Abstract
Background/Aims
Hypertriglyceridemia (HTG) is the third most common cause of acute pancreatitis. In patients with severe HTG (TG level>1000 mg/dL), it may be beneficial to immediately lower the levels of triglyceride (TG) and chylomicrons. In this study, we present one of the largest case series on the use of therapeutic plasma exchange (TPE) for hypertriglyceridemia-induced acute pancreatitis (HTG-AP).
Materials and Methods
Overall, 33 patients who were admitted to our clinic for HTG-AP and underwent TPE between January 2007 and July 2017 were included in the study. Clinical data and outcomes and the reduction of triglyceride levels were examined retrospectively.
Results
The TG level decreased by 54.4%, and the total cholesterol level decreased by 52.1% after one TPE session. The TG decrease after the second TPE session was found to be 79.4%. There were 20 (60.6%) patients with mild acute pancreatitis, 10 (30.3%) patients with moderetaly severe acute pancreatitis, and 3 (9.1%) patients with severe acute pancreatitis based on the categorization according to the revised Atlanta criteria. Regarding local complications, the acute peripancreatic fluid collection was observed in 13 (39.4%) patients, acute necrotic collection was observed in 1 (3%) patient, walled-off necrosis was observed in 1 (3%) patient, and pancreatic pseudocyst was not observed in any patient. Mortality was not determined in patients with mild and moderately severe acute pancreatitis, and its rate was 33.3% in patients with severe acute pancreatitis. The overall mortality rate was 3%. No significant complications related to TPE were noted.
Conclusion
TPE is a safe and helpful therapeutic treatment method for patients with HTG-AP and may be considered particularly in patients with severe acute pancreatitis.
Keywords: Hypertriglyceridemia-induced acute pancreatitis, therapeutic plasma exchange, hypertriglyceridemia
INTRODUCTION
Acute pancreatitis (AP) is an acute inflammatory process of the pancreas. Hypertriglyceridemia (HTG) is the third most common cause of AP, following gallstones and alcohol. It is responsible for 1%–4% of AP cases, and it seems to be more serious than AP caused by other etiologies (1–3). There is risk of acute pancreatitis if TG level is greater than 500 mg/dL, and the frequency of AP is higher especially when TG levels are 1000 mg/dL or higher (4). HTG may be either primary (Types I, IV, and V, according to the Fredrickson classification) or secondary. Secondary HTG may occur because of alcohol consumption, diabetes mellitus (DM), pregnancy, obesity, hypothyroidism, nephrotic syndrome, or taking certain medications (β-blockers, oral retinoids, etc.) (1–3,5).
The mechanism of hypertriglyceridemia-induced acute pancreatitis (HTG-AP) is probably caused by excess TGs being hydrolyzed by the pancreatic lipase, which leaks out of the acinar cells in the vascular bed of the pancreas. This is the probable reason for the accumulation of free fatty acids and lysolecithin at high concentrations. Free fatty acids are toxic, and they can damage acinar cells and capillary endothelium (6,7). Furthermore, the elevated concentrations of chylomicrons increase the viscosity in the veins, and the impaired pancreatic blood flow leads to ischemia and acidosis in the pancreas (8). Free fatty acids in acidosis activate trypsinogen and initiate acute edema and necrotizing pancreatitis (6).
In patients with severe HTG (TG level>1000 mg/dL), it may be beneficial to lower the triglyceride (TG) levels without delay. Plasmapheresis has been shown to be effective in reducing the triglyceride levels in patients with severe HTG (9). This can be achieved by the rapid removal of TGs from plasma, leading to the reduction of free fatty acids, which can cause further damage to the pancreas. In this study, we present one of the largest case series on the use of therapeutic plasma exchange (TPE) for HTG-AP.
MATERIALS AND METHODS
Study population
In this study, 33 patients (7 females and 26 males, mean age of 40.8±7.0 [range 28–50] years) who had been diagnosed as HTG-AP and treated by TPE in our Gastroenterology Clinic between January 2007 and July 2017 were included in the study. The diagnosis of HTG-AP was based on TG levels being greater than 1000 mg/dL and the presence of at least 2 out of 3 diagnostic criteria for acute pancreatitis (abdominal pain, serum lipase>3-fold, characteristic findings in imaging modalities). The diagnosis of HTG-AP was achieved by exclusion of other causes, such as acute biliary pancreatitis.
Data of patients were recorded, which included age, gender, body mass index, blood pressure, pulse, fever, alcohol consumption and smoking, presence of DM, previous exacerbations of AP and related therapeutic apheresis, previous medical treatment for hyperlipidemia, length of exacerbation-free period after therapeutic apheresis (by reassessment of patients). In addition, the levels of fasting blood glucose, blood urea nitrogen (BUN), creatinine, calcium (Ca), alanine amino transferase (ALT), gamma glutamyl transferase, lactic dehydrogenase (LDH), thyroid stimulating hormone (TSH), amylase (at admission and the day after TPE), lipase (at admission and the day after TPE), TG (at admission and the day after TPE), cholesterol (at admission and the day after TPE), C-reactive protein (CRP), sedimentation, leukocyte, hemoglobin, and platelet counts were recorded for each patient. The time between the initiation of abdominal pain or admission to hospital and plasma exchange procedure were also recorded. The reduction in TG and total cholesterol were calculated using the values at admission and the day after TPE. The Acute Physiology and Chronic Health Evaluation II (APACHE score) (at admission), Ranson score (at 48th hour), and Balthazar classification score based on abdominal computed tomography (CT) were recorded to be able to grade the severity of acute pancreatitis. According to the 2012 Atlanta criteria, the severity of AP is categorized into 3 levels as mild acute pancreatitis (MAP), moderately severe acute pancreatitis (MSAP), and severe acute pancreatitis (SAP). MAP lacks organ failure and local/systemic complications. MSAP manifests transient organ failure (≤48 hours), local complications, and/or exacerbation of coexisting disease. SAP is defined by the presence of persistent organ failure (≥48 hours). Organ failure includes pulmonary failure, which is defined as an arterial PO2<60 mm Hg on the room air or the requirement for mechanical ventilation. Cardiovascular failure is defined as the development of shock (systolic pressure<90 mm Hg) that persists after fluid resuscitation. Renal failure is defined as a serum creatinine level>2 mg/dL after rehydration or the need for hemodialysis in patients without pre-existing renal disease. Local complications include acute peripancreatic fluid collection (APFC), pancreatic pseudocyst, acute necrotic collection, and walled-off necrosis. The systemic complication was defined as an exacerbation of a pre-existing comorbidity precipitated by AP (10). Patients were categorized according to the 2012 Atlanta criteria. Mortality rates of the MAP, MSAP, and SAP groups were compared.
Compliance with ethical requirements
All patient data were retrospectively obtained through the electronic medical records. The study protocol was approved by the ethical committee of the Adnan Menderes University School of Medicine (October 27, 2017; approval #1253). Informed consent could not be obtained because of the retrospective design of the study.
Conservative treatment and plasma exchange procedures
In addition to the restriction of oral intake, intravenous hydration, and pain medication in conservative treatment, immediate therapeutic plasma exchange was also performed with the purpose of reducing TG levels (in our institution, TPE therapy is available 24 hours a day). Low-molecular-weight heparin (LMWH) was only used for thrombosis prophylaxis. Insulin was administered when the glucose level was >150 mg/dL. TPE was performed daily until the TG level was reduced to <1000 mg/dL.
A Haemonetics MCS+ (Haemonetics Corp. Braintree, USA) device was used in all TPE procedures. Vascular access was obtained with a double- or single-lumen catheter, usually placed in the femoral vein; peripheral veins were used for the return of blood in some cases. A central venous catheter was used in cases with inappropriate vasculature. During each TPE, one (rarely up to 2) estimated plasma volume was exchanged and replaced with a 30 g/I albumin added bicarbonate-based electrolyte solution. Anticoagulation during TPE was achieved with unfractionated heparin or trisodium citrate (either 4% or 15% solution; the protocol included intravenous calcium substitution). The plasma waste of a patient after TPE is shown in Figure 1.
Figure 1.

Plasma waste of a patient’s after TPE
Statistical analysis
The data were processed by the Statistical Package for Social Sciences, Version 17.0 (SPSS Inc.; Chicago, IL, USA). The Kolmogorov–Smirnov test was used to determine whether the distribution of variables was normal. Data were presented as a percent, mean±standard deviation, and median. The Mann–Whitney U-test and Kruskal–Wallis test were used for nonparametric tests for variables without normal distribution. A chi-squared test was performed to compare qualitative data; the descriptive statistics were shown (in %), and p-values less than 0.05 were considered as significant.
RESULTS
Regarding the patient data collected between 2007 and 2017, 43 (6.1%) of 705 patients diagnosed with acute pancreatitis had HTG-AP. All 10 patients who did not receive TPE had MAP with TG levels ranging between 500 mg/dL and 1000 mg /dL. No complications and mortality were determined. Overall, 33 of these patients underwent TPE; 26 of these patients were male (78.8%), and 7 were female (22.2%) with the mean age of 40.8±7.0 (range 28–50) years. Of all the patients, 17 were nonsmokers (51.4%), 8 were ex-smokers (24.3%), and 8 were current smokers (24.3%). Six (18.2%) patients reported a regular alcohol use, whereas other patients used no alcohol. Thyroid function tests were within the normal reference range for all patients. Hyperlipidemia associated with secondary hypothyroidism was not determined in any of the patients. Calcium (Ca) levels of all patients were within the normal reference range. High-level BUN-creatinine developed in 2 patients after AP. Overall, 16 patients (48.5%) had DM, whereas 4 patients (12.1%) had impaired glucose tolerance. Insulin infusion treatment was administered before TPE treatment to all 16 patients with DM. LMWH was given in prophylactic doses for venous thromboembolism. In total, 12 patients (36.4%) had a known history of hyperlipidemia, and 5 of these used fenofibrate 267 mg per day, whereas 7 used gemfibrozil 600 mg per day irregularly.
Eight patients (24.2%) experienced repeated AP events following TPE for the treatment of HTG-AP. All these patients had problems such as incompatibility related to drug use, drug withdrawal, and dietary guidelines. Among these, 2 patients had HTG, and AP occurred during pregnancy, 6 had DM. Overall, 25 patients (75.8%) showed no HTG-AP attacks throughout the mean follow-up period of 4.5±2.3 years (range 1–10 years) (Table 1). The time between the initiation of abdominal pain or hospital admission and plasma exchange procedure was 12±4 (range 6–24) hours on average.
Table 1.
Demographic data
| Parameter | Value |
|---|---|
| N | 33 |
| Gender | |
| • Male | 26 (78.8%) |
| • Female | 7 (21.2%) |
| Age (years) | 40.8±7.0 (range 28–50) |
| Diabetes Mellitus | 16 (48.5%) |
| Impaired glucose tolerance | 4 (12.1%) |
| Alcohol abuse | 6 (18.2%) |
| Pregnancy | 2 (6.1%) |
| Previously known HTG story | 12 (36.4%) |
| Recurrent HTG-AP | 8 (24.2%) |
| - Irregular drug use | 4 (100%) |
| - Diabetes mellitus | 3 (75%) |
| - Pregnancy | 2 (50%) |
N: number of patients; HTG: hypertriglyceridemia; HTG-AP: hypertriglyceridemia-induced acute pancreatitis
Therapeutic plasma exchange was well tolerated. Occurrence of vomiting in 5 patients, palpitation and tachycardia in 4 patients, and asymptomatic hypotension in 3 patients was observed. Two patients had hypervolemia, which was successfully treated with intravenous furosemide. Hemolysis was not detected in any of the patients. The occlusion of the catheter was seen in 1 patient. TPE was discontinued in none of these patients.
According to the 2012 Atlanta criteria, 20 (60.6%) patients had MAP, 10 (30.3%) patients had MSAP, and 3 (9.1%) patients had SAP (Table 2). Regarding local complications, the APFC was observed in 13 (39.4%) patients, acute necrotic collection was observed in 1 (3%) patient, walled-off necrosis was observed in 1 (3%) patient, and pancreatic pseudocyst was identified in no patient. Systemic complications were observed in 2 (6%) patients (Table 2). Renal failure occurred in 2 patients who had SAP. The complication of necrotizing pancreatitis occurred in 2 patients who had SAP; one of these patients underwent endoscopic necrosectomy (CT findings are shown in Figure 2), whereas the other died from pulmonary failure, cardiovascular failure, and sepsis. Only one session of TPE had been performed for both patients with necrotizing pancreatitis. Mortality rate was 33.3% in the SAP patients, and 0% in the MAP and MSAP patients. The total mortality rate was 3%.
Table 2.
Clinical course
| Parameter | Value |
|---|---|
| The time from initiation of | 12±4 (range 10–24) |
| abdominal pain to first TPE (hours) | |
| pre-TPE APACHE score | 3 (2–4.5) |
| post-TPE APACHE score | 1 (1–2) |
| Ranson score | 2.2±1.7 (range 0–8) |
| Grading of pancreatitis (Balthazar score) | 2.2±1.6 (range 1–9) |
| - Stage B | 2 (6.0%) |
| - Stage C | 16 (48.5%) |
| - Stage D | 12 (36.4%) |
| - Stage E | 3 (9.1%) |
| According to the 2012 Atlanta criteria | |
| - MAP | 20 (60.6%) |
| - MSAP | 8 (30.3%) |
| - SAP | 3 (9.1%) |
| Local Complication | |
| -APFC | 13 (39.4%) |
| - Pancreatic pseudocyst | 0 (0%) |
| - Acute necrotic collection | 1 (3%) |
| -Walled-off necrosis | 1 (3%) |
| Systemic Complications | 2 (6.0%) |
| Organ Failure | |
| - Renal failure | 2 (6.1%) |
| - Respiratory failure | 1 (3%) |
| - Cardiovascular failure | 1(3%) |
| Hospital mortality | 3% |
APACHE: Acute Physiology and Chronic Health Evaluation II; MAP: mild acute pancreatitis; MSAP: moderately severe acute pancreatitis; SAP: severe acute pancreatitis; APFC: acute peripancreatic fluid collection
Figure 2.
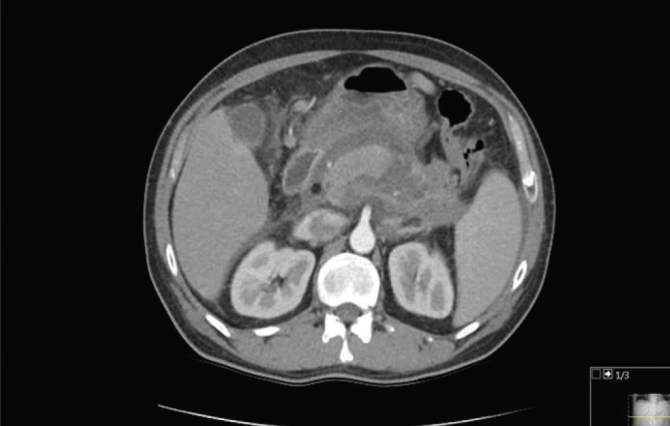
The CT findings
Regarding the mean pre- and post-TPE levels, TG was 1506 (1406–3615) mg/dL and 686 (442.5–1334.5) mg/dL with a decrease of 54.4%, and cholesterol was 529 (344.5–655) mg/dL and 253 (182–316) mg/dL with a decrease of 52.1%, respectively. Morever, when the mean amylase and lipase values determined before and 3 days after TPE were compared, amylase was 189.5 (70–528.5) U/L and 67.5 (35.7–163.5) U/L with a decrease of 64.4%, whereas lipase was 1597 (844–2482) U/L and 256 (158.5–521) U/L with a decrease of 84.9%, respectively (Table 3).
Table 3.
Comparison of serum lipids, amylase and lipase before and after therapeutic plasma exchange treatment in each patient
| Before TPE | After TPE | % reduction | p | |
|---|---|---|---|---|
| Triglyceride | 1506 [1406–3615] | 686 [442.5–1334.5] | ||
| (0–149 mg/dL) | (min–max: 1040–24293) | (min–max: 111–4241) | 54.4 | <0.001 |
| Total cholesterol | 529 [344.5–655] | 253 [182–316] | ||
| (0–149 mg/dL) | (min–max: 173–765) | (min–max: 134–458) | 52.1 | <0.001 |
| Amylase | 189.5 [70–528,5] | 67.5 [35.7–136.5 | ||
| (25–125 U/L) | (min–max: 33–1675) | ](range 13–1120 ) | 64.4 | 0.001 |
| Lipase | 1697 [844–2482] | 256 [158.5–521] | ||
| (8–78 U/L) | (min–max: 89–3770) | (min–max: 41–1614) | 84.9 | <0.001 |
N: number of patients; HTG: hypertriglyceridemia; HTG-AP: hypertriglyceridemia-induced acute pancreatitis
TG levels decreased below 1000 mg/dL with single TPE session in 27 patients (81.8%) and with 2 TPE sessions in 6 patients (18.2%).
The median pre-TPE APACHE score of the patients was 3 (2–4.5), whereas the median post-TPE APACHE score was 1 (1–2) (p<0.01). There was no correlation between the decrease in TG and pre- and post-APACHE scores.
The mean Ranson score was 2.2±1.7 (range 0–8). The mean Balthazar classification score based on abdominal CT of the patients was 2.2±1.6 (range 1–9). Overall, 15 (45.4%) patients had a Balthazar score of ≥3.
There was no significant difference determined between the CRP values obtained at the first 24 hours before TPE and 3 days after TPE.
DISCUSSION
Our series provide data on 33 patients who underwent plasmapheresis because of hypertriglyceridemia-induced acute pancreatitis. The majority of our patients were male (78.8%), and most were in their 30s and 40s. In total, 16 (48.5%) patients had DM. Regular alcohol consumption was relatively low (18.2%) among the patients. Overall, 12 patients (36.4%) had a known history of hyperlipidemia. TPE proved to be very effective, and TG levels decreased to <1000 mg/dL after one- or two-session TPE in all patients.
Severe hypertriglyceridemia is responsible for 1%–4% of AP cases (1). In our study, severe hyperlipidemia was responsible for 6.1% of the cases. The mortality rate was 3% in patients with interstitial edematous pancreatitis, whereas 17% in patients with pancreatic necrosis (11, 12).
Early clinical recognition of HTG-AP is highly important for the initiation of appropriate treatment and prevention of complications. Elevated TG levels and medical history of hypertriglyceridemia suggest the consideration of HTG as an etiological factor. Clinical findings, complications, and course of the disease in HTG-associated acute pancreatitis do not differ from non-HTG acute pancreatitis. Clinically, acute, recurrent, and rarely chronic pancreatitis can be diagnosed. Pathologically, however, pancreatitis can be edematous, hemorrhagic, and necrotizing. The optimal treatment of active HTG-AP is not completely clear. The standard treatment of AP is the restriction of oral intake, maintenance of intravenous hydration, and administration of pain medications. Furthermore, specific heparin and insulin treatments can be given and TPE to reduce TG levels can be performed (13). At the same time, renal functions should be observed. Thus, we carried out the conventional treatment and follow-up protocol for all our patients diagnosed with AP.
Since the first use of plasmapheresis in the treatment of HTG-AP in 1978 by Betteridge et al. (14), numerous reports and several case studies have been published revealing its efficacy (15,16). Plasmapheresis is indicated in AP patients displaying excessively elevated TG levels (TG>1000 mg/dL) (17). TPE rapidly reduces TG and total cholesterol levels; thus, it is recommended (18).
The beneficial effects of plasmapheresis are the removal of TGs, active enzymes, and inflammatory plasma mediators, as well as the supplementation of free fatty acids, apolipoproteins, and lipoprotein lipase from a healthy donor. Accessibility issues in many centers and its high cost limit the use of plasmapheresis. This leads to the most recently established guidelines prepared by the American Society for Apheresis, which considers HTG-AP as a Category-III indication (in the case of disorders for which the optimum role of apheresis therapy is not established, individualized decision is necessary) (19).
Most HTG cases are secondary, and they occur by the precipitating conditions affecting lipid metabolism (e.g., DM, excessive alcohol consumption, hypothyroidism, pregnancy, or some medications such as steroids, estrogen, and tamoxifen) (20).
In our study group, some patients displayed secondary risk factors that can potentially precipitate HTG (DM in 16 patients [48.5%], impaired glucose tolerance in 4 patients [12.1%], excessive alcohol consumption in 6 patients [18.2%], and pregnancy in 2 patients [6.1%]). In the follow-up period, 8 patients (24.2%) manifested a recurrence of HTG-AP. The evaluation of these patients revealed their lack of compliance with the given diet program and fibrate treatment. Overall, 6 patients had DM, and 2 patients were pregnant. A lack of compliance with the treatment and diet plan as well as the presence of DM and pregnancy may be significant risk factors for the recurrence of HTG-AP.
In a larger retrospective study, Chen et al. (21) divided their patients with HTG-AP into 2 groups, those who had undergone plasma exchange and those who had not. They compared the mortality and morbidity rates; however, the comparison revealed no significant difference between these 2 groups. Nonetheless, the authors stated that the apheresis time could be a critical point. In their study involving TPE implementation, Gubensek et al. (22) found that TPE reduced serum triglycerides faster and more effectively than it could be expected with conservative treatment. The delay in TPE therapy had no influence on survival rates. They found no correlation between TG levels and severity of the disease (i.e., APACHE).
However, in a few reports, the implementation of apheresis as soon as possible was shown to maximize the reduction of mortality/morbidity rates and to decrease the duration of hospital stay (23,24).
Performing a single plasma exchange session can reduce the serum TG level by 49%–80%; however, 2 or more sessions may be required in some patients to acquire a TG level lower than 1000 mg/dL. Yeh et al. (18) and Lennertz et al. (25) achieved improvement in their clinical and laboratory results with TG levels decreasing to 70% in one session. In their study, Kadikoylu et al. (26) obtained a TG reduction of 48% in a session, whereas Gavva et al. (24) found a reduction of 84% in TG within a few hours following one single session of TPE. Similarly, in our study, one TPE session decreased the TG level by 54.4% and total cholesterol level by 52.1%. After the second TPE session, the decrease in TG level was 79.4%. The TG level decreased below 1000 mg/dL after one single session in 27 (81.8%) patients and after 2 sessions in 6 patients (18.2%). There was no significant difference between the pre- and post-TPE levels of the inflammation marker, CRP. However, a significant difference between the pre- and post-APACHE scores was shown (p<0.01). In a study by Nakhoda et al. (27), although TG levels were quickly reduced by TPE, there was no statistically significant difference between the pre- and post-TPE APACHE scores.
Some centers use plasmapheresis only in patients with severe HTG-AP, whereas others use it in patients with a low APACHE score (28,29). In a study by Ramírez-Bueno et al. (28), the TG level was reduced to <1000 mg/dL after one TPE session in 8 out of 11 patients, and death occurred in 3 patients with a poor severity index. In study by Gubensek et al. (29), the mortality rate was determined as 4% in patients with moderate pancreatitis (APACHE score<8) and as 42% in patients with severe pancreatitis (APACHE score≥8). The overall mortality rate was reported as 15%. Kyriakidis et al. (30) reported that 8 of 10 patients with HTG-AP who had undergone TPE within 48 hours were successfully treated, whereas 2 of them were operated because of the infection of the necrotic segments, and one of them was lost. In the study by He et al. (31), in which they compared the patients treated with high-volume hemofiltration (HVHF) or LMWH combined with insulin (LMWH-insulin), HVHF was found to reduce TGs more effectively than the LMWH+insulin treatment. However, no differences were found between the 2 treatments regarding local pancreatic complications (p>0.05), the requirement of surgical intervention (p=0.49), mortality rate (p=0.49), and the duration of hospitalization (p=0.144).
Moderately severe and SAP were observed in 91 (63.1%) patients in the study conducted by Wang et al. (32), in which 144 HTG-AP patients were managed by conservative treatment only. Regarding local complications, APFC was observed in 66 (45.8%) patients, acute necrotic collections were observed in 13 (9%) patients, and pancreatic pseudocyst was observed in 5 (3.5%) patients. Systemic complications were observed in 6 (4.2%) patients. Mortality was observed in 8 (5.5%) patients. Patients were divided and compared according to the TG level as less and more than 2648. The ratios of MSAP and SAP were 50% in patients with TG levels below 2648 and 74.3% in patients with TG levels above 2648 (p=0.004). The mortality rate was 1.5% in patients with TG levels below 2648 and 8.4% in patients with TG levels above 2648 (p=0.07) (32).
We included all patients regardless of their severity of AP. In all patients, apheresis was performed for 12±4 hours on average (range 6–24 hours), following admission and appropriate clinical diagnosis. Overall, 20 patients (60.6%) had MAP, 10 (30.3%) had MSAP, and 3 (9.1%) had SAP. APFC was observed in 13 (39.4%) patients, acute necrotic collection was observed in 1 (3%) patient, walled-off necrosis was observed in 1 (3%) patient, and pancreatic pseudocyst was not observed in any patient. Acute necrotic collections developed in 2 of 3 patients displaying SAP. One of them died from respiratory failure, cardiovascular failure, and sepsis. The other patient was successfully treated with endoscopic necrosectomy. The complication of renal insufficiency occurred in 2 of the patients who had SAP. No complications or mortality were encountered in the patients with MAP diagnosis. Mortality rate was 33.3% in SAP patients; the overall mortality rate was 3% in our study group. When compared with Wang et al.’s (32) study, the ratio of MSAP and SAP was 39.4% in our TPE treatment group and 66.1% in the conservative treatment group; the mortality rates were 3% and 5%, respectively. Stefanutti et al. (33) evaluated the therapeutic efficacy of TPE in patients with HTG, and 17 patients who had not responded to conventional medical therapy (fat-free diet plus pharmacological treatment) were referred for TPE in a multicenter case series study. The removal of TG-rich lipoproteins prevented relapses of AP (15). According to that experience, it was said that the outcome of each case might be altered depending on the initiation time of the TPE treatment (33). TPE therapy should be continued until TG levels decrease below <500 mg/dL (3). We performed TPE in one or 2 sessions until the TG level was reduced below 1000 mg/dL because of the high costs.
The limitation of our study is that we had no case with pancreatitis due to hyperlipidemia not treated by TPE as control group.
The HTG-AP treatment involves the restriction of oral intake, maintenance of intravenous hydration and administration of pain medication, insulin treatment in patients with plasma glucose level of >150 mg/dL, LMWH, and TPE. A lipid-lowering diet (fat-free diet) and drugs (fenofibrate, gemfibrozil, and omega-3-fatty acid) are utilized in reducing HTG. TPE is a safe and helpful therapeutic treatment method for patients with HTG-AP. Potential candidates for apheresis may be the patients with predicted severe or severe AP who continue to have TG levels greater than 1000 mg/dL after the first 24–48 hours. Further studies that compare the apheresis+conservative treatment and only conservative treatment in the HTG-AP patients are required.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of the Adnan Menderes University School of Medicine (Decision Date: October 27, 2017; Decision No: #1253).
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - A.K., İ.Y.; Design - A.K., İ.Y.; Supervision - G.K., Z.B., M.Ü., M.H.Y.; Materials - İ.Y., Z.B.; Data Collection and/or Processing - M.Ü., M.H.Y.; Analysis and/or Interpretation - M.Ü., A.C.; Literature Search - A.C., A.D.; Writing Manuscript - A.K., A.C.; Critical Reviews - G.K., Z.B.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Fortson MR, Freedman SN, Webster PD., 3rd Clinical assessment of hyperlipidemic pancreatitis. Am J Gastroenterol. 1995;90:2134–9. [PubMed] [Google Scholar]
- 2.Deng LH, Xue P, Xia Q, Yang XN, Wan MH. Effect of admission hypertriglyceridemia on the episodes of severe acute pancreatitis. World J Gastroenterol. 2008;14:4558–61. doi: 10.3748/wjg.14.4558. https://doi.org/10.3748/wjg.14.4558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stefanutti C, Labbadia G, Morozzi C. Severe hypertriglyceridemia-related acute pancreatitis. Ther Apher Dial. 2013;17:130–7. doi: 10.1111/1744-9987.12008. https://doi.org/10.1111/1744-9987.12063 [DOI] [PubMed] [Google Scholar]
- 4.Berglund L, Brunzell JD, Goldberg AC, Goldberg IJ, Stalenhoef A. Treatment options for hypertriglyceridemia: from risk reduction to pancreatitis. Best Pract Res Clin Endocrinol Metab. 2014;28:423–37. doi: 10.1016/j.beem.2013.10.002. https://doi.org/10.1016/j.beem.2013.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunzell JD, Schrott HG. The interaction of familial and secondary causes of hypertriglyceridemia: role in pancreatitis. J Clin Lipidol. 2012;6:409–12. doi: 10.1016/j.jacl.2012.06.005. https://doi.org/10.1016/j.jacl.2012.06.005 [DOI] [PubMed] [Google Scholar]
- 6.Halangk W, Lerch MM, Brandt-Nedelev B, et al. Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. J Clin Invest. 2000;106:773–81. doi: 10.1172/JCI9411. https://doi.org/10.1172/JCI9411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimura W, Mossner J. Role of hypertriglyceridemia in the pathogenesis of experimental acute pancreatitis in rats. Int J Pancreatol. 1996;20:177–84. doi: 10.1007/BF02803766. https://doi.org/10.1007/BF02803766 [DOI] [PubMed] [Google Scholar]
- 8.Zeng Y, Wang X, Zhang W, Wu K, Ma J. Hypertriglyceridemia aggravates ER stress and pathogenesis of acute pancreatitis. Hepatogastroenterology. 2012;59:2318–26. doi: 10.5754/hge12042. https://doi.org/10.5754/hge12042 [DOI] [PubMed] [Google Scholar]
- 9.Click B, Ketchum AM, Turner R, Whitcomb DC, Papachristou GI, Yadav D. The role of apheresis in hypertriglyceridemia-induced acute pancreatitis: A systematic review. Pancreatology. 2015;15:313–20. doi: 10.1016/j.pan.2015.02.010. https://doi.org/10.1016/j.pan.2015.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis-2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–11. doi: 10.1136/gutjnl-2012-302779. https://doi.org/10.1136/gutjnl-2012-302779 [DOI] [PubMed] [Google Scholar]
- 11.Singh VK, Bollen TL, Wu BU, et al. An assessment of the severity of interstitial pancreatitis. Clin Gastroenterol Hepatol. 2011;9:1098–103. doi: 10.1016/j.cgh.2011.08.026. https://doi.org/10.1016/j.cgh.2011.08.026 [DOI] [PubMed] [Google Scholar]
- 12.van Santvoort HC, Bakker OJ, Bollen TL, et al. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology. 2011;141:1254–63. doi: 10.1053/j.gastro.2011.06.073. https://doi.org/10.1053/j.gastro.2011.06.073 [DOI] [PubMed] [Google Scholar]
- 13.Tsuang W, Navaneethan U, Ruiz L, Palascak JB, Gelrud A. Hypertriglyceridemic pancreatitis: presentation and management. Am J Gastroenterol. 2009;104:984–91. doi: 10.1038/ajg.2009.27. https://doi.org/10.1038/ajg.2009.27 [DOI] [PubMed] [Google Scholar]
- 14.Betteridge DJ, Bakowski M, Taylor KG, Reckless JP, de Silva SR, Galton DJ. Treatment of severe diabetic hypertriglyceridaemia by plasma exchange. Lancet. 1978;1:1368. doi: 10.1016/s0140-6736(78)92450-9. https://doi.org/10.1016/S0140-6736(78)92450-9 [DOI] [PubMed] [Google Scholar]
- 15.Stefanutti C, Di Giacomo S, Vivenzio A, et al. Therapeutic plasma exchange in patients with severe hypertriglyceridemia: a multicenter study. Artif Organs. 2009;33:1096–102. doi: 10.1111/j.1525-1594.2009.00810.x. https://doi.org/10.1111/j.1525-1594.2009.00810.x [DOI] [PubMed] [Google Scholar]
- 16.Syed H, Bilusic M, Rhondla C, Tavaria A. Plasmapheresis in the treatment of hypertriglyceridemia-induced pancreatitis: A community hospital’s experience. J Clin Apher. 2010;25:229–34. doi: 10.1002/jca.20232. https://doi.org/10.1002/jca.20232 [DOI] [PubMed] [Google Scholar]
- 17.Ewald N, Kloer HU. Severe hypertriglyceridemia: an indication for apheresis? Atheroscler Suppl. 2009;10:49–52. doi: 10.1016/S1567-5688(09)71810-0. https://doi.org/10.1016/S1567-5688(09)71810-0 [DOI] [PubMed] [Google Scholar]
- 18.Yeh JH, Chen JH, Chiu HC. Plasmapheresis for hyperlipidemic pancreatitis. J Clin Apher. 2003;18:181–5. doi: 10.1002/jca.10063. https://doi.org/10.1002/jca.10063 [DOI] [PubMed] [Google Scholar]
- 19.Schwartz J, Padmanabhan A, Aqui N, et al. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice-Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Seventh Special Issue. J Clin Apher. 2016;31:149–62. doi: 10.1002/jca.21470. https://doi.org/10.1002/jca.21470_c [DOI] [PubMed] [Google Scholar]
- 20.Valdivielso P, Ramirez-Bueno A, Ewald N. Current knowledge of hypertriglyceridemic pancreatitis. Eur J Intern Med. 2014;25:689–94. doi: 10.1016/j.ejim.2014.08.008. https://doi.org/10.1016/j.ejim.2014.08.008 [DOI] [PubMed] [Google Scholar]
- 21.Chen JH, Yeh JH, Lai HW, Liao CS. Therapeutic plasma exchange in patients with hyperlipidemic pancreatitis. World J Gastroenterol. 2004;10:2272–4. doi: 10.3748/wjg.v10.i15.2272. https://doi.org/10.3748/wjg.v10.i15.2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gubensek J, Buturovic-Ponikvar J, Romozi K, Ponikvar R. Factors affecting outcome in acute hypertriglyceridemic pancreatitis treated with plasma exchange: an observational cohort study. PLoS One. 2014;9:e102748. doi: 10.1371/journal.pone.0102748. https://doi.org/10.1371/journal.pone.0102748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furuya T, Komatsu M, Takahashi K, Hashimoto N, Hashizume T, Wajima N, et al. Plasma exchange for hypertriglyceridemic acute necrotizing pancreatitis: report of two cases. Ther Apher. 2002;6:454–8. doi: 10.1046/j.1526-0968.2002.00461.x. https://doi.org/10.1046/j.1526-0968.2002.00461.x [DOI] [PubMed] [Google Scholar]
- 24.Gavva C, Sarode R, Agrawal D, Burner J. Therapeutic plasma exchange for hypertriglyceridemia induced pancreatitis: A rapid and practical approach. Transfus Apher Sci. 2016;54:99–102. doi: 10.1016/j.transci.2016.02.001. https://doi.org/10.1016/j.transci.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 25.Lennertz A, Parhofer KG, Samtleben W, Bosch T. Therapeutic plasma exchange in patients with chylomicronemia syndrome complicated by acute pancreatitis. Ther Apher. 1999;3:227–33. doi: 10.1046/j.1526-0968.1999.00158.x. https://doi.org/10.1046/j.1526-0968.1999.00158.x [DOI] [PubMed] [Google Scholar]
- 26.Kadikoylu G, Yavasoglu I, Bolaman Z. Plasma exchange in severe hypertriglyceridemia a clinical study. Transfus Apher Sci. 2006;34:253–7. doi: 10.1016/j.transci.2005.11.009. https://doi.org/10.1016/j.transci.2005.11.009 [DOI] [PubMed] [Google Scholar]
- 27.Nakhoda S, Zimrin AB, Baer MR, Law JY. Use of the APACHE II score to assess impact of therapeutic plasma exchange for critically ill patients with hypertriglyceride-induced pancreatitis. Transfus Apher Sci. 2017;56:123–6. doi: 10.1016/j.transci.2016.10.005. https://doi.org/10.1016/j.transci.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 28.Ramirez-Bueno A, Salazar-Ramirez C, Cota-Delgado F, de la Torre-Prados MV, Valdivielso P. Plasmapheresis as treatment for hyperlipidemic pancreatitis. Eur J Intern Med. 2014;25:160–3. doi: 10.1016/j.ejim.2013.08.701. https://doi.org/10.1016/j.ejim.2013.08.701 [DOI] [PubMed] [Google Scholar]
- 29.Gubensek J, Buturovic-Ponikvar J, et al. Treatment of hyperlipidemic acute pancreatitis with plasma exchange: a single-center experience. Ther Apher Dial. 2009;13:314–7. doi: 10.1111/j.1744-9987.2009.00731.x. https://doi.org/10.1111/j.1744-9987.2009.00731.x [DOI] [PubMed] [Google Scholar]
- 30.Kyriakidis AV, Raitsiou B, Sakagianni A, et al. Management of acute severe hyperlipidemic pancreatitis. Digestion. 2006;73:259–64. doi: 10.1159/000095425. https://doi.org/10.1159/000095425 [DOI] [PubMed] [Google Scholar]
- 31.He WH, Yu M, Zhu Y, et al. Emergent Triglyceride-lowering Therapy With Early High-volume Hemofiltration Against Low-Molecular-Weight Heparin Combined With Insulin in Hypertriglyceridemic Pancreatitis: A Prospective Randomized Controlled Trial. J Clin Gastroenterol. 2016;50:772–8. doi: 10.1097/MCG.0000000000000552. https://doi.org/10.1097/MCG.0000000000000552 [DOI] [PubMed] [Google Scholar]
- 32.Wang SH, Chou YC, Shangkuan WC, Wei KY, Pan YH, Lin HC. Relationship between Plasma Triglyceride Level and Severity of Hypertriglyceridemic Pancreatitis. PLoS One. 2016;11:e0163984. doi: 10.1371/journal.pone.0163984. https://doi.org/10.1371/journal.pone.0163984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stefanutti C, Di Giacomo S, Labbadia G. Timing clinical events in the treatment of pancreatitis and hypertriglyceridemia with therapeutic plasmapheresis. Transfus Apher Sci. 2011;45:3–7. doi: 10.1016/j.transci.2011.06.013. https://doi.org/10.1016/j.transci.2011.06.013 [DOI] [PubMed] [Google Scholar]


