Abstract
Background/Aims
A definitive biopsy-based diagnosis of gastric cancer is sometimes difficult, and some cases are pathologically diagnosed as gastric indefinite neoplasia (GIN). The most appropriate forceps size for gastric biopsy has yet to be determined. In this study, we investigated the relation between the forceps size and the frequency of GIN diagnosis.
Materials and Methods
The records of patients from two historical groups were reviewed. The first group comprised patients evaluated during the period when standard biopsy forceps (StF) were used (April 2010–March 2011), and the second group comprised patients evaluated during the period when small biopsy forceps (SmF) were used (April 2011–March 2013). Patients in whom GIN lesions were diagnosed with biopsy were identified, and pertinent data were compared between the two groups of patients.
Results
Among the 8,420 patients who underwent esophagogastroduodenoscopy (EGD) during the first period, 2,584 (30.7%) underwent gastric biopsy with StF. Among the 15,968 patients who underwent EGD during the second period, 4,204 (26.3%) underwent gastric biopsy with SmF. GIN was diagnosed in a significantly greater number of patients in the SmF group than in the StF group (52 [1.25%] vs. 19 [0.73%]; p=0.048). The mean minor-axis lengths of the biopsy samples were 1.50±0.50 mm and 1.38±0.40 mm in the StF group and the SmF group, respectively, with the SmF group samples tending to be shorter (p=0.088).
Conclusion
Because the SmF use may increase the rate of GIN diagnosis, the use of SmF with a standard-caliber endoscope should be avoided.
Keywords: Endoscopic biopsy forceps, pathology, gastric indefinite neoplasia, upper GI
INTRODUCTION
Gastric cancer is the most common type of gastrointestinal cancer in East Asian countries, such as Japan and South Korea. Endoscopic forceps biopsy (EFB) is the gold standard for diagnosing gastric epithelial tumors. However, definitive diagnosis is often difficult, and some tumors are incorrectly diagnosed as gastric indefinite neoplasia (GIN), which corresponds to Category 2 in the revised Vienna Classification (1). GIN lesions require a short-interval follow-up.
Biopsy forceps come in various sizes and are used differently depending on the size of the endoscope and their intended purpose. The most appropriate size for gastric biopsy forceps has yet to be determined. According to the Japanese Classification of Gastric Cancer, a diagnosis of GIN is attributed, at least partly, to the small size of the biopsy specimens (2). Because specimens obtained by small biopsy forceps are small, the use of small biopsy forceps is expected to increase the rate of GIN diagnoses. Therefore, we investigated the relation between the forceps size and diagnosis of GIN.
MATERIALS AND METHODS
Patients
Patients to be included in the study were identified from the medical records of two historical groups of patients who underwent esophagogastroduodenoscopy (EGD) at our hospital. The first group comprised patients evaluated during the period when standard biopsy forceps (StF) were used (April 2010–March 2011), and the second group comprised patients evaluated during the period when small biopsy forceps (SmF) were used (April 2011–March 2013). The study patients were those who underwent gastric biopsy after EGD and in whom GIN (Category 2 in the Vienna Classification of GIN), was diagnosed.
Endoscopes and biopsy forceps
The EGD was performed either by gastroenterologists in the Department of Internal Medicine or by gastrointestinal surgeons. Standard-caliber endoscopes (GIF-H260, GIF-Q260, and GIF-H260Z; Olympus, Tokyo, Japan) were used. The StF and SmF used for biopsy were the Radial Jaw 4 standard-capacity single-use biopsy forceps (oval-shaped; cup external diameter, 2.2 mm; cup maximum opening width, 7.1 mm) and Radial Jaw 4 single-use pediatrics biopsy forceps (oval-shaped; cup external diameter, 1.6 mm; cup maximum opening width, 5.4 mm), respectively (Boston Scientific Japan; Tokyo, Japan).
Biopsy samples and pathological evaluation
In all cases, protruded lesions were biopsied at the center, and depressed or ulcerative lesions were biopsied at the margin. Biopsy samples were fixed in 10% formalin solution and stained with hematoxylin-eosin for pathologic evaluation. Diagnosis was subsequently performed by two pathologists, including one pathologist certified by the Japanese Society of Pathology. Specimens were subjected to additional pathological review (e.g., deep cuts or immunostaining), based on the initial diagnosis.
A single author used a stereoscopic microscope to determine the sizes of biopsy samples. If GIN was diagnosed from samples taken from multiple points within a single lesion, the mean biopsy sample size was used for analysis.
Study endpoints
The number of GIN diagnoses, patient characteristics, lesion characteristics (e.g., site, macroscopic appearance, and color tone), the endoscopist’s experience level, biopsy sample size, occurrence of additional pathological investigation, and the main reason for a diagnosis of GIN were investigated in both patient groups. In all cases in which GIN was diagnosed, the clinical course was followed for 3 years, and the timing of EGD after the GIN diagnosis and the final pathological result were investigated. For background information, the number of patients diagnosed with gastric carcinoma as a result of EFB and the number of patients who experienced hemorrhage after EFB were also investigated in both groups.
Definitions
The Japanese Classification of Gastric Cancer was used to classify the lesion sites (2). This classification divides the stomach into three equal-size sections: the upper, middle, and lower thirds. Lesions distributed throughout the stomach and cases involving a gastric remnant were classified as “other.”
The macroscopic appearance of the lesion was judged to resemble either early gastric cancer (EGC-like) or advanced gastric cancer (AGC-like). An EGC-like appearance corresponded to Type 0 (superficial neoplastic lesion) in the Paris classification, whereas an AGC-like appearance corresponded to Types 1–5 (3).
The color tone of the lesion was classified as reddened if the surface appeared redder than the surrounding mucosa.
Additional pathological investigation was defined as additional deep cutting or immunostaining of the sample.
The endoscopist was classified by his or her level of experience as a non-expert (having less than 2 years of experience) or an expert (having 2 or more years of experience).
The reasons for a GIN diagnosis were classified as (a) small number of atypical cells; (b) erosion and/or inflammation; and (c) tissue damage (2).
Statistical analysis
The -test, chi-squared test, and G-test were used for statistical analyses. A p-value of <0.05 was considered significant. The SPSS Statistics 13.0 (SPSS, Inc., Chicago, IL, USA) was used for all statistical analyses.
Ethics committee approval
This study was approved by our hospital ethics committee (No. 3345). Because this was a retrospective study, informed consent was not necessary.
RESULTS
Study patients, the number of GIN diagnoses, and the number of biopsy samples
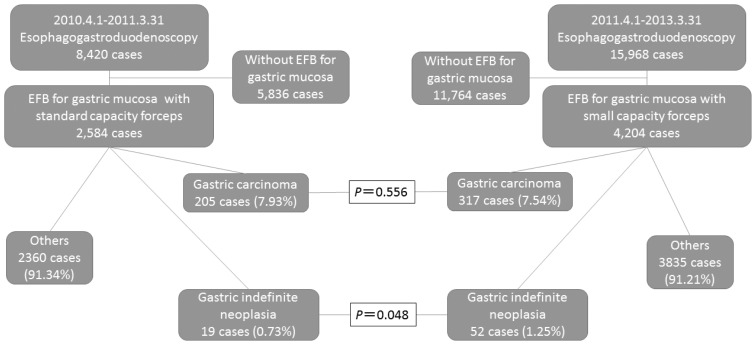
Of the 8,420 patients who underwent EGD between April 2010 and March 2011, 2,584 (30.7%) underwent gastric biopsy (StF group). Of the 15,968 patients who underwent EGD between April 2011 and March 2013, 4,204 (26.3%) underwent gastric biopsy (SmF group).
GIN was diagnosed in 19 (0.73%) of the 2,584 patients in the StF group and 52 (1.25%) of the 4,204 patients in the SmF group (Figure 1). The difference in the diagnostic rate was significant (p=0.048). Thirty-five biopsy specimens had been obtained from the 19 patients in the StF group, and 109 biopsy specimens had been obtained from the 52 patients in the SmF group. The mean±SD numbers of biopsy specimens per GIN diagnosis were 1.84±1.18 and 2.10±1.23, respectively (p=0.465).
Figure 1.
Flow diagram of patient selection for the study
The forceps size, frequency of gastric carcinoma, and gastric carcinoma-to-GIN ratio
Gastric carcinoma was diagnosed via EFB in 205 patients from the initially identified StF group and 317 patients from the initially identified SmF group. From the number of EFBs performed, gastric carcinoma was diagnosed in 7.93% and 7.54% of the StF group and SmF group, respectively (p=0.556). The gastric carcinoma-to-GIN ratios were 10.8:1 and 6.1:1 in the StF group and SmF group, respectively, with the ratio being significantly higher in the SmF group (p0.041).
Patient and lesion characteristics
The two groups did not differ significantly in terms of the mean age or sex. There was also no difference in terms of the lesion site, color tone, macroscopic appearance, or endoscopists’ experience (Table 1).
Table 1.
Patient characteristics and endoscopic features of lesions diagnosed as gastric indefinite neoplasia, per study group
| Standard-Capacity Forceps (n=19) | Small-Capacity Forceps (n=52) | Total (n=71) | p | |
|---|---|---|---|---|
| Age (mean±SD, years) | 70.1±8.3 | 72.6±7.7 | 71.9±8.0 | 0.239* |
| Males, no. | 15 (78.9%) | 30 (58.3%) | 45 (63.3%) | 0.100** |
| Location | ||||
| Upper | 3 (15.8%) | 10 (19.2%) | 14 | 0.128*** |
| Middle | 10 (52.6%) | 13 (25.0%) | 23 | |
| Lower | 5 (26.3%) | 27 (52.0%) | 32 | |
| Other | 1 (5.3%) | 2 (3.8%) | 2 | |
| Macroscopic type | ||||
| EGC-like | 14 (73.7%) | 42 (69.2%) | 56 | 0.914** |
| AGC-like | 5 (26.3%) | 16 (30.8%) | 21 | |
| Surface color | ||||
| White to yellow | 4 (21.1%) | 11 (21.2%) | 15 | 0.750** |
| Red | 15 (78.9%) | 41 (78.8%) | 56 | |
| Endoscopic experience | ||||
| Non-expert | 1 (5.3%) | 3 (5.8%) | 4 | 0.617** |
| Expert | 18 (94.7%) | 49 (94.2%) | 67 | |
Upper, Middle, Lower: the upper, middle, lower third of the stomach; EGC: early gastric cancer; AGC: advanced gastric cancer
t-test,
chi-squared test,
G-test
Characteristics of the endoscopic biopsy specimens
The mean major axis lengths of the GIN biopsy samples were 2.82±0.83 mm and 2.48±0.80 mm in the StF group and SmF group, respectively, with no significant difference between them (p=0.127). The mean minor axis lengths of the biopsy samples were 1.50±0.50 mm and 1.38±0.40 mm in the StF group and SmF group, respectively, with samples in the SmF group tending to be shorter than those in the StF group (p=0.088). Eight biopsy samples (42.1%) obtained with StF were subjected to additional pathological examination—all to immunostaining. Seventeen biopsy samples (32.7%) obtained with SmF were subjected to additional pathological examination, 2 samples and 15 samples by deep cutting and immunostaining, respectively. There were no between-group differences in the percentages of cases subjected to additional pathological examination (Table 2).
Table 2.
Size and further examination of the endoscopic biopsy samples, per study group
| Standard-Capacity Forceps (n=19) | Small-Capacity Forceps (n=52) | Total (n=71) | p | |
|---|---|---|---|---|
| Size of specimen | ||||
| Major axis (average±SD, mm) | 2.82±0.83 | 2.48±0.80 | 2.58±0.81 | 0.127* |
| Minor axis (average±SD, mm) | 1.50±0.50 | 1.38±0.40 | 1.41±0.42 | 0.088* |
| Additional pathological investigation | 8 (42.1%) | 17 (32.7%) | 25 (35.2%) | 0.496** |
t-test,
chi-squared test
Reasons for GIN diagnosis
The reasons for the diagnosis of GIN in the StF group were as follows: low number of atypical cells (5 cases [26.3%]), erosion and/or inflammation (12 cases [63.2%]), and tissue damage (2 cases (10.5%). The reasons in the SmF group were a low number of atypical cells (15 cases [28.8%]), erosion and/or inflammation (34 cases [65.4%]), and tissue damage (3 cases [5.8%]). The reasons did not differ significantly between the two groups (Table 3).
Table 3.
Main reasons for the diagnosis of gastric indefinite neoplasia, per study group
| Standard-Capacity Forceps (n=19) | Small-Capacity Forceps (n=52) | Total (n=71) | p | |
|---|---|---|---|---|
| Small number of atypical cells | 5 (26.3%) | 15 (28.8%) | 20 | 0.811* |
| Erosion and/or inflammation | 12 (63.2%) | 34 (65.4%) | 46 | |
| Tissue damage | 2 (10.5%) | 3 (5.8%) | 5 |
G-test
Clinical courses of cases diagnosed as GIN
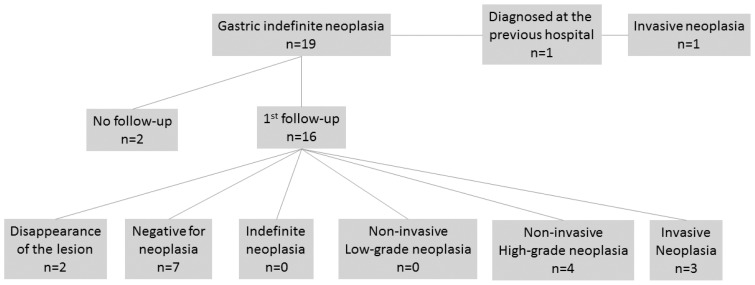
The clinical courses of cases in the StF group that were diagnosed as cases of GIN are shown in Figure 2. The follow-up was not possible for 2 of the 19 patients. In 1 patient, GIN was diagnosed by a previous doctor; therefore, a follow-up EGD was not performed. A follow-up EGD was performed in 16 cases, and none resulted in a GIN re-diagnosis.
Figure 2.
Flow diagram of the clinical course after diagnosis of gastric indefinite neoplasia from lesion samples obtained by standard biopsy forceps
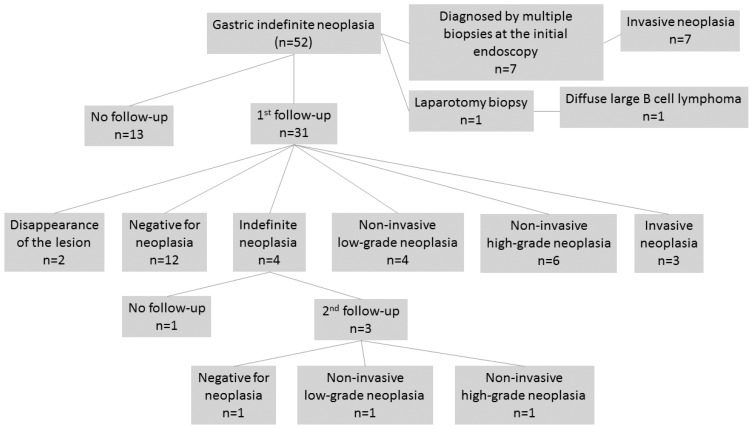
The clinical courses of cases in the SmF group diagnosed as GIN cases are shown in Figure 3. The follow-up was not performed for 13 of the 52 patients. For 7 patients, diagnosis was made based on multiple biopsy samples obtained during the initial EGD. A biopsy was performed via laparotomy in 1 patient. The follow-up endoscopy was performed in 31 cases, and 4 (12.9%) were re-diagnosed as GIN cases.
Figure 3.
Flow diagram of the clinical course after diagnosis of gastric indefinite neoplasia from lesion samples obtained by small-capacity biopsy forceps
For the 47 patients who underwent the follow-up EGD, the median time from the first EGD to the first follow-up EGD was 64 days (range, 7–626 days).
In both groups, approximately 40% of the final diagnoses were of gastrointestinal epithelial neoplasia, according to the Vienna Classification (1), and no significant between-group difference in the final diagnosis was observed (Table 4).
Table 4.
Final diagnoses from the endoscopic forceps biopsy specimens, surgical specimens, and clinical course, per study group
| Standard-Capacity Forceps (n=19) | Small-Capacity Forceps (n=52) | Total (n=71) | p | |
|---|---|---|---|---|
| Negative for neoplasia or lesion disappearance | 9 (47.4%) | 15 (28.9%) | 24 | 0.378* |
| Epithelial neoplasia | 8 (42.1%) | 22 (42.3%) | 30 | |
| Malignant lymphoma | 0 (0%) | 1 (1.9%) | 1 | |
| Indefinite neoplasia | 2 (10.5%) | 14 (26.9%) | 16 |
G-test
Bleeding after the endoscopic forceps biopsy
Overall, 6,788 patients underwent gastric mucosal biopsy during the 3-year study period, and no post-biopsy bleeding was observed.
DISCUSSION
Endoscopic-targeted biopsy is a primary diagnostic approach for most gastrointestinal diseases. Forceps of various shapes and sizes have been developed. Various types of standard-capacity forceps (with or without a needle and various cup shapes) have been evaluated in studies that have examined the adequacy of specimens for histological interpretation (4,5).
SmF can be used with all types of gastrointestinal endoscopes for EGD, including small-caliber endoscopes <6 mm in diameter with small channels. Because of such advantages, SmF are used routinely not only for small-caliber endoscopy, but also for standard-caliber endoscopy. The question remains whether forceps that are “too small” are better than forceps that are “too big.”
In the study described herein, forceps of two different sizes, SmF and StF, were used during the standard-caliber endoscopy for EGD. Significantly more cases were diagnosed as GIN cases when EFB was performed with SmF than when EFB was performed with StF.
The two study groups did not differ significantly in terms of patient or lesion characteristics. However, in comparison to the StF biopsy samples, the SmF biopsy samples tended to be approximately 10% shorter with respect to both the major and minor axes. Although the reasons for a GIN diagnosis, including the small number of atypical cells, did not differ between the two groups, the smaller sample size was presumed to make pathological evaluation difficult and contribute to the high rate of GIN diagnosis in the SmF group.
GIN was not ultimately diagnosed in 16 patients in the StF group who underwent a follow-up EGD after an initial diagnosis of GIN. In the SmF group, however, GIN was re-diagnosed upon a follow-up EGD in 4 (12.9%) of 31 patients in whom GIN was diagnosed initially. We believe that when the follow-up biopsy of lesions diagnosed as GIN is performed, the forceps used should be equal to or greater in size than the StF (Figure 2, 3). Thus, we conclude that the endoscopic biopsy performed with an SmF may increase the rate of GIN diagnosis.
One disadvantage of a GIN diagnosis is the obligatory increase in the number of EGDs. A short-interval follow-up period is recommended after a diagnosis of GIN (1,2). Our study patients in whom GIN was diagnosed underwent a follow-up EGD after approximately 60 days. We believe that the increased incidence of a GIN diagnosis attributed to SmF use will increase the frequency of endoscopy, the risks of endoscopic adverse events, and the associated costs.
Comparisons similar to our have focused on the relation between the biopsy forceps size and pathological evaluation. Elmunzer et al. (6) performed colonic mucosal biopsies in patients with inflammatory bowel disease and reported the following criteria for an adequate biopsy specimen: length (≥3 mm), penetration into the muscularis mucosa, and <20% crush artifact. Compared to standard large-capacity forceps, jumbo forceps were found to yield a significantly higher rate of adequate biopsy samples. Thus, “the greater embraces the less.”
Certain reports state that the possibility of obtaining large, easily evaluable biopsies with large forceps contributes to an accurate diagnosis; such conclusions seem intuitive. Other reports, however, state that the forceps size does not contribute to diagnosis. Before performing ESD to treat gastric epithelial tumors, Jeon et al. (7) used StF (opening width of 6.8 mm) and jumbo forceps (opening width of 8.0 mm) to perform biopsies and investigated diagnostic concordance between the biopsy samples and ESD samples. They found no difference related to forceps size in diagnostic concordance between the two groups. However, they did report that a larger number of biopsies contributed to the diagnostic concordance. They noted that no difference in diagnostic ability attributable to forceps size was found because both forceps were of sufficient size to obtain full-thickness mucosal samples, a factor that they believe eliminated the advantages of the jumbo forceps. Forceps as large as jumbo forceps may not be necessary for gastric biopsy. When we consider this possibility together with our study findings, we conclude that it may be possible to obtain samples that fulfill the requirements for pathological evaluation with the use of an StF.
SmF are frequently used during small-caliber endoscopy, including transnasal endoscopy. One disadvantage of small-caliber endoscopes, however, is the small size (2.0 mm) of the forceps channel, which limits the biopsy forceps options to SmF (8,9). In a previously reported study that compared patients who underwent small-caliber endoscopy with SmF against patients who underwent standard-caliber endoscopy with StF, the authors found that, although the biopsy sample size was significantly smaller in the former group, the groups did not differ in terms of the ability of pathologists to diagnose Barrett’s mucosa and dysplasia (10). By comparison, in our study, standard endoscopy was performed in both groups, and by a simple comparison of diagnostic ability based on the forceps size, we found that the inability to use forceps other than SmF during transnasal endoscopy may represent a disadvantage in terms of the biopsy-based diagnosis of gastric epithelial tumors.
Our study findings should be interpreted in light of the study limitations. Our study was conducted as a single-center retrospective study, and thus we could not control for differences in the experience of endoscopists and pathologists or for factors that rendered measurement impossible.
Regardless of our study limitations, our data lead us to conclude that when gastric mucosa is biopsied, the pathological diagnostic yield of StF may be lower than that of StF, and the use of SmF may increase the incidence of GIN diagnosis. Thus, an SmF should be avoided for use with a standard-caliber endoscope.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from our hospital ethics committee (Decision No: 3345).
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - Y.M.; Design - Y.M.; Supervision - M.T., F.I.; Resources - Y.M.; Materials - Y.M.; Data Collection and/or Processing - M.K., H.K., M.O., Y.S., Y.I., S.O., M.Y.; Analysis and/or Interpretation - H.Y., T.F.; Literature Search - Y.M., H.Y.; Writing Manuscript - Y.M., H.Y.; Critical Review - M.T., F.I.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut. 2002;51:130–1. doi: 10.1136/gut.51.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–12. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 3.Endoscopic Classification Review Group. Update on the Paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005;37:570–8. doi: 10.1055/s-2005-861352. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein DE, Barkin JS, Reiner DK, et al. Standard biopsy forceps versus large-capacity forceps with and without needle. Gastrointest Endosc. 1995;41:573–6. doi: 10.1016/S0016-5107(95)70193-1. [DOI] [PubMed] [Google Scholar]
- 5.Danesh BJ, Burke M, Newman J, et al. Comparison of weight, depth, and diagnostic adequacy of specimens obtained with 16 different biopsy forceps designed for upper gastrointestinal endoscopy. Gut. 1985;26:227–31. doi: 10.1136/gut.26.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elmunzer BJ, Higgins PD, Kwon YM, et al. Jumbo forceps are superior to standard large-capacity forceps in obtaining diagnostically adequate inflammatory bowel disease surveillance biopsy specimens. Gastrointest Endosc. 2008;68:273–8. doi: 10.1016/j.gie.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 7.Jeon HK, Ryu HY, Cho MY, et al. A randomized trial to determine the diagnostic accuracy of conventional vs. jumbo forceps biopsy of gastric epithelial neoplasias before endoscopic submucosal dissection; open-label study. Gastric Cancer. 2014;17:661–8. doi: 10.1007/s10120-013-0322-2. [DOI] [PubMed] [Google Scholar]
- 8.Ai ZL, Lan CH, Fan LL, et al. Unsedated transnasal upper gastrointestinal endoscopy has favorable diagnostic effectiveness, cardiopulmonary safety, and patient satisfaction compared with conventional or sedated endoscopy. Surg Endosc. 2012;26:3565–72. doi: 10.1007/s00464-012-2367-4. [DOI] [PubMed] [Google Scholar]
- 9.Atar M, Kadayifci A. Transnasal endoscopy: technical considerations, advantages and limitations. World J Gastrointest Endosc. 2014;6:41–8. doi: 10.4253/wjge.v6.i2.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jobe BA, Hunter JG, Chang EY, et al. Office-based unsedated small-caliber endoscopy is equivalent to conventional sedated endoscopy in screening and surveillance for Barrett’s esophagus: a randomized and blinded comparison. Am J Gastroenterol. 2006;101:2693–703. doi: 10.1111/j.1572-0241.2006.00890.x. [DOI] [PubMed] [Google Scholar]