Abstract
Background/Aims
Endoscopic papillectomy (EP) has emerged as an alternative to surgery in the management of ampullary lesions. The aim of this study is to evaluate feasibility, efficacy, safety, outcome, and impact of EP in the management of benign ampullary lesions.
Materials and Methods
This is a multicenter, retrospective study of 44 patients who had EP of benign ampullary lesions.
Results
Over the 11-year period, 44 (55.7%) of 79 patients underwent EP for benign ampullary lesions. Complete resection was achieved in 40 patients (91%). An underlying adenocarcinoma was the only risk factor for incomplete resection. Twenty-eight lesions (63.6%) were resected en-bloc and 16 lesions (36.4%) were resected in piecemeal fashion. Post-papillectomy histopathologic diagnoses were tubular adenoma in 14 patients (32%), invasive adenocarcinoma in 9 patients (20.5%), tubullovillous adenoma in 7 patients (16%), tubullovillous adenoma with carcinoma limited to the mucosal layer in 5 patients (11.3%), adenoma with high-grade dysplasia in 4 patients (9%), neuroendocrine tumor in 1 patient (2.3%), ganglioneuroma in 1 patient (2.3%), hamartomatous polyp in 1 patient (2.3%), adenofibroma in 1 patients (2.3%), and Brunner gland hyperplasia in 1 patient (2.3%). Seven (15.9%) procedure-related complications occurred: 3 (6.8%) bleeding, 2 (4.5%) pancreatitis, 1 (2.3%) abdominal pain, and 1 (2.3%) stent migration to the pancreatic duct. Seven patients (17%) had recurrence.
Conclusion
Endoscopic papillectomy is a safe and effective method and can be considered as a first-line approach in patients with benign ampullary lesions with intent for cure. It also allows for correct histological diagnosis and staging.
Keywords: Endoscopic papillectomy, endoscopic ampullectomy, ampulla
INTRODUCTION
In clinical practice, neoplasms of the ampulla of Vater are rare, but expanding access to gastrointestinal endoscopy is increasing the rate of detection (1). Fortunately, this rate includes premalignant lesions and early malignancies. However, benign lesions do arise at this location as well. In fact, adenomas (sporadic or hereditary) and tubullovillous adenomas outnumber other duodenal papillary lesion histologies (2). Nonetheless, all ampullary lesions warrant resection due to a finite risk of malignant transformation as well as a known rate of sampling error whereby biopsies may miss carcinoma foci, also described in this report (3–7).
Currently, the three treatment options for ampullary lesions are pancreaticoduodenectomy (Whipple surgery), surgical ampullectomy, and endoscopic papillectomy (EP). Whipple surgery is the historic gold standard but reports of considerable postoperative morbidity and mortality have driven a search for less invasive options (8,9). The resulting advent of surgical ampullectomy (open duodenal papillectomy with reimplantation of the common bile duct and pancreatic duct into the duodenal wall) has brought a less invasive, still open surgical option at the expense of higher recurrence rates (10,11). The next evolution of minimally invasive ampullary intervention is purely EP. EP requires pancreatobiliary endoscopic expertise and is a technically rigorous procedure with a known range of complications, which also require operator expertise for management (12).
Here, we report our experience from four centers in Turkey. The aim of this study is to describe clinical and demographic characteristics, indications, feasibility, safety, outcomes, and impact of EP in the management of benign tumors involving ampulla of Vater.
MATERIALS AND METHODS
Patients
All consecutive EP procedures performed at two large volume centers (1000–1500 endoscopic retrograde cholangiopancreatographies (ERCP)/y) from January 2004 to April 2015 and two small volume centers (100–150 ERCPs/y) from January 2013 to April 2015 were reviewed. The research protocol was approved by the ethics committee for biomedical research (Protocol no: 2016.026.IRB2.006). Standard endoscopic consent form was signed prior to each procedure. Procedures performed for presumptively benign ampullary lesions were analyzed. Patients with a malignant histology at pre-papillectomy biopsies and/or endoscopic appearance suggestive of an underlying malignancy (friable, ulcerating, spontaneously bleeding lesions) were excluded from the study. The demographics, clinical presentations, and procedural information (instruments, sedation, findings, interventions, outcome, and complications) were retrospectively analyzed. Procedures were performed on inpatient basis. Patients with advanced-stage disease on imaging studies (computed tomography, magnetic resonance imaging, endoscopic ultrasound (EUS)) and malignancy on prior endoscopic biopsies were excluded.
Equipment and procedure
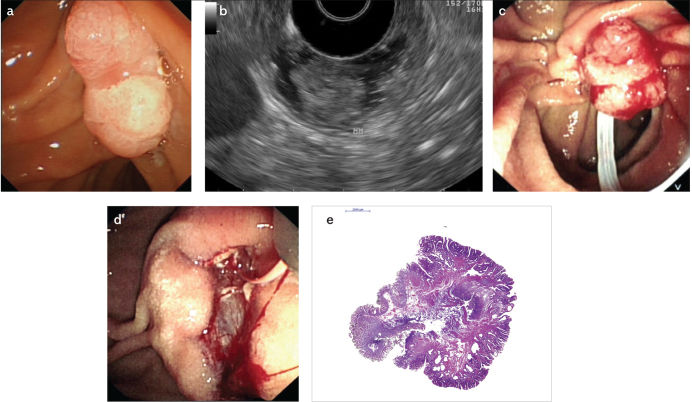
An EUS evaluation of an ampullary lesion prior to a papillectomy attempt was performed, depending on the preference of the endoscopist upon the size and endoscopic appearance of the lesions and/or institutional availability of EUS at the time of the procedure. All EP procedures were performed by experienced pancreatobiliary therapeutic endoscopists. The procedures were performed with patients in the prone position in endoscopy suites with fluoroscopy. All patients had deep sedation with fentanyl, midazolam, and propofol administered by an anesthesiologist. Prophylactic antibiotics were not routinely administered. The procedures were performed with a side viewing duodenoscope (Olympus TJF 240, Olympus TJF Q180V, Japan; Fujinon ED 530XT, Japan). Cholangiography and pancreatography were performed to assess proximal duct extension of the ampullary lesion. An oval-shaped polypectomy snare was used to resect ampullary lesions. The snare was positioned with intent for en-bloc resection. Pure cut or blend current electrocautery were used. In case of failure to perform an en-bloc resection, the remnant lesion was resected with a polypectomy snare and/or biopsy forceps and/or ablated with argon plasma coagulation. Pre-papillectomy submucosal injection, pancreatic duct methylene blue injection, as well as post-procedure pancreatic and biliary stent insertion were performed at the discretion of the endoscopist. Endoscopic appearance of an ampullary lesion, endosonographic evaluation, EP, as well as histopathology of the resected specimen is presented in Figure 1a–e.
Figure 1. a–e.
Endoscopic view of an ampullary lesion without worrisome features for malignancy (a); endosonographically, the hypoechoic mucosal lesion is not invading into the muscularis mucosa (b); ampullary lesion is grasped with a polypectomy snare for resection (c); post-papillectomy view with a guide-wire advanced into the pancreatic duct prior to pancreatic duct stent placement (d); histopathologic evaluation (H&E stain) revealed a tubulovillous adenoma with high-grade dysplasia foci and negative margins (e)
RESULTS
Patient population
Over the 11-year period, 79 patients underwent EP. Thirty-five patients (44.3%) with a malignant histopathology or endoscopic features suggestive of an underlying malignancy (friable, ulcerating, spontaneously bleeding lesions) prior to EP were excluded from the study. Forty-four patients (55.7%) underwent EP for benign ampullary lesions (Table 1). Thirty-eight procedures (86.3%) were performed at high-volume centers and six procedures (12.7%) were performed at low-volume centers. There were 29 female (66%) and 15 male (44%) patients. Patients’ age ranged from 33 to 84 y, with a median age of 64 y.
Table 1.
Patient characteristics
| Patient Characteristics | N (%) |
|---|---|
| Female | 29 (66%) |
| Male | 15 (44%) |
| Median age | 64 (range: 33–84) |
| Presenting complaints | |
| Pancreatitis | 16 (36%) |
| Incidentally during evaluation of unrelated GI complaints | 10 (23%) |
| Asymptomatic bile duct dilation | 9 (20.5%) |
| Jaundice | 5 (11.5%) |
| FAP screening | 2 (4.5%) |
| Biliary stone | 1 (2.3%) |
| Hemoccult positive stool | 1 (2.3%) |
Ampullary lesions were discovered during evaluation for pancreatitis in 16 patients (36%), upper gastrointestinal complaints unrelated to ampullary lesions (gastroesophageal reflux, dyspepsia) in 10 patients (23%), asymptomatic bile duct dilation in 9 patients (20.5%), jaundice in 5 patients (11.5%), screening of 2 patients (4.5%) with familial adenomatosis polyposis, 1 patient (2.3%) with bile duct stone, and work-up of 1 patient (2.3%) with hemoccult-positive stool.
Pre-endoscopic ampullectomy evaluation
Sixteen patients (36%) had an endosonographic assessment prior to the endoscopic ampullectomy. EUS evaluation revealed T1N0 lesions in all assessed patients who underwent EUS. Post-papillectomy histopathology revealed 8 precancerous mucosal lesions (50%), 7 cancers limited to the mucosal layer and 1 cancer with submucosal invasion. The patient who had a cancer-invading submucosal layer had positive resection margins. This patient underwent a Whipple procedure, which did not reveal any residual malignancy. Pre-papillectomy endosonographic evaluation was accurate in 100% of patients in ruling out a >T1 lesion. In one patient, submucosal invasion was present but not evident at EUS.
Among 28 patients who did not have a pre-papillectomy endosonographic evaluation, six patients had cancer. Three patients had positive resection margins: two of them did not undergo a surgical resection secondary to being poor operative candidates and one of them subsequently developed obstructive symptoms requiring metal stent placement. One patient underwent Whipple surgery, which revealed a T1 adenocarcinoma.
Endoscopic papillectomy
The average size of the resected lesions was 18.5 mm (range, 5–55 mm). Twenty patients (45%) had submucosal injection prior to EP. Twenty-eight lesions (63.6%) were resected en-bloc and 16 lesions (36.4%) were resected in piecemeal fashion. In nine patients, argon plasma coagulation was used to ablate remnant tissue. Twenty-seven pancreatic (61.3%) and 8 biliary (18%) stents were placed.
Histopathology
Histopathologic diagnoses included tubular adenoma in 14 patients (32%), invasive adenocarcinoma in 9 patients (20.5%), tubullovillous adenoma in 7 patients (16%), tubullovillous adenoma with carcinoma limited to the mucosal layer in 5 patients (11.3%), adenoma with high-grade dysplasia in 4 patients (9%), neuroendocrine tumor in 1 patient (2.3%), ganglioneuroma in 1 patient (2.3%), hamartomatous polyp in 1 patient (2.3%), adenofibroma in 1 patient (2.3%), and Brunner gland hyperplasia in 1 patient (2.3%).
Patients who were referred to participating institutions with established diagnoses did not undergo routine repeat endoscopic biopsies for histopathologic confirmation, unless otherwise indicated with endoscopic appearance of the lesions. Fourteen patients (32%) were diagnosed with adenocarcinoma, which was missed on prior endoscopic biopsies. Five non-adenomatous lesions (11.3%) were erroneously diagnosed as adenoma at biopsies obtained prior to papillectomies.
The resection was complete with negative margins in 40 patients (91%). Four (28.5%) of 14 patients with adenocarcinoma had positive resection margins. Two of them underwent a Whipple procedure: One had no tumor and the other one had adenocarcinoma limited to the mucosa. The other two patients were not operative candidates.
Complications
No procedure-related mortality occurred. Seven patients (15.9%) had procedure-related complications: 3 patients had bleeding (6.8%) (two patients required endoscopic therapeutic intervention without any blood transfusion and one patient had bleeding requiring blood transfusion), 2 patients (4.5 %) had pancreatitis (one had a prophylactic pancreatic stent and the other one did not have a prophylactic pancreatic stent), 1 patient (2.3%) had pain prolonging hospital stay (discharged home on post-op day 5), and 1 (2.3%) patient had stent migration to the pancreatic duct that was removed endoscopically. Five complications (13.1%) occurred in cases performed at high-volume centers, and two complications (33.3%) (bleeding requiring blood transfusion and abdominal pain) occurred in cases performed at low-volume centers.
Follow-up
Patients had endoscopic follow-ups (every 3 to 6 months for the first 2 years). If no recurrences were noted, patients were recommended to have annual or bi-annual follow-ups). Median follow-up was 24 mo (range, 6–84 mo). Seven patients (17%) had recurrence” six recurrences (85.7%) were diagnosed within the first 6 mo, and one recurrence was diagnosed 1 y after papillectomy. Six of the recurrences were adenomas that were managed endoscopically. Three of these lesions were initially removed in piecemeal fashion. The index histopathology of these lesions was tubullovillous adenoma in 2 patients, tubullovillous adenoma with intramucosal carcinoma in 2 patients, adenoma with high-grade dysplasia in 1 patient, and adenoma in 1 patient. One patient who was found to have an adenocarcinoma on the follow-up was referred to surgery. The index histopathology of this lesion was tubullovillous adenoma with intramucosal carcinoma. This lesion was initially resected in a piecemeal fashion. Two patients developed biliary stones (12 and 60 mo after the papillectomy).
DISCUSSION
Lesions involving the ampulla of Vater need to be resected completely due to concurrent or future malignant potential. The historic gold-standard surgical treatment options carry significant morbidity as well as mortality and have prompted a search for less invasive options. EP has emerged as a minimally invasive, safe, and efficacious therapeutic alternative to surgery in order to resect tumors involving the ampulla of Vater (12).
The literature on EP is relatively sparse for several reasons, including the limited number of centers that can offer this procedure (requiring special expertise in pancreatobiliary endoscopy). As a result, the majority of the literature is retrospective, often single-center studies with short-term follow-up. In contrast, the current study is a multicenter study with longer follow-up to 2 years, which is intended to demonstrate the durability of EP as a minimally invasive cancer therapy.
All ampullary lesions warrant resection due to malignant potential and undersampling (13–16) at biopsy. In our cohort, despite efforts to exclude an underlying malignancy by endoscopic appearance and forceps biopsies of ampullary lesions, post-papillectomy histopathological evaluation revealed adenocarcinoma in 32% of cases and non-adenomatous lesions in 11.3% of cases. Diagnostic concordance between the prepapillectomy biopsy and papillectomy specimen can be as low as 64% (14). A review of the available literature reveals 12.3% carcinomas, 7.6% non-neoplastic histopathologies, and 1% neuroendocrine tumors in patients who had undergone EP with a presumptive diagnosis of adenomatous ampullary lesions (Table 2) (17–40). EP plays an important role in guiding any future management steps, and with long-term follow-up studies such as the current report, will likely be accepted as a durable primary therapeutic modality for lesions in this location (41).
Table 2.
Summary of published studies on endoscopic ampullectomy
| Study | Year | Patients (N) | Success N (%) | Histology | Complications (%) | Recurrence N (%) | Surgery N (%) | Mortality |
|---|---|---|---|---|---|---|---|---|
| Binmoeller (17) | 1993 | 25 | 23 (92%) | 25 adenomas (18 MGD, 1 HGD) | Pancreatitis (12%) Bleeding (8%) |
6 (26%) | 3 (12%) | 0 |
| Vogt (18) | 2000 | 18 | 12 (67%) | 18 adenomas | Pancreatitis (11%) Bleeding (11%) Stent occlusion (6%) |
6 (33%) | 3 (17%) | |
| Park (19) | 2000 | 6 | 5 (83%) | 4 adenomas 2 carcinomas |
Pancreatitis (33%) | 0 | 1 (17%) | |
| Zadorova (20) | 2001 | 16 | 13 (81%) | 16 adenomas | Pancreatitis (13%) Bleeding (13%) |
3 (19%) | 1 (6%) | |
| Desilets (21) | 2001 | 13 | 12 (92%) | 13 adenomas (1 HGD) | Pancreatitis (8%) | 0 | 1 (8%) | |
| Norton (22) | 2002 | 26 | 26 (100%) | 25 adenoma 1 carcinoma 1 inflammatory polyp 1 normal papilla |
Bleeding (8%) Acute pancreatitis (15%) Pancreatitis due to PD stenosis (8%) Perforation (4%) |
2(10%) | 1 (4%) | |
| Cheng (23) | 2004 | 55 | 39 (74%) | 45 adenoma (7 HGD) 5 carcinoma 2 carcinoid 1 gastric heterotopia 2 normal histology |
Pancreatitis (9%) Bleeding (7%) Perforation (2%) |
9 (16%) | 7 (13%) | |
| Catalano (24) | 2004 | 103 | 83 (80%) | 97 adenoma (14 HGD) 6 carcinoma |
Pancreatitis (5%) Papillary stenosis (3%) Bleeding (2%) |
20 (20%) | 16 (16%) | |
| Moon (25) | 2005 | 6 | 6 (100%) | 6 adenoma | Pancreatitis (17%) Cholangitis (17%) |
0 | 0 | |
| Han (26) | 2005 | 22 | 16 (73%) | 14 adenoma (3 HGD) 2 adenocarcinoma 1 carcinoid 3 chronic inflammation 1 adenomatous hyperplasia 1 cavernous lymphangioma |
Bleeding (18%) Papillary stenosis (% 5) Perforation (%5) Cholangitis (%5) Abnormal LTs (%5) |
1 (4.5%) | 0 | 0 |
| Bohnaker (27) | 2005 | 106 | 73 (73%) | 92 adenoma (18 HGD) 4 carcinoma 1 lymphangioma 12 hyperplastic |
Bleeding (13%) Pancreatitis (6%) |
15 (15%) | 19 (19%) | |
| Harewood (28) | 2005 | 19 | NR | NR | Pancreatitis (16%) Cholangitis (5%) Abdominal pain (5%) |
NR | NR | |
| Katsinelos (29) | 2006 | 14 | 11 (79%) | 11 adenoma 3 carcinoma |
Pancreatitis (7%) Bleeding (7%) |
2 (18%) | 3 (21%) | |
| Boix (30) | 2008 | 21 | 6 (28.5%) | 11 adenoma (4 HGD) 10 carcinoma |
Pancreatitis (19%) Bleeding (5%) |
1 (16.6%) | 15 (71.4%) | |
|
| ||||||||
| Jung (31) | 2008 | 22 | 12 (55%) | 11 adenoma (2 HGD) 9 carcinoma 1 lymphoma 1 inflammation |
Pancreatitis (18%) Bleeding (5%) Perforation (5%) |
2 (16.7%) | 6 (27%) | |
| Irani (32) | 2009 | 102 | 86 (84%) | 94 adenoma 8 carcinoma |
Pancreatitis (10%) Bleeding (5%) Perforation (2%) Cholangitis (1%) Papillary stenosis (3%) |
8 (8%) | 16 (16%) | |
| Yamao (33) | 2010 | 36 | 29 (81%) | 26 adenoma 8 carcinoma 1 hyperplastic 1 inflammation |
Pancreatitis (8%) Bleeding (8%) Biliary stenosis (3%) |
1 (3%) | 1 (3%) | |
| Malet (34) | 2011 | 42 | 39 (93%) | 30 adenoma (10 HGD and CIS) 7 inflammatory 2 somatostatinoma |
Pancreatitis (14%) Bleeding (7%) Cholangitis (2%) |
4 (10%) | 4 (%10) | |
| Patel (35) | 2011 | 38 | 38 (100%) | 38 adenoma (6 HGD) | Pancreatitis (8%) Bleeding (5%) Infection (3%) |
6 (16%) | 0 | |
| Salmi (36) | 2012 | 61 | 56 (82%) | 33 adenoma (11 HGD) 10 adenocarcinoma 3 endocrine carcinoma 16 no dysplasia |
Pancreatitis (10%) Bleeding (5%) Perforation (3%) |
3 (5 %) | 5 (8%) | |
| Laleman (37) | 2013 | 91 | 71 (78%) | 65 adenoma (19 HGD) 16 carcinoma 12 non-specific |
Pancreatitis (15%) Bleeding (12%) Cholangitis (4%) |
13 (18%) | 14 (15%) | |
| Napoleon (38) | 2014 | 93 | 84 (90 %) | 66 adenoma (32 HGD) 13 carcinoma 7 Inflammation 3 Adenomyomatosis 3 Brunner gland 1 neuroendocrine tumor |
Pancreatitis (20%) Bleeding (10%) Perforation (3.6%) Biliary (7 %) Papillary stenosis (1.8%) |
5 (5.3%) | 5 (5.3%) | 1 |
| De Palma (39) | 2015 | 27 | 25 (93%) | 22 adenoma (4 HGD) 3 adenocarcinoma |
Pancreatitis (11%) Bleeding (7%) |
1 (4%) | 2 (7%) | |
| Tsuji (40) | 2015 | 115 | 113 (98.2%) | 85 adenoma 13 cancer in adenoma 10 cancer 7 hyperplasia |
Pancreatitis (10.2 %) Bleeding (18 %) Perforation (2.6%) Cholangitis (1.7%) Papillary stenosis (4.3%) |
NR | 1 (0.9%) | 1 |
| Current Study | 44 | 40 (91%) | 18 adenoma (4 HGD) 9 adenocarcinoma 7 tubullovillous adenoma 5 tubullovillous adenoma with mucosal carcinoma 1 neuroendocrine tumor 1 ganglioneuroma 1 hamartomatous polyp 1 adenofibroma 1 Brunner gland hyperplasia |
Bleeding (6.8%) Pancreatitis (4.5%) Abdominal pain (2.3%) Stent migration (2.3%) |
7 (17%) | 3 (6.8%) | 0 | |
| Total | 1121 | 918 (81.8%) | 872 adenoma (79%) 137 carcinoma (12.3%) 84 non-neoplastic (7.6%) 11 NET (1 %) 1 Lymphoma |
Pancreatitis (10.3%) Bleeding (8.9%) Perforation (1.7%) Stenosis (1.5%) Cholangitis (1%) |
115 (11.6%) | 127 (11.3%) | 2 (% 0.1) | |
MGD: moderate grade dysplasia; HGD: high grade dysplasia; CIS: carcinoma in situ; PD: pancreatic duct; LTs: liver tests; NR: not reported; NET: neuroendocrine tumor
Our technical success (complete resection) rate was 91%, consistent or slightly better than previously published series (mean: 81.8 %) (Table 2) (17–40). None of the patients with a benign histopathology had positive resection margins. In our study, an underlying malignancy was the only risk factor for the incomplete resection of ampulla of Vater lesions (p=0.002). Submucosal injection did not have any impact on the completeness of resection or complication rates. Incomplete resection only occurred in four patients, of which, 2 were suitable for surgical intervention. Surgery found no residual tumor (false diagnosis of incomplete resection), while the other patient had adenocarcinoma limited to the mucosa. A review of the literature shows variable surgical resection rates (0%71.4%) (Table 2). The low rate of surgical referral (6.8%) in this study is likely due to strict exclusion of highly suspicious lesions (by endoscopic inspection and/or pre-papillectomy biopsy) from the study. In our study, seven patients (17%) experienced local recurrence and were managed endoscopically, except for one patient who had an adenocarcinoma at follow-up endoscopy who was referred to surgery. Given the retrospective study design and limited number of recurrences, it is not possible to reach a conclusion on the predictive parameters for recurrence (such as resection method, underlying histopathology, submucosal invasion). The reported cumulative recurrence rate of published series was 11.6% (range, 0%–33%) (Table 2) (17–40). Although majority of the recurrences in our patients (85.7%) occurred within the first 6 mo, post-papillectomy follow-up should be extended at least for 18 to 24 mo due to delayed recurrences reported in the literature. The outcome of patients with benign ampullary lesions as well as carcinoma in-situ undergoing EP has been excellent (Table 2). However, there has been concern about the adequacy of EP alone in patients with T1 ampullary tumors. Histopathological evaluation of surgically resected T1 ampullary cancers showed 56.7% of lymphovascular invasion and 22 % of lymph node metastasis as well as an association between tumor grade and lymph node metastasis (42,43). Since long-term survival of carcinoma of the ampulla of Vater depends on lymph node metastasis, pancreaticoduodenectomy remains the gold-standard treatment, as it is currently believed to better address the high rate of lymphovascular invasion and lymph node metastasis observed for this disease (44). However, forthcoming prospective studies may establish risk stratification protocols allowing EP to be safely extended to patients with low-risk tumor biology in this setting (e.g., well-differentiated tumors without lymphovascular invasion).
Although EUS provides useful information on the locoregional staging of ampullary tumors, it is not routinely used prior to endoscopic papillectomies. The overall accuracy of EUS and intraductal EUS in T-staging of ampullary neoplasms is found to be 63% and 73%, respectively (45). In our study, endosonographic evaluation depended on the preference of the endoscopist as well as the availability of EUS during the time of the procedure. Thirty-six percent of our patients had endosonographic evaluation prior to EP. Histopathologic examination of resected specimens confirmed that EUS was 100% accurate in excluding extension beyond the submucosal layer but missed submucosal invasion in one patient (6.2%). In a recently published guideline, endosonography was suggested to be used in the evaluation of large (>1 cm) lesions as well as lesions with features suggestive of an underlying malignancy (12). Our observation concurs with this recommendation. In our study, the EUS findings were consistent with early-stage disease; therefore, it did not change our plan to pursue with EP. However, our management plan would be different if the EUS findings would have shown more advanced disease. We suggest surgical resection, rather than EP, for advanced-stage disease (>T1).
Cholangiography and pancreatography were performed in all our patients to evaluate proximal duct extension of the ampullary lesions prior to papillectomy attempt. Given the stringent inclusion criteria, we did not encounter intraductal tumor extension in our patients. We think that this practice is important for risk stratification prior to papillectomy attempt.
The average complication rate in published series is 23.4% (Table 2) (17–40). Pancreatitis is the most commonly encountered complication (10.3%) (range, 4.5%–33%), followed by bleeding (8.9%), perforation (1.7%), stenosis (1.5%), and cholangitis (1%). Only three procedure-related deaths were reported in the literature (38,40,46). Although only seven patients experienced procedure-related complications (15.9%), three of them required endoscopic re-intervention (2 bleeding and 1 stent migration into the pancreatic duct). Post-papillectomy pancreatitis was seen in two patients, one of them had a prophylactic pancreatic duct stent placed during the papillectomy while the other patient did not. Although pancreatitis is one of the most common and potentially serious complications (Table 2), few studies have focused on its prevention (17–40). Even though the current practice is prophylactic pancreatic stenting, its impact in the prevention of post-papillectomy pancreatitis remains unsubstantiated in the literature (28–47). There is no study assessing the impact of pharmacologic intervention, as well as comparing suppository non-steroidal anti-inflammatory medications with prophylactic pancreatic stent placement to prevent pancreatitis in post-papillectomy patients. Our pancreatitis rate is relatively lower than previously published series. This may be due to a high rate of prophylactic pancreatic stent placement and/or evaluation of pancreatic enzyme levels only in patients with typical abdominal pain suggestive of acute pancreatitis. One patient with post-procedure abdominal pain had normal pancreatic enzymes and abdominal tomography. No procedure-related mortality occurred. There was no statistically significant difference between complications occurring in high- and low-volume centers (p=0.209).
There are several limitations to the current study that are inherent to a retrospective study design. The main limitation of this study was its retrospective nature, lack of predefined algorithm, reliance on data that was not designed for the study, unequal contribution of each institution in terms of number of patients, and a relatively small sample size, as well as reliance on histopathologic results of biopsy samples obtained prior to papillectomy without histopathologic confirmation of the biopsy slides at institutions where papillectomy was performed.
In summary, EP is effective and safe in expert hands and should be considered as a first-line modality allowing correct diagnosis and staging of benign ampullary lesions with a curative intent. An underlying adenocarcinoma is a risk factor for incomplete resection of ampullary lesion. Further studies are needed to determine the appropriate evaluation of ampullary lesions prior to endoscopic resection, the role of rectal administration of non-steroidal anti-inflammatory drugs in the prevention of pancreatitis, and appropriate patient selection for adequate oncological resection of T1 ampullary tumors with EP.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Koç University School of Medicine (Decision No: 2016.026.IRB2.006).
Informed Consent: Written informed consent was obtained from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - T.A., E.P., E.A., S.D., B.Ç., B.Ö.; Design - T.A., E.P., E.A., S.D., B.Ç., B.Ö.; Supervision - T.A., E.P., E.A., S.D., B.Ç., B.Ö.; Resource - T.A., E.P., E.A., S.D., B.Ç., B.Ö.; Materials - T.A., E.P., E.A., S.D., B.Ç., B.Ö.; Data Collection and/or Processing - T.A., E.P., E.A., S.D., B.Ç., B.Ö.; Analysis and/or Interpretation - T.A., E.P., E.A., S.D., B.Ç., B.Ö.; Literature Search - T.A., E.P., E.A., S.D., B.Ç., B.Ö.; Writing - T.A., E.P., E.A., S.D., B.Ç., B.Ö.; Critical Reviews - T.A., E.P., E.A., S.D., B.Ç., B.Ö.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Perzin KH, Bridge MF. Adenomas of the small intestine: a clinico pathologic review of 51 cases and a study of their relationship to carcinoma. Cancer. 1981;48:799–819. doi: 10.1002/1097-0142(19810801)48:3<799::aid-cncr2820480324>3.0.co;2-q. https://doi.org/10.1002/1097-0142(19810801)48:3<799::AID-CNCR2820480324>3.0.CO;2-Q [DOI] [PubMed] [Google Scholar]
- 2.Bohnacker S, Seitz U, Nguyen D, et al. Endoscopic resection ofbenign tumors of the duodenal papilla without and with intraductal growth. Gastrointest Endosc. 2005;62:551–60. doi: 10.1016/j.gie.2005.04.053. https://doi.org/10.1016/j.gie.2005.04.053 [DOI] [PubMed] [Google Scholar]
- 3.Stolte M, Pscherer C. Adenoma-carcinoma sequence in the papilla of Vater. Scand J Gastroenterol. 1996;31:376–82. doi: 10.3109/00365529609006414. https://doi.org/10.3109/00365529609006414 [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi K, Enjoji M, Kitamura K. Endoscopic biopsy has limited accuracy in diagnosis of ampullary tumors. Gastrointest Endosc. 1990;36:588–92. doi: 10.1016/s0016-5107(90)71170-4. https://doi.org/10.1016/S0016-5107(90)71170-4 [DOI] [PubMed] [Google Scholar]
- 5.Bellizzi AM, Kahaleh M, Stelow EB. The assessment of specimensprocured by endoscop ic ampullectomy. Am J Clin Pathol. 2009;132:506–13. doi: 10.1309/AJCPUZWJ8WA2IHBG. https://doi.org/10.1309/AJCPUZWJ8WA2IHBG [DOI] [PubMed] [Google Scholar]
- 6.Elek G, Gyori S, Toth B, Pap A. Histological evaluation of preoperative biopsies from ampulla vateri. Pathol Oncol Res. 2003;9:32–41. doi: 10.1007/BF03033712. [DOI] [PubMed] [Google Scholar]
- 7.Roggin KK, Yeh JJ, Ferrone CR, et al. Limitations of ampullectomyin the treatment of nonfamilial ampullary neoplasms. Ann Surg Oncol. 2005;12:971–80. doi: 10.1245/ASO.2005.03.009. https://doi.org/10.1245/ASO.2005.03.009 [DOI] [PubMed] [Google Scholar]
- 8.Diener MK, Fitzmaurice C, Schwarzer G, et al. Pylorus-preservingpancreaticoduodenectomy (pp Whipple) versus pancreaticoduodenectomy (classic Whipple) for surgical treatment of periampullary and pancreatic carcinoma. Cochrane Database Syst Rev. 2011;11:CD006053. doi: 10.1002/14651858.CD006053.pub4. https://doi.org/10.1002/14651858.CD006053.pub4 [DOI] [PubMed] [Google Scholar]
- 9.de Castro SM, van Heek NT, Kuhlmann KF, et al. Surgical management of neoplasms of the ampulla of Vater: local resection or pancreatoduodenectomy and prognostic factors for survival. Surgery. 2004;136:994–1002. doi: 10.1016/j.surg.2004.03.010. https://doi.org/10.1016/j.surg.2004.03.010 [DOI] [PubMed] [Google Scholar]
- 10.Clary BM, Tyler DS, Dematos P, Gottfried M, Pappas TN. Localampullary resection with careful intraoperative frozen section evaluation for presumed benign ampullary neoplasms. Surgery. 2000;127:628–33. doi: 10.1067/msy.2000.106532. https://doi.org/10.1067/msy.2000.106532 [DOI] [PubMed] [Google Scholar]
- 11.Posner S, Colletti L, Knol J, Mulholland M, Eckhauser F. Safety and long-term efficacy of transduodenal excision for tumors of the ampulla of Vater. Surger. 2000;128:694–701. doi: 10.1067/msy.2000.108218. https://doi.org/10.1067/msy.2000.108218 [DOI] [PubMed] [Google Scholar]
- 12.ASGE Standards of Practice Committee. Chathadi KV, Khashab MA, et al. The role of endoscopy in ampullary and duodenal adenomas. Gastrointest Endosc. 2015;82:773–81. doi: 10.1016/j.gie.2015.06.027. https://doi.org/10.1016/j.gie.2015.06.027 [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi K, Enjoji M, Kitamura K. Endoscopic biopsy has limited accuracy in diagnosis of ampullary tumors. Gastrointest Endosc. 1990;36:588–92. doi: 10.1016/s0016-5107(90)71170-4. https://doi.org/10.1016/S0016-5107(90)71170-4 [DOI] [PubMed] [Google Scholar]
- 14.Bellizzi AM, Kahaleh M, Stelow EB. The assessment of specimens procured by endoscopic ampullectomy. Am J Clin Pathol. 2009;132:506–13. doi: 10.1309/AJCPUZWJ8WA2IHBG. https://doi.org/10.1309/AJCPUZWJ8WA2IHBG [DOI] [PubMed] [Google Scholar]
- 15.Elek G, Gyori S, Toth B, Pap A. Histological evaluation of preoperative biopsies from ampulla vateri. Pathol Oncol Res. 2003;9:32–41. doi: 10.1007/BF03033712. https://doi.org/10.1007/BF03033712 [DOI] [PubMed] [Google Scholar]
- 16.Roggin KK, Yeh JJ, Ferrone CR, et al. Limitations of ampullectomy in the treatment of nonfamilial ampullary neoplasms. Ann Surg Oncol. 2005;12:971–80. doi: 10.1245/ASO.2005.03.009. https://doi.org/10.1245/ASO.2005.03.009 [DOI] [PubMed] [Google Scholar]
- 17.Binmoeller KF, Boaventura S, Ramsperger K, Soehendra N. Endoscopic snare excision of benign adenomas of the papilla of Vater. Gastrointest Endosc. 1993;39:127–31. doi: 10.1016/s0016-5107(93)70051-6. https://doi.org/10.1016/S0016-5107(93)70051-6 [DOI] [PubMed] [Google Scholar]
- 18.Vogt M, Jakobs R, Benz C, Arnold JC, Adamek HE, Riemann JF. Endoscopic therapy of adenomas of the papilla of Vater. A retrospective analysis with long-term follow-up. Dig Liver Dis. 2000;32:339–45. doi: 10.1016/s1590-8658(00)80028-6. https://doi.org/10.1016/S1590-8658(00)80028-6 [DOI] [PubMed] [Google Scholar]
- 19.Park SW, Song SY, Chung JB, et al. Endoscopic snare resectionfor tumors of the ampulla of Vater. Yonsei Med J. 2000;41:213–8. doi: 10.3349/ymj.2000.41.2.213. https://doi.org/10.3349/ymj.2000.41.2.213 [DOI] [PubMed] [Google Scholar]
- 20.Zádorová Z, Dvofák M, Hajer J. Endoscopic therapy of benign tumors of the papilla of Vater. Endoscopy. 2001;33:345–7. doi: 10.1055/s-2001-13693. https://doi.org/10.1055/s-2001-13693 [DOI] [PubMed] [Google Scholar]
- 21.Desilets DJ, Dy RM, Ku PM, Hanson BL, Elton E, Mattia A, et al. Endoscopic management of tumors of the major duodenal papilla: Refined techniques to improve outcome and avoid complications. Gastrointest Endosc. 2001;54:202–8. doi: 10.1067/mge.2001.116564. https://doi.org/10.1067/mge.2001.116564 [DOI] [PubMed] [Google Scholar]
- 22.Norton ID, Gostout CJ, Baron TH, Geller A, Petersen BT, Wiersema MJ. Safety and outcome of endoscopic snare excision of the major duodenal papilla. Gastrointest Endosc. 2002;56:239–43. doi: 10.1016/s0016-5107(02)70184-3. https://doi.org/10.1067/mge.2002.126064 [DOI] [PubMed] [Google Scholar]
- 23.Cheng CL, Sherman S, Fogel EL, et al. Endoscopic snare papillectomy for tumors of the duodenal papillae. Gastrointest Endosc. 2004;60:757–64. doi: 10.1016/s0016-5107(04)02029-2. https://doi.org/10.1016/S0016-5107(04)02029-2 [DOI] [PubMed] [Google Scholar]
- 24.Catalano MF, Linder JD, Chak A, et al. Endoscopic managementof adenoma of the major duodenal papilla. Gastrointest Endosc. 2004;59:225–32. doi: 10.1016/s0016-5107(03)02366-6. https://doi.org/10.1016/S0016-5107(03)02366-6 [DOI] [PubMed] [Google Scholar]
- 25.Moon JH, Cha SW, Cho YD, et al. Wire-guided endoscopic snarepapillectomy for tumors of the major duodenal papilla. Gastrointest Endosc. 2005;61:461–6. doi: 10.1016/s0016-5107(04)02649-5. https://doi.org/10.1016/S0016-5107(04)02649-5 [DOI] [PubMed] [Google Scholar]
- 26.Han J, Lee SK, Park DH, et al. Treatment outcome after endoscopic papillectomy of tumors of the major duodenal papilla. Korean J Gastroenterol. 2005;46:110–9. [PubMed] [Google Scholar]
- 27.Bohnacker S, Seitz U, Nguyen D, et al. Endoscopic resection ofbenign tumors of the duodenal papilla without and with intraductal growth. Gastrointest Endosc. 2005;62:551–60. doi: 10.1016/j.gie.2005.04.053. https://doi.org/10.1016/j.gie.2005.04.053 [DOI] [PubMed] [Google Scholar]
- 28.Harewood GC, Pochron NL, Gostout CJ. Prospective, randomized, controlled trial of prophylactic pancreatic stent placement for endoscopic snare excision of the duodenal ampulla. Gastrointest Endosc. 2005;62:367–70. doi: 10.1016/j.gie.2005.04.020. https://doi.org/10.1016/j.gie.2005.04.020 [DOI] [PubMed] [Google Scholar]
- 29.Katsinelos P, Paroutoglou G, Kountouras J, et al. Safety and long-term follow-up of endoscopic snare excision of ampullary adenomas. Surg Endosc. 2006;20:608–13. doi: 10.1007/s00464-004-2278-0. https://doi.org/10.1007/s00464-004-2278-0 [DOI] [PubMed] [Google Scholar]
- 30.Boix J, Lorenzo-Zuniga V, Moreno de Vega V, Domenech E, Gassull MA. Endoscopic resection of ampullary tumors: 12-year review of 21 cases. Surg Endosc. 2009;23:45–9. doi: 10.1007/s00464-008-9866-3. https://doi.org/10.1007/s00464-008-9866-3 [DOI] [PubMed] [Google Scholar]
- 31.Jung MK, Cho CM, Park SY, et al. Endoscopic resection of ampullary neoplasms: a single-center experience. Surg Endosc. 2009;23:2568–74. doi: 10.1007/s00464-009-0464-9. https://doi.org/10.1007/s00464-009-0464-9 [DOI] [PubMed] [Google Scholar]
- 32.Irani S, Arai A, Ayub K, et al. Papillectomy for ampullary neoplasm: results of a single referral center over a 10-year period. Gastrointest Endosc. 2009;70:923–32. doi: 10.1016/j.gie.2009.04.015. https://doi.org/10.1016/j.gie.2009.04.015 [DOI] [PubMed] [Google Scholar]
- 33.Yamao T, Isomoto H, Kohno S, et al. Endoscopic snare papillectomy with biliary and pancreatic stent placement for tumors of the major duodenal papilla. Surg Endosc. 2010;24:119–24. doi: 10.1007/s00464-009-0538-8. https://doi.org/10.1007/s00464-009-0538-8 [DOI] [PubMed] [Google Scholar]
- 34.Jeanniard-Malet O, Caillol F, Pesenti C, Bories E, Monges G, Giovannini M. Short-term results of 42 endoscopic ampullectomies: a single-center experience. Scand J Gastroenterol. 2011;46:1014–9. doi: 10.3109/00365521.2011.571711. https://doi.org/10.3109/00365521.2011.571711 [DOI] [PubMed] [Google Scholar]
- 35.Patel R, Davitte J, Varadarajulu S, Wilcox CM. Endoscopic resection of ampullary adenomas: complications and outcomes. Dig Dis Sci. 2011;56:3235–40. doi: 10.1007/s10620-011-1826-4. https://doi.org/10.1007/s10620-011-1826-4 [DOI] [PubMed] [Google Scholar]
- 36.Salmi S, Ezzedine S, Vitton V, et al. Can papillary carcinomas betreated by endoscopic ampullectomy? Surg Endosc. 2012;26:920–5. doi: 10.1007/s00464-011-1968-7. https://doi.org/10.1007/s00464-011-1968-7 [DOI] [PubMed] [Google Scholar]
- 37.Laleman W, Verreth A, Topal B, et al. Endoscopic resection of ampullary lesions: a single-center 8-year retrospective cohort study of 91 patients with long-term follow-up. Surg Endosc. 2013;27:3865–76. doi: 10.1007/s00464-013-2996-2. https://doi.org/10.1007/s00464-013-2996-2 [DOI] [PubMed] [Google Scholar]
- 38.Napoleon B, Gincul R, Ponchon T, et al. Endoscopic papillectomy for early ampullary tumors: long-term results from a large multicenter prospective study. Endoscopy. 2014;46:127–34. doi: 10.1055/s-0034-1364875. https://doi.org/10.1055/s-0034-1364875 [DOI] [PubMed] [Google Scholar]
- 39.De Palma GD, Luglio G, Maione F, et al. Endoscopic snare papillectomy: a single institutional experience of a standardized technique. A retrospective cohort study. Int J Surg. 2015;13:180–3. doi: 10.1016/j.ijsu.2014.11.045. https://doi.org/10.1016/j.ijsu.2014.11.045 [DOI] [PubMed] [Google Scholar]
- 40.Tsuji S, Itoi T, Sofuni A, Mukai S, Tonozuka R, Moriyasu F. Tips and tricks in endoscopic papillectomy of ampullary tumors: single-center experience with large case series. J Hepatobiliary Pancreat Sci. 2015;22:E22–7. doi: 10.1002/jhbp.207. https://doi.org/10.1002/jhbp.207 [DOI] [PubMed] [Google Scholar]
- 41.Ogawa T, Ito K, Fujita N, et al. Endoscopic papillectomy as a method of total biopsy for possible early ampullary cancer. Digest Endosc. 2012;24:291. doi: 10.1111/j.1443-1661.2011.01214.x. https://doi.org/10.1111/j.1443-1661.2011.01214.x [DOI] [PubMed] [Google Scholar]
- 42.Lee SY, Jang KT, Lee KT, et al. Can endoscopic resection be applied for early stage ampulla of Vater cancer? Gastrointest Endosc. 2006;63:783–8. doi: 10.1016/j.gie.2005.09.015. https://doi.org/10.1016/j.gie.2005.09.015 [DOI] [PubMed] [Google Scholar]
- 43.Amini A, Miura JT, Jayakrishnan TT, et al. Is local resection adequate for T1 stage ampullary cancer? HPB. 2015;17:66–71. doi: 10.1111/hpb.12297. https://doi.org/10.1111/hpb.12297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qiao QL, Zhao YG, Ye ML, et al. Carcinoma of the ampulla of Vater: factors influencing long-term survival of 127 patients with resection. World J Surg. 2007;31:137–143. doi: 10.1007/s00268-006-0213-3. https://doi.org/10.1007/s00268-006-0213-3 [DOI] [PubMed] [Google Scholar]
- 45.Ito K, Fujita N, Noda Y, et al. Preoperative evaluation of ampullary neoplasm with EUS and transpapillary intraductal US: a prospective and histopathologically controlled study. Gastrointest Endosc. 2007;66:740–7. doi: 10.1016/j.gie.2007.03.1081. https://doi.org/10.1016/j.gie.2007.03.1081 [DOI] [PubMed] [Google Scholar]
- 46.Kahaleh M, Shami VM, Brock A, et al. Factors predictive of malignancy and endoscopic resectability in ampullary neoplasia. Am J Gastroenterol. 2004;99:2335–9. doi: 10.1111/j.1572-0241.2004.40391.x. https://doi.org/10.1111/j.1572-0241.2004.40391.x [DOI] [PubMed] [Google Scholar]
- 47.Chang WI, Min YW, Yun HS, Lee KH, Lee KT, Rhee PL. Prophylactic pancreatic stent placement for endoscopic duodenal ampullectomy: a single-center retrospective study. Gut Liver. 2014;8:306–12. doi: 10.5009/gnl.2014.8.3.306. https://doi.org/10.5009/gnl.2014.8.3.306 [DOI] [PMC free article] [PubMed] [Google Scholar]