Abstract
BACKGROUND
In uremic animals, vitamin D receptor (VDR) agonists like paricalcitol (Pc) attenuate cardiac hypertrophy, but this effect has not been replicated consistently in humans with chronic kidney disease. Elevated fibroblast growth factor 23 (FGF23) levels cause cardiac hypertrophy with activation of the myocardial calcineurin/nuclear factor of activated T cell (NFAT) axis and may antagonize the cardioprotective effects of VDR agonist therapy. We hypothesized that the effectiveness of Pc may depend on the prevailing circulating levels of FGF23 and could be potentiated by the combined administration of a pan-FGF23 receptor (FGFR) blocker agent (PD173074).
METHODS
In rats with 5/6 nephrectomy treated with Pc or PD173074 or both agents concurrently, myocardial mRNA expression of renin–angiotensin system, VDR, FGFR4, and calcineurin/NFAT target genes was determined. In adolescents on hemodialysis, we analyzed sequential echocardiograms, blood pressures and serial FGF23 measurements, and their relations to the cumulative administered dose of parenteral Pc.
RESULTS
The ratio of Pc dose/plasma levels of FGF23 correlated inversely (P < 0.005) with the cardiac mass in uremic rats and in hemodialysis patients, independently of hypertension. Despite persistently elevated FGF23 levels and myocardial FGFR4 activation, Pc suppressed upregulated myocardial calcineurin/NFAT target genes, and the effects were amplified by coadministration of PD173074.
CONCLUSIONS
The beneficial effects of Pc on uremic cardiac hypertrophy are counterbalanced by the increased FGF23 levels. Blockade of FGF23-mediated signaling increased the Pc-induced suppression of the myocardial calcineurin/NFAT system. Higher doses of Pc should be considered in the treatment of patients with uremic cardiomyopathy.
Keywords: blood pressure, cardiac hypertrophy, CKD, FGF23, hypertension, NFAT/calcineurin axis, paricalcitol, renin–angiotensin-system, vitamin D
Left ventricular hypertrophy (LVH) is common in chronic kidney disease (CKD) and is associated with high cardiovascular disease mortality.1,2 LVH in association with3 or independent of hypertension4 is multifactorial including suppressed vitamin D activity and elevated circulating levels of the phosphaturic hormone fibroblast growth factor (FGF) 23.5–8 Levels of FGF23 reach extremely high concentrations with advanced stages of CKD on dialysis and strongly associate with LVH in both adults9 and in younger pediatric patients.10,11
Experimentally, 1,25 dihydroxyvitamin D (calcitriol) and other vitamin D receptor (VDR) agonists like 19-nor-1,25-dihydroxyvitamin D2 (paricalcitol, Pc) suppress the myocardial renin–angiotensin system (RAS)12–14 and abrogate cardiac hypertrophy, with reduction14,15 or no changes in blood pressure (BP).16 Hypertension-independent cardiac hypertrophy induced by FGF23 results from activation of FGF receptor isoform 4 (FGFR4) that upregulates calcineurin/nuclear factor of activated T cell (NFAT) signaling.8,17 These effects may be blocked by a pan-FGFR inhibitor8 or by a specific FGFR4 inhibitor17 and thus attenuate cardiac hypertrophy. VDR agonists, while attenuating myocardial hypertrophy, also stimulate FGF23 secretion by the osteocytes,18,19 which promotes hypertrophy and antagonizes the VDR-induced cardioprotective effects. Calcitriol was initially noted to suppress NFAT signaling in human T cells20 and more recently to reduce the 2 NFAT target genes regulator of calcineurin 1 (RCAN1) and transient receptor potential channel 6 (TRPC6) in the kidney or the myocardium of rats without21,22 and with uremia.23 In the latter experiments, however, neither BP nor circulating FGF23 levels were measured, and calcitriol treatment resulted in reduced myocardial expression of the FGFR4.23 Here, we hypothesized that the VDR agonist Pc would downregulate myocardial calcineurin/NFAT axis and thus improve cardiac hypertrophy in experimental uremia despite important elevations of circulating FGF23 levels and hypertension. We further speculated that Pc cardioprotection would be amplified by the coadministration of a FGFR blocker capable of reducing bone FGF23 secretion24 and myocardial FGFR4 activation. Our data demonstrate that despite elevated BP, Pc attenuates cardiac hypertrophy with downregulation of myocardial NFAT signaling and that these effects are potentiated by the concurrent administration of a FGF23 blocking agent.
Studies in patients with CKD have shown inconsistent effects of VDR agonist therapy on LV mass, but none of the studies included FGF23 measurements.25,26 Since young patients on maintenance hemodialysis with high FGF23 levels and LVH10 often require higher doses of Pc to control their hyperparathyroidism,27 the preclinical observations alluded to in the above paragraph led us to hypothesize that the effectiveness of Pc to improve LV mass in these patients could be dependent on the administered dose relative to the prevailing levels of circulating FGF23.
MATERIAL AND METHODS
Experimental animals and design
Male Sprague–Dawley rats (250–395 g), underwent 5/6 nephrectomy (Nx) or sham operation as previously described,15 were fed standard rat chow containing 1% calcium and 0.74 % phosphorus and free access to water. Four days after surgery, the animals were randomly assigned to 5 groups: (i) sham controls; (ii) 5/6Nx (untreated controls) receiving vehicle 3 times per week by intraperitoneal injection; (iii) 5/6Nx + Pc receiving intraperitoneal Pc, 0.3 μg/kg, 3 times per week; (iv) 5/6Nx+pan-FGFR blocker PD173074 (PD) intraperitoneal 1 mg/kg/day; and (v) 5/6Nx with Pc+PD combined, at the above stated doses. The dose of Pc employed has been shown to suppress renal and myocardial RAS genes expression and to effectively control hyperparathyroidism without elevation in serum ionized calcium in the 5/6 Nx rat model14,15 and is within the therapeutic dose range currently used in clinical practice.27,28 We opted to use a pan-FGFR blocker, and not the FGFR4-specific inhibitor, in order to evaluate its known attenuating effects on bone-derived secretion of FGF23 and the expected reduction of circulating FGF23 levels.24 Following 4 weeks of treatment, blood and urine were collected, and the retrieved hearts were weighted, and prepared for further analysis. Systolic BPs were determined by tail-cuff plethysmography (IITC Life Science, Woodland Hills, CA), and all routine biochemical analytes measured as described previously.14,15 FGF23 was determined by a mouse C-terminal 2-site enzyme-linked immunosorbent (ELISA) assay (Immutopics, San Clemente, CA).
Quantitative real-time polymerase chain reaction.
Total RNA was purified from rat hearts using 1 ml of TRizol (G Biosciences) per 100 mg of tissue following the manufacturers’ protocol. Prior to quantitative real-time PCR (qPCR), RNA samples were digested with DNase I (Roche), and mRNA was reverse-transcribed into cDNA using qScript cDNA SuperMix (Quanta Biosciences). qPCR was conducted as previously described.29 Briefly, 50 ng of cDNA, PerfeCTA SYBR Green FastMix ROX (Quanta Biosciences), and gene-specific primers were run on a StepOne plus Real-Time PCR System (Applied Biosystems). Raw data were quantified via StepOneTM software v2.3 from Life Technologies. Relative gene expression was normalized to expression levels of GAPDH and evaluated using the 2−ΔΔCt method. Primer sequences are listed in Table 1 in the Supplementary Material, Table S1.
Table 1.
Baseline and follow-up biochemical and echocardiographic parameters in hemodialysis patients
| Parameter | Baseline | Follow-up | P value |
|---|---|---|---|
| Systolic blood pressure, Z-score | 1.87 ± 0.27 | 2.33 ± 0.34 | 0.1 |
| Diastolic blood pressure, Z-score | 1.50 ± 0.25 | 1.52 ± 0.28 | 0.9 |
| Serum calcium, corrected, mg/dl | 9.3 (8.6–9.7) | 9.3 (8.8–9.8) | 0.4 |
| Serum phosphorus, mg/dl | 6.9 (5.7–7.4) | 7.1 (5.1–7.9) | 0.7 |
| Intact PTH, pg/ml | 479 (233–569) | 325 (189–583) | 0.3 |
| 25-OH vitamin D, ng/ml | 30 (19–40) | 32 (24–41) | 0.2 |
| FGF23, RU/ml | 25,238 ± 30,244 (6,204; 1,167–62,420) | 19,153 ± 25,190 (7,104; 1,249–23,711) | 0.2 |
| Log FGF23 | 3.85 (3.09–4.51) | 3.74 (3.07–4.83) | 0.8 |
| Dose paricalcitol, mcg/week | 15.2 (4.8–23.3) | ||
| Dose paricalcitol, mcg/kg/week | 0.33 (0.11–0.49) | ||
| LVMI, Z-score | 1.78 (0.67–2.78) | 1.64 (0.73–3.45) | 0.2 |
| IVST, Z-score | 0.68 (−0.30 to −0.97) | 0.39 (−0.10–0.69) | 0.9 |
Data expressed as median (25–75th percentile) or average ± SEM. P value calculated by paired t-testing. Abbreviations: FGF, fibroblast growth factor; IVST, interventricular septal thickness; LVMI, left ventricular mass index (see text for calculation); PTH, parathyroid hormone.
Patients
This retrospective, single center, study included 20 patients aged 16 ± 4 years (50% female, 12 African American and 8 Hispanic) receiving maintenance hemodialysis treatment at the Holtz Children’s/Jackson Memorial Medical Center, University of Miami, in Miami, FL. The diseases causing end-stage renal disease were predominantly glomerulopathies (14/20). To avoid potential confounding effects of other cardiovascular risk factors frequently prevalent in older patients, we excluded patients with diabetes, history of smoking, evidence of peripheral vascular disease, clinical or echocardiographic evidence of coronary heart disease, and sustained poorly controlled hypertension. Additional exclusion criteria were congenital cardiomyopathy, primary myocardial disease, or historic heart transplantation. Our final analysis included only patients <21 years of age treated with Pc, who underwent sequential echocardiograms and concurrent serial plasma FGF23 measurements. All patients received standard in-center hemodialysis 3 times weekly were prescribed phosphate binders, and all received parenteral Pc as the only VDR agonist, and the administered dose was recorded and documented during the hemodialysis treatment sessions. The study was approved by the local Institutional Review Board.
Clinical data and laboratory methods.
Baseline data were obtained in the month preceding the initial echocardiographic studies (Baseline, Table 1). Anthropometric measurements were expressed as percentiles or Z-scores according to standardized national charts.30 The BP employing an automated oscillometric device (Dynamap; GE Healthcare, Waukesha, WI) were obtained in the sitting position after 5 minutes of resting prior to initiation of hemodialysis, in the opposite arm of the working graft or arteriovenous fistula needed for dialysis, and the average of 3 sequential measurements was recorded as the predialysis BP for that date. Time-averaged predialysis BP measurements were calculated from the readings obtained the first day of each dialysis week during the 4 weeks preceding the first echocardiogram (baseline) and similarly during the subsequent weeks preceding the second echocardiogram (follow-up); these readings were indexed to age, gender, and height percentile to define hypertension (>95th percentile) and to calculate Z-scores.31 Time-averaged monthly routine blood chemistries and quarterly intact PTH levels were calculated from the 6-month aggregate periods preceding the second sequential echocardiogram (Follow-up, Table 1) and compared with values obtained within 1 month of the initial echocardiogram (Baseline, Table 1). The Pc doses were variable among patients due to periodic adjustments according to PTH and serum Ca levels following reported guidelines.27,28 The cumulative dose represents the aggregate of the administered Pc doses, properly documented during each hemodialysis session, between the initial and second echocardiogram. Plasma iPTH concentrations were measured by a commercially available electrochemiluminescence immunoassay recognizing the intact (1–84) molecule and serum 25(OH) D levels by an immunochemiluminometric assay (DiaSorin) previously described.10 Plasma levels of FGF23 were measured by a second-generation C-terminal ELISA that recognizes both the intact FGF23 and its C-terminal fragments (Immutopics). Values in 33 local children aged 5–21 years with normal glomerular filtration rate were median 97 RU/ml, range 55–220 RU/ml, lower quartile 70 RU/ml.32 FGF23 measurements employing the C-terminal (Immutopics) or an intact molecule assay in patients with CKD are highly correlated and accurately reflect the biologically active hormone.33
Echocardiography.
All echocardiograms were performed by the same echocardiogram laboratory staff at Holtz Children’s Hospital, supervised and interpreted by a single pediatric cardiologist (SS). Patients on maintenance dialysis at our center undergo routine echocardiograms once yearly, as part of a comprehensive cardiovascular risk assessment. Interventricular septal thickness (IVST) and LV mass (LVM) were calculated following routine guidelines as previously reported.10 In brief, the LVM was indexed (LVMI) to the power of 2.7 of the patient’s height (mass [g]/height [m]2.7), and LVH defined as LVMI >95th percentile for age and gender, further adjusted to height and age rather than chronological age to mitigate potential effects of short stature on height-based calculations, and thus LVMI was also expressed as Z-scores.10,11
Statistical analysis.
Data are expressed as mean ± SEM and median with ranges. Two-tailed Student’s t-test, or Mann–Whitney (when not normally distributed) compared baseline and follow-up data in the dialysis patients. ANOVA followed by Tukey post-testing for multiple comparisons between all treatment groups were used for the animal data. Correlations were assessed by Pearson or Spearman tests as appropriate and by regression analysis. FGF23 concentrations were log10 transformed prior to calculation of their relationships to heart weight and LVMI, and when used as the denominator to calculate the ratio of the administered Pc dose (average weekly dose in µg/kg) and the FGF23 level (Pc/logFGF23 ratio). Graph Pad Prism version 5.00 for Windows (Graph Pad Software, San Diego, CA) was used, and P values of <0.05 were considered significant.
RESULTS
Antihypertrophic effect of Pc in a rat model of CKD is dependent on serum FGF23 levels
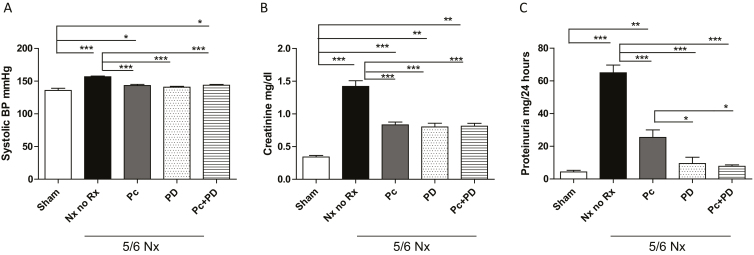
Baseline BP, renal function, and mineral markers were normal and similar in all animal groups (Supplementary Material, Table S2). After 4 weeks of renal ablation, hypertension most prominent in the untreated Nx group was similarly attenuated by either treatment but remained higher compared with sham (Figure 1a). Plasma creatinine (Figure 1b) and proteinuria (Figure 1c) were higher in the untreated group and attained significantly lower values with all treatment modalities. Of note, PD alone or combined with Pc resulted in lower proteinuria than Pc alone (Figure 1c). Serum calcium levels were comparable in all groups. A nonsignificant trend toward higher phosphorus levels occurred with the PD alone (7.92 ± 1.47 mg/dl; n = 5) or PD combined with Pc (7.30 ± 1.90 mg/dl; n = 6), compared with sham (6.1 ± 0.6 mg/dl; n = 5) and the untreated Nx group (6.02 ± 0.90 mg/dl; n = 5), probably reflecting reduced urinary phosphorus excretion by the effects of the PD.
Figure 1.
Systolic blood pressure, plasma creatinine and proteinuria in rats following 4 weeks of renal ablation. 5/6Nx, 5/6 Nephrectomy; No Rx, untreated uremic control group; Pc, paricalcitol; blocker, PD, PD173074 pan-FGFR blocker. (a) Hypertension, (b) elevated creatinine, and (c) proteinuria were similarly attenuated by either treatment. Compared with sham (n = 5), elevated BP levels (a) most prominently in untreated Nx rats (n = 14), remained higher in all 3 treated groups (Pc, n = 12; PD, n = 6; Pc+PD, n = 8). Untreated Nx rats (n = 14) displayed marked elevations of serum creatinine (b) compared with sham (n = 5) and sustained similar declines following treatment with Pc (n = 12), PD (n = 6) or Pc+PD (n = 8). Proteinuria (c), significantly higher in untreated Nx rats (n = 8) compared with sham (n = 5), sustained amplified improvement with PD alone (n = 6) or in tandem with Pc (n = 8) compared with Pc alone (n = 5). ANOVA: P < 0.0001 for systolic blood pressure and proteinuria, P < 0.001 for creatinine. Tukey post-testing: ***P < 0.001. **P < 0.01, and *P < 0.05 compared with the groups sharing the vertical connecting line. Values represent mean ± SEM. Abbreviations: BP, blood pressure; FGFR, fibroblast growth factor
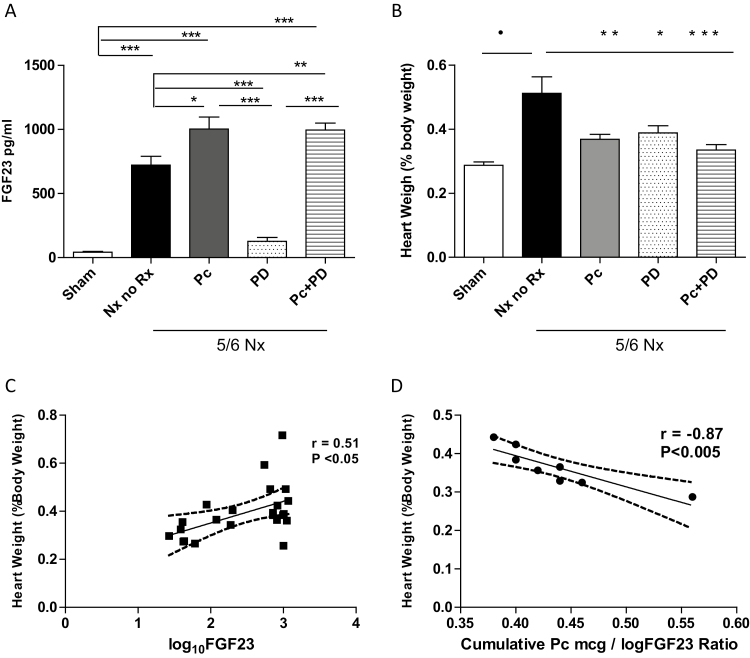
FGF23 concentrations, changes in cardiac hypertrophy, and their relationships with the Pc doses are displayed in Figure 2. Markedly elevated FGF23 levels in untreated 5/6Nx rats were suppressed to sham levels by PD alone (Figure 2a). Pc alone or in combination with PD further increased FGF23 levels (Figure 2a). Cardiac hypertrophy (Figure 2b) in untreated Nx rats was attenuated by all treatment modalities, most prominently in the animals given combined Pc+PD (Figure 2b). In the treated groups, the heart weight was unrelated to BP (r = 0.18) but correlated positively with FGF23 levels (Figure 2c) and correlated inversely with the Pc/log FGF23 ratio (Figure 2d). Myocardial brain natriuretic peptide and atrial natriuretic peptide, 2 established markers of ventricular stress and hypertrophy,8,13,15,21 were significantly elevated in untreated 5/6Nx rats (both P < 0.01 vs. sham) and reduced by all treatment modalities, particularly by PD alone or in combination with Pc (both, P < 0.001 vs. untreated Nx rats) (not shown). Heart weight correlated positively with the myocardial expression of atrial natriuretic peptide (r = 0.4) and brain natriuretic peptide (r = 0.72, P < 0.001). Taken together, the data are consistent with the interpretation that Pc attenuates cardiac hypertrophy more effectively at higher doses relative to prevailing circulating FGF23 concentrations. Furthermore, PD added to Pc induced a reduction in cardiac hypertrophy that was more pronounced than the reduction observed with each agent alone.
Figure 2.
FGF23 concentrations, cardiac hypertrophy, and their relationships with the administered dose of paricalcitol in 5/6 nephrectomy rats. Nx, 5/6 Nephrectomy; No Rx, untreated uremic control group; Pc, paricalcitol; PD, PD17307 pan-FGFR blocker. (a) FGF23 levels compared with sham (n = 5) were elevated after 4 weeks of renal ablation in No Rx (n = 8), and sustained further elevations after treatment with Pc alone (n = 5) or Pc in tandem with blocker (Pc+PD, n = 8), while levels failed to increase in those treated with PD alone (n = 6) remaining comparable with the sham group. ANOVA: P < 0.0001. Tukey post-testing: ***P < 0.001. **P < 0.01, and *P < 0.05 compared with the groups sharing the vertical connecting line. Data are mean ± SEM. (b) Cardiac hypertrophy compared with sham (n = 5) was most prominent in untreated 5/6Nx (n = 6). While Pc treatment alone (n = 10) or PD treatment alone (n = 6) provided prevention of cardiac hypertrophy, Pc treatment in tandem with PD (n = 8) provided superior effects on attenuating cardiac hypertrophy. ANOVA: P < 0.0001. Tukey post-testing: •P < 0.0001 compared with sham; ***P < 0.001, **P < 0.01, and *P < 0.05 compared with 5/6 Nx no Rx, respectively. Data are mean ± SEM. (c) Cardiac weight correlated significantly with logFGF23 values in 5/6 Nx rats (n = 23), r = 0.5, P < 0.05. (d) Cardiac weight correlated inversely with the ratio of the administered dose of Pc divided by the log10 FGF23 value (Pc/log FGF23 ratio) (n = 8), r = −0.87, P < 0.005.
Myocardial VDR and AGT expression are modified by Pc administration and pan-FGF receptor blockade in the uremic heart
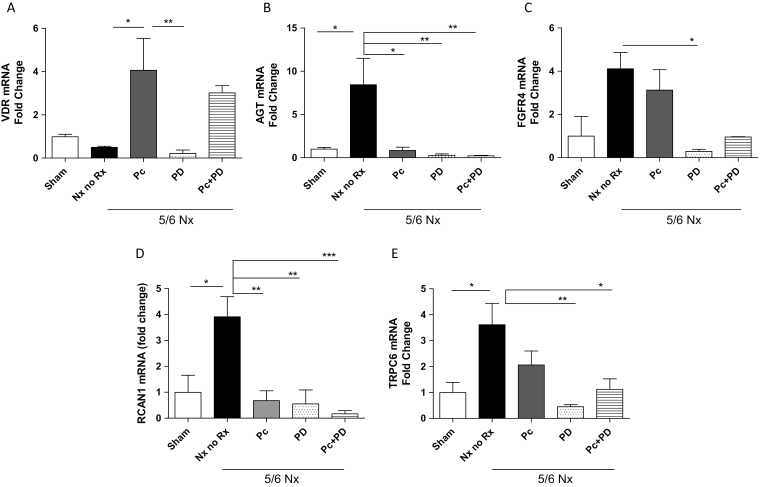
As expected, VDR expression was reduced in untreated Nx rats and remained suppressed with PD alone, while Pc alone or in combination with PD increased VDR (Figure 3a). Angiotensinogen (AGT), a key component of the cardiac RAS,14 increased 8.5-fold above sham in untreated rats and was reduced by all treatment modalities (Figure 3b). Changes in angiotensin II type 1a receptor (ATR1a) mirrored those of AGT (not shown). The reduced AGT expression by PD alone may indicate a crosstalk of FGF23 with the cardiac RAS, a contributor to myocardial hypertrophy.
Figure 3.
mRNA expression of hypertrophy-modulating genes VDR, AGT, FGFR4, and calcineurin/NFAT target genes RCAN1 and TRPC6 in the myocardium of rats after 4 weeks of renal ablation. 5/6Nx, 5/6 Nephrectomy; No Rx, untreated uremic control group; Pc, paricalcitol; PD, PD173074 pan-FGFR blocker. (a) The reduced VDR expression in the Nx untreated group (n = 5) compared with sham (n = 4) was corrected by treatment with Pc alone (n = 5) (*P < 0.05 vs. untreated Nx) or Pc in tandem with PD (n = 5), while PD blocker alone (n = 5) had no measured effects on VDR mRNA levels. (b) AGT mRNA levels, markedly upregulated by renal ablation in untreated animals (n = 4) compared with sham (n = 4), were suppressed by Pc alone (n = 4) (*P < 0.05) or when administered in tandem with PD (n = 4) (**P < 0.01) compared with untreated Nx animals, as well as by blocker alone (n = 4) (**P < 0.01 vs. untreated Nx). (c) FGFR4 expression, markedly elevated in the myocardium of untreated uremic rats (n = 4) compared with sham (n = 5), was effectively downregulated by PD alone (n = 5) (*P < 0.05 vs. untreated Nx), remained essentially unmodified by Pc alone (n = 5) and declined less robustly by Pc in tandem with PD (n = 4). (d) Compared with sham (n = 6), RCAN1 was upregulated in untreated Nx (n = 6), and effectively and similarly attenuated by treatment with Pc alone (n = 5) and PD alone (n = 5) (both **P < 0.01 vs. untreated Nx). Downregulation of RCAN1 mRNA was amplified when both Pc and PD were administered in tandem (n = 5) (***P < 0.001). (e) Upregulated TRPC6 mRNA levels in the untreated Nx animals (n = 8) compared with sham (n = 5) (*P < 0.05 vs. sham) were reduced most effectively with PD alone (n = 6) (**P < 0.01) or in tandem with Pc (n = 6) (*P < 0.05), and more modestly with Pc alone (n = 5). Data are mean ± SEM. Abbreviations: AGT, Angiotensinogen; FGFR, fibroblast growth factor; VDR, vitamin D receptor.
Pc administration and pan-FGF receptor blockade have distinctive effects on the expression of FGFR4 and calcineurin/NFAT target genes in the uremic heart
Cardiac expression of FGFR4 (Figure 3c), the receptor isoform mediating FGF23 effects on cardiac myocytes, and of the NFAT target genes, RCAN1 (Figure 3d) and TRPC6 (Figure 3e), was all upregulated in untreated Nx rats. PD, alone or combined with Pc, markedly reduced their levels to values similar to those in sham-operated rats. Pc alone did not modify FGFR4 expression, while it did attenuate both RCAN1 and TRPC6 expression. These effects were more pronounced by the combined administration of Pc with PD (Figure 3d). Thus, while PD did reduce FGFR4 activation and inhibited NFAT target genes, Pc did not modify the upregulated cardiac FGFR4 levels but induced a profound reduction in the expression of NFAT target genes.
Antihypertrophic effect of Pc is also dependent on serum FGF23 levels in young CKD patients
Dialysis vintage was 24 ± 17 months, and the average duration between sequential echocardiograms was 7 ± 2.1 months. BPs were similarly elevated at both time points reflecting systolic hypertension in 60% (n = 12) and diastolic hypertension in 55% (n = 11) of patients on follow-up (Table 1). Mineral markers were normal at both time points, only 2/20 patients had evidence of vitamin D deficiency [25(OH) D <20 ng/ml], and PTH levels, albeit not statistically different, declined by 30% at follow-up indicating improvement of secondary hyperparathyroidism. Plasma FGF23 levels, over 60-fold above that of local controls with normal glomerular filtration rate, exhibited further increases on the second measurement in 8 patients.
Overall, the baseline median LVMI was 41.3 g/m2.7 (IQR 32–48), and the height-and age-adjusted baseline LVMI Z-score (Table 1) reflected LVH in 11/20 (55%) of patients. The LVMI Z-score sustained a nonsignificant downward trend in 7 patients (35%). More prominent changes were observed in the IVST Z-scores (Table 1), with improvement in 12 patients (60%). The posterior wall thickness Z-score also sustained improvement in 9 (45%) of patients (not shown). Baseline LVH improved significantly (P < 0.05) in 4/11 (36%) on sequential echocardiograms. None of the 9 patients without evidence of baseline LVH developed de-novo LVH on the sequential echocardiogram despite persistent hypertension, indicating no LVMI progression. Neither LVMI nor IVST correlated significantly with systolic or diastolic BP Z-scores, dialysis vintage, interdialytic weight gain, serum calcium, phosphorus, PTH, or 25(OH) D values. FGF23 levels correlated significantly with the IVST (r = 0.6, P < 0.05), serum calcium (r = 0.6, P < 0.005), serum phosphorus (r = 0.6, P < 0.05), and the calcium × phosphorus (mg2) product (r = 0.7, P < 0.05).
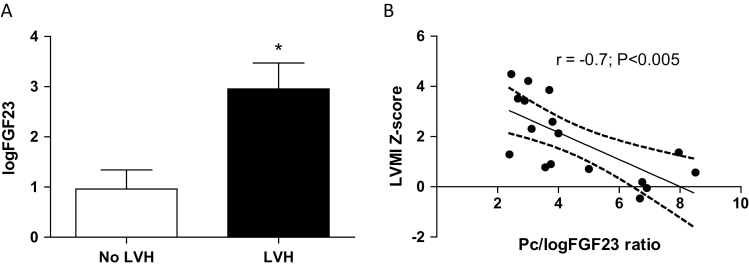
The administered Pc dose averaged 0.33 µg/kg/week amounting to a total cumulative average dose of 15.2 µg/week (Table 1). Of note, 9/20 (45%) of the patients received doses >0.3 µg/kg/week (range 0.31–1.03 µg/kg/week); the weekly dose, unadjusted (µg/week) and adjusted for body weight (µg/kg/week), correlated inversely with LVMI Z-scores (r = −0.5, P < 0.05) and IVST Z-scores (r = −0.5, P < 0.05). Patients with baseline LVH had significantly higher serum FGF23 levels compared with patients without LVH (Figure 4a). Notably, LVMI progressed modestly in only 3/8 patients and improved in 5/8 (on average by 18%) in those with elevations of FGF23 on serial measurements, and the average weekly dose of Pc unadjusted or adjusted for weight did not correlate with the logFGF23 levels. To evaluate whether the beneficial effects of Pc on LVMI were relative to the existing FGF23 levels, we examined the relationship between LVMI Z-scores and the ratio of Pc dose (µg/kg/week) to the log-transformed level of FGF23 (log10FGF23). This Pc/logFGF23 ratio correlated strongly with the LVMI Z-score (Figure 4b), confirming the relation observed in the experimental animals as described above. Multiple regression analysis adjusted for anemia, serum phosphorus, PTH, 25(OH) D levels, dialysis vintage, systolic and diastolic BP Z-scores, and interdialytic weight gain showed that only the Pc/logFGF23 ratio correlated significantly (P < 0.05) with the follow-up LVMI Z-score. These associations, taken together with the higher serum FGF23 levels in patients with LVH, suggest that the cardioprotective effects of Pc depend on the administered dose relative to the existing levels of circulating FGF23 elevations independently of BP levels.
Figure 4.
FGF23 values and baseline LVH, and the relationship between the administered paricalcitol (Pc) dose/logFGF23 and the LVMI Z-score in hemodialysis patients. (a) Patients with LVH (n = 11) on baseline echocardiograms (closed bars) displayed significantly higher log10 FGF23 values (2.9 ± 0.5) compared with those in patients (n = 9) without LVH (0.9 ± 0.4) (open bars); *P < 0.005. Data represent mean ± SEM. (b) The negative correlation (r = –0.7) suggests that the improvement of LVMI Z-scores depends on the administered dose of Pc relative to the prevailing FGF23 levels (the Pc/logFGF23 ratio); n = 17 determinations. Abbreviations: LVH, left ventricular hypertrophy; LVMI, left ventricular mass index.
DISCUSSION
Our experimental and clinical studies show that improvement of LVH in CKD by Pc is modulated by the existing levels of FGF23. In the experimental model, the protective effects of Pc involve suppression of myocardial calcineurin/NFAT signaling, and these effects are incremented by coadministration of a FGFR pan-blocker. In the dialysis patients, protection against myocardial hypertrophy when circulating FGF23 concentrations were very high, required doses of Pc substantially higher than those recommended in clinical practice. Undoubtedly hypertension remains an important determinant of LVH in young patients with CKD and elevated FGF23 levels11 and its treatment is essential, but even when BP is under control LVH may progress,34 and upregulation of FGF23-mediated calcineurin/NFAT signaling constitutes an important etiologic factor.
Our observations in the murine 5/6 Nx model confirm the beneficial effects of Pc on renal function as evidenced by the reduction in serum creatinine and proteinuria in the treated animals.14,15,35 Calcitriol and analogues such as Pc downregulate the expression of gene products involved in renal fibrogenesis such as transforming growth factor β, vascular endothelial growth factor, and components of the RAS resulting in the improvement of glomerular and tubulointerstitial changes with attenuation of renal dysfunction.15,36 In contrast with our previous observations,8 we also demonstrate improved renal function and BP following the administration of the FGFR pan-blocker PD. However, the studies are not strictly comparable because the length of uremia was 50% shorter and the degree milder in the present studies. Nonetheless, the improvement induced by PD alone, or in combination with Pc, raises the possibility that FGF23 may contribute to the development of hypertension and renal dysfunction in experimental CKD. FGF23 was shown to increase distal tubular reabsorption of sodium with upregulation of the sodium chloride cotransporter (NCC) involving the FGFR1c/αKlotho complex, with consequent volume expansion, hypertension, and cardiac hypertrophy, providing an additional mechanism by which FGF23 may contribute to renal and cardiac dysfunction,37 a potential target that may have been affected by the PD administration.
In the uremic rats, we demonstrate a strong inverse relation between cardiac hypertrophy and the ratio of Pc dose/FGF23 levels, suggesting that Pc attenuates cardiac hypertrophy more effectively with higher doses relative to the circulating levels of FGF23. At the same time and as predicted, FGF23 levels increased following VDR agonist treatment,19,36 known to be mediated by VDR binding to a vitamin D responsive element on osteoblasts near the proximal promoter of the FGF23 gene.18 In contrast, the pan-FGFR blocker PD alone resulted in the lowest FGF23 levels as expected from studies demonstrating the role of the FGFR on the osteocyte in mediating the increased expression of FGF23 in experimental uremia.24 Of note, although the suppression of FGF23 secretion from the osteocyte by PD appeared to be overridden by Pc (Figure 2a), cardiac hypertrophy remained significantly attenuated (Figure 2b) and coincided with the reduced mRNA expression of hypertrophy-modulating genes observed in this group given combined Pc+PD (Figures 3b,d,e). The precise mechanisms by which Pc seems to be capable of antagonizing considerably the suppressive effects of PD on FGF23 secretion were not addressed in the present experiments and will require additional studies. Nevertheless, the blocking effects of PD on cardiac FGFR activation17 may have contributed to the attenuated cardiac hypertrophy in the Pc+PD group despite the persistently elevated circulating FGF23 levels. Suppression of myocardial RAS components by Pc (Figure 3b), in agreement with our earlier observations,14 also likely contributed to the attenuation of cardiac hypertrophy. All the components required for angiotensin II (ANG II) production are normally present in the heart,38 and upregulated cardiac RAS components including renin and AGT have been demonstrated in the myocardium of uremic animals with cardiac hypertrophy and fibrosis.14,36 AGT, the initial component of the RAS cascade, was markedly upregulated in the cardiac tissue of the uremic animals. While downregulation of AGT mRNA expression following Pc administration was predicted and confirms our previous observations,14 the downregulation of AGT by PD alone was unexpected, and to our knowledge, it has not been described before, suggesting VDR-independent effects of FGFR signaling on the cardiac RAS. AGT upregulation was recently reported in autopsy heart tissues of deceased patients with advanced CKD and in cultured rat neonatal cardiomyocytes and fibroblasts exposed to recombinant FGF23.39 The mechanisms by which FGF23 potentially activates cardiac AGT and other RAS components including ANG II are not entirely clear and may involve suppression of angiotensin-converting-enzyme-2 (ACE2), an enzyme highly expressed in the kidney and heart that prevents cardiac remodeling and hypertrophy by converting ANG II to the inactive ANG 1–7.40 Rodents with CKD and FGF23 excess display reduced renal tubular expression of ACE2, a negative regulator of the RAS, which favors ANG II activation and thus promotes cardiac hypertrophy.41 Therefore, PD interference with the FGF23-mediated suppression of ACE2 and concurrent activation of the classical RAS cascade provides an explanation for the cardiac AGT downregulation and the attenuation of cardiac hypertrophy observed in the animals given PD alone. Furthermore, administration of recombinant FGF23 reverses the induction of renal ACE2 and prevents angiotensin receptor blocker-induced klotho upregulation in murine CKD,42 and paracrine activation of FGFR1 in the distal tubule provided unexpected cardioprotective effects with attenuation of hypertension and cardiac hypertrophy.43 More recent observations have demonstrated upregulation of FGF23 mRNA by stimulation with ANG II and aldosterone of cardiac myocytes in vitro, suggesting that RAS activation may contribute to local cardiac induction of FGF23.39 We speculate that the administration of Pc in tandem with PD, by suppressing AGT and other RAS genes,14 could have also contributed to reduce FGF23 stimulation in the myocardium. These observations have provided additional layers of complexity to the intricate crosstalk between the FGF23-klotho axis, vitamin D, and the RAS.44
Like others, we also observed that the profound downregulation of the myocardial VDR following 5/6Nx can be corrected by Pc.6,36 However, VDR downregulation was not modified by PD alone, a finding not previously reported with potential clinical relevance when considering the development of future therapies involving selective myocardial FGF-receptor blockers. The VDR is expressed in murine cardiac myocytes,45 a functional VDR has been identified in primary human cardiac fibroblasts,46 VDR downregulation or its absence are linked to cardiac hypertrophy,21 and its integrity possesses potent antihypertrophic properties.6,21,36 The latter appears to be independent of BP and hemodynamic effects because cardiac hypertrophy can improve with significant, moderate, or no changes in BP levels.13,14,36,45 In the present study, BP was similarly reduced in all 3 treated animal groups (Figure 1a), but the attenuation of cardiac hypertrophy was variable (Figure 2b), and the BPs did not correlate with the cardiac weights. The mechanisms underlying the antihypertrophic effects of VDR agonists are not entirely understood and include not only suppression of the myocardial RAS13,14 but also seem to involve renin-independent suppression of the myocardial calcineurin/NFAT target gene RCAN 1, as first demonstrated in nonuremic mice.45
Elevated FGFR4 expression has been reported in myocardial tissues of animals and in stored myocardial samples of deceased humans with CKD.17,47 The present study demonstrates in vivo (i) marked upregulation of mRNA FGFR4 in the myocardium of untreated 5/6Nx animals, (ii) effective downregulation with the pan-FGFR blocker PD alone, and (iii) persistently upregulated expression of FGFR4 following treatment with Pc alone. The latter finding differs from the reported decline of cardiac FGFR4 mRNA in 5/6Nx rats treated with calcitriol.23 While these discrepancies in FGFR4 expression remain unexplained, persistent FGFR4 activation, unaffected by Pc, as a result of elevated FGF23 levels seem to be more closely in line with the expected changes observed following administration of Pc and other VDR agonists.36 Direct transcriptional inhibition of NFAT activation has been described for VDR agonists in interleukin 2 immunocompetent cells,48 and inhibition of the NFAT target gene RCAN1 was demonstrated in the cardiomyocyte of nonuremic mice infused with ANG II.45 We observed reduced expression of RCAN1 and TRPC6 levels in the myocardium of Pc-treated animals, an additional mechanism by which Pc, like calcitriol23 and conceivable other VDR agonists, exerts antihypertrophic effects by direct suppression of the myocardial NFAT target genes in uremia despite concurrent FGF23/FGFR4 activation. All in all, our findings provide a plausible explanation for the improved cardiac hypertrophy observed with VDR agonist therapy despite cardiac FGFR4 activation and persistently elevated FGF23 levels.
Our observations in the patients with advanced CKD confirm the inverse relation between LVH and the ratio of Pc dose/FGF23 levels described in the uremic animals, supporting the notion that higher doses of Pc are required to attenuate LVH when FGF23 levels are substantially elevated. Serial FGF23 measurements may help identify CKD patients with exceptionally high risk of death.49 Our observations in young dialysis-dependent patients with CKD represent, to our knowledge, the first studies demonstrating that Pc treatment was associated with improved ventricular mass, indicated by decreased LVMI in 35% of the patients, and 43% reduction of IVST in 60% of the patients, despite elevated FGF23 levels on serial measurements. Furthermore, 22% of patients sustained reduction of LVH, and LVH did not develop in any patient with normal baseline LVMI despite persistently elevated BP levels. Unlike our cohort, older CKD patients have longer exposures to multiple factors promoting LVH5,7,26,50 and frequently receive lower doses of Pc than usually required by young patients to attain recommended PTH targets.27,28 Paradoxically, VDR agonists stimulate bone production of FGF23,18 further elevating circulating FGF23 levels in CKD,19 potentially counteracting the cardioprotective effects of Pc. The opposing effects of VDR agonists and FGF23 on the myocardium17,51 could explain the lack of LVMI attenuation after Pc treatment reported in previous trials.26,50 FGF23 levels were not determined in any of these trials,26,50 and therefore, it is not possible to determine whether patients with higher FGF23 levels and more prominent LVH would have benefitted from higher doses of Pc. Nonetheless, it appears that cardioprotection requires a Pc dose higher than the usual doses prescribed to maintain parathyroid hormone levels within recommended limits.52 Increasing the Pc dose may be particularly important with rising FGF23 levels in serial determinations because of their association with particularly high mortality risks.49 Raising FGF23 levels is not a rare event, as documented in 40% of our cohort of patients. The lack of a significant correlation between the FGF23 levels and the administered dose of Pc suggests that other factors, such as serum Ca and phosphate, also contribute to the elevation of circulating FGF23, as previously noted in young patients on dialysis53 and confirmed in the present cohort.
The average administered dose of Pc at 0.33 µg/kg/week, 2–3-fold higher as 0.16 µg/kg/week50 and 0.10 µg/kg/week26 given in the adult trials, may partially explain the improvements in LVMI and IVST. Notably, nearly one-half of our patients received even higher Pc doses ranging from 0.31 to 1.0 µg/kg/week. In agreement with this suggestion, young hemodialysis adults receiving Pc at vitamin D equivalent doses twice as high as those treated with calcitriol showed no progression of LVMI at 12 months while those on calcitriol did.52 Future studies will be needed to elucidate the effects of longer periods of higher Pc dosing on myocardial changes in patients on hemodialysis with persistently elevated levels of FGF23.
The present study has limitations and strengths. The clinical portion is retrospective at a single center and includes few patients. Although the average elapsed time between serial echocardiograms was shorter than in the previously reported studies,26,50 the cumulative weight-adjusted dose of Pc received by our cohort probably exceeded that given in those trials lasting nearly twice longer than the present study. A controlled-placebo study would have been ideal, but ethical considerations prevented us from withholding Pc treatment from patients displaying prominent secondary hyperparathyroidism.
Even though Pc failed to significantly reduce LVM in larger randomized studies of adults with moderate CKD,26,50 it did reduce left atrial volume54 and arrested progression of the LVM.52 Larger, more prolonged, and prospective studies, including older adults, may be needed to confirm our findings, including the use of the Pc/log FGF23 ratio as a tool to adjust the Pc dose to the prevailing levels of circulating FGF23.
In summary, we demonstrate in experimental uremia that the combined administration of Pc and a FGF23 blocker antagonizes the effects of elevated FGF23 on cardiac hypertrophy in uremia and that when given alone, Pc effectiveness is dependent on the dose relative to the levels of FGF23. In young patients with advanced CKD on maintenance hemodialysis, we show that administration of high therapeutic doses of Pc is associated with improved LV mass enlargement and LVH despite hypertension and persistently elevated serum levels of FGF23, and we confirm, as noted in the animal studies, that the cardioprotective effects of Pc are incremental with higher doses proportional to the prevailing levels of circulating FGF23.
Supplementary Material
ACKNOWLEDGMENT
This study was partly supported by National Institute of Health grant number 12 RO1HL128714 and by a grant from the Fondo Nacional de Ciencia y Tecnologia (FONACIT) grant 13 2005000283, Venezuela.
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1. Foley RN, Parfrey PS, Harnett JD, Kent GM, Martin CJ, Murray DC, Barre PE. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int 1995; 47:186–192. [DOI] [PubMed] [Google Scholar]
- 2. Mitsnefes MM. Cardiovascular disease in children with chronic kidney disease. J Am Soc Nephrol 2012; 23:578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kupferman JC, Aronson Friedman L, Cox C, Flynn J, Furth S, Warady B, Mitsnefes M; CKiD Study Group . BP control and left ventricular hypertrophy regression in children with CKD. J Am Soc Nephrol 2014; 25:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Siedlecki AM, Jin X, Muslin AJ. Uremic cardiac hypertrophy is reversed by rapamycin but not by lowering of blood pressure. Kidney Int 2009; 75:800–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Freundlich M, Lipshultz SE, Filler G. Chronic kidney disease and cardiac morbidity-what are the possible links?Prog Ped Cardiol 2016; 41:89–95. [Google Scholar]
- 6. Mizobuchi M, Nakamura H, Tokumoto M, Finch J, Morrissey J, Liapis H, Slatopolsky E. Myocardial effects of VDR activators in renal failure. J Steroid Biochem Mol Biol 2010; 121:188–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pilz S, Tomaschitz A, Drechsler C, de Boer RA. Vitamin D and heart disease. Kidney Int Suppl 2011; 1:S111–S115. [Google Scholar]
- 8. Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M. FGF23 induces left ventricular hypertrophy. J Clin Invest 2011; 121:4393–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gutiérrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, Wang TJ, deFilippi C, Wolf M. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 2009; 119:2545–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seeherunvong W, Abitbol CL, Chandar J, Rusconi P, Zilleruelo GE, Freundlich M. Fibroblast growth factor 23 and left ventricular hypertrophy in children on dialysis. Pediatr Nephrol 2012; 27:2129–2136. [DOI] [PubMed] [Google Scholar]
- 11. Mitsnefes MM, Betoko A, Schneider MF, Salusky IB, Wolf MS, Jüppner H, Warady BA, Furth SL, Portale AA. FGF23 and left ventricular hypertrophy in children with CKD. Clin J Am Soc Nephrol 2018; 13:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 2002; 110:229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bodyak N, Ayus JC, Achinger S, Shivalingappa V, Ke Q, Chen YS, Rigor DL, Stillman I, Tamez H, Kroeger PE, Wu-Wong RR, Karumanchi SA, Thadhani R, Kang PM. Activated vitamin D attenuates left ventricular abnormalities induced by dietary sodium in Dahl salt-sensitive animals. Proc Natl Acad Sci USA 2007; 104:16810–16815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Freundlich M, Li YC, Quiroz Y, Bravo Y, Seeherunvong W, Faul C, Weisinger JR, Rodriguez-Iturbe B. Paricalcitol downregulates myocardial renin-angiotensin and fibroblast growth factor expression and attenuates cardiac hypertrophy in uremic rats. Am J Hypertens 2014; 27:720–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Freundlich M, Quiroz Y, Zhang Z, Zhang Y, Bravo Y, Weisinger JR, Li YC, Rodriguez-Iturbe B. Suppression of renin-angiotensin gene expression in the kidney by paricalcitol. Kidney Int 2008; 74:1394–1402. [DOI] [PubMed] [Google Scholar]
- 16. Husain K, Ferder L, Mizobuchi M, Finch J, Slatopolsky E. Combination therapy with paricalcitol and enalapril ameliorates cardiac oxidative injury in uremic rats. Am J Nephrol 2009; 29:465–472. [DOI] [PubMed] [Google Scholar]
- 17. Grabner A, Amaral AP, Schramm K, Singh S, Sloan A, Yanucil C, Li J, Shehadeh LA, Hare JM, David V, Martin A, Fornoni A, Di Marco GS, Kentrup D, Reuter S, Mayer AB, Pavenstädt H, Stypmann J, Kuhn C, Hille S, Frey N, Leifheit-Nestler M, Richter B, Haffner D, Abraham R, Bange J, Sperl B, Ullrich A, Brand M, Wolf M, Faul C. Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell Metab 2015; 22:1020–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, Pi M, Quarles LD. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol 2006; 17:1305–1315. [DOI] [PubMed] [Google Scholar]
- 19. Wesseling-Perry K, Pereira RC, Sahney S, Gales B, Wang HJ, Elashoff R, Jüppner H, Salusky IB. Calcitriol and doxercalciferol are equivalent in controlling bone turnover, suppressing parathyroid hormone, and increasing fibroblast growth factor-23 in secondary hyperparathyroidism. Kidney Int 2011; 79:112–119. [DOI] [PubMed] [Google Scholar]
- 20. Takeuchi A, Reddy GS, Kobayashi T, Okano T, Park J, Sharma S. Nuclear factor of activated T cells (NFAT) as a molecular target for 1alpha,25-dihydroxyvitamin D3-mediated effects. J Immunol 1998; 160:209–218. [PubMed] [Google Scholar]
- 21. Chen S, Law CS, Grigsby CL, Olsen K, Hong TT, Zhang Y, Yeghiazarians Y, Gardner DG. Cardiomyocyte-specific deletion of the vitamin D receptor gene results in cardiac hypertrophy. Circulation 2011; 124:1838–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sonneveld R, Ferrè S, Hoenderop JG, Dijkman HB, Berden JH, Bindels RJ, Wetzels JF, van der Vlag J, Nijenhuis T. Vitamin D down-regulates TRPC6 expression in podocyte injury and proteinuric glomerular disease. Am J Pathol 2013; 182:1196–1204. [DOI] [PubMed] [Google Scholar]
- 23. Leifheit-Nestler M, Grabner A, Hermann L, Richter B, Schmitz K, Fischer DC, Yanucil C, Faul C, Haffner D. Vitamin D treatment attenuates cardiac FGF23/FGFR4 signaling and hypertrophy in uremic rats. Nephrol Dial Transplant 2017; 32:1493–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hassan A, Durlacher K, Silver J, Naveh-Many T, Levi R. The fibroblast growth factor receptor mediates the increased FGF23 expression in acute and chronic uremia. Am J Physiol Renal Physiol 2016; 310:F217–F221. [DOI] [PubMed] [Google Scholar]
- 25. Park CW, Oh YS, Shin YS, Kim CM, Kim YS, Kim SY, Choi EJ, Chang YS, Bang BK. Intravenous calcitriol regresses myocardial hypertrophy in hemodialysis patients with secondary hyperparathyroidism. Am J Kidney Dis 1999; 33:73–81. [DOI] [PubMed] [Google Scholar]
- 26. Wang AY, Fang F, Chan J, Wen YY, Qing S, Chan IH, Lo G, Lai KN, Lo WK, Lam CW, Yu CM. Effect of paricalcitol on left ventricular mass and function in CKD–the OPERA trial. J Am Soc Nephrol 2014; 25:175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Seeherunvong W, Nwobi O, Abitbol CL, Chandar J, Strauss J, Zilleruelo G. Paricalcitol versus calcitriol treatment for hyperparathyroidism in pediatric hemodialysis patients. Pediatr Nephrol 2006; 21:1434–1439. [DOI] [PubMed] [Google Scholar]
- 28. Freundlich M, Abitbol CL. Oral paricalcitol: expanding therapeutic options for pediatric chronic kidney disease patients. Pediatr Nephrol 2017; 32:1103–1108. [DOI] [PubMed] [Google Scholar]
- 29. Singh S, Grabner A, Yanucil C, Schramm K, Czaya B, Krick S, Czaja MJ, Bartz R, Abraham R, Di Marco GS, Brand M, Wolf M, Faul C. Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int 2016; 90:985–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Center of Disease Control and Prevention. CDC Growth Chart <http://wwwcdcgov/growthcharts/clinical_chartshtm> 2011. Accessed 15 December 2011.
- 31. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004; 114:555–576. [PubMed] [Google Scholar]
- 32. Cuervo C, Abitbol CL, Zilleruelo GE, Freundlich M. Fibroblast growth factor-23 and renin-angiotensin system levels in vitamin-D-dependent rickets type I. Pediatr Nephrol 2016; 31:1189–1193. [DOI] [PubMed] [Google Scholar]
- 33. Wesseling-Perry K, Wang H, Elashoff R, Gales B, Jüppner H, Salusky IB. Lack of FGF23 response to acute changes in serum calcium and PTH in humans. J Clin Endocrinol Metab 2014; 99:E1951–E1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Seifert ME, de Las Fuentes L, Ginsberg C, Rothstein M, Dietzen DJ, Cheng SC, Ross W, Windus D, Dávila-Román VG, Hruska KA. Left ventricular mass progression despite stable blood pressure and kidney function in stage 3 chronic kidney disease. Am J Nephrol 2014; 39:392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mizobuchi M, Morrissey J, Finch JL, Martin DR, Liapis H, Akizawa T, Slatopolsky E. Combination therapy with an angiotensin-converting enzyme inhibitor and a vitamin D analog suppresses the progression of renal insufficiency in uremic rats. J Am Soc Nephrol 2007; 18:1796–1806. [DOI] [PubMed] [Google Scholar]
- 36. Panizo S, Barrio-Vázquez S, Naves-Díaz M, Carrillo-López N, Rodríguez I, Fernández-Vázquez A, Valdivielso JM, Thadhani R, Cannata-Andía JB. Vitamin D receptor activation, left ventricular hypertrophy and myocardial fibrosis. Nephrol Dial Transplant 2013; 28:2735–2744. [DOI] [PubMed] [Google Scholar]
- 37. Andrukhova O, Slavic S, Smorodchenko A, Zeitz U, Shalhoub V, Lanske B, Pohl EE, Erben RG. FGF23 regulates renal sodium handling and blood pressure. EMBO Mol Med 2014; 6:744–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wollert KC, Drexler H. The renin-angiotensin system and experimental heart failure. Cardiovasc Res 1999; 43:838–849. [DOI] [PubMed] [Google Scholar]
- 39. Leifheit-Nestler M, Kirchhoff F, Nespor J, Richter B, Soetje B, Klintschar M, Heineke J, Haffner D. Fibroblast growth factor 23 is induced by an activated renin-angiotensin-aldosterone system in cardiac myocytes and promotes the pro-fibrotic crosstalk between cardiac myocytes and fibroblasts. Nephrol Dial Transplant 2018; 33:1722–1734. doi: 10.1093/ndt/gfy006 [DOI] [PubMed] [Google Scholar]
- 40. Kovesdy CP, Quarles LD. FGF23 from bench to bedside. Am J Physiol Renal Physiol 2016; 310:F1168–F1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dai B, David V, Martin A, Huang J, Li H, Jiao Y, Gu W, Quarles LD. A comparative transcriptome analysis identifying FGF23 regulated genes in the kidney of a mouse CKD model. PLoS One 2012; 7:e44161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. de Jong MA, Mirkovic K, Mencke R, Hoenderop JG, Bindels RJ, Vervloet MG, Hillebrands JL, van den Born J, Navis G, de Borst MH; NIGRAM consortium . Fibroblast growth factor 23 modifies the pharmacological effects of angiotensin receptor blockade in experimental renal fibrosis. Nephrol Dial Transplant 2017; 32:73–80. [DOI] [PubMed] [Google Scholar]
- 43. Han X, Ross J, Kolumam G, Pi M, Sonoda J, King G, Quarles LD. Cardiovascular effects of renal distal tubule deletion of the FGF receptor 1 gene. J Am Soc Nephrol 2018; 29:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. de Borst MH, Vervloet MG, ter Wee PM, Navis G. Cross talk between the renin-angiotensin-aldosterone system and vitamin D-FGF-23-klotho in chronic kidney disease. J Am Soc Nephrol 2011; 22:1603–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen S, Gardner DG. Liganded vitamin D receptor displays anti-hypertrophic activity in the murine heart. J Steroid Biochem Mol Biol 2013; 136:150–155. [DOI] [PubMed] [Google Scholar]
- 46. Meredith A, Boroomand S, Carthy J, Luo Z, McManus B. 1,25 Dihydroxyvitamin D3 inhibits TGFβ1-mediated primary human cardiac myofibroblast activation. PLoS One 2015; 10:e0128655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Leifheit-Nestler M, Große Siemer R, Flasbart K, Richter B, Kirchhoff F, Ziegler WH, Klintschar M, Becker JU, Erbersdobler A, Aufricht C, Seeman T, Fischer DC, Faul C, Haffner D. Induction of cardiac FGF23/FGFR4 expression is associated with left ventricular hypertrophy in patients with chronic kidney disease. Nephrol Dial Transplant 2016; 31:1088–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Towers TL, Staeva TP, Freedman LP. A two-hit mechanism for vitamin D3-mediated transcriptional repression of the granulocyte-macrophage colony-stimulating factor gene: vitamin D receptor competes for DNA binding with NFAT1 and stabilizes c-Jun. Mol Cell Biol 1999; 19:4191–4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Isakova T, Cai X, Lee J, Xie D, Wang X, Mehta R, Allen NB, Scialla JJ, Pencina MJ, Anderson AH, Talierco J, Chen J, Fischer MJ, Steigerwalt SP, Leonard MB, Hsu CY, de Boer IH, Kusek JW, Feldman HI, Wolf M; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators . Longitudinal FGF23 trajectories and mortality in patients with CKD. J Am Soc Nephrol 2018; 29:579–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H, Bhan I, Agarwal R, Zoccali C, Wanner C, Lloyd-Jones D, Cannata J, Thompson BT, Andress D, Zhang W, Packham D, Singh B, Zehnder D, Shah A, Pachika A, Manning WJ, Solomon SD. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA 2012; 307:674–684. [DOI] [PubMed] [Google Scholar]
- 51. Ky B, Shults J, Keane MG, Sutton MS, Wolf M, Feldman HI, Reese PP, Anderson CA, Townsend RR, Deo R, Lo J, Gadegbeku C, Carlow D, Sulik MJ, Leonard MB; CRIC Study Investigators . FGF23 modifies the relationship between vitamin D and cardiac remodeling. Circ Heart Fail 2013; 6:817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sezer S, Tutal E, Bal Z, Uyar ME, Bal U, Cakir U, Acar NO, Haberal M. Differential influence of vitamin D analogs on left ventricular mass index in maintenance hemodialysis patients. Int J Artif Organs 2014; 37:118–125. [DOI] [PubMed] [Google Scholar]
- 53. Cano FJ, Freundlich M, Ceballos ML, Rojo AP, Azocar MA, Delgado IO, Ibacache MJ, Delucchi MA, Lillo AM, Irarrázabal CE, Ugarte MF. Longitudinal FGF23 and Klotho axis characterization in children treated with chronic peritoneal dialysis. Clin Kidney J 2014; 7:457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tamez H, Zoccali C, Packham D, Wenger J, Bhan I, Appelbaum E, Pritchett Y, Chang Y, Agarwal R, Wanner C, Lloyd-Jones D, Cannata J, Thompson BT, Andress D, Zhang W, Singh B, Zehnder D, Pachika A, Manning WJ, Shah A, Solomon SD, Thadhani R. Vitamin D reduces left atrial volume in patients with left ventricular hypertrophy and chronic kidney disease. Am Heart J 2012; 164:902–9.e2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.