Abstract
Background/Aims
In 80% of the patients, Acute pancreatitis (AP) occurs as a self-limiting disease that does not require any specific treatment; however, in 20% of the cases it occurs in its clinically severe form that may lead to local or systemic complications. The aim of this prospective study was to examine the relationship between the neutrophil to lymphocyte ratio (NLR) and the systemic complications and severity of AP.
Materials and Methods
This prospective study included 100 patients with AP. Age, sex, NLR, Ranson scores and the revised Atlanta classification of the patients were recorded. The patients were divided into two groups according to the Ranson scores as mild and severe AP. According to the Revised Atlanta classification, the patients were divided into two groups as mild and moderate+severe AP.
Results
According to the Ranson score, NLR at the time of admission and at the 48th hour in the severe group was found to be statistically higher than the mild AP group (p<0.01). The receiver operating characteristic (ROC) curve analysis was performed to determine the cut-off value of NLR at the emergency department in order for it to be used for distinguishing AP patients with and without systemic complications. The area under the ROC curve was 0.81. Sensitivity and specificity were 87.50% and 69.05%, respectively, when the NLR cut-off value was >7.13.
Conclusion
Neutrophil to lymphocyte ratio is associated with severe AP. We also regard NLR as a valuable parameter for predicting the development of systemic complications in patients with AP.
Keywords: Per oral endoscopic myotomy, achalasia, aflatoxin, lower esophageal sphincter
INTRODUCTION
Acute pancreatitis (AP) is an inflammatory disease of the pancreas that may involve the surrounding tissue and distant organ systems (1–3). AP is one of the most common gastrointestinal system diseases that require hospitalization. Annually, 270,000 patients are hospitalized due to AP just in the United States, and the in-hospital treatment costs are higher than US$2.5 billion/year (4). In 80% to 90% of the patients, AP occurs as a self-limiting disease that does not require any specific treatment; however, in 10% to 20% of the cases, it occurs in its clinically severe form that may cause local or systemic complications (5). Our treatment goal must be to diagnose severe cases early and to limit the complications (5). Early diagnosis of severe AP is critical for starting supportive treatment in time, recognizing complications as soon as possible, and referring the patients to suitable centers (6). In order to plan treatment according to the severity of the disease scoring systems, multiple criteria and some serum markers are used to recognize patients at risk for severe disease and the development of complications. One of these indicators could be the neutrophil-to-lymphocyte ratio (NLR). The NLR is calculated by dividing the number of neutrophils in the peripheral blood by the number of lymphocytes. According to the current literature, the NLR is accepted as a parameter that reflects the negative effects of high neutrophil numbers that indicate an acute inflammatory response and the effects of low lymphocyte numbers that indicate the deterioration in the general health condition and physiological stress together (7,8). High NLRs have been associated with poor prognosis in benign and malignant clinical conditions (9). The NLR can be calculated quickly and simply using a complete blood count in the emergency department, and studies have shown that the NLR provides valuable information for interventions during the critical hours (10). The aim of this prospective study was to evaluate the association of the NLR with the severity and systemic complications of AP.
MATERIALS AND METHODS
Study population and study protocol
This prospective observational cohort study was conducted in the emergency department of a tertiary hospital that treats 300,000 patients/year. A total of 100 consecutive patients with a diagnosis of AP between June 2014 and January 2015 were included in the study.
Inclusion criteria
All patients diagnosed with AP were included in the study. The diagnosis of AP was based on the presence of two or more of the following (11): abdominal pain consistent with AP (acute onset, often radiating to the back, continuous, and severe pain), elevation over three times the upper normal limit of serum amylase/lipase, and characteristic findings of AP on contrast-mediated computed tomography (CT), magnetic resonance imaging, or abdominal ultrasonography (USG).
Exclusion criteria
Exclusion criteria were patients <18 years old, pregnant patients, patients with onset of symptoms >48 h ago, patients with hemoproliferative disease, patients receiving chemotherapy (in the previous month), patients with chronic liver disease, patients on steroids or antibiotics, patients who have received blood transfusion (in the previous month), and patients with findings or symptoms of infections of other organ systems.
The vital findings, demographic characteristics, NLR calculated on admission and at 48 h (NLR48), and laboratory findings were recorded. According to the revised Atlanta classification, the AP cases were divided into three groups: mild, moderate, and severe AP. The revised Atlanta Score was calculated for all patients. Patients with mild AP according to the revised Atlanta classification were given 1 point, patients with moderate AP were given 2 points, and patients with severe AP were given 3 points. Owing to the fact that there were less than five patients in the severe risk group, patients in this group were included in the moderate risk group. The Ranson scores of the patients were calculated and recorded on the study forms. A total of two groups were formed. Patients with Ranson scores of <3 points were classified as mild, and patients with Ranson scores of ≥3 points were grouped as severe AP. Patients were divided into AP with and without systemic complications. Patients were grouped into quartiles according to their NLR values on admission, and four groups were formed. Patients with an NLR lower than the first quartile (NLR ≤3.68; n=25) were categorized in Group 1, patients with an NLR value between the first and the second quartiles (3.69≤NLR≤6.03; n=25) in Group 2, patients with an NLR value between the second and the third quartiles (6.04≤NLR≤10.28; n=25) in Group 3, and patients with an NLR value higher than the third quartile (NLR >10.28; n=25) in Group 4.
The duration of hospital stay, the need for intensive care, the need for surgical treatment, and the development of complications were assessed. One month after having been discharged from the hospital, patients were examined by abdominal USG for local complications and, if clinically necessary, with additional CT scans. Samples for complete blood count were obtained from the peripheral blood. Samples of peripheral blood were placed in Ethylene diamine tetra acetic acid (EDTA) tubes. A complete blood count was performed by an automated analyzer (Siemens ADVIA 120 hematology analyzer; Siemens, Eschborn, Germany). The NLR values were calculated by dividing the neutrophil number in the peripheral blood by the lymphocyte number.
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences version 15 for Windows (SPSS Inc.; Chicago, IL, USA). Both visual (histogram and probability graphs) and analytical (Kolmogorov-Smirnov and Shapiro-Wilk tests) methods were used to determine whether data were normally or non-normally distributed. Descriptive variables were expressed as mean±standard deviation (SD) for data that were normally distributed and as median and interquartile range (IQR) for variables that were not normally distributed. For comparison of the differences between the groups, the Mann-Whitney U test and independent t-test were used for quantitative variables, and the chi-square test and Fisher’s exact test were used for categorical variables. Patients were divided into four groups according to their NLR values on admission. The differences between these groups were analyzed using the Kruskal-Wallis test. The comparison between the two groups was made using the Mann-Whitney U test and the Bonferroni correction. The usability of the NLR on admission and at 48 h for predicting the development of systemic complications was assessed using a receiver operating characteristic (ROC) curve. The cut-off value was calculated using the Youden’s J index. A p value of <0.05 was accepted as statistically significant with 95% confidence interval (CI).
Written informed consent was obtained from all patients prior to enrollment to the study. Ethics Committee of Konya Training and Research Hospital (2014/74) approved the study protocol in accordance with the Declaration of Helsinki and Good Clinical Practices.
RESULTS
A total of 100 patients including 60 (60%) females and 40 (40%) males were included in the study. The youngest patient was 19 years old, and the oldest was 92 years old. The mean age of the patients was 58.55±18.42 years. Whereas 51 (51%) patients presented with abdominal pain typical for pancreatitis, 47 (47%) presented with non-specific abdominal pain, and 2 (2%) presented with deterioration of the general condition. Five (5%) patients required intensive care, and 4 (4%) patients required mechanical ventilation. Ninety-seven (97%) patients were discharged with recovery, but 3 (3%) died due to multiple organ failure. Table 1 shows the demographic characteristics of the patients.
Table 1.
Demographic findings of the patients
| Age, year, mean±SD | 58.55±18.42 |
|---|---|
| Gender, n (%) | |
| Male | 40 (40%) |
| Female | 60 (60%) |
| Etiology, n (%) | |
| Gallbladder stone | 61 (61%) |
| Other causes | 39 (39%) |
| Complaint, n (%) | |
| Typical epigastric pain | 51 (51%) |
| Non-specific abdominal pain | 47 (47%) |
| Other | 2 (2%) |
| Duration of hospital stay, days, median (IQR) | 4 (3) |
| Ranson score at 48 h, median (IQR) | 2 (2) |
| CTSI score | 1 (2) |
CTSI score: computed tomography severity index
Local complications developed in 10 (10%) patients, and systemic complications developed in 16 (16%) patients (Table 2). Patients were divided into two groups according to the Ranson scores: mild (<3 points) and severe (≥3 points) AP. A statistically significant difference was determined with respect to the NLR of the two groups (p<0.001; 4.56 (4.74) and 9.60 (13.68) values of median (IQR) NLR in the mild and severe groups, respectively).
Table 2.
Systemic and local complications that the patients developed
| Systemic complications | MOF | Renal failure | Metabolic acidosis | Pleural effusion | Hypocalcemia | ARDS |
|---|---|---|---|---|---|---|
| Patients (n=16, 16%) | 3 (3%) | 7 (7%) | 4 (4%) | 7 (7%) | 4 (4%) | 2 (2%) |
| Local complications | Pancreatic necrosis | Pancreatic pseudocyst | Intra-abdominal abscess | Pancreatic ascites | Splenic vein thrombosis | |
| Patients (n=10, 10%) | 2 (2%) | 3 (3%) | 1 (1%) | 7 (7%) | 1 (1%) |
Some patients developed more than one systemic and local complications
MOF: multiple organ failure; ARDS: acute respiratory distress syndrome
According to the revised Atlanta classification, 81 (81%) patients had mild AP and 19 (19%) had moderate or severe AP. These two groups were compared with respect to demographic characteristics, laboratory results on presentation, surgical treatment, and local and systemic complications. A statistically significant difference was observed between the NLR calculated for the two groups formed according to the revised Atlanta classification (p<0.001) (Table 3).
Table 3.
Demographic, laboratory, and clinical properties according to the revised Atlanta classification
| Revised Atlanta classification | p | ||
|---|---|---|---|
|
| |||
| Mild AP (n=81) | Moderate+severe AP (n=19) | ||
| Gender, n (%) | |||
| Male | 28 (34.6%) | 12 (63.2%) | 0.022 |
| Female | 53 (65.4%) | 7 (36.8%) | |
| Etiology, n (%) | |||
| Gallstone | 48 (59.3%) | 13 (68.4%) | 0.46 |
| Other causes | 33 (40.7%) | 6 (31.6%) | |
| Procalcitonin, ng/mL | 0.10 (0.17) | 0.52 (1.28) | <0.001 |
| Procalcitonin48, ng/mL | 0.06 (0.18) | 1.07 (5.04) | <0.001 |
| CRP, mg/L | 7.8 (14.90) | 32 (101.60) | 0.001 |
| CRP48, mg/L | 28 (74.29) | 170 (73) | <0.001 |
| NLR | 5.01 (5.10) | 16 (13.81) | <0.001 |
| NLR48 | 2.42 (2) | 12 (7.24) | <0.001 |
| Amylase, IU/L | 1459 (1516) | 1865 (1295) | 0.255 |
| Local complication, n (%) | 0 (0%) | 10 (52.6%) | <0.001 |
| Systemic complication, n (%) | 0 (0%) | 16 (84.2%) | <0.001 |
| Surgical treatment, n (%) | 21 (25.9%) | 8 (42.1%) | 0.162 |
NLR: neutrophil-to-lymphocyte ratio; CRP: C-reactive protein
Parameters were expressed as median (IQR) and mean±SD; parameters with 48 written beside them are values measured at 48 h
For the correlation analyses, we identified the presence of a statistically significant and positive correlation between the admission NLR, the Ranson score, the revised Atlanta score, and the duration of hospitalization (p<0.001; r=0.551, 0.451, and 0.495, respectively). Systemic complications developed in 4% (1 patient) of the patients in the first NLR quartile. This rate increased up to 40% (10 patients) in the fourth NLR quartile (p<0.001). The Ranson score was ≥3 in 16% (4 patients) of the patients in the first NLR quartile, whereas this rate increased up to 60% (15 patients) in the fourth NLR quartile (p<0.001) (Table 4). The NLR was higher in all biliary and non-biliary AP types with systemic complications than in AP without complications (p<0.05) (Table 5). A ROC curve analysis was performed to determine the cut-off value of the NLR in emergency service in order for it to be used to distinguish patients with AP with and without systemic complications. The area under the ROC curve (AUC) was 0.81. Sensitivity and specificity were 87.50% and 69.05%, respectively, when the NLR cut-off value was >7.13 (positive predictive value (PPV): 35%, negative predictive value (NPV): 96.7%, positive likelihood ratio (+LR): 2.83, and negative likelihood ratio (−LR): 0.18; p<0.001) (Figure 1). A ROC curve analysis was performed to determine the cut-off value of the NLR48 in order to be used to distinguish patients with AP with and without systemic complications. The AUC was 0.93. Sensitivity and specificity were 93.75% and 88.10%, respectively, when the NLR48 cut-off value was >6.2 (PPV: 60%, NPV: 98.7, +LR: 7.87, and −LR: 0.07; p<0.001) (Figure 2). The performance of the inflammation markers in predicting the development of systemic complications was assessed. The AUC of the white blood cell (WBC), NLR, C-reactive protein, and procalcitonin were 0.80, 0.81, 0.79, and 0.81, respectively (Table 6).
Table 4.
Complications and scores of the NLR quartiles
| Group 1 (n=25) | Group 2 (n=25) | Group 3 (n=25) | Group 4 (n=25) | ||
|---|---|---|---|---|---|
| NLR | NLR | NLR | NLR | ||
| ≤3.68 | 3.69–6.03 | 6.04–10.28 | >10.28 | p | |
| Ranson score, n (%) | |||||
| <3 | 21 (84%) | 22 (88%) | 14 (56%) | 10 (40%) | <0.001 |
| ≥3 | 4 (16%) | 3 (12%) | 11 (44%) | 15 (60%) | |
| CTSI score, n (%) | |||||
| 0–1 | 17 (68%) | 16 (64%) | 12 (48%) | 8 (32%) | 0.011 |
| 2–3 | 8 (32%) | 9 (36%) | 11 (44%) | 11 (44%) | |
| 4–6 | 0 (0%) | 0 (0%) | 2 (8%) | 6 (24%) | |
| Surgical treatment, n (%) | 6 (24%) | 7 (28%) | 8 (32%) | 8 (32%) | 0.91 |
| Local complications, n (%) | 0 (0%) | 0 (0%) | 4 (16%) | 6 (24%) | 0.007 |
| Systemic complications, n (%) | 1 (4%) | 0 (0%) | 5 (20%) | 10 (40%) | <0.001 |
NLR: neutrophil-to-lymphocyte ratio; CTSI score: computed tomography severity index
Table 5.
Comparison of NLR and NLR48 in patients with and without systemic complications
| Systemic complicationspresent (n=16) | Systemic complicationsabsent (n=84) | p | |
|---|---|---|---|
| Acute gallstone pancreatitis (n=61) | n=11 | n=50 | |
| NLR | 19.2 (20.10) | 5.89 (5.41) | 0.001 |
| NLR48 | 12.7 (6.28) | 2.41 (2.10) | <0.001 |
| Acute pancreatitis due to other causes (n=39) | n=5 | n=34 | |
| NLR | 9.3 (13.60) | 4.15 (5.63) | 0.04 |
| NLR48 | 9.76 (4.44) | 2.79 (2.39) | 0.002 |
| All acute pancreatitis (n=100) | n=16 | n=84 | |
| NLR | 14.15 (18.93) | 5.15 (5.49) | <0.001 |
| NLR48 | 11.65 (6.27) | 2.53 (2.46) | <0.001 |
The NLRs with 48 written beside them are values calculated at 48 h; parameters were expressed as median (IQR)
Figure 1.
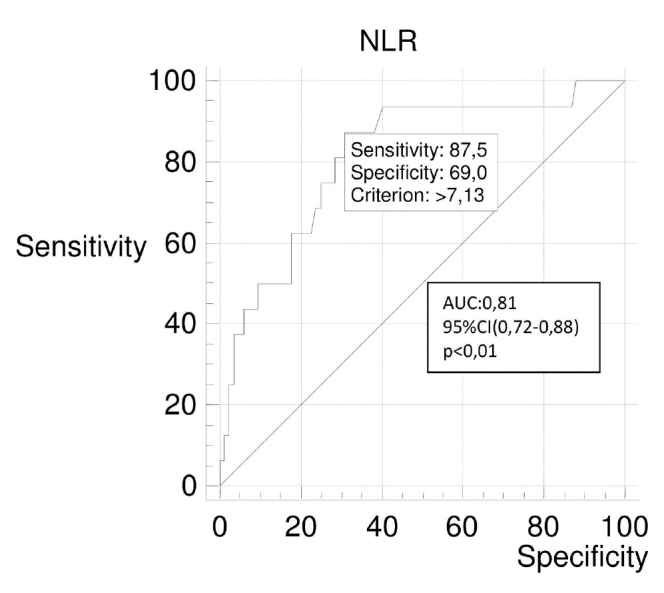
ROC curve for NLR for systemic complications in patients with acute pancreatitis
Figure 2.
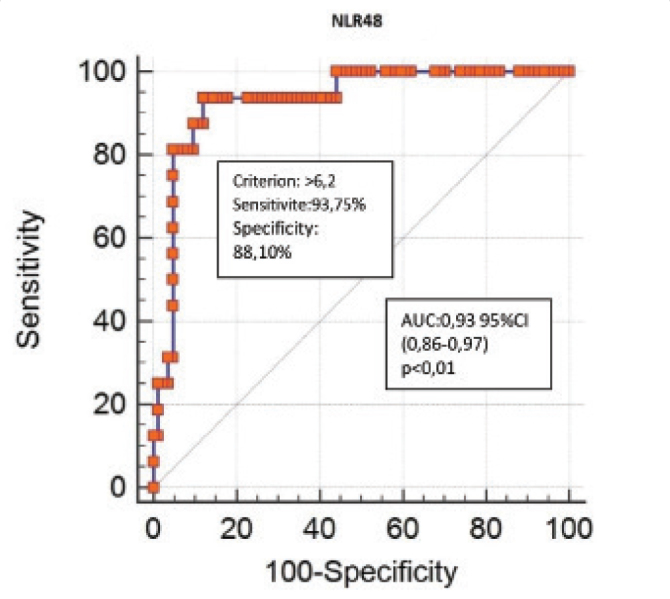
ROC curve for NLR48 for systemic complications in patients with acute pancreatitis
Table 6.
The ROC analysis of inflammation markers in predicting the development of systemic complications
| AUC (95% CI) | Cut-off | Sensitivity (%) | Specificity (%) | +LR | −LR | PPV (%) | NPV (%) | p | |
|---|---|---|---|---|---|---|---|---|---|
| WBC, ×103/μL | 0.80 (0.71–0.87) | 12.7 | 81.25 | 76.19 | 3.41 | 0.25 | 39.4 | 95.5 | <0.001 |
| WBC48, ×103/μL | 0.89 (0.82–0.95) | 11.1 | 87.5 | 84.52 | 5.65 | 0.15 | 51.9 | 97.3 | <0.001 |
| NLR | 0.81 (0.72–0.88) | 7.13 | 87.5 | 69.05 | 2.83 | 0.18 | 35.0 | 96.7 | <0.001 |
| NLR48 | 0.93 (0.86–0.97) | 6.2 | 93.75 | 88.10 | 7.87 | 0.07 | 60.0 | 98.7 | <0.001 |
| CRP, mg/L | 0.79 (0.70–0.86) | 29.7 | 62.50 | 88.10 | 5.25 | 0.43 | 50.0 | 92.5 | <0.001 |
| CRP48, mg/L | 0.83 (0.75–0.90) | 97.0 | 87.50 | 76.19 | 3.67 | 0.16 | 41.2 | 97.0 | <0.001 |
| PCT, ng/mL | 0.81 (0.72–0.88) | 0.21 | 87.50 | 75.0 | 3.50 | 0.17 | 40.0 | 96.9 | <0.001 |
| PCT48, ng/mL | 0.82 (0.73–0.88) | 0.15 | 93.75 | 70.24 | 3.15 | 0.08 | 37.5 | 98.3 | <0.001 |
ROC: receiver operating characteristic curve; AUC: area under the ROC curve; +LR: positive likelihood ratio; −LR: negative likelihood ratio; PPV: positive predictive value; NPV: negative predictive value; NLR: neutrophil-to-lymphocyte ratio; WBC: white blood cell count; CRP: C-reactive protein; PCT: procalcitonin
The parameters with 48 written beside them are values measured at 48 h
DISCUSSION
In most patients, AP runs a mild clinical course without complications, requiring only short-term hospitalization. However, the remaining 20% of the patients have a complicated clinical course. These patients may suffer long-term intensive care admission, long-term hospitalization, and invasive interventions, and significant mortality rates may occur (12). The scoring systems that have been used to determine the prognosis and severity of AP have various limitations. The search for new parameters to add to these scoring systems is one of the active discussions at present. The NLR value can be calculated quickly and simply using a complete blood count in the emergency department. We examined its association with the severity and systemic complications of AP in the present study. According to the data obtained in our study, the NLR was significantly higher in the severe AP groups than in the mild AP groups according to both Ranson scoring and revised Atlanta classification (p<0.001). Furthermore, the systemic complications of AP could be detected with 87.50% sensitivity and 69.05% specificity in our study, when the cut-off level for NLR was 7.13.
Neutrophils provoke the inflammatory cytokine cascades (interleukin (IL)-6, IL-8, and tumor necrosis factor alpha), proteolytic enzymes (myeloperoxidase, elastase, collagenase, and β-glucuronidase), and free oxygen radicals and stimulate inflammation and tissue destruction (13). It has been observed that these inflammatory mediators have important effects on the systemic inflammatory response during AP (14). An increase in the neutrophil count indicates the development of systemic inflammatory response syndrome (SIRS) and multiple organ failure syndrome, which are indicators of severe AP (9). Neutrophils provoke the inflammatory cascade and SIRS in AP, leading to a decrease in the lymphocyte count during severe sepsis, and this is associated with a poor prognosis (15–17). SIRS, multiple organ failure, and severe sepsis are systemic complications of AP. Studies have also shown that there is an association between the low peripheral lymphocyte count and the severity of the disease in AP cases (18,19).
It has been determined that the NLR is associated with various abdominal diseases. A previous study published in 2014 has shown that the NLR is a useful parameter for the diagnosis of acute appendicitis. In the present study, among patients who had undergone appendectomy due to appendicitis, patients with a histopathologically confirmed diagnosis of appendicitis had higher NLR values than those with normal pathology results. In the same study, patients with complicated appendicitis (perforated or gangrenous) had higher NLR values than those with uncomplicated appendicitis (20). It was also shown that a high NLR was correlated with the severity of the disease in acute cholecystitis, one of the causes of acute abdomen. In patients who had undergone cholecystectomy for acute cholecystitis, an NLR of >3 on admission was seen to be associated with severe cholecystitis (changes secondary to cholecystitis, such as bleeding, gangrene, emphysema, and perforation), long duration of surgery, and prolonged hospitalization. An NLR value <3 is associated with simple cholecystitis, short surgery duration, and short hospitalization (21,22). It has also been identified that the rate of intensive care admission and the length of hospitalization of patients with AP increase with an increase in the NLR (23).
In the study they conducted between 2007 and 2011 including 629 patients treated with the diagnosis of AP, Jones et al. (24) examined the association between the severity of pancreatitis, disease prognosis, in-hospital mortality, and NLR. They reported that they detect a significant association between “the NLR and lymphocyte count” and the severity and prognosis of pancreatitis and in-hospital mortality. A total of 283 patients were analyzed in the study conducted by Azab et al. (23). They demonstrated that the NLR value is significantly higher in severe AP cases than in mild-moderate AP cases. They showed that the NLR is superior to the WBC for predicting intensive care admission on their ROC curve for admission to the intensive care unit. They also reported the NLR cut-off value for predicting intensive care admission and duration of hospitalization as ≥4.7. On the other hand, Suppiah et al. (9) divided patients diagnosed with AP into groups of mild and severe AP according to the 1992 Atlanta symposium criteria and compared the NLR values of these groups on admission, day 1, and day 2. They reported the admission NLR cut-off value as 10.6, day 1 NLR cut-off value as 8.1, and day 2 NLR cut-off value as 4.8 in their ROC analysis to predict patients with mild and severe AP.
In the present study, we performed the ROC analysis in order to predict the risk of systemic complications in patients with AP and determined the cut-off levels on both admissions to the emergency unit and at 48 h. In our study, the admission NLR cut-off value (>7.13) obtained for prediction of systemic complications was close to the average of the admission NLR cut-off values obtained by Azab et al. (23) (cut-off ≥4.7) and Suppiah et al. (9) (cut-off=10.6). This demonstrates that our study supports the studies published by Azab et al. and Suppiah et al. The ROC analyses performed according to the NLR values on admission were calculated as AUC 0.65 in the study by Azab et al. (23) (95% CI; p value was not mentioned in their study) and as AUC 0.68 in the study by Suppiah et al. (9) (95% CI: 0.56–0.80; p<0.01). In our study, the ROC analysis performed according to the NLR value on admission was calculated as AUC 0.81 (95% CI: 0.72–0.88; p<0.01). The AUC value observed in our study was higher than those observed in the previous studies; therefore, the validity of the cut-off level obtained in our study is higher. On the other hand, the NLR48 cut-off value (>6.2) obtained in our study to predict systemic complications was higher than that obtained in the study by Suppiah et al. (9) (cut-off=4.8). However, the sensitivity, specificity, positivity, and NPVs obtained in our study were statistically more significant.
In a retrospective study conducted in South Korea, the NLR on admission and on day 2 was found to be significantly higher in the moderate+severe AP group classified according to the revised Atlanta classification than in the mild AP group (p=0.01 and p=0.001, respectively) (25). In the ROC analysis of the same study conducted to predict progression into organ failure, the cut-off value for NLR on admission was calculated to be 5.03 (AUC: 0.62, 95% CI: 0.51–0.72; p<0.05). In our study, both NLR on admission and NLR48 were found to be significantly higher in the moderate+severe AP group classified according to the revised Atlanta classification than in the mild AP group (p<0.001). Additionally, the NLR on admission calculated according to the Ranson score was observed to be significantly higher in the severe AP group than in the mild AP group (median (IQR): 9.60 (13.68) vs 4.56 (4.74), respectively; p<0.001). In our study, parallel to the religious beliefs of the society, no case of alcoholic pancreatitis was observed, and gallstones were observed in the etiology of 61% of the patients. In the study by Jeon et al. (25), the etiology of AP comprised alcohol in 51% of the patients and gallstones in 27.8%. The outcomes of the study conducted in South Korea (25) and our study were parallel despite the etiologies of AP being different.
Similar to the studies by Suppiah et al. (9) and Jeon et al. (25), we investigated the predictive value of the NLR for systemic complications in patients with AP. In our study, the cut-off value for prediction of systemic complications in patients with AP was calculated to be >7.13, which was different than those observed in the studies by Jeon et al. (25) (cut-off=5.03) and Suppiah et al. (9) (cut-off=10.6). This may be due to the varying values of the NLR between races (23). Patient selection may affect the outcome as well. Our study is different to other studies with regard to the patient selection method. Patients with AP were included in the studies by both Suppiah et al. (9) and Jeon et al. (25) without any selection criteria. In our study, some of the patients (those using steroid or antibiotic medication and those with hemoproliferative diseases or chronic hepatic disorders) were not included in the study since the WBC and, therefore, the NLR values may be affected. Antibiotherapy may reduce the inflammation and may affect the WBC and NLR in the treatment of AP. Thus, the NLR value on admission is more important because patients with AP, and especially cases with severe AP, may undergo antibiotherapy following hospitalization, and hence, the NLR may be affected. On the other hand, the NLR is re-calculated 2–3 days after hospitalization, and the response to the treatment may be assessed by comparison of this measurement with those on admission.
The relationship between mortality and the NLR value in AP has been reported in several studies. Gülen et al. (22) published a retrospective study in 2015 that divided 332 patients with AP into two groups of alive and dead. They identified that the NLR is significantly higher (p=0.041) in the group that had died than in the group that had survived. In the retrospective study by Li et al. (26), the NLR was reported to be independently related to mortality in AP according to the outcomes of the univariate and multivariate analyses (hazard ratio=4.726, 95% CI: 1.627–13.726; p=0.004). Similar to the study by Gülen et al. (22), higher NLR values were observed on admission in patients who lost their lives due to AP in the present study (26) than those survivors (p<0.001). Furthermore, the ROC analysis performed for the 100-day mortality prediction revealed an optimal cut-off value of 16.64 for NLR on admission, and the sensitivity and specificity values were 82.4% and 75.6%, respectively (AUC: 0.80, 95% CI: 0.74–0.86; p<0.001). Since we aimed to investigate the relationship between development of systemic complications in AP and NLR in our study, and since our mortality rate was low, we did not investigate the relationship between NLR and mortality in AP.
Our study has some limitations. The first is the single-center nature of our study. The second is the lack of patients with alcoholic pancreatitis in parallel with the religious beliefs of the community living in the proximity of our hospital. Our low number of severe AP cases and low mortality rates are also limitations. The relatively low number of cases may also be considered a limitation.
In recent years, the association between the NLR and the severity of AP has become prominent, and our study has also demonstrated this association with strong statistical results. Based on the evidence we presented in our study, we believe that the NLR as a single parameter is capable of predicting the systemic complications of AP. We believe that emergency department physicians should also consider NLR in addition to the multifactorial scoring systems for predicting the prognosis of AP in the emergency department.
Footnotes
Part of this study was presented as an oral presentation at the 3rd International Critical Care and Emergency Medicine Congress, May 20, 2016, Antalya, Turkey.
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of Konya Training and Research Hospital (2014/74).
Informed Consent: Written informed consent was obtained from all the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - K.K., Y.K.G.,B.C.; Design - K.K., Y.K.G., B.C., R.K., N.B.A.,Ö.K.; Supervision - K.K., N.B.A., R.K., Ö.K.; Materials - K.K., Y.K.G., E.T.S., R.K., B.C., N.B.A.; Data Collection and/or Processing - K.K., Y.K.G., Ö.K., N.B.A., E.T.S., R.K., B.C.; Analysis and/or Interpretation - K.K., Y.K.G., N.B.A.; Literature Review - K.K., Y.K.G.; Writer - K.K., Y.K.G.; Critical Review - K.K., Y.K.G., Ö.K., N.B.A., E.T.S., R.K., B.C.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Banks PA, Freeman ML. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379–400. doi: 10.1111/j.1572-0241.2006.00856.x. https://doi.org/10.1111/j.1572-0241.2006.00856.x [DOI] [PubMed] [Google Scholar]
- 2.Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132:1127–51. doi: 10.1053/j.gastro.2007.01.055. https://doi.org/10.1053/j.gastro.2007.01.055 [DOI] [PubMed] [Google Scholar]
- 3.Bollen TL, Van Santvoort HC, Besselink MG, et al. The Atlanta Classification of acute pancreatitis revisited. Br J Surg. 2008;95:6–21. doi: 10.1002/bjs.6010. https://doi.org/10.1002/bjs.6010 [DOI] [PubMed] [Google Scholar]
- 4.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179–87. doi: 10.1053/j.gastro.2012.08.002. https://doi.org/10.1053/j.gastro.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forsmark CE, Baillie J. AGA Institute technical review on acute pancreatitis. Gastroenterology. 2007;132:2022–44. doi: 10.1053/j.gastro.2007.03.065. https://doi.org/10.1053/j.gastro.2007.03.065 [DOI] [PubMed] [Google Scholar]
- 6.Bülbüller N, Doğru O, Ayten R, Akbulut H, İlhan YS, Çetinkaya Z. Procalcitonin is a predictive marker for severe acute pancreatitis. Ulus Travma Acil Cerrahi Derg. 2006;12:115–20. [PubMed] [Google Scholar]
- 7.Gibson PH, Cuthbertson BH, Croal BL, et al. Usefulness of neutrophil to lymphocyte ratio as predictor of new onset atrial fibrillation after coronary artery bypass grafting. Am J Cardiol. 2010;105:186–91. doi: 10.1016/j.amjcard.2009.09.007. https://doi.org/10.1016/j.amjcard.2009.09.007 [DOI] [PubMed] [Google Scholar]
- 8.Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M. The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology. 2007;73:215–20. doi: 10.1159/000127412. https://doi.org/10.1159/000127412 [DOI] [PubMed] [Google Scholar]
- 9.Suppiah A, Malde D, Arab T, et al. The prognostic value of the neutrophil-lymphocyte ratio (NLR) in acute pancreatitis: identification of an optimal NLR. J Gastrointest Surg. 2013;17:675–81. doi: 10.1007/s11605-012-2121-1. https://doi.org/10.1007/s11605-012-2121-1 [DOI] [PubMed] [Google Scholar]
- 10.Akıllı NB, Yortanlı M, Mutlu H, et al. Prognostic importance of neutrophil-lymphocyte ratio in critically ill patients: short-and long-term outcomes. Am J Emerg Med. 2014;32:1476–80. doi: 10.1016/j.ajem.2014.09.001. https://doi.org/10.1016/j.ajem.2014.09.001 [DOI] [PubMed] [Google Scholar]
- 11.Banks PA, Bollen TL, Dervenis C, et al. Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2012;62:102–11. doi: 10.1136/gutjnl-2012-302779. https://doi.org/10.1136/gutjnl-2012-302779 [DOI] [PubMed] [Google Scholar]
- 12.Papachristou GI, Clermont G, Sharma A, Yadav D, Whitcomb DC. Risk and markers of severe acute pancreatitis. Gastroenterol Clin North Am. 2007;36:277–96. doi: 10.1016/j.gtc.2007.03.003. https://doi.org/10.1016/j.gtc.2007.03.003 [DOI] [PubMed] [Google Scholar]
- 13.Felderbauer P, Muller C, Bulut K, et al. Pathophysiology and treatment of acute pancreatitis: new therapeutic targets-a ray of hope. Basic Clin Pharmacol Toxicol. 2005;97:342–50. doi: 10.1111/j.1742-7843.2005.pto_274.x. https://doi.org/10.1111/j.1742-7843.2005.pto_274.x [DOI] [PubMed] [Google Scholar]
- 14.Wittel UA, Rau B, Gansauge F, et al. Influence of PMN leukocyte-mediated pancreatic damage on the systemic immune response in severe acute pancreatitis in rats. Dig Dis Sci. 2004;49:1348–57. doi: 10.1023/b:ddas.0000037833.16433.77. https://doi.org/10.1023/B:DDAS.0000037833.16433.77 [DOI] [PubMed] [Google Scholar]
- 15.de Jager CP, van Wijk PT, Mathoera RB, de Jongh-Leuvenink J, van der Poll T, Wever PC. Lymphocytopenia and neutrophil-lymphocyte count ratio predict bacteremia better than conventional infection markers in an emergency care unit. Crit Care. 2010;14:R192. doi: 10.1186/cc9309. https://doi.org/10.1186/cc9309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zahorec R. Ratio of neutrophil to lymphocyte counts-rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102:5–14. [PubMed] [Google Scholar]
- 17.Le Tulzo Y, Pangault C, Gacouin A, et al. Early circulating lymphocyte apoptosis in human septic shock is associated with poor outcome. Shock. 2002;18:487–94. doi: 10.1097/00024382-200212000-00001. https://doi.org/10.1097/00024382-200212000-00001 [DOI] [PubMed] [Google Scholar]
- 18.Pavlov P, Uchikov P, Murdzheva M, Tuleva S, Tsvetkova T. Main lymphocyte populations and their subpopulations in patients with acute pancreatitis studied in the course of disease. Khirurgiia (Sofiia) 2001;57:4–11. [In Bulgarian, English abstract] [PubMed] [Google Scholar]
- 19.Pezzilli R, Billi P, Beltrandi E, et al. Circulating lymphocyte subsets in human acute pancreatitis. Pancreas. 1995;11:95–100. doi: 10.1097/00006676-199507000-00010. https://doi.org/10.1097/00006676-199507000-00010 [DOI] [PubMed] [Google Scholar]
- 20.Kahramanca Ş, Özgehan G, Şeker D, et al. Neutrophil-to-lymphocyte ratio as a predictor of acute appendicitis. Ulus Travma Acil Cerrahi Derg. 2014;20:19–22. doi: 10.5505/tjtes.2014.20688. https://doi.org/10.5505/tjtes.2014.20688 [DOI] [PubMed] [Google Scholar]
- 21.Lee SK, Lee SC, Park JW, Kim SJ. The utility of the preoperative neutrophil-to-lymphocyte ratio in predicting severe cholecystitis: a retrospective cohort study. BMC Surg. 2014;14:100. doi: 10.1186/1471-2482-14-100. https://doi.org/10.1186/1471-2482-14-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gülen B, Sonmez E, Yaylacı S, et al. Effect of harmless acute pancreatitis score, red cell distribution width and neutrophil to lymphocyte ratio on the mortality of patients with nontraumatic acute pancreatitis at the emergency department. World J Emerg Med. 2015:629–33. doi: 10.5847/wjem.j.1920-8642.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azab B, Jaglall N, Atallah JP, et al. Neutrophil-lymphocyte ratio as a predictor of adverse outcomes of acute pancreatitis. Pancreatology. 2011;11:445–52. doi: 10.1159/000331494. https://doi.org/10.1159/000331494 [DOI] [PubMed] [Google Scholar]
- 24.Jones MJ, Neal CP, Ngu WS, Dennison AR, Garcea G. Examination of the prognostic value of leucocyte subsets and neutrophil-to-lymphocyte ratio in patients with acute pancreatitis. Pancreatology. 2014;14:S63. https://doi.org/10.1016/j.pan.2014.05.595 [Google Scholar]
- 25.Jeon TJ, Park JY. Clinical significance of the neutrophil-lymphocyte ratio as an early predictive marker for adverse outcomes in patients with acute pancreatitis. World J Gastroenterol. 2017;23:3883–9. doi: 10.3748/wjg.v23.i21.3883. https://doi.org/10.3748/wjg.v23.i21.3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Zhao Y, Feng L, Guo R. Comparison of the prognostic values of inflammation markers in patients with acute pancreatitis: a retrospective cohort study. BMJ Open. 2017;7:e013206. doi: 10.1136/bmjopen-2016-013206. https://doi.org/10.1136/bmjopen-2016-013206 [DOI] [PMC free article] [PubMed] [Google Scholar]


