Abstract
Background/Aims
Non-alcoholic fatty liver disease (NAFLD) is a growing public health problem worldwide and is associated with increased morbidity and mortality. Currently, there is no definitive treatment for this disease. δ-Tocotrienol has potent anti-inflammatory and antioxidant properties and may reduce liver injury in NAFLD. The present study aims to evaluate the efficacy and safety of δ-tocotrienol in the treatment of NAFLD.
Materials and Methods
The present study was a randomized, double-blind, placebo-controlled pilot study conducted in patients aged >20 years, belonging to both sexes, having ultrasound-proven fatty liver disease, having a fatty liver index (FLI) of ≥60, and persistent elevation of alanine transaminase. A total of 71 patients were assigned to receive either oral δ-tocotrienol (n=35, 300 mg twice daily) or placebo (n=36) for 12 weeks. At the baseline and at the end of the study, clinical and biochemical parameters, including lipid profile, liver function tests, high-sensitivity C-reactive protein (hs-CRP), and malondialdehyde (MDA) were measured. Body mass index and FLI were calculated, and ultrasound grading of hepatic steatosis was performed.
Results
Out of 71 enrolled patients, 64 patients, 31 in the δ-tocotrienol group and 33 in the placebo group, completed the study. After 12 weeks of supplementation, δ-tocotrienol showed greater efficacy than placebo by decreasing serum aminotransferases, hs-CRP, MDA, and FLI score (p<0.001). However, it did not improve hepatic steatosis on ultrasound examination. No adverse effects were reported.
Conclusion
δ-Tocotrienol was safe, and it effectively improved aminotransferase levels and inflammatory and oxidative stress markers in patients with NAFLD. Large-scale randomized clinical trials are warranted to further support these findings.
Keywords: δ-Tocotrienol, non-alcoholic fatty liver disease, aminotransferase, C-reactive protein, malondialdehyde, fatty liver index
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) represents a clinico-histopathological spectrum of liver disorders characterized by histological features resembling alcoholic liver disease. However, by definition, NAFLD occurs in patients with little or no history of alcohol intake (threshold <30 g/day in men and <20 g/day in women). Alcohol consumption above these limits indicates alcoholic liver disease (1). NAFLD is now recognized as a leading cause of liver-related morbidity and mortality worldwide. Its prevalence is likely to increase over time owing to the epidemics of obesity and diabetes. In addition to the development of fatty liver, NAFLD may progress to hepatocellular injury, inflammation, and necrosis, which are known as non-alcoholic steatohepatitis (NASH). NASH may progress to fibrosis, cirrhosis, and hepatocellular carcinoma. It is expected to surpass hepatitis C virus infection as the leading cause of cirrhosis and hepatocellular carcinoma in a decade or so (2).
Lifestyle modifications, including reducing weight, eating a healthy diet, and performing regular physical activity, are commonly recommended for treating NAFLD. However, this approach alone has not been able to decelerate the increasing prevalence of the disease. Currently, there is no definitive treatment for NAFLD, and the need to find appropriate therapy now is more urgent than ever before (2).
Liver biopsy is the current gold standard for NAFLD diagnosis. However, it is an invasive and costly procedure and is impractical owing to high disease prevalence. It is highly operator-dependent, is associated with sampling error, and major complications occur in 0.1%–2.3% of cases. Thus, this method is unsuitable for screening and follow-up of patients with NAFLD (3). Alternately, non-invasive diagnostic methods, e.g., liver ultrasonography (USG) and fatty liver index (FLI), have been developed to determine liver fat content (4). Several studies have shown that USG has adequate sensitivity and specificity for detecting hepatosteatosis; the sensitivity and specificity have been found to be 60%–94% and 84%–95%, respectively (3,4). FLI is a clinical and metabolic parameter of liver fat content, which correlates with liver USG. It is a validated algorithm derived from body mass index (BMI), waist circumference (WC), triglycerides (TG), and gamma-glutamyl transferase (γ-GT). It has an accuracy of 0.84 (95% CI: 0.81–0.87) for non-invasive detection of hepatosteatosis (4,5).
Oxidative stress and inflammation are documented factors in the pathogenesis of NAFLD. Vitamin E has potent anti-inflammatory and antioxidant properties and may reduce liver injury in NAFLD. It is composed of eight isoforms: four tocopherols (alpha, beta, gamma, and delta) and four tocotrienols (alpha, beta, gamma, and delta). Tocopherols and tocotrienols have a saturated and an unsaturated side chain, respectively. The unsaturated side chain of tocotrienol allows for more efficient penetration into the tissues with saturated fat layers, such as liver (6). Comparative studies between tocopherols and tocotrienols showed that tocotrienols are significantly more potent than tocopherols against inflammation and oxidative stress. In a study, δ-tocotrienol is the most potent isoform of vitamin E (7). A recent study has revealed that δ-tocotrienol has better bioavailability when supplemented at higher doses (8). To date, no data is available on human intervention studies involving supplementation of δ-tocotrienol in NAFLD (9). We conducted this pilot study using high dose (600 mg/day) δ-tocotrienol to evaluate its efficacy and safety in the treatment of NAFLD.
MATERIALS AND METHODS
Study design
This double-blind, randomized-controlled pilot study was conducted at a tertiary care institute between October 2015 and March 2016. The study conformed to the Declaration of Helsinki and was approved by the institutional ethical review committee. All patients provided written informed consent before enrolment.
Selection of participants
The inclusion criteria for enrolling patients were as follows: >20 years of age, belonging to both sexes, having ultrasound-proven fatty liver, FLI of ≥60, and mild to moderate persistent elevation of alanine transaminase (ALT), that is, not greater than four times the upper limit of 42 IU/L (10). Patients with chronic hepatitis B or C, alcoholic liver disease, autoimmune liver disease, hemochromatosis, Wilson’s disease, and those receiving medications, such as hepatotoxic, lipid lowering, herbal, or vitamin supplements, were excluded. Criteria used for exclusion of alcoholic liver disease was a history of an average alcohol consumption of >30 g/day in men and >20 g/day in women, either currently or for a period of more than three consecutive months in 2 years prior to screening (1). As the present study was conducted in Pakistan, which is a Muslim country where alcohol consumption rate is very low, none of the participants enrolled in the present study had a history of alcohol consumption.
Randomization and masking
Patients were randomly drawn and assigned to δ-tocotrienol and placebo group. The same opaque capsules containing either δ-tocotrienol or placebo (sucrose) were administered to the patients by a research assistant blinded to the contents of the capsules. Our δ-tocotrienol capsules (American River Nutrition, Inc., Hadley, MA, USA) were extracted from annatto seeds according to the present standard procedures and consisted of 90% δ-tocotrienol and 10% γ-tocotrienol. The patients were asked to take one capsule containing 300 mg of either δ-tocotrienol or identically matched placebo twice daily for 12 weeks. The patients were advised to eat a fat-reduced diet and perform regular physical activity.
Procedure
All patients underwent a complete work-up consisting of a clinical examination, anthropometric measurements, laboratory analysis, and a liver USG.
Clinical assessment
Personal characteristics, including demographic and disease history, were obtained. Anthropometric measurements, such as weight, height, and WC, were obtained using standard protocols. Systolic and diastolic blood pressure was measured using an electronic blood pressure manometer. BMI was calculated as weight (kg) divided by the square of height (m).
Laboratory analysis
Laboratory test results obtained as part of screening included tests for hepatitis B and C, antinuclear antibody, anti-mitochondrial antibody, serum ferritin, ceruloplasmin, fasting serum glucose, complete blood count, serum ALT, aspartate transaminase (AST), γ-GT, alkaline phosphatase (ALP), total bilirubin, albumin, urea, creatinine, uric acid, and fasting lipid profile. Then, blood samples were extracted after 12–14 h of overnight fasting at baseline and at the end of intervention. The samples were centrifuged at 1500 ×g for 15 min to separate the serum. Serum was stored at −80 °C until analysis at the end of the trial to avoid analytical variation.
Serum ALT, AST, γ-GT, ALP, and TG were measured by ADVIA® 1800 (Siemens, Tarrytown, NY, USA). Serum high-sensitivity C-reactive protein (hs-CRP) was measured by chemiluminescence assay using IMMULITE® 1000 (Siemens, Tarrytown, NY, USA). Serum malondialdehyde (MDA) was measured using thiobarbituric acid reactive substance assay kit (Cayman Chemical, Ann Arbor, MI, USA) on a microplate reader.
Ultrasonography and FLI evaluation
All patients underwent ultrasonography at baseline and at the end of intervention to assess the degree of liver steatosis by a single sonographist using Toshiba Xario™ 200 (Toshiba Medical Systems Corporation, Tokyo, Japan) equipped with a 3.0–5.0 MHz probe. Steatosis was scored semi-quantitatively on a scale of 0–3 (0, absent; 1, mild; 2, moderate; and 3, severe). Steatosis is graded on the basis of abnormally intense, high-level echoes arising from the liver parenchyma, hepatorenal echodiscrepancy, echo penetration into the deeper portion of the liver, and portal vein wall clarity in accordance with the methodology described by Saverymuttu et al. (11).
In addition, FLI was calculated in all subjects. FLI varies between 0 and 100; an index of <30 rules out and ≥60 rules in fatty liver (4,5). FLI is calculated by using the software “The Medical Algorithms” (The Medical Algorithms Company, TX, USA) (12).
Outcomes
The outcome measures included mean reduction in ALT, AST, hs-CRP, MDA, and FLI score and improvement in hepatic steatosis grade on USG from baseline to the end of intervention. Consults were performed every 2 weeks from baseline to the end of the study in order to identify and treat any potential adverse effects.
Statistical analysis
The sample size was selected based on Teare et al. (13) and Whitehead et al. (14) who recommend a sample size of 70 for a pilot randomized trial. Statistical analyzes were performed using the Statistical Package for Social Sciences (SPSS) software version 21.0 (IBM Corp.; Armonk, NY, USA). Normality of continuous variables was tested by Kolmogorov-Smirnov test. Data are expressed as mean±SD for normally distributed variables. Mean reduction in the study parameters over the trial period was calculated by after intervention value minus the baseline value. Differences in the means of continuous variables between the two groups were tested using analysis of covariance with pretest (baseline) value as covariate and post-test value as outcome. Within the groups, differences in the means were analyzed using a paired t-test. A p-value of <0.05 was considered statistically significant, and all reported p-values are two-tailed.
RESULTS
Demographics and baseline characteristics
Of a total of 109 screened patients, 71 patients met the eligibility criteria, consented to inclusion, were enrolled in the study, and randomly assigned: 35 to δ-tocotrienol and 36 to placebo. Of these, 64 patients completed the study (n=31 and n=33 for δ-tocotrienol and placebo groups, respectively) (Figure 1). Table 1 shows the baseline characteristics of the study participants. No significant between-group differences in these characteristics were found at baseline.
Figure 1.
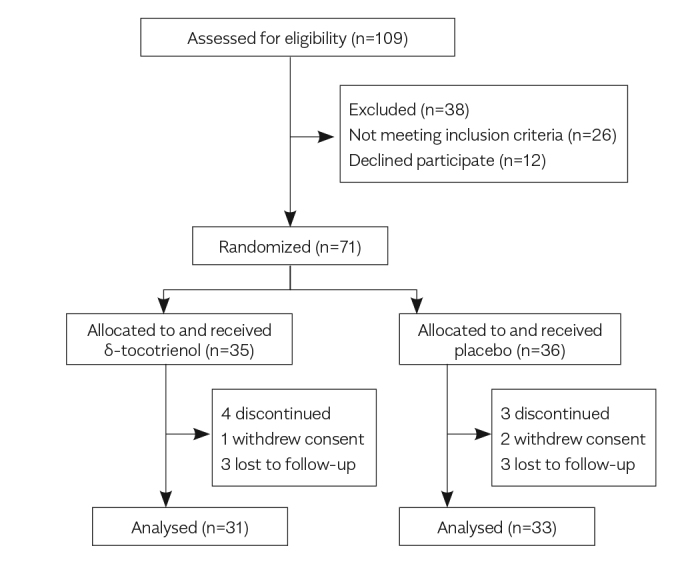
Trial flow diagram
Table 1.
Baseline characteristics of patients with NAFLD in each group
| Variable | Tocotrienol (n=31) | Placebo (n=33) | p |
|---|---|---|---|
| Sex (%) | |||
| Female | 16 (51.6) | 19 (57.6) | |
| Male | 15 (48.4) | 14 (42.4) | 0.410 |
| Age (years) | 44.7±8.8 | 43.9±9.2 | 0.713 |
| Height (m) | 1.68±0.068 | 1.66±0.068 | 0.437 |
| Weight (kg) | 86.93±13.4 | 87.2±12.9 | 0.937 |
| BMI (kg/m2) | 30.68±2.9 | 31.30±3.1 | 0.342 |
| WC (cm) | 100.2±3.4 | 99.96±3.5 | 0.767 |
| GGT (IU/L) | 51.45±9.2 | 49.66±9.2 | 0.440 |
| TG (mmol/L) | 2.43±0.48 | 2.46±0.45 | 0.775 |
| FLI | 84.58±8.46 | 85.42±8.9 | 0.663 |
| hs-CRP (mg/L) | 4.28±1.49 | 4.41±1.46 | 0.718 |
| MDA (μmol/L) | 6.36±1.47 | 6.25±1.45 | 0.752 |
| ALT (IU/L) | 83.61±18.1 | 82.36±18.2 | 0.784 |
| AST (IU/L) | 61.06±10.25 | 60.39±10.50 | 0.797 |
| ALP (IU/L) | 85.90±13.81 | 87.21±12.92 | 0.697 |
| Grading of hepatic steatosis n (%) | |||
| Mild | 14 (45.2) | 17 (51.5) | |
| Moderate | 13 (41.9) | 11 (33.3) | 0.388 |
| Severe | 4 (12.9) | 5 (15.2) | |
Data are expressed as mean±SD or number (%)
BMI: body mass index; WC: waist circumference; GGT: gamma-glutamyl transferase; TG: triglycerides; FLI: fatty liver index; hs-CRP: high-sensitivity C-reactive protein; MDA: malondialdehyde; ALT: alanine aminotransferase; AST: aspartate aminotransferase; ALP: alkaline phosphatase; NAFLD, non-alcoholic fatty liver disease
Statistical analysis was performed by independent t-test or chi-square test
Efficacy assessment
Within-group comparison of serum biochemical levels and metabolic factors showed that ALT, AST, hs-CRP, MDA, and FLI scores decreased in both groups after 12 weeks of intervention. However, the mean reduction in the δ-tocotrienol group was highly significant (p<0.001) for all these parameters (Table 2, Figure 2 and 3). Between-group comparison revealed that the mean reduction in ALT, AST, hs-CRP, MDA, and FLI score in the δ-tocotrienol group was significantly greater than that in the placebo group (p<0.001 for all) (Table 3, Figure 2 and 3). However, we did not observe any improvement in the degree/grade of hepatic steatosis following USG at the end of intervention.
Table 2.
Within-group comparison of serum biochemical levels and metabolic factors
| δ-tocotrienol (n=31) | Placebo (n=33) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Variable | Before | After | p | Before | After | p |
| Weight (kg) | 86.93±13.4 | 82.52±12.8 | <0.001 | 87.2±12.9 | 85.2±11.6 | 0.008 |
| BMI (kg/m2) | 30.68±2.9 | 29.20±2.6 | <0.001 | 31.30±3.1 | 30.27±2.9 | 0.006 |
| WC (cm) | 100.2±3.4 | 97.98±3.2 | <0.001 | 99.96±3.5 | 99.04±3.1 | 0.013 |
| GGT (IU/L) | 51.45±9.2 | 43.33±9.6 | <0.001 | 49.66±9.2 | 46.08±8.7 | 0.002 |
| TG (mmol/L) | 2.43±0.48 | 2.19±0.44 | <0.001 | 2.46±0.45 | 2.34±0.43 | 0.001 |
| FLI | 84.58±8.46 | 75.16±12.19 | <0.001 | 85.42±8.9 | 81.34±10.44 | <0.001 |
| hs-CRP (mg/L) | 4.28±1.49 | 3.51±1.37 | <0.001 | 4.41±1.46 | 4.16±1.42 | 0.006 |
| MDA (μmol/L) | 6.36±1.47 | 5.45±1.31 | <0.001 | 6.25±1.45 | 5.90±1.37 | 0.003 |
| ALT (IU/L) | 83.61±18.1 | 70.56±17.54 | <0.001 | 82.36±18.2 | 78.70±17.81 | 0.001 |
| AST (IU/L) | 61.06±10.25 | 52.16±9.76 | <0.001 | 60.39±10.5 | 56.55±10.34 | 0.001 |
| ALP (IU/L) | 85.90±13.81 | 80.06±13.87 | <0.001 | 87.21±12.9 | 83.61±13.56 | 0.001 |
Data are expressed as mean±SD
BMI: body mass index; WC: waist circumference; GGT: gamma-glutamyl transferase; TG: triglycerides; FLI: fatty liver index; hs-CRP: high-sensitivity C-reactive protein; MDA: malondialdehyde; ALT: alanine aminotransferase; AST: aspartate aminotransferase; ALP: alkaline phosphatase
Statistical analysis was performed by paired t-test
Figure 2.
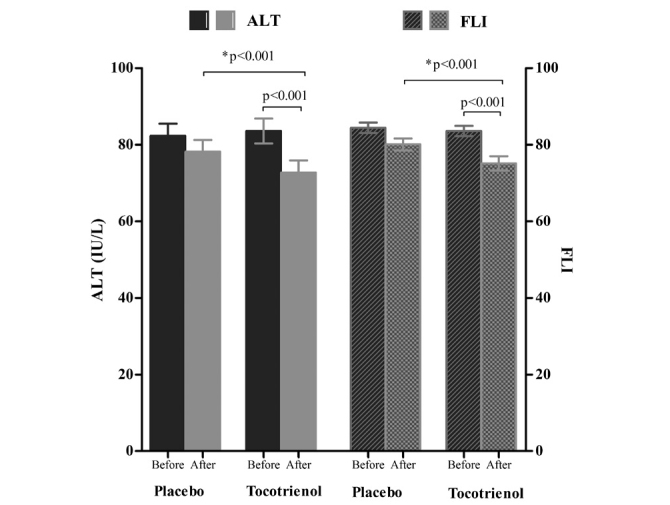
Serum alanine transaminase (ALT) and fatty liver index (FLI) before and after intervention. p<0.001 for comparison of baseline value to the 12 week value in the δ-tocotrienol group; *p<0.001 for comparison of the δ-tocotrienol group with the placebo group at the end of intervention; T bars denote standard error of means (SEM)
Figure 3.
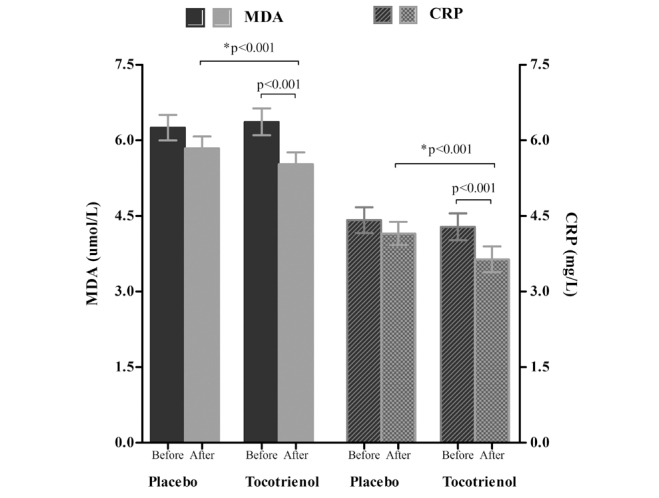
High-sensitivity C-reactive protein (hs-CRP) and malondialdehyde (MDA) before and after intervention; p<0.001 for comparison of baseline value to week 12 value in the δ-tocotrienol group; *p<0.001 for comparison of the δ-tocotrienol group with the placebo group at the end of intervention; T bars denote standard error of means (SEM)
Table 3.
Between-group comparison of serum biochemical levels and metabolic factors
| δ-tocotrienol (n=31) | Placebo (n=33) | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Variable | Before | After | Mean difference (95% CI) | Before | After | Mean difference (95% CI) | p |
| Weight (kg) | 86.93±13.4 | 82.52±12.8 | −4.52 (−4.78 to −4.25) | 87.2±12.9 | 85.2±11.6 | −1.94 (−2.21 to −1.58) | <0.001 |
| BMI (kg/m2) | 30.68±2.9 | 29.20±2.6 | −1.50 (−1.67 to −1.38) | 31.30± 3.1 | 30.27± 2.9 | −0.99 (−1.16 to −0.83) | <0.001 |
| WC (cm) | 100.2±3.4 | 97.8±3.2 | −2.34 (−2.62 to −2.13) | 99.96±3.5 | 99.04±3.1 | −0.90 (−1.28 to −0.77) | <0.001 |
| GGT (IU/L) | 51.45±9.2 | 42.33±9.6 | −9.02 (−9.61 to −8.33) | 49.66±9.2 | 46.08±8.7 | −3.42 (−3.99 to −3.15) | <0.001 |
| TG (mmol/L) | 2.43±0.48 | 2.19±0.44 | −0.24 (−0.28 to −0.21) | 2.46±0.45 | 2.34±0.43 | −0.12 (−0.16 to −0.10) | <0.001 |
| FLI | 84.58±8.46 | 75.16±12.19 | −9.41 (−10.57 to −8.26) | 85.42±8.9 | 81.34±10.44 | −4.04 (−5.04 to −3.29) | <0.001 |
| hs-CRP (mg/L) | 4.28±1.49 | 3.51±1.37 | −0.74 (−0.85 to −0.64) | 4.41±1.46 | 4.16±1.42 | −0.26 (−0.37 to −0.16) | <0.001 |
| MDA (μmol/L) | 6.36±1.47 | 5.45±1.31 | −0.89 (−0.97 to −0.81) | 6.25±1.45 | 5.90±1.37 | −0.33 (−0.45 to −0.26) | <0.001 |
| ALT (IU/L) | 83.61±18.1 | 70.56±17.54 | −13.0 (−13.96 to −11.83) | 82.36±18.2 | 78.70±17.81 | −3.62 (−5.09 to −3.08) | <0.001 |
| AST (IU/L) | 61.06±10.25 | 52.16±9.76 | −8.77 (−9.21 to −7.13) | 60.39±10.5 | 56.55±10.34 | −3.67 (−5.25 to −3.29) | <0.001 |
| ALP (IU/L) | 85.90±13.81 | 80.06±13.87 | −5.83 (−6.52 to −5.15) | 87.21±12.9 | 83.61±13.56 | −3.56 (−4.36 to −2.84) | <0.001 |
Data are expressed as mean±SD.
BMI: body mass index; WC: waist circumference; GGT: gamma-glutamyl transferase; TG: triglycerides; FLI: fatty liver index; hs-CRP: high-sensitivity C-reactive protein; MDA: malondialdehyde; ALT: alanine aminotransferase; AST: aspartate aminotransferase; ALP: alkaline phosphatase
Statistical analysis was performed by analysis of covariance adjusted for baseline
Safety
The treatment was well tolerated, and no adverse events were reported.
DISCUSSION
The objective of the present study was to conduct a pilot clinical trial to evaluate the efficacy and safety of δ-tocotrienol in patients with NAFLD. The results from this 12-week pilot study provide evidence that δ-tocotrienol is safe and has significantly greater efficacy than placebo in improving serum aminotransferases, hs-CRP, MDA, and FLI score (p<0.001) in patients with NAFLD. Within the placebo group, values were significantly lower than baseline but not in a similar magnitude as the tocotrienol group. As the study participants were advised to consume low-fat foods and increase physical activity, the improvement observed in the placebo group may be due to increased patients’ adherence to fat-reduced diet, exercise, and motivation because of study participation (15). Nevertheless, the improvement in the tocotrienol group was significantly greater than that in the placebo group.
A recent study employing a δ-tocotrienol preparation as used in the present study has shown it to be effective in improving serum hs-CRP, MDA, and γ-GT in subjects with hypercholesterolemia (16). On the other hand, a recent study conducted by Magosso et al. (17) in 87 patients with hypercholesterolemic NAFLD on a tocotrienol-rich fraction (TRF) (mixture of 61.5 mg of α-, 112.8 mg of γ-, and 25.7 mg of δ-tocotrienol and 61.1 mg of α-tocopherol) twice daily for 1 year did not show any statistically significant improvement in weight, BMI, lipid profile, liver enzymes, and C-reactive protein (CRP). This discrepancy between the results by Magosso’s study and our study can be because of the presence of 61.1 mg α-tocopherol in TRF preparation. Animal studies have demonstrated that the presence of >20% α-tocopherol in TRF preparation attenuates γ-tocotrienol action to suppress 3-hydroxy-3-methylglutaryl coenzyme A reductase activity, the rate limiting enzyme in cholesterol synthesis (18). The reason for attenuation of tocotrienol’s efficacy in the presence of α-tocopherol is that α-tocopherol depresses the bioavailability of tocotrienols after oral administration. α-Tocopherol has higher affinity than tocotrienols to α-tocopherol transfer protein in the liver. This results in preferential secretion of α-tocopherol to the peripheral tissues and inhibition in the secretion of tocotrienols. Entrapment of tocotrienols inside the liver cells leads to preferential metabolism of tocotrienols in the liver microsomes and thus, decreases the response to tocotrienol therapy and affects the treatment outcomes (9,19). Moreover, recent studies, involving human subjects, have confirmed that pure tocotrienols (e.g., annatto tocotrienols with 90% δ-tocotrienol3 and 10% γ-tocotrienol) have superior bioavailability than TRF owing to the absence of α-tocopherol’s interference in absorption (9).
In the present study, we used FLI along with USG to diagnose NAFLD. In our study population, FLI tightly correlated with the presence/degree of hepatic steatosis detected by USG at baseline. At the end of the study, FLI significantly reduced in the tocotrienol group than in the placebo group (p<0.001), suggesting a greater reduction in intrahepatic fat in the tocotrienol group. However, we did not observe any improvement in the degree/grade of hepatic steatosis using USG at the end of the study, suggesting that the reduction in intrahepatic fat in the tocotrienol group was not sufficient enough to bring a radiological improvement in steatosis grade. This is because the trial period was too short to accomplish a radiologically detectable change in hepatic steatosis. Admittedly, only biochemical and clinical changes were detected in this setting. FLI is based on biochemical and anthropometric measures that are more sensitive than USG for detecting response to therapy during short interval.
The reduced FLI in our study was accounted by a decrease in all components of FLI. The reduction in weight, BMI, WC, and TG levels observed in the present study was consistent with previously published studies demonstrating the anti-obesity effects of tocotrienol in laboratory animals. Zhao et al. (9) identified that 90% pure γ-tocotrienol supplementation for 4 weeks leads to a significant reduction in diet-induced weight gain in young mice. Wong et al. (20) reported that 90% pure δ-tocotrienol supplementation for 8 weeks leads to a significant reduction in abdominal adiposity and fat cell masses in obese rats. These studies demonstrated that tocotrienols decrease body weight and reduce body fat mass in vivo. In addition to the direct effects of body weight reduction, the anti-obesity effect of tocotrienol is also reflected by the reduction in biochemical parameters, such as serum TG and cholesterol levels. The possible mechanisms involved in the weight-reducing effects of tocotrienols have been studied in different cell lines and tissues. These include modulation of adipogenesis and fat cell differentiation, modulation of energy sensing, and induction of apoptosis in preadipocytes (9).
In the present study, the δ-tocotrienol group showed a significantly greater reduction in inflammatory (hs-CRP) and oxidative stress (MDA) parameters than the placebo group (p<0.001). Regarding mechanisms by which δ-tocotrienol exerts its anti-inflammatory effects, several studies have demonstrated that δ-tocotrienol modulates different signaling pathways and transcriptional factors involved in inflammation (16,21). The antioxidant effects of δ-tocotrienol are mediated through induction of different antioxidant enzymes. The major types include superoxide dismutase, nicotinamide adenine dinucleotide phosphate, quinone oxidoreductase, and glutathione peroxidase. These enzymes quench reactive oxygen species (ROS), such as superoxide radicals (6). Oxidative stress is an important contributing factor involved in the progression of hepatic steatosis to NASH. ROS overproduction induces nuclear factor-κB (NF-κB) activation leading to increased production of inflammatory cytokines, for example, tumor necrosis factor-alpha and interleukin-6. These inflammatory mediators induce CRP production in the liver. CRP has pro-inflammatory potential. δ-Tocotrienol inhibits inflammatory cytokines and prevents ROS-induced NF-κB expression by quenching free radicals (16,17).
Our study has some limitations. First, although USG is a practical approach commonly used for non-invasive diagnosis of hepatic steatosis, it is not the gold standard technique for quantitative hepatic fat assessment. The non-invasive diagnosis of hepatic steatosis should rely on magnetic resonance spectroscopy or magnetic resonance imaging proton density fat fraction as gold standards, which were not used in the present study (22). Second, the duration of the study was too short to allow an accurate evaluation of the potential clinical utility of δ-tocotrienol in NAFLD. Indeed, only biochemical and anthropometric outcomes were meaningful in this setting. Third, the sample size was small, and therefore, the generalizability of findings is unclear. Clinical trials with larger sample size and longer duration are needed to fully assess the efficacy and safety of δ-tocotrienol in NAFLD.
In conclusion, the present study has demonstrated that patients with NAFLD assigned to δ-tocotrienol significantly benefited in terms of improvement in aminotransferase levels and inflammatory, oxidative stress, and hepatic steatosis markers compared with placebo during the 12-week period. Furthermore, at the dosage of 600 mg/day, δ-tocotrienol was generally safe and well tolerated, and no adverse events were reported. Although the results of the present study are very encouraging, they provide a platform only for future planning of large-scale randomized clinical trials, which will provide a complete picture of the utility of this compound. Taken together, these results provide a proof-of-principle for the superiority of δ-tocotrienol and strongly favor the safe and effective use of this compound for the management of NAFLD in the future.
Acknowledgements
We thank the patients who agreed to participate in this study. We thank all our colleagues at Armed Forces Institute of Pathology/Armed Forces Institute of Radiology who cared for the patients enrolled in this study. We thank Prof. Asaf A Qureshi, Department of Basic Medical Sciences, University of Missouri-Kansas, for donating the Delta-tocotrienol capsules.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of Armed Forces Institute of Pathology (Decision Date: 28.05.2015/Decision No: ERC/AFIP-5-22-15).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - D.A.K.; Design - D.A.K., M.A.P., A.I., S.K.; Supervision - D.A.K.; Resource - D.A.K., A.I., S.K.; Materials - A.I., S.K.; Data Collection and/or Processing - M.A.P., S.K.; Analysis and/or Interpretation - M.A.P., D.A.K., A.I., S.K.; Literature Search - M.A.P., D.A.K., A.I., S.K.; Writing - M.A.P., D.A.K., A.I., S.K.; Critical Reviews - D.A.K., M.A.P.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: This study is funded by the Armed Forces Institute of Pathology, Rawalpindi and Higher Education Commission, Government of Pakistan, Islamabad.
REFERENCES
- 1.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–609. doi: 10.1053/j.gastro.2012.04.001. https://doi.org/10.1053/j.gastro.2012.04.001 [DOI] [PubMed] [Google Scholar]
- 2.Oseini AM, Sanyal AJ. Therapies in non-alcoholic steatohepatitis (NASH) Liver Int. 2017;37:97–103. doi: 10.1111/liv.13302. https://doi.org/10.1111/liv.13302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koplay M, Sivri M, Erdogan H, Nayman A. Importance of imaging and recent developments in diagnosis of nonalcoholic fatty liver disease. World J Hepatol. 2015;7:769–76. doi: 10.4254/wjh.v7.i5.769. https://doi.org/10.4254/wjh.v7.i5.769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.AlShaalan R, Aljiffry M, Al-Busafi S, Metrakos P, Hassanain M. Nonalcoholic Fatty Liver Disease: Noninvasive Methods of Diagnosing Hepatic Steatosis. Saudi J Gastroenterol. 2015;21:64–70. doi: 10.4103/1319-3767.153812. https://doi.org/10.4103/1319-3767.153812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zelber-Sagi S, Webb M, Assy N, et al. Comparison of fatty liver index with noninvasive methods for steatosis detection and quantification. World J Gastroenterol. 2013;19:57–64. doi: 10.3748/wjg.v19.i1.57. https://doi.org/10.3748/wjg.v19.i1.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahsan H, Ahad A, Iqbal J, Siddiqui WA. Pharmacological potential of tocotrienols: a review. Nutr Metab. 2014;11:52. doi: 10.1186/1743-7075-11-52. https://doi.org/10.1186/1743-7075-11-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasanthi HR, Parameswari RP, Das DK. Multifaceted role of tocotrienols in cardioprotection supports their structure: function relation. Genes Nutr. 2012;7:19–28. doi: 10.1007/s12263-011-0227-9. https://doi.org/10.1007/s12263-011-0227-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qureshi AA, Khan DA, Silswal N, Saleem S, Qureshi N. Evaluation of Pharmacokinetics, and Bioavailability of Higher Doses of Tocotrienols in Healthy Fed Humans. J Clin Exp Cardiolog. 2016;7:434. doi: 10.4172/2155-9880.1000434. https://doi.org/10.4172/2155-9880.1000434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao L, Fang X, Marshall M, Chung S. Regulation of Obesity and Metabolic Complications by Gamma and Delta Tocotrienols. Molecules. 2016;21:344. doi: 10.3390/molecules21030344. https://doi.org/10.3390/molecules21030344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwenger KJ, Allard JP. Clinical approaches to non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:1712–23. doi: 10.3748/wjg.v20.i7.1712. https://doi.org/10.3748/wjg.v20.i7.1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saverymuttu SH, Joseph AE, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J (Clin Res Ed) 1986;292:13–5. doi: 10.1136/bmj.292.6512.13. https://doi.org/10.1136/bmj.292.6512.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Medical Algorithms. Fatty Liver Index (FLI) of Bedogni et al for Predicting Hepatic Steatosis [Software] n.d.. [Accessed March 15, 2017]. Available at: http://www.medicalalgorithms.com/fatty-liver-index-fli-of-bedogni-et-al-for-predicting-hepatic-steatosis.
- 13.Teare MD, Dimairo M, Shephard N, Hayman A, Whitehead A, Walters SJ. Sample size requirements to estimate key design parameters from external pilot randomised controlled trials: a simulation study. Trials. 2014;15:264. doi: 10.1186/1745-6215-15-264. https://doi.org/10.1186/1745-6215-15-264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitehead AL, Julious SA, Cooper CL, Campbell MJ. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat Methods Med Res. 2016;25:1057–73. doi: 10.1177/0962280215588241. https://doi.org/10.1177/0962280215588241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zelber-Sagi S, Godos J, Salomone F. Lifestyle changes for the treatment of nonalcoholic fatty liver disease: a review of observational studies and intervention trials. Therapeutic Advances in Gastroenterology. 2016;9:392–407. doi: 10.1177/1756283X16638830. https://doi.org/10.1177/1756283X16638830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qureshi AA, Khan DA, Mahjabeen W, Trias AM, Silswal N, Qureshi N. Impact of δ-Tocotrienol on Inflammatory Biomarkers and Oxidative Stress in Hypercholesterolemic Subjects. J Clin Exp Cardiolog. 2015;6:367. [Google Scholar]
- 17.Magosso E, Ansari MA, Gopalan Y, et al. Tocotrienols for normalization of hepatic echogenic response in nonalcoholic fatty liver: a randomised placebo-controlled clinical trial. Nutr J. 2013;12:166. doi: 10.1186/1475-2891-12-166. https://doi.org/10.1186/1475-2891-12-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qureshi AA, Pearce BC, Nor RM, Gapor A, Peterson DM, Elson CE. Dietary α-tocopherol attenuates the impact of γ-tocotrienol on hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in chickens. J Nutr. 1996;126:389–94. doi: 10.1093/jn/126.2.389. https://doi.org/10.1093/jn/126.2.389 [DOI] [PubMed] [Google Scholar]
- 19.Sontag TJ, Parker RS. Influence of major structural features of tocopherols and tocotrienols on their ω-oxidation by tocopherol-ω-hydroxylase. J Lipid Res. 2007;48:1090–8. doi: 10.1194/jlr.M600514-JLR200. https://doi.org/10.1194/jlr.M600514-JLR200 [DOI] [PubMed] [Google Scholar]
- 20.Wong W-Y, Ward LC, Fong CW, Yap WN, Brown L. Anti-inflammatory γ- and δ-tocotrienols improve cardiovascular, liver and metabolic function in diet-induced obese rats. Eur J Nutr. 2015:1–18. doi: 10.1007/s00394-015-1064-1. [DOI] [PubMed] [Google Scholar]
- 21.Qureshi AA, Tan X, Reis JC, et al. Suppression of nitric oxide induction and pro-inflammatory cytokines by novel proteasome inhibitors in various experimental models. Lipids Health Dis. 2011;10:177. doi: 10.1186/1476-511X-10-177. https://doi.org/10.1186/1476-511X-10-177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kramer H, Pickhardt PJ, Kliewer MA, et al. Accuracy of Liver Fat Quantification with Advanced CT, MRI, and Ultrasound Techniques: Prospective Comparison with MR Spectroscopy. AJR Am J Roentgenol. 2017;208:92–100. doi: 10.2214/AJR.16.16565. https://doi.org/10.2214/AJR.16.16565 [DOI] [PMC free article] [PubMed] [Google Scholar]


