Aquitalea sp. strain MWU14-2217 was isolated from wild cranberry bog soils in the Cape Cod National Seashore.
ABSTRACT
Aquitalea sp. strain MWU14-2217 was isolated from wild cranberry bog soils in the Cape Cod National Seashore. The draft genome is 4.3 Mbp with 4,133 coding sequences and contains predicted genes for phenazines, colicins, siderophores, and putative exporters of these compounds and genes responsible for motility and biofilm formation.
ANNOUNCEMENT
The genus Aquitalea was described in 2006 as a member of the Neisseriales, now placed in the family Chromobacteriaceae, and is closely related to the genus Chromobacterium (1). This genus includes three recognized species, all of which were isolated from aquatic environments (1–4). Despite the importance of wetland bacteria in geochemical processes, little is known about their taxonomy or environmental functions. As part of a culture-dependent survey of bacteria from wetland bogs, MWU14-2217 was isolated along with a number of previously uncharacterized Pseudomonas spp. and Chromobacterium spp. from wild cranberry bogs in the Cape Cod National Seashore in Massachusetts. The discovery of new species within well-established genera suggests an important if underappreciated example of microbial evolution in these critical ecosystems.
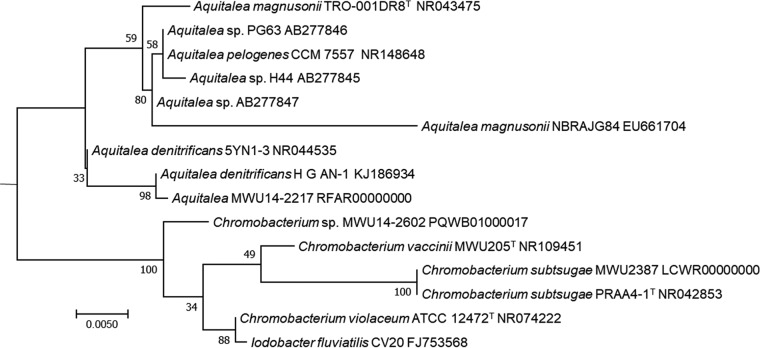
Soil samples were plated onto King’s medium B (KMB) supplemented with ampicillin and cycloheximide, and then single colonies were purified three times on KMB. MWU14-2217 was provisionally placed in the genus Aquitalea by 16S rRNA gene similarity (∼99%) with other members of the genus by phylogenetic analysis (Fig. 1). For genome sequencing, MWU14-2217 was grown in KMB broth overnight for genomic DNA (gDNA) isolation (DNeasy blood and tissue kit, Qiagen). gDNA was sheared to ∼600 bp to generate libraries on an Apollo 384 liquid handler (Wafergen, Kapa Biosystems KK8201). Resulting DNA fragments were end repaired, A tailed, ligated to indexes/adapters (catalog number 520999; Bioo), and cleaned using AMPure beads (Agencourt Bioscience/Beckman Coulter). Samples were amplified with Kapa HiFi enzyme, and the resultant libraries were assessed by quantitative PCR (Kapa library quantification kit KK4835), pooled, and sequenced on the Illumina MiSeq platform in 2 × 300-bp and 2 × 150-bp paired-end flow cells. All read file data sets were combined, trimmed, partially assembled, and annotated using the Comprehensive Genome Analysis feature of the PATRIC (version 3.5.26) website (https://patricbrc.org/) with default parameters (5). The sequence consisted of 4,335,744 bp (G+C content, 60.26%) within 25 contigs. The largest contig is 755,022 bp, and the N50 value is 448,300 bp, with a sequence coverage of 151×. The orthologous average nucleotide identity (ANI) was less than 86% (6, 7), and the digital DNA-DNA hybridization (dDDH) (8, 9) was <30% with Aquitalea magnusonii (GenBank accession number AP018823) (2) and Aquitalea pelogenes (GenBank accession number LNQV00000000) (4), the only two genomic sequences available for this genus. The closest relative based on the 16S rRNA sequence is Aquitalea denitrificans (Fig. 1), but at this time, no whole-genome sequence is available.
FIG 1.
16S rRNA phylogeny of Aquitalea sp. strain MWU14-2217. The evolutionary history of this Aquitalea sp. strain was inferred in MEGA7 (13) by maximum likelihood based on the Kimura 2-parameter model (14) with a discrete gamma distribution (5 categories; +G parameter, 0.7410), a complete deletion of gaps and missing data, and a rate variation model that allowed for some evolutionarily invariable sites (+I, 82.42% of sites). There were a total of 969 positions in the final data set. Initial trees for the heuristic search were obtained from Neighbor-Join and BioNJ algorithms applied to a matrix of pairwise distances estimated using maximum composite likelihood. The tree with the highest log likelihood (−1,883.98) was drawn to scale with branch lengths measured in the number of substitutions per site. Bootstrap values from 500 samplings are indicated next to branches. Aquitalea sp. sequences were retrieved from GenBank by taking the bacterial 16S rRNA RefSeq targeted locus project sequence for each species. Chromobacterium and Iodobacter were included as outgroups. MWU 14-2217 clusters with the A. denitrificans subgroup, but in the absence of a genomic sequence for A. denitrificans, the exact taxonomic placement of MWU 14-2217 cannot be determined.
Gene number and functional predictions were made using the RASTtk function of PATRIC with default settings (6). MWU14-2217 contained 4,133 predicted protein-coding genes (4,680 by NCBI annotation), with 76 tRNA and 9 rRNA operons, 23 chemotaxis (che) and 5 aerotaxis genes, the multidrug efflux pump genes mdtABC (10) and emrAB (11), the macrolide-specific macAB (12) efflux pump, and an exoprotease exporter gene. Although the bacterium is not fluorescent, a phenazine-like biosynthesis gene (phzF) is present, as well as a colicin V production protein and a homoserine lactone efflux protein.
Data availability.
This whole-genome shotgun project has been deposited at DDBJ/EMBL/GenBank under the accession number RFAR00000000 and in the SRA database under accession number SRP167089.
ACKNOWLEDGMENTS
This research was supported by the College of Health Sciences and Biomedical Sciences Program and the Office of Research and Sponsored Programs, Midwestern University.
Genomic sequencing was performed using an Illumina MiSeq instrument at the Arizona State University CLAS Genomics Core facility.
REFERENCES
- 1.Lau H-T, Faryna J, Triplett EW. 2006. Aquitalea magnusonii gen. nov., sp. nov., a novel Gram-negative bacterium isolated from a humic lake. Int J Syst Evol Microbiol 56:867–871. doi: 10.1099/ijs.0.64089-0. [DOI] [PubMed] [Google Scholar]
- 2.Ishizawa H, Kuroda M, Ike M. 2017. Draft genome sequence of Aquitalea magnusonii strain H3, a plant growth-promoting bacterium of duckweed (Lemna minor). Genome Announc 5:e00812-17. doi: 10.1128/genomeA.00812-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee C-M, Weon H-Y, Kim Y-J, Son J-A, Yoon S-H, Koo B-S, Kwon S-W. 2009. Aquitalea denitrificans sp. nov., isolated from a Korean wetland. Int J Syst Evol Microbiol 59:1045–1048. doi: 10.1099/ijs.0.002840-0. [DOI] [PubMed] [Google Scholar]
- 4.Sedláček I, Kwon S-W, Švec P, Mašlanˇová I, Kýrová K, Holochová P, Černohlávková J, Busse H-J. 2016. Aquitalea pelogenes sp. nov., isolated from mineral peloid. Int J Syst Evol Microbiol 66:962–967. doi: 10.1099/ijsem.0.000819. [DOI] [PubMed] [Google Scholar]
- 5.Wattam AR, Davis JJ, Assaf R, Boisvert S, Brettin T, Bun C, Conrad N, Dietrich EM, Disz T, Gabbard JL, Gerdes S, Henry CS, Kenyon RW, Machi D, Mao C, Nordberg EK, Olsen GJ, Murphy-Olson DE, Olson R, Overbeek R, Parrello B, Pusch GD, Shukla M, Vonstein V, Warren A, Xia F, Yoo H, Stevens RL. 2017. Improvements to PATRIC, the all-bacterial Bioinformatics Database and Analysis Resource Center. Nucleic Acids Res 45:D535–D542. doi: 10.1093/nar/gkw1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konstantinidis KT, Tiedje JM. 2005. Genomic insights that advance the species definition for prokaryotes. Proc Natl Acad Sci U S A 102:2567–2572. doi: 10.1073/pnas.0409727102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoon S-H, Ha S-M, Lim J, Kwon S, Chun J. 2017. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie Van Leeuwenhoek 110:1281–1286. doi: 10.1007/s10482-017-0844-4. [DOI] [PubMed] [Google Scholar]
- 8.Meier-Kolthoff JP, Auch AF, Klenk H-P, Göker M. 2013. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics 14:60. doi: 10.1186/1471-2105-14-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wayne LG, Brenner DJ, Colwell RR, Grimont PAD, Kandler O, Krichevsky MI, Moore LH, Moore WEC, Murray RGE, Stackebrandt E, Starr MP, Truper HG. 1987. Report of the Ad Hoc Committee on Reconciliation of Approaches to Bacterial Systematics. Int J Syst Evol Microbiol 37:463–464. doi: 10.1099/00207713-37-4-463. [DOI] [Google Scholar]
- 10.Horiyama T, Nishino K. 2014. AcrB, AcrD, and MdtABC multidrug efflux systems are involved in enterobactin export in Escherichia coli. PLoS One 9:e108642. doi: 10.1371/journal.pone.0108642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heacock-Kang Y, Sun Z, Zarzycki-Siek J, Poonsuk K, McMillan IA, Chuanchuen R, Hoang TT. 2018. Two regulators, PA3898 and PA2100, modulate the Pseudomonas aeruginosa multidrug resistance MexAB-OprM and EmrAB efflux-pumps and biofilm formation. Antimicrob Agents Chemother doi: 10.1128/AAC.01459-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greene NP, Kaplan E, Crow A, Koronakis V. 2018. Antibiotic resistance mediated by the MacB ABC transporter family: a structural and functional perspective. Front Microbiol 9:950. doi: 10.3389/fmicb.2018.00950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimura M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This whole-genome shotgun project has been deposited at DDBJ/EMBL/GenBank under the accession number RFAR00000000 and in the SRA database under accession number SRP167089.