients who were principally treated with standard 5-fluorouracil + mitomycin combination chemoradiotherapy were eligible for analysis. The 3 -and 5-year PFS rates were 92.4% and 63%, respectively. The lower T stage was found to be associated with a prolonged PFS (p=0.001). The 3 -and 5-year CFS rates were 84.3% and 74.9%, respectively. A longer CFS was observed with lower T stages (p=0.05). At the last follow-up, 75% of the patients with anal cancer were alive, and 71.4% of the patients were disease free. The median OS was not reached with a median follow-up of 54 months (range, 6–115 months). The 3 -and 5-year OS rates were 82% and 71.1%, respectively. No late toxicity was observed during the follow-up period.
Discussion
The short -and long-term prognoses of HIV-negative patients with anal squamous cell carcinoma were good, and low-grade toxicity was rare, thereby demonstrating that these patients can be successfully treated in a real-life setting with favorable outcomes.
Keywords: Anal cancer, squamous cell carcinoma, mitomycin, chemoradiation, human immunodeficiency virus
INTRODUCTION
Anal cancer is one of the rarest malignancies of the digestive system. In 2016, 8,080 new cases of anal cancers and 1,080 deaths from anal cancers were estimated in the United States (1). An analysis of registry data from Western countries revealed that the incidence is increasing, which draws attention to this rare malignancy. In Canada, there were 515 new cases per year reported in 2014, with an age-adjusted annual incidence rate of 1.3 per 100,000. European data indicate that 2,000 males and 2,300 females are diagnosed with anal cancer every year (2). Similarly, anal cancer has an age-specific incidence of 1.2 per 100,000 person-years for men and 1.6 per 100,000 person-years for women. According to the Turkish Cancer 2010 statistics between 2009 and 2013, 316 new cases of anal cancer were diagnosed, and the age-adjusted incidence rate was reported to be 0.3 per 100,000 for males and 0.2 per 100,000 for females (3).
The anal canal is lined with three different cell types craniocaudally: glandular, transitional, and squamous cells. Hence, adenocarcinomas, melanomas, small cell carcinomas, and carcinoids are the most frequent pathological diagnoses in malignancies of the anal canal region. However, the majority of histologies are non-keratinizing squamous cell carcinomas (SCCs) and its cloacogenic, transitional, and basaloid variants.
Anal SCC has been historically treated with surgery. In modern medicine, preservation of the structural and functional integrity of the anal sphincter, while not compromising the curability, is important for the treatment of anal SCC. Hence, an organ-preserving approach with chemoradiation (CRT) was established and has been validated for more than 40 years (4). The commonly preferred regimen for CRT comprises 5-fluorouracil (5-FU) and mitomycin (MMC). A cure is possible with CRT for the majority of patients, and surgery is considered as salvage therapy for those who relapsed or have residual disease after the first round of CRT.
Cervical and anal cancers are commonly associated with human papillomavirus (HPV) causality. Chronic infection with HPV and an increased risk of proto-oncogenicity can result in the development of squamous cell carcinoma. Along with HPV infection, other sexually transmitted diseases, such as human immunodeficiency virus (HIV) infections, are also associated with increased risk. Furthermore, chronic immunosuppression is also a risk factor for stage progression (5,6). With the development of highly active antiretroviral therapy, the prognosis of many cancer types has improved for HIV-positive patients, and several studies have reported successful treatment of HIV-positive patients. However, the risk of opportunistic infections and intolerance to chemotherapy due to low performance status and variations in CRT protocols are primary concerns. The present study has a different patient profile for anal SCC because the HIV prevalence, anoreceptive intercourse, and male homosexuality were low or reported to be low due to cultural and religious reasons (7).
Only a few randomized clinical trials have been performed due to the rarity of this tumor type. Therefore, epidemiological and clinical data from real-life experiences and follow-ups have a significant value for understanding the management of anal SCC. This trial was designed as a retrospective cohort analysis in a single but comprehensive cancer institute that served as a major reference center.
MATERIALS AND METHODS
The hospital electronic database was searched for patients with anal SCC who were consulted in either the medical oncology or radiation oncology department between January 1, 1995 and January 1, 2015. A local ethical committee approved the trial. Written informed consent was signed by the patients, and the principles outlined in the Declaration of Helsinki were followed. A chart review was conducted to access the demographic and clinical data. Baseline patient characteristics (age, gender, occupation, smoking status, marital status, stage at presentation, symptoms at presentation, diagnostic methods, clinical management strategies, adverse events, salvage surgeries, and colostomy surgeries) were recorded. Study endpoints included overall survival (OS), progression-free survival (PFS), colostomy rates, colostomy-free survival (CFS), and treatment-related toxicity.
Treatment protocol
Patients were treated intravenously with 5-FU/MMC (750 mg/m2/d of 5-FU via continuous infusion on days 1 to 5 and 29 to 32 with 15 mg/m2/d of MMC bolus on day 1). In 6 patients, due to the shortage of MMC, cisplatin (Cis)/5FU (100 mg/m2/d of cisplatin on day 1 with the same 5-FU application) was administered. All patients were treated with chemoradiotherapy with curative intent after the treatment volume was defined using three-dimensional computed tomography (CT)-based planning. Patients were simulated in the supine position with a full bladder and empty rectum. An intravenous contrast material was used. Target volumes consisted of the primary tumor, metastatic lymph nodes, and elective regional lymphatics, including perirectal, presacral, internal iliac, external iliac, and inguinal lymph nodes. Radiotherapy (RT) was administered using linear accelerators, typically with 6–25 MV photons, 1.8–2 Gy daily fractions, and 5 fractions per week with a median dose of 50.4 Gy (range, 43.2–64.4 Gy).
Statistical analysis
This study was designed as a retrospective cohort study, which included detailed clinical characteristics and treatment data of all cases at a single comprehensive cancer institute. Hospital records were searched for descriptive analyses and outcome data. CFS, PFS, and OS were the main outcome measures. OS was defined as the time from diagnosis to death from any cause, and PFS was defined as the time from diagnosis to disease progression or recurrence. CFS was defined as the time from diagnosis of anal cancer to the date of colostomy surgery. Comparative statistical analyses between independent groups were performed using the chi-square test for categorical variables and the Mann-Whitney test for continuous variables. The confidence interval (CI) was set as 95% throughout the analyses. Survival outcomes were assessed using the Kaplan-Meier approach, and subgroup analyses were performed using the log-rank test. All statistical analyses were performed using the IBM Statistical Package for Social Sciences (SPSS) software version 21 (IBM Inc.; Armonk, NY, USA).
RESULTS
Patient and tumor characteristics
A search of the institute’s cancer records yielded 28 patients with anal cancer who were treated with CRT in the past 20 years (between January 1995 and January 2015). The main characteristics of the patients and tumors are listed in Table 1. The median follow-up time was 54 months (range, 6–115 months). The median age of the patients was 58 years (range, 39–71 years). There were 46.4% (13) female and 53.6% (15) male patients. The patient population was entirely HIV negative. A recent or past smoking history was present in 78% of patients. Tumor stages were as follows: 25% as stage I, 42.9% as stage II, 17.8% as stage IIIA, and 14.3% as stage IIIB.
Table 1.
Main characteristics of patients and their tumors
| N | % | |
|---|---|---|
| Median age, years | 54 (range, 39–74) | |
| Gender distribution | ||
| Female | 13 | 46.4 |
| Male | 15 | 53.6 |
| HIV serology | ||
| Positive | - | - |
| Negative | 28 | 100 |
| HCV serology | ||
| Positive | 1 | 3.6 |
| Negative | 27 | 96.4 |
| ECOG performance status | ||
| ECOG 0 | 23 | 82.1 |
| ECOG 1 | 5 | 17.9 |
| T stage | ||
| T1 | 10 | 35.7 |
| T2 | 12 | 42.9 |
| T3 | 6 | 21.4 |
| TNM stage | ||
| Stage I | 7 | 25 |
| Stage II | 12 | 42.9 |
| Stage IIIA | 5 | 17.8 |
| Stage IIIB | 4 | 14.3 |
HBV: Hepatitis B virus; HCV: Hepatitis C virus; HIV: Human immunodeficiency virus; ECOG: Eastern Cooperative Oncology Group; TNM: Classification of Malignant Tumours
Treatment and response
The entire study population had been treated with CRT with curative intent, and all patients completed RT except for 1 patient. CRT was performed without interruptions in 20 (69%) patients. The median treatment interruption was 4 days (range, 2–10 days). Intensity-modulated radiotherapy (IMRT) was administered to 1 (3.6%) patient, 13 (46.4%) patients were treated with conventional RT, and 14 (50%) were treated with three-dimensional conformal RT (3DCRT). Six patients (21.4%) received radiation concomitantly with cisplatin, and 22 patients received standard 5-FU + MMC chemotherapy (details explained later). There was no delay in chemotherapy administration, except in 1 patient who developed grade 4 hematologic toxicity and died due to febrile neutropenia.
Initial tumor responses were assessed radiologically with pelvic magnetic resonance imaging (MRI) 60 days after CRT. All patients showed a complete response (CR). At a median follow-up of 54 months, 4 disease progressions and 8 deaths were recorded. In 3 of 4 patients, disease relapsed in the anal canal, and in one patient, disease progression occurred both locally and in the regional (inguinal) lymphatics. Overall, 5 patients underwent abdominoperineal resection (APR), 4 patients had disease progression, and 1 patient developed non-healing anocutaneous fistula and underwent APR. In 2 of 4 patients who developed disease progression, the initial chemotherapy regimen comprised cisplatin +5-FU.
The 3 -and 5-year PFS rates were 92.4% and 63%, respectively (Figure 1A). The initial T stage was found to be associated with prolonged PFS (p=0.001) (Figures 1B and 1C). The 3 -and 5-year CFS rates were 84.3% and 74.9%, respectively (Figure 2A). Longer CFS was observed with lower T stages (p=0.05) (Figures 2b, c.).
Figure 1. a–c.
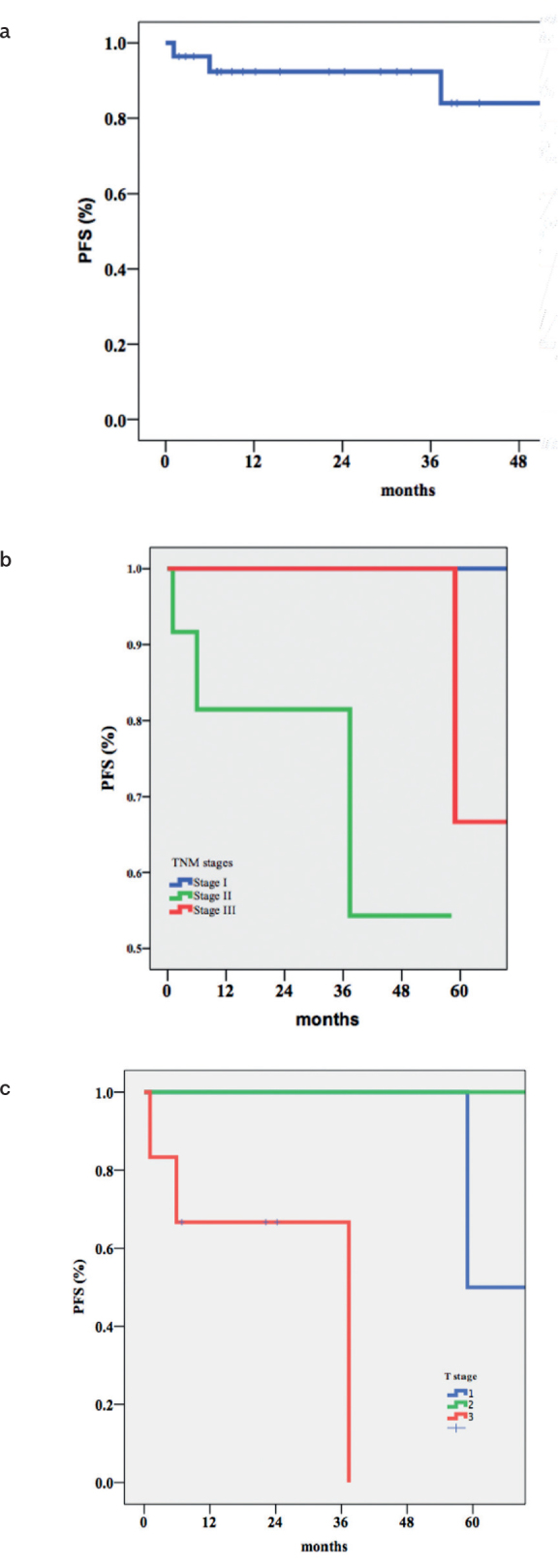
Overall PFS (a); PFS according to TNM stages (b); -PFS according to T stages (c)
Figure 2. a–c.
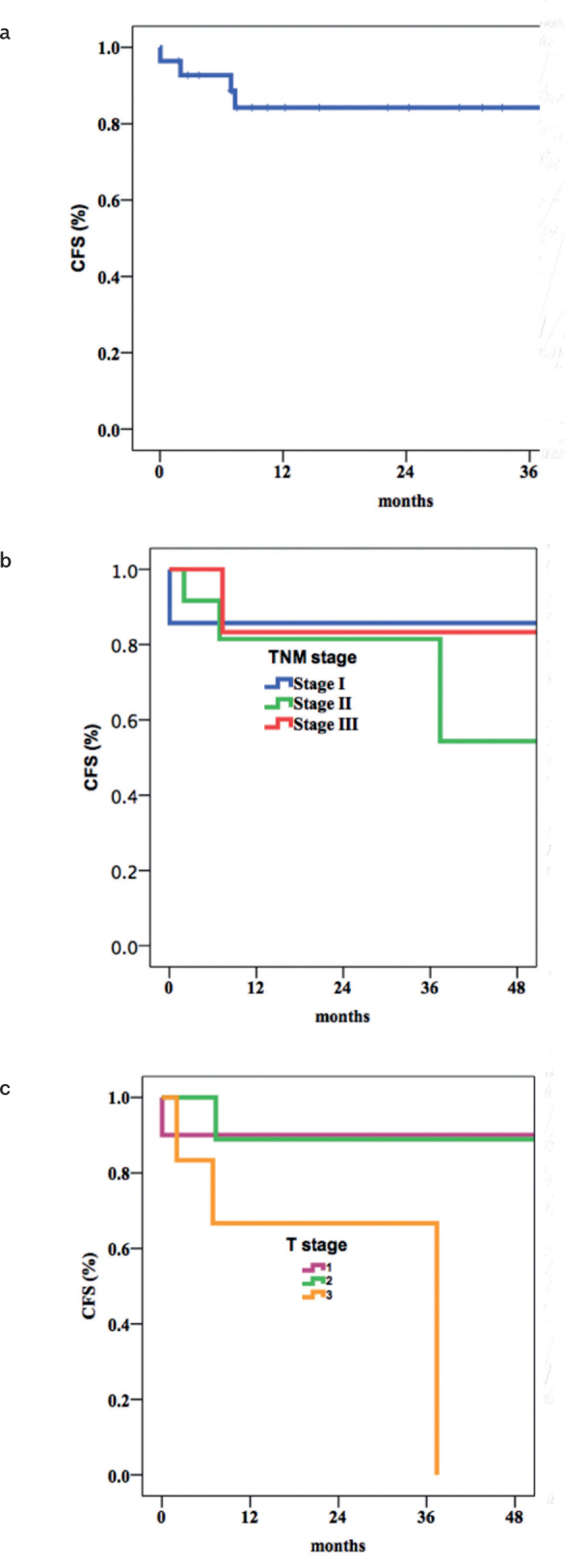
Overall CFS (a); CFS according to TNM stages (b); CFS according to T stages (c)
At the last follow-up, 75% of patients with anal cancer were alive, and 71.4% of patients were disease free. One cancer-specific death occurred due to progressive lung metastasis of the anal SCC. During the follow-up, one patient was diagnosed with metastatic pancreatic cancer as a second primary malignancy, and this patient died due to pancreatic cancer. In 3 patients, ischemic cardiac disease was the cause of death: one case was due to trauma due to a traffic accident, and one case was a cerebrovascular event. In one of the patients who developed relapse of anal SCC, lung metastasis developed in the long term; however, the patient was still alive after 34 months. The median OS was not reached within the median follow-up of 54 months (range, 6–115 months). Currently available data reveal that the mean survival is 152.4+ months (SE: 19.2; 95% CI: 114.8–189.9 months). The 3 -and 5-year OS rates were 82% and 71.1%, respectively.
Toxicity
The acute toxicities are listed in Table 2. One toxic death occurred due to febrile neutropenia after MMC chemotherapy. Grade 2 neutropenia developed in 32.1% of patients, and grade 2 thrombocytopenia occurred in 14.3% of patients. No dose adjustment was needed for hematologic toxicity. Dermatologic toxicity was fairly common; 17.9% developed grade 2 erythema, and 7.1% experienced grade 3 erythema. For 1 patient, grade 3 radiation enteritis developed, and CRT had to be interrupted for 5 days and started again. This patient developed grade 2 enteritis at the time of completion of CRT with palliative medications and without any other treatment delay. For the performance status, no statistically significant deterioration was noted during the acute term after CRT. No renal or neurologic toxic events occurred among patients treated with cisplatin.
Table 2.
Acute toxicities (n/%)
| Toxicity | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|
| Dermatologic | 5 (17.9) | 2 (7.1) | - | - |
| Gastrointestinal | 1 (3.6) | 1 (3.6) | - | - |
| Genitourinary | 2 (7.1) | - | - | - |
| All hematologic | 13 (46.4) | - | - | 1 (3.6) |
| Neutropenia | 9 (32.2) | - | - | 1 (3.6) |
| Thrombocytopenia | 4 (14.3) | - | - | - |
No late toxicity was observed during the follow-up period. All patients except those who received a colostomy retained their sphincter functions. However, 1 patient developed chronic enterocutaneous fistula and subsequently underwent APR.
DISCUSSION
There are a limited number of randomized controlled studies on anal cancer. Hence, long-term follow-up data can provide important information to enhance our understanding of the course of this disease and its management. Herein, we presented an HIV-negative patient population with anal SCC treated at a high-volume cancer institute.
Surgery has historically been the choice of treatment for anal cancer. The 5-year OS outcomes of surgery ranged between 40% and 70% (8–10). However, APR resulted in permanent colostomy and major debility. The fact that SCC is radiosensitive has lead physicians to focus on less debilitating modalities, such as radiation and chemotherapy, as sphincter-sparing treatment options. The first results from an attempt at an organ-sparing approach were from Nigro et al. (4). In the preliminary study by Nigro et al. (4), 3 patients with anal SCC were concomitantly treated with radiation comprising 5FU/MMC. Following the reported success of Nigro et al. (4), patients with anal SCC were treated with radiation alone and with concomitant CRT comprising 5FU/MMC in two randomized controlled trials (RCTs) conducted by the European Organization for Research and Treatment of Cancer and the United Kingdom Coordinating Committee for Cancer Research (11–13). The OS did not differ between the groups in both trials; however, CRT was found to be better in terms of local failure rates (3 years, 30–32%) and colostomy rates (3 years, 28–24%). In the Radiation Therapy Oncology Group 87–04 trial, the disease-free survival at 4 years was superior for the 5FU/MMC group at 73% vs. 51% for the 5FU only group (14). Our results demonstrated that the survival outcomes were comparable and even superior to the results of RCTs. The 3-year PFS rate was 92.4%, and the 3-year CFS rate was 84.3% in our trial, which is consistent with previously published results. Additionally, the 5-year PFS was 63%, and the 5-year CFS was 74.9%. The CR rate was 100%.
We demonstrated that the initial T stage of a tumor was associated with PFS and CFS rates as expected. This finding is in agreement with previous reports (15–17). However, we found that the TNM stage was not a prognostic factor for PFS and CFS. These results may represent the impact of the tumor size on the prognosis of anal carcinoma. The low patient population may lead to a bias for representing the association between the TNM stage and prognosis.
The majority of anal carcinoma cases have been diagnosed in early non-metastatic stages and treated with a multimodal approach. However, optimal chemotherapy and optimal radiation techniques are topics of an ongoing debate.
The most common regimens have been 5-FU as a backbone, in combination with either MMC or cisplatin. However, points of discussion have included the dosage and timing of MMC and the replacement of infusional FU with cisplatin (Table 3). First, doses have not been standardized in RCTs (11,14,18,19). MMC was assumed to be stored in hypoxic cells and may have a continued efficacy beyond the first cycle, and the utility of a second dosage is questionable. In our patient group, we used MMC in a 15 mg/m2 dose and omitted the second cycle of MMC because the major toxicity of CRT was hematologic and associated with MMC.
Table 3.
Summary of major trials and chemotherapy regimens
| Mitomycin | Cisplatin | 5-Fluorouracil | Radiation | Outcomes (%)& | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Treatment day | Dose in mg/m2# | Treatment day | Dose in mg/m2# | Treatment day | Dose in mg/m2# | Technique | Dose^ | CFS | PFS | OS | |
| Nigro et al. (4) (Preliminary study) | 1 | 0.5 mg/kg bolus | - | - | Days 1–5 CI | 25 mg/kg CI | CoRT | 3000 r 15 F. | NA | NA | NA |
| Bartelink et al. (11) | 1 | 15 bolus | - | - | Days 1–5 CI | 750 | CoRT | 45 Gy 25 F. | 71 | 60 | NA |
| UKCCCR trial (ACT-I) (12, 13)* | 1 | 12 bolus | - | - | Days 1–4 (5) CI | 750–1000 | CoRT | 45 Gy 20–25 F. | NA | NA | 65.3 |
| Flam et al. (14) | Days 1 and 29 | 10 | - | - | Days 1–4, 29–32 | 1000 | CoRT | 45 Gy 25 F. | 71! | 731 | 51 |
| ACT-II (19) | 1 | 12 bolus | Days 1 and 29 | 60 | Days 1–4, 29–32 | 1000 | DCRT | 50.4 Gy 28 F. | 68 | 69 | 79 |
| RTOG 98–11 (18–30) | Days 1 and 29 | 10 | Days 1 and 29 | 75 | Days 1–4, 29–32 | 1000 | CoRT | 45 Gy 25 F. | 71.9 | 67.8 | 78.3 |
| ACCORD 03 Trial(20) | - | - | Days 1 and 29 | 80 | Days 1–4, 29–32 | 800 | CoRT | 45 Gy 25 F. | 82.4 | NA | NA |
| Current Study | 1 | 15 | 1 | 60 | Days 1–5, 29–32 | 750 | 50% DCRT 46.4% CoRT 3.6% IMRT |
50.4 Gy 28 F. | 63 | 74.9 | 71.1& |
5-year outcomes in percentages;
UKCCCR anal cancer trial working party;
mg/m2;
cGy, median dose;
estimated 4-year rates of survival;
median OS was not reached
CFS: colostomy-free survival; PFS: progression-free survival; OS: overall survival; CoRT: conventional radiotherapy (RT); DCRT: 3-dimensional RT; IMRT: intensity-modulated RT; F: fraction; NR: not reached; NA: not available; CI: continuous infusion
Cisplatin is the backbone of chemotherapies for many cancer types, and it is an important radiosensitizer that is efficiently used in many squamous cancer types, such as head, neck, and cervical carcinoma. In an ACT II trial, James et al. (19) tested the utility of cisplatin instead of MMC to defer hematological adverse events. The largest anal carcinoma trial, ACT II, failed to show the non-inferiority of cisplatin to MMC. Additionally, the cumulative toxicities of cisplatin-added and MMC-added groups were found to be similar. Only grade 3 hematologic toxicities were found to be significantly higher in MMC-treated patients (p<0.001). The CFS rates were 72% for cisplatin/FU and 75% for MMC/FU. The PFS rates did not significantly differ between the MMC -and cisplatin-treated groups (73% vs. 72%, hazard ratio: 0.95, 95% CI: 0.75–1.19, p=0.63). In the long-term results of the RTOG 98–11 trial, the DFS and OS rates were in favor of the 5FU/MMC group regarding the increased hematologic toxicity grade from 3 to 4 in the MMC arm, although the overall toxicity did not differ between the cisplatin and MMC groups (18). In our patient cohort, 6 patients were treated with cisplatin due to a shortage of MMC, and 2 patients developed disease progression. However, our study was not designed to show the difference or statistically set up to make a conclusion regarding the implications of cisplatin. Moreover, due to the available scientific evidence, cisplatin was not used at our institute as a substitute for MMC for CRT for anal cancer unless there was a shortage of MMC.
Another potential role of chemotherapeutics as a treatment strategy can be as an induction chemotherapy. Many trials have shown inefficacy or even worse outcomes (18,20,21). Herein, at our institute, neoadjuvant chemotherapy or induction chemotherapy is not standard for treatment of patients with anal carcinoma.
Another agent that is offered as a substitute for CRT is capecitabine. Infusional FU has drawbacks: it requires inpatient care or the placement of a central venous line, and there are CT scheduling problems. Capecitabine is an oral prodrug of FU that has been shown to be as efficacious as infusional FU for other colorectal and non-colorectal cancers. Hence, capecitabine instead of infusional FU was tested for the treatment of anal SCC in Phase I and II studies (22,23). The results showed that capecitabine may be a safe and effective substitute for FU for the treatment of anal SCC. However, this treatment course should be further justified to implement a wide usage. At our institute, we did not offer capecitabine instead of FU to our patients.
The known risk factors for anal cancer include a positive HIV status, a history of anoreceptive sexual intercourse, HPV infection, smoking, and female gender. The patient population in our study was different from that in prior studies (24–26). First, all patients with anal SCC were tested for HIV and were negative. In prior studies, HIV was reported in 6.7%–15.1% of patients (15,27). Although the HIV infection rate has increased in recent decades, the overall HIV prevalence is still low compared with that in Western countries (28–30). This discrepancy may be due to the low prevalence of HIV in general (7,29). There is no national screening program for anal cancer and anal precancerous lesions in HIV-positive patients. Hence, real-world data on the prevalence of anal cancer have not been reported. Similarly, the HPV status was incompletely tested in the patient population of this study. HPV was detected in the tumor tissue of 2 patients (7%), whereas anal warts were present in 28% of the patients. This discordance may have been associated with changes in the pathologic techniques over the long term. This study lacks demographic data regarding the preference of gender in sexual acts and anal intercourse. Sex with the same gender is a cultural and religious taboo in Turkey. Therefore, our patients hesitated to answer questions on sexual acts and behaviors.
We investigated a group of patients with anal SCC at our institute as well as their long-term follow-up outcome results and toxicities. Our results are important because the combined patient population was from a high-volume referral cancer center and may be a good representation of the entire country. The results showed that outcomes in the real-world setting are comparable with those of published clinical trials and showed the quality of care and high standards of treatment for a rare malignancy, such as anal cancer.
In conclusion, patients with anal cancer may have a long, disease-free lifespan without morbid surgeries and functional organ loss when treated at high-volume, experienced cancer centers via a multidisciplinary approach.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from Hacettepe University Ethical Committee of Clinical Trials (Decision No: GO 16/365-10).
Informed Consent: Written informed consent was obtained from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept -E.E., F.Y., Ş.Y.; Design -E.E., F.Y., Ş.L., Y.K., M.G., Ö.D., Ş.Y. Supervision -Ş.Y., F.Y.; Resources -Ş.Y., F.Y.; Materials -E.E., F.Y., Ş.L., Y.K., M.G., Ö.D., Ş.Y. Data Collection and/or Processing -E.E., Ş.L., Y.K., M.G.; Analysis and/or Interpretation -E.E., Ö.D., Y.K., Ş.Y., Ş.L.; Literature Search -E.E., Y.K., Ş.L.; Writing -E.E., Ö.D., Ş.Y., M.G.; Critical Reviews -E.E., Y.K., Ş.Y., Ş.L., Ö.D., F.Y.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Glynne-Jones R, Nilsson PJ, Aschele C, et al. Anal cancer: ESMO-ESSO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii10–20. doi: 10.1093/annonc/mdu159. [DOI] [PubMed] [Google Scholar]
- 3.Gultekin MBG, Utku E, Ergun A, et al. In: Turkiye Kanser Istatistikleri. Sencan IIG, editor. Ankara: 2013. [Google Scholar]
- 4.Nigro ND, Vaitkevicius VK, Considine B., Jr Combined therapy for cancer of the anal canal: a preliminary report. Dis Colon Rectum. 1974;17:354–6. doi: 10.1007/BF02586980. [DOI] [PubMed] [Google Scholar]
- 5.Chiao EY, Krown SE, Stier EA, Schrag D. A population-based analysis of temporal trends in the incidence of squamous anal canal cancer in relation to the HIV epidemic. J Acquir Immune Defic Syndr. 2005;40:451–5. doi: 10.1097/01.qai.0000159669.80207.12. [DOI] [PubMed] [Google Scholar]
- 6.Adami J, Gabel H, Lindelof B, et al. Cancer risk following organ transplantation: a nationwide cohort study in Sweden. Br J Cancer. 2003;89:1221–7. doi: 10.1038/sj.bjc.6601219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sargin F, Yildiz D, Aydin OA, et al. Changes in HIV demographic patterns in a low prevalence population: no evidence of a shift towards men who have sex with men. Int J Infect Dis. 2016;48:52–6. doi: 10.1016/j.ijid.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Boman BM, Moertel CG, O’Connell MJ, et al. Carcinoma of the anal canal. A clinical and pathologic study of 188 cases. Cancer. 1984;54:114–25. doi: 10.1002/1097-0142(19840701)54:1<114::aid-cncr2820540124>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 9.Klas JV, Rothenberger DA, Wong WD, Madoff RD. Malignant tumors of the anal canal: the spectrum of disease, treatment, and outcomes. Cancer. 1999;85:1686–93. doi: 10.1002/(sici)1097-0142(19990415)85:8<1686::aid-cncr7>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 10.Dougherty BG, Evans HL. Carcinoma of the anal canal: a study of 79 cases. Am J Clin Pathol. 1985;83:159–64. doi: 10.1093/ajcp/83.2.159. [DOI] [PubMed] [Google Scholar]
- 11.Bartelink H, Roelofsen F, Eschwege F, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol. 1997;15:2040–9. doi: 10.1200/JCO.1997.15.5.2040. [DOI] [PubMed] [Google Scholar]
- 12.Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research. Lancet. 1996;348:1049–54. doi: 10.1016/S0140-6736(96)03409-5. [DOI] [PubMed] [Google Scholar]
- 13.Northover J, Glynne-Jones R, Sebag-Montefiore D, et al. Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomised UKCCCR Anal Cancer Trial (ACT I) Br J Cancer. 2010;102:1123–8. doi: 10.1038/sj.bjc.6605605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flam M, John M, Pajak TF, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol. 1996;14:2527–39. doi: 10.1200/JCO.1996.14.9.2527. [DOI] [PubMed] [Google Scholar]
- 15.Abunassar M, Reinders J, Jonker DJ, Asmis T. Review of anal cancer patients at the Ottawa hospital. Eur J Surg Oncol. 2015;41:653–8. doi: 10.1016/j.ejso.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Leon O, Guren M, Hagberg O, et al. Anal carcinoma -Survival and recurrence in a large cohort of patients treated according to Nordic guidelines. Radiother Oncol. 2014;113:352–8. doi: 10.1016/j.radonc.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Ajani JA, Winter KA, Gunderson LL, et al. US intergroup anal carcinoma trial: tumor diameter predicts for colostomy. J Clin Oncol. 2009;27:1116–21. doi: 10.1200/JCO.2008.19.6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gunderson LL, Winter KA, Ajani JA, et al. Long-term update of US GI intergroup RTOG 98-11 phase III trial for anal carcinoma: survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin. J Clin Oncol. 2012;30:4344–51. doi: 10.1200/JCO.2012.43.8085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James RD, Glynne-Jones R, Meadows HM, et al. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, open-label, 2 × 2 factorial trial. Lancet Oncol. 2013;14:516–24. doi: 10.1016/S1470-2045(13)70086-X. [DOI] [PubMed] [Google Scholar]
- 20.Peiffert D, Tournier-Rangeard L, Gerard JP, et al. Induction chemotherapy and dose intensification of the radiation boost in locally advanced anal canal carcinoma: final analysis of the randomized UNICANCER ACCORD 03 trial. J Clin Oncol. 2012;30:1941–8. doi: 10.1200/JCO.2011.35.4837. [DOI] [PubMed] [Google Scholar]
- 21.Meropol NJ, Niedzwiecki D, Shank B, et al. Induction therapy for poor-prognosis anal canal carcinoma: a phase II study of the cancer and Leukemia Group B (CALGB 9281) J Clin Oncol. 2008;26:3229–34. doi: 10.1200/JCO.2008.16.2339. [DOI] [PubMed] [Google Scholar]
- 22.Deenen MJ, Dewit L, Boot H, Beijnen JH, Schellens JH, Cats A. Simultaneous integrated boost-intensity modulated radiation therapy with concomitant capecitabine and mitomycin C for locally advanced anal carcinoma: a phase 1 study. Int J Radiat Oncol Biol Phys. 2013;85:e201–7. doi: 10.1016/j.ijrobp.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Glynne-Jones R, Meadows H, Wan S, et al. EXTRA--a multicenter phase II study of chemoradiation using a 5 day per week oral regimen of capecitabine and intravenous mitomycin C in anal cancer. Int J Radiat Oncol Biol Phys. 2008;72:119–26. doi: 10.1016/j.ijrobp.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 24.White EC, Goldman K, Aleshin A, Lien WW, Rao AR. Chemoradiotherapy for squamous cell carcinoma of the anal canal: Comparison of one versus two cycles mitomycin-C. Radiother Oncol. 2015;117:240–5. doi: 10.1016/j.radonc.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Walsh T, Bertozzi-Villa C, Schneider JA. Systematic review of racial disparities in human papillomavirus-associated anal dysplasia and anal cancer among men who have sex with men. Am J Public Health. 2015;105:e34–45. doi: 10.2105/AJPH.2014.302469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ugurluer G, Ballerini G, Moeckli R, Matzinger O, Bourhis J, Ozsahin M. Helical tomotherapy for the treatment of anal canal cancer: a dosimetric comparison with 3D conformal radiotherapy. Tumori. 2015;101:268–72. doi: 10.5301/tj.5000269. [DOI] [PubMed] [Google Scholar]
- 27.Salama JK, Mell LK, Schomas DA, et al. Concurrent chemotherapy and intensity-modulated radiation therapy for anal canal cancer patients: a multicenter experience. J Clin Oncol. 2007;25:4581–6. doi: 10.1200/JCO.2007.12.0170. [DOI] [PubMed] [Google Scholar]
- 28.Cerci P, Inkaya AC, Alp S, Tumer A, Unal S. Evaluation of 255 HIV/AIDS cases: Hacettepe cohort, Ankara, Turkey. Mikrobiyol Bul. 2016;50:94–103. doi: 10.5578/mb.10610. [DOI] [PubMed] [Google Scholar]
- 29.Koksal MO, Beka H, Lubke N, et al. HIV-1 subtypes and drug resistance profiles in a cohort of heterosexual patients in Istanbul, Turkey. Med Microbiol Immunol. 2015;204:551–5. doi: 10.1007/s00430-015-0419-9. [DOI] [PubMed] [Google Scholar]
- 30.Ajani JA, Winter KA, Gunderson LL, et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA. 2008;299:1914–21. doi: 10.1001/jama.299.16.1914. [DOI] [PubMed] [Google Scholar]


