Abstract
Mild cognitive impairment (MCI) has been extensively investigated in recent decades to identify groups with a high risk of dementia and to establish effective prevention methods during this period. Neuropsychological performance and cortical thickness are two important biomarkers used to predict progression from MCI to dementia. This study compares the cortical thickness and neuropsychological performance in people with MCI and cognitively healthy older adults. We further focus on the relationship between cortical thickness and neuropsychological performance in these two groups. Forty-nine participants with MCI and 40 cognitively healthy older adults were recruited. Cortical thickness was analysed with semiautomatic software, Freesurfer. The analysis reveals that the cortical thickness in the left caudal anterior cingulate (p=0.041), lateral occipital (p=0.009) and right superior temporal (p=0.047) areas were significantly thinner in the MCI group after adjustment for age and education. Almost all neuropsychological test results (with the exception of forward digit span) were significantly correlated to cortical thickness in the MCI group after adjustment for age, gender and education. In contrast, only the score on the Category Verbal Fluency Test and the forward digit span were found to have significant inverse correlations to cortical thickness in the control group of cognitively healthy older adults. The study results suggest that cortical thinning in the temporal region reflects the global change in cognition in subjects with MCI and may be useful to predict progression of MCI to Alzheimer’s disease. The different pattern in the correlation of cortical thickness to the neuropsychological performance of patients with MCI from the healthy control subjects may be explained by the hypothesis of MCI as a disconnection syndrome.
Keywords: cortical thickness, dementia, mild cognitive impairment, neuropsychological performance, magnetic resonance imaging
The significant growth in the population with dementia has been highlighted as a public health priority [1]. A wide range of cognitive impairment is the core symptom of dementia and determines the loss of independent functioning. Mild cognitive impairment (MCI) is a transitional state between normal ageing and dementia [2]. MCI has been extensively investigated in recent decades to identify those with a high risk of dementia and to establish effective prevention methods during this period. Neuropsychological performance and cortical thickness are two important biomarkers used to predict progression from MCI to dementia.
Sub-normative neuropsychological performance is one of the core diagnostic criteria for MCI. A wide range of cognitive impairment, including memory, attention and executive functions, can be found in patients with MCI. In addition to its diagnostic value, neuropsychological assessment also provides a possible means of differentiating high-risk groups for different types of dementia [3].
Along with the rapid development of neuroimaging techniques, the use of cortical thickness as measured on T1-weighted magnetic resonance imaging (MRI) as a biomarker to predict or facilitate early diagnosis of dementia has become a research direction of great interest. Compared to voxel-based morphology (VBM), the measurement of cortical thickness allows more precise measurement in deep sulci and analysis of the morphology as a cortical sheet [4]. Convergent findings strongly suggest a significant difference in cortical thickness amongst normal control patients, those with MCI and those with dementia [5-7]. Furthermore, a longitudinal study of 382 participants who were followed up for 24 months suggested that cortical thickness was sensitive for the early diagnosis of Alzheimer’s disease [8]. Another study reported that a decrease in cortical thickness could be detected in cognitively normal individuals several years before the onset of clinical symptoms [9].
Cortical thickness was suggested to have a close relationship with neuropsychological performance [10]. Despite the consistent evidence in support of this hypothesis, large variations were found across studies in the correlation of cortical thickness to neuropsychological performance amongst normal older adults and those with MCI and AD. Verbal memory performance was found to be associated with the medial temporal cortical thickness in normal subjects [11]. In subjects with MCI, the thickness of the entorhinal and praecuneus cortices predicted learning, whereas the posterior cingulate cortical thickness predicted learning in subjects with AD [12]. Another study suggested that MCI entails a specific cortical thinning relationship with high-level executive outcomes that is qualitatively different from that observed in healthy older adults [13]. This variation in the correlational patterns may shed light on the underlying differences in the cognitive processes and compensatory mechanisms between people with MCI and normal older adults. There is a paucity of research into differences between people with MCI and healthy subjects in the relationship between neuropsychological performance and cortical thickness. Therefore, we conducted this study to compare the cortical thickness and neuropsychological performance between subjects with MCI and healthy older adults. The relationship between the cortical thickness and neuropsychological performance in these two groups was also examined. We hypothesised that subjects with MCI would have thinner cortices and would display worse neuropsychological performance than healthy older adults. The correlation between the brain cortical thickness and a specific neuropsychological performance may have different patterns in these two groups.
MATERIALS AND METHODS
Subjects
Forty-nine patients with MCI and 40 cognitively healthy elderly control subjects (healthy controls; HC) were recruited. All of the participants were recruited from local elderly community centres. The study was approved by the Clinical Research Ethics Committee of The Chinese University of Hong Kong (NTEC-CUHK ethics committee). Written informed consent was obtained from all of the participants.
All of the participants underwent a battery of neuropsychological tests to evaluate their cognitive functions.
The Cantonese version of the Mini-Mental State Examination (CMMSE) [14, 15] was used to evaluate general cognitive function. The Clinical Dementia Rating (CDR) [16] scale was used to measure the severity of dementia. The Chinese version of the Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog) [17, 18] was used to assess the global cognitive deficit in patients with MCI. In addition, the forward and backward digit span tests from the Wechsler Adult Intelligence Scale [19] were used to assess the function of short-term memory and working memory, respectively. The Category Verbal Fluency Test (CVFT) [20, 21] was used to examine executive and semantic memory functions. The diagnosis of MCI was made by expert neurologists based on the Mayo Clinic Criteria [2], which includes (1) subjective memory complaints, (2) objective memory impairment (i.e., delayed recall scores of at least 1.5 standard deviations below age- and education-matched persons with a CDR of 0), (3) intact daily life activities, (4) a CDR score of 0.5 and (5) no clinical dementia (CMMSE score > 22 for older adults with more than 2 years of education, CMMSE score > 20 for older adults with less than 2 years of education and CMMSE score > 19 for older adults with no education [22]. Participants with profound sensory deficits or psychiatric (i.e., dependence on alcohol or other substances) and/or neurological disorders other than dementia (i.e., head trauma, multiple sclerosis and Parkinson’s disease) were excluded.
MRI acquisition
The MRI images were acquired using a 3 Tesla Philips MRI scanner (Achieva TX, Philips Medical Systems, Best, the Netherlands) with an eight-channel SENSE head coil. A 3D high-resolution T1-weighted anatomical image was obtained for each participant (repetition time [TR] = 7.4 ms; echo time [TE] = 3.4 ms; flip angle = 8°; voxel size = 1.04 × 1.04 × 0.6 mm3).
Cortical thickness analysis
The image data were exported from the MRI scanner to a personal computer for morphometric analysis. Before analysis, all images were checked for severe head motion. Semi-automatic software, the FreeSurfer version 5.3 software package (http://surfer.nmr.mgh.harvard.edu), was used to obtain estimates of cortical thickness, which was measured by reconstructing representations of the grey/white matter boundary and the cortical surface and then calculating the distance between those surfaces at numerous points (vertices) across the cortical mantle [23, 24]. Failures in FreeSurfer’s initial Talairach alignments were identified by visual inspection of all images and were rectified before reconstruction of the cortical surfaces. Topological defects in the automatically determined grey/white matter boundary were manually corrected. The cortical thickness values of 68 structures based on the Desikan-Killiany atlas were extracted from FreeSurfer [25]. All analyses were performed without knowledge of the subjects' identity.
Statistical analysis
Linear regression adjusted for age and education was used for statistical analyses of the mean cortical thickness of region of interests (ROIs) between the subjects in the MCI and normal control groups, and p values of less than 0.05 were considered to indicate statistical significance. Partial correlations between neuropsychological scores and mean cortical thickness, adjusted for age, sex and years of education, were calculated for both MCI and control groups. Bonferroni correction was applied to correct for multiple comparisons, and p values of less than 0.01 were considered to indicate statistical significance after correction.
RESULTS
Demographic and baseline data
Table 1 shows significant differences in age and education between the MCI group and the HC group. Compared with those with MCI, the participants in the HC group were younger (mean [SD], 69.45 [4.56] vs. 75.92 [5.39]) and had more years of education (mean [SD], 8.00 [4.00] vs. 4.13 [4.04]). No significant difference was found in the gender ratio. The participants with MCI had significantly lower scores on the CMMSE, CDR sum of boxes, ADAS-Cog, CVFT and forward and backward digit span tests than the subjects in the HC group (p<0.05). The mean CMMSE score in the MCI group was 24.94, and that in the HC group was 27.68.
Table 1.
Participant demographics and neuropsychological performance.
| Healthy Controls (n=40) Mean (SD) |
MCI (n=49) Mean (SD) |
p-value | |
|---|---|---|---|
| Age | 69.45 (4.56) | 75.92 (5.39) | <0.001 |
| Gender (Male: Female) | 15:25 | 26:23 | 0.143 |
| Education (years) | 8.00 (4.00) | 4.13 (4.04) | <0.001 |
| CMMSE | 27.68 (2.51) | 24.94 (2.85) | <0.001 |
| CDR - sum of boxes | 0.16 (0.43) | 1.02 (1.04) | <0.001 |
| ADAS-Cog | 6.46 (2.57) | 13.59 (3.61) | <0.001 |
| Delayed recall | 6.58 (1.47) | 2.29 (1.46) | <0.001 |
| CVFT | 40.10 (7.58) | 31.27 (8.03) | <0.001 |
| Digit span test (forward) | 7.50 (1.36) | 6.80 (1.44) | 0.021 |
| Digit span test(backward) | 3.93 (1.65) | 2.59 (1.39) | <0.01 |
ADAS-Cog - Chinese version of the Alzheimer’s Disease Assessment Scale–Cognitive Subscale; CDR - Clinical Dementia Rating; CMMSE - Cantonese version of the Mini-Mental State Examination; CVFT - Category Verbal Fluency Test
Difference in cortical thickness between MCI and HC groups
The mean cortical thicknesses of all areas in the brain are shown in Table 2 for the MCI group and the HC group. Analysis reveals significantly less cortical thickness in the left caudal anterior cingulate (p=0.041), left lateral occipital (p=0.009) and right superior temporal (p=0.047) areas in the MCI group after adjustment for age and education.
Table 2.
Cortical thickness in healthy control and mild cognitive impairment (mean +/- S.D., mm, adjusted for age and education).
| Healthy Control | MCI | |||
|---|---|---|---|---|
|
|
||||
| Brain region | Left | Right | Left | Right |
| Caudal anterior cingulate gyrus | 2.689 (0.315) * | 2.599 (0.296) | 2.502 (0.378)* | 2.512 (0.290) |
| Caudal middle frontal gyrus | 2.258 (0.168) | 2.262 (0.148) | 2.218 (0.131) | 2.243 (0.145) |
| Cuneus | 1.618 (0.125) | 1.619 (0.118) | 1.612 (0.125) | 1.606 (0.117) |
| Entorthinal area | 3.403 (0.392) | 3.605 (0.487) | 3.288 (0.340) | 3.522 (0.413) |
| Fusiform gyrus | 2.639 (0.148) | 2.603 (0.156) | 2.577 (0.158) | 2.554 (0.188) |
| Inferior parietal lobe | 2.164 (0.123) | 2.115 (0.113) | 2.142 (0.135) | 2.122 (0.148) |
| Inferior temporal gyrus | 2.695 (0.161) | 2.681 (0.154) | 2.613 (0.158) | 2.636 (0.184) |
| Isthmus cingulate gyrus | 2.416 (0.187) | 2.302 (0.225) | 2.267 (0.229) | 2.195 (0.206) |
| Lateral occipital gyrus | 1.902 (0.130)* | 1.879 (0.126) | 1.899 (0.152)* | 1.874 (0.147) |
| Lateral orbitofrontal gyrus | 2.522 (0.140) | 2.469 (0.153) | 2.510 (0.164) | 2.430 (0.166) |
| Lingual gyrus | 1.787 (0.118) | 1.810 (0.087) | 1.782 (0.144) | 1.779 (0.167) |
| Medial orbitofrontal gyrus | 2.283 (0.170) | 2.369 (0.164) | 2.289 (0.181) | 2.612 (0.165) |
| Middle temporal gyrus | 2.670 (0.172) | 2.746 (0.139) | 2.660 (0.142) | 2.715 (0.169) |
| Parahippocampal gyrus | 2.535 (0.230) | 2.557 (0.256) | 2.378 (0.303) | 2.489 (0.264) |
| Paracentral gyrus | 2.271 (0.179) | 2.270 (0.158) | 2.223 (0.179) | 2.222 (0.158) |
| Pars opercularis | 2.357 (0.173) | 2.366 (0.135) | 2.351 (0.120) | 2.352 (0.142) |
| Pars orbitalis | 2.539 (0.217) | 2.509 (0.235) | 2.471 (0.221) | 2.494 (0.247) |
| Pars triangularis | 2.245 (0.134) | 2.279 (0.148) | 2.202 (0.134) | 2.213 (0.162) |
| Periphery calcarine | 1.385 (0.878) | 1.427 (0.103) | 1.414 (0.123) | 1.446 (0.128) |
| Postcentral gyrus | 1.819 (0.132) | 1.765 (0.104) | 1.779 (0.123) | 1.787 (0.118) |
| Posterior cingulate gyrus | 2.440 (0.221) | 2.395 (0.198) | 2.345 (0.175) | 2.325 (0.177) |
| Precentral gyrus | 2.364 (0.151) | 2.343 (0.124) | 2.312 (0.136) | 2.284 (0.144) |
| Precuneus | 2.128 (0.141) | 2.064 (0.119) | 2.086 (0.161) | 2.047 (0.141) |
| Rostral anterior cingulate gyrus | 2.820 (0.199) | 2.882 (0.248) | 2.744 (0.223) | 2.802 (0.286) |
| Rostral middle frontal gyrus | 2.110 (0.137) | 2.154 (0.120) | 2.090 (0.141) | 2.139 (0.139) |
| Superior frontal gyrus | 2.518 (0.146) | 2.540 (0.142) | 2.475 (0.141) | 2.503 (0.137) |
| Superior parietal lobe | 1.884 (0.135) | 1.843 (0.122) | 1.863 (0.126) | 1.831 (0.121) |
| Superior temporal gyrus | 2.563 (0.146) | 2.596 (0.177)* | 2.491 (0.161) | 2.574 (0.155)* |
| Supramarginal gyrus | 2.298 (0.126) | 2.229 (0.149) | 2.219 (0.141) | 2.201 (0.135) |
| Frontal pole | 2.671 (0.263) | 2.634 (0.210) | 2.597 (0.256) | 2.593 (0.275) |
| Temporal pole | 3.638 (0.267) | 3.759 (0.301) | 3.513 (0.283) | 3.625 (0.293) |
| Transverse temporal gyrus | 2.148 (0.252) | 2.106 (0.254) | 2.070 (0.197) | 2.107 (0.203) |
| Insula | 2.891 (0.157) | 2.879 (0.175) | 2.861 (0.158) | 2.800 (0.165) |
p<0.05
Correlation between cortical thickness and neuropsychological performance in MCI group
Almost all neuropsychological performance, except for the forward digit span, was significantly correlated with the cortical thickness (Table 3). The CMMSE score showed a significant correlation with the right inferior temporal gyrus (r=0.508; p<0.01; Fig. 1). The CDR sum of boxes score showed a significant correlation with the left parahippocampal gyrus (r=-0.413; p<0.01; Fig. 2). The performance on the ADAS-Cog showed a significant correlation with the left entorhinal area (r=-0.413; p<0.01). The CVFT score showed a significant correlation with the right rostral middle gyrus (r=0.398; p<0.01). Scores on the backward digit span test showed significant correlations with the right pars orbitalis (r=0.408; p<0.01). A thicker cortex in these regions was associated with better performance on the CVFT and on the backward digit span test.
Table 3.
Correlation between neuropsychological performance and cortical thickness in mild cognitive impairment.
| CMMSE | CDR-Sum of boxes | ADAS-Cog | CVFT | Forward digit span |
Backward Digit span |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Brain region | Left | Right | Left | Right | Left | Right | Left | Right | Left | Right | Left | Right |
| Caudal anterior cingulate gyrus | -.077 | .075 | -.005 | .042 | -.139 | -.047 | .041 | .075 | -.108 | -.213 | -.104 | .172 |
| Caudal middle frontal gyrus | -.202 | -.171 | .309 | .302 | -.061 | .142 | -.108 | -.152 | -.022 | -.184 | -.059 | .018 |
| Cuneus | -.050 | .032 | -.062 | -.051 | .062 | -.097 | .209 | .221 | -.151 | -.090 | .065 | .120 |
| Entorthinal area | .173 | .323 | -.262 | -.366 | *-.413 | -.259 | .349 | .335 | .101 | .246 | -.301 | -.228 |
| Fusiform gyrus | .213 | .239 | -.159 | -.337 | -.137 | -.204 | .106 | .178 | .156 | .162 | -.125 | -.016 |
| Inferior parietal lobe | .096 | .029 | .091 | .083 | .003 | .058 | .163 | .117 | -.109 | -.110 | -.059 | .074 |
| Inferior temporal gyrus | .191 | *.508 | -.023 | -.198 | -.216 | -.369 | .350 | .262 | .023 | .214 | -.102 | .101 |
| Isthmus cingulate gyrus | .336 | .201 | -.118 | -.116 | -.277 | -.193 | -.043 | -.032 | .115 | .134 | .120 | .246 |
| Lateral occipital gyrus | .075 | -.017 | -.133 | .005 | -.035 | -.040 | .085 | -.016 | .007 | .092 | .085 | .225 |
| Lateral orbitofrontal gyrus | -.028 | -.040 | .202 | .003 | -.053 | -.044 | .231 | .125 | .085 | -.038 | .176 | -.021 |
| Lingual gyrus | .108 | .185 | -.111 | -.060 | -.119 | -.226 | .172 | .209 | .048 | .125 | .165 | .191 |
| Medial orbitofrontal gyrus | .076 | .046 | -.023 | .033 | .030 | -.148 | .334 | .376 | .038 | .066 | .118 | .047 |
| Middle temporal gyrus | .212 | .359 | .084 | -.182 | -.025 | .048 | .131 | .137 | -.072 | -.083 | -.137 | .180 |
| Parahippocampal gyrus | .215 | .200 | *-.413 | -.317 | -.061 | -.193 | -.111 | -.012 | .004 | .131 | -.337 | -.190 |
| Paracentral gyrus | -.144 | .043 | .122 | .197 | -.073 | -.044 | .005 | .048 | -.244 | -.098 | .005 | .054 |
| Pars opercularis | .031 | .001 | .160 | .101 | -.031 | -.117 | .114 | .126 | -.117 | .131 | .028 | -.187 |
| Pars orbitalis | -.013 | .059 | .311 | .221 | .029 | .099 | -.175 | -.050 | .277 | .245 | .315 | *.408 |
| Pars triangularis | .045 | .051 | .058 | -.009 | -.124 | -.155 | .170 | .159 | .013 | .138 | .227 | .302 |
| Pericalcarine | -.029 | -.251 | .048 | .072 | -.069 | -.117 | .173 | .194 | .010 | .010 | .194 | .038 |
| Postcentral gyrus | -.170 | -.188 | .131 | .107 | -.013 | .035 | .049 | .247 | -.114 | -.146 | .042 | .081 |
| Posterior cingulate gyrus | .039 | .040 | -.012 | .096 | -.116 | .057 | .100 | -.036 | -.046 | -.120 | -.067 | .181 |
| Precentral gyrus | -.044 | -.165 | .011 | .093 | .012 | -.088 | -.040 | .003 | -.193 | -.066 | -.090 | -.044 |
| Precuneus | .102 | .134 | -.032 | .060 | -.151 | -.115 | .203 | .184 | -.021 | -.106 | .032 | .022 |
| Rostral anterior cingulate gyrus | -.028 | -.070 | .284 | .239 | .024 | .214 | .016 | -.148 | -.096 | -.090 | .087 | -.014 |
| Rostral middle frontal gyrus | -.267 | -.100 | .185 | .116 | .071 | -.006 | .260 | *.398 | .017 | -.103 | .053 | .023 |
| Superior frontal gyrus | -.196 | -.190 | .391 | .255 | .032 | -.002 | .008 | .048 | -.210 | -.098 | .007 | -.119 |
| Superior parietal lobe | .002 | .029 | -.024 | .020 | -.089 | -.028 | .151 | .188 | -.008 | -.004 | .128 | .260 |
| Superior temporal gyrus | .247 | .232 | -.142 | -.242 | -.089 | .036 | .324 | .235 | .086 | .084 | .127 | .050 |
| Supramarginal gyrus | .092 | .026 | .167 | -.027 | -.174 | -.039 | .175 | .112 | -.021 | .109 | .062 | .149 |
| Frontal pole | .104 | .176 | -.150 | -.089 | .042 | -.080 | .047 | .324 | .056 | -.049 | .323 | -.005 |
| Temporal pole | .115 | .256 | -.215 | -.175 | -.187 | -.209 | .356 | 252 | .208 | .085 | .021 | -.041 |
| Transverse temporal gyrus | -.267 | -.188 | .198 | .089 | .131 | .251 | .029 | -.170 | -.173 | -.002 | .254 | .156 |
| Insula | .092 | .116 | .237 | -.012 | -.162 | -.299 | .276 | .322 | -.051 | .079 | .007 | -.078 |
p<0.01. ADAS-Cog - Chinese version of the Alzheimer’s Disease Assessment Scale-Cognitive Subscale; CDR - Clinical Dementia Rating; CMMSE - Cantonese version of the Mini-Mental State Examination; CVFT - Category Verbal Fluency Test
Figure 1.
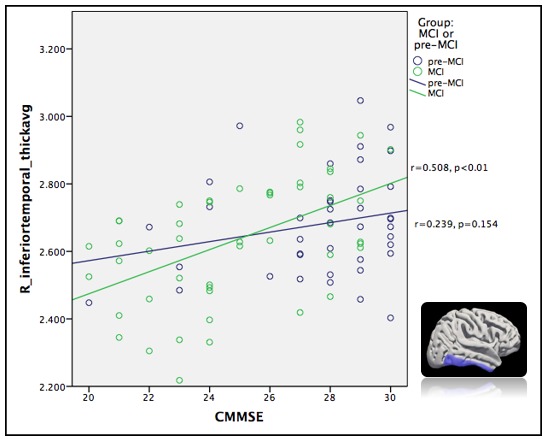
Correlation between right temporal gyrus and Cantonese version of the Mini-Mental State Examination (CMMSE).
Figure 2.
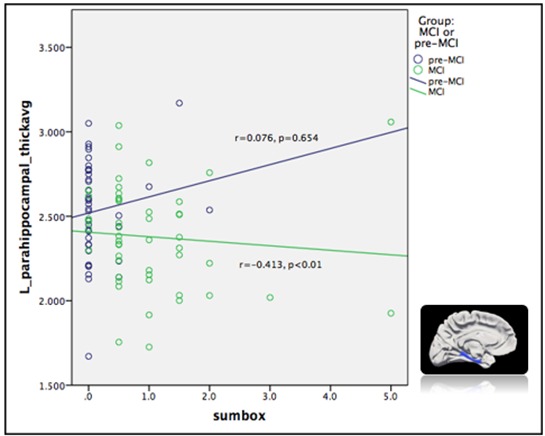
Correlation between left parahippocampal gyrus and Clinical Dementia Rating (CDR)- sum of boxes.
Correlation between cortical thickness and neuropsychological performance in HC group
Only the scores on the CVFT and the forward digit span test were found to have significant correlations with cortical thickness in the HC group (Table 4). The CVFT score showed an inverse correlation with the left middle temporal gyrus (r=-0.445; p<0.01), whilst the forward digit span test score showed a significant inverse correlation with the left pars opercularis (r=-0.496; p<0.01), the left rostral middle frontal gyrus (r=-0.422; p<0.01) and the right orbitofrontal cortex (r=-0.456; p<0.01). A thicker cortex in these regions was associated with poorer performance on the CVFT and on the forward digit span test.
Table 4.
Correlation between neuropsychological performance and cortical thickness in healthy control.
| CMMSE | CDR-Sum of boxes | ADAS-Cog | CVFT | Forward digit span |
Backward Digit span |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Brain region | Left | Right | Left | Right | Left | Right | Left | Right | Left | Right | Left | Right |
| Caudal anterior cingulate gyrus | .010 | -.062 | -.159 | -.177 | .104 | .257 | .131 | -.166 | .110 | -.063 | -.076 | -.133 |
| Caudal middle frontal gyrus | -.066 | .116 | .220 | .164 | .182 | .106 | -.031 | -.043 | -.314 | -.253 | .116 | .072 |
| Cuneus | -.060 | .041 | .185 | .025 | .169 | .095 | .021 | .056 | -.230 | -.057 | .155 | .143 |
| Entorthinal area | -.252 | -.175 | .161 | .162 | .272 | .211 | -.229 | -.227 | -.091 | -.069 | -.096 | -.210 |
| Fusiform gyrus | -.015 | -.027 | .122 | .131 | .268 | .171 | -.081 | -.100 | -.183 | -.242 | -.053 | -.100 |
| Inferior parietal lobe | -.055 | .128 | .158 | -.012 | .145 | .176 | -.024 | .006 | -.156 | -.009 | .081 | .182 |
| Inferior temporal gyrus | -.006 | .239 | .002 | -.230 | .073 | -.154 | -.199 | -.090 | -.104 | -.008 | .228 | .013 |
| Isthmus cingulate gyrus | -.139 | -.137 | .377 | .249 | .271 | .342 | -.148 | -.232 | -.293 | -.130 | .058 | .247 |
| Lateral occipital gyrus | .229 | .277 | -.108 | -.105 | .018 | .088 | .124 | .197 | .028 | -.009 | .259 | .277 |
| Lateral orbitofrontal gyrus | -.278 | -.234 | .389 | .284 | .153 | .126 | -.300 | -.049 | -.396 | -.416 | .127 | .076 |
| Lingual gyrus | -.079 | .222 | .105 | .001 | .197 | .131 | .122 | .246 | -.405 | .017 | -.004 | .216 |
| Medial orbitofrontal gyrus | -.191 | -.068 | .263 | -.046 | .122 | .121 | -.227 | -.182 | -.393 | *-.456 | -.003 | .079 |
| Middle temporal gyrus | -.125 | .043 | .309 | .006 | .248 | .085 | *-.445 | -.306 | -.195 | -.133 | .205 | .174 |
| Parahippocampal gyrus | -.180 | -.151 | .076 | -.007 | .109 | -.029 | -.293 | -.201 | -.241 | -.235 | -.046 | -.131 |
| Paracentral gyrus | .096 | -.086 | .036 | .124 | .233 | .116 | .130 | .044 | -.139 | -.235 | .287 | .065 |
| Pars opercularis | -.211 | .117 | .303 | .193 | .340 | .153 | -.094 | .042 | *-.496 | -.258 | .043 | -.005 |
| Pars orbitalis | -.228 | -.187 | .261 | .040 | -.043 | .064 | .064 | -.045 | -.225 | -.355 | .075 | .011 |
| Pars triangularis | -.200 | -.038 | .333 | .207 | .261 | .041 | -.008 | -.031 | -.367 | -.116 | -.116 | -.080 |
| Pericalcarine | -.177 | -.109 | .249 | .097 | .187 | .091 | .036 | .020 | -.237 | -.331 | -.040 | -.093 |
| Postcentral gyrus | .144 | -.103 | -.017 | .057 | -.011 | .181 | -.004 | .039 | -.009 | -.093 | .145 | -.027 |
| Posterior cingulate gyrus | -.061 | -.046 | .000 | .064 | .306 | .379 | -.074 | -.039 | -.044 | -.144 | .125 | .095 |
| Precentral gyrus | -.049 | .016 | .145 | .220 | .110 | .183 | -.031 | -.015 | -.326 | -.183 | .217 | .132 |
| Precuneus | .059 | -.028 | -.060 | .179 | .199 | .170 | .077 | .001 | -.091 | -.143 | .323 | .204 |
| Rostral anterior cingulate gyrus | -.108 | -.117 | -.111 | -.086 | .068 | -.035 | -.142 | .023 | -.189 | -.201 | -.171 | -.090 |
| Rostral middle frontal gyrus | -.276 | .054 | .267 | .064 | .037 | -.003 | -.181 | -.092 | *-.422 | -.162 | -.183 | .247 |
| Superior frontal gyrus | .063 | -.001 | .023 | .158 | .137 | .196 | -.025 | -.117 | -.166 | -.295 | .092 | .088 |
| Superior parietal lobe | .092 | -.037 | .092 | .100 | .168 | .173 | .039 | .021 | -.074 | -.173 | .267 | .271 |
| Superior temporal gyrus | .136 | .213 | .093 | -.188 | .202 | .177 | .008 | -.029 | -.142 | .205 | .011 | .126 |
| Supramarginal gyrus | .093 | -.020 | .134 | -.105 | .187 | .045 | -.096 | -.103 | -.084 | -.092 | .056 | .038 |
| Frontal pole | -.051 | .030 | -.051 | -.074 | .037 | -.174 | -.238 | .074 | -.132 | -.086 | .231 | .259 |
| Temporal pole | -.007 | .131 | -.037 | -.069 | .080 | -.021 | -.198 | -.226 | .025 | .076 | .105 | .101 |
| Transverse temporal gyrus | -.033 | .232 | .051 | -.289 | .131 | .051 | .022 | .235 | -.118 | .214 | .026 | .260 |
| Insula | .057 | -.078 | .038 | .206 | -.060 | .132 | -.220 | -.274 | -.155 | -.229 | -.008 | -.083 |
p<0.01 ADAS-Cog - Chinese version of the Alzheimer’s Disease Assessment Scale-Cognitive Subscale; CDR - Clinical Dementia Rating; CMMSE - Cantonese version of the Mini-Mental State Examination; CVFT - Category Verbal Fluency Test
DISCUSSION
In this study, we compared the differences in cortical thickness between participants with MCI and those in an HC group. We also examined the association between neuropsychological performance and cortical thickness. The neuropsychological performance of the MCI group was significantly worse than that of the HC group, which was expected. We found significant thinning in the anterior cingulate and superior temporal regions in participants with MCI compared with those in the HC group. This result is in line with the results of previous studies [26]. It was suggested that cortical thinning begins in the temporal region and spreads to other areas [27]. In addition, the anterior cingulate region was reported in a previous study to be more sensitive comparing to other brain regions to early AD-related changes [6]. Both features were noted in our findings. The cortical thicknesses of these two areas may be useful for early identification of subjects with MCI. In addition to these two areas, the left lateral occipital region was found to be significantly thinner in the MCI group. It was relatively uncommon to note atrophy in the occipital region in subjects with MCI, but studies have nonetheless shown significant increases in the atrophy rate of the occipital region in subjects with AD and MCI [28].
Correlation between cortical thickness and neuropsychological performance in subjects with MCI
Global cognition as measured by the CMMSE and the CDR sum of boxes showed a moderate correlation with the temporal area in participants with MCI; temporal atrophy is a hallmark of early AD-related changes. Therefore, our finding supports the notion that cortical thinning in this region is directly linked to a decline in global cognition. This may further support the use of the cortical thickness of the temporal area to predict the progression of MCI to AD.
Difference in correlational patterns
The participants with MCI showed significant correlations between the cortical thickness in various brain areas and each of the neuropsychological performance measures, with the exception of the forward digit span test, but normal older adults showed significant correlations between cortical thickness and two neuropsychological measures only. No global cognition scores such as those on the CMMSE or the CDR sum of boxes were found to have a significant correlation with the cortical thickness in the HC group. One possible explanation for this finding is the ceiling effect of neuropsychological measures in the HC group. However, it could not explain the lack of correlation in tests such as the ADAS-Cog, CVFT and the forward digit span test, in which no prominent ceiling effects were noted. Another postulation is that the participants in the HC group had better connectivity across the whole brain and, therefore, a better compensatory mechanism. When one brain area appeared to be dysfunctional due to the loss of grey matter, other brain areas could compensate so that neuropsychological performance and global cognition are relatively maintained. In participants with MCI, due to the lower degree of connectivity across the whole brain, neuropsychological performance and global cognition directly reflected the severity of cortical thinning without compensation by other brain areas.
The second possible explanation is supported by recent research findings suggesting that MCI and AD represent a disconnection syndrome and that the cognitive impairment results from a decrease in the effectiveness of whole-brain connectivity [29, 30]. A growing body of evidence shows an alteration of functional connectivity in patients with MCI and AD, compared with health control subjects [31, 32]. The connectivity is usually increased in the local area or lobe but significantly decreased across different lobes of the brain [33]. In addition to the functional connectivity, alteration of the structural connectivity, as measured by white matter integrity, has also been reported in patients with prodromal AD [34]. Such Weakening of both functional and structural connectivity may affect the compensatory mechanism. The efficiency of the brain’s function as a single unit may then decrease. Cognitive functions become compartmentally dependent upon one or two areas and are more susceptible to degeneration and loss of neuronal cells. Further study that involves concomitant structural and functional connectivity investigation is needed to verify that the difference in the relationship between regional cortical thickness and neuropsychological performance between healthy and MCI subjects is due to changes in connectivity.
In our study, scores on the CVFT and the digit backward span test showed a positive correlation with cortical thickness in the MCI group. This means that a decrease in cortical thickness is associated with poorer performance on neuropsychological tests, which is compatible with our previous hypothesis. The neuropsychological performance may be more dependent upon the integrity of grey matter in specific brain regions in subjects with MCI due to the impairment of whole-brain connectivity. However, the HC group members had the opposite result: the CVFT and the forward digit span test scores showed a negative correlation with cortical thickness, which means that an increase in cortical thickness is associated with poorer performance on neuropsychological tests. The Previous study also showed that the positive correlation between brain volume and cognition was not found in healthy subjects [35]. One of the possibilities is that the neuropsychological tests were not sensitive enough to reflect the changes in the preclinical phase. The healthy subjects may have AD pathology without symptoms. The previous study found neuronal hypertrophy in the hippocampus and anterior cingulate gyrus neurons among asymptomatic AD patients compared with MCI and control, which may be due to compensation at the local level [36]. Such local compensation may increase cortical thickness but have limited effect on the neuropsychological performance, causing the negative correlation between cortical thickness and neuropsychological performance. However, we could not confirm this explanation in the current study without measurement of AD pathology in our subjects.
Most cognitive training targets deficits in individual cognitive domains. For example, if someone was noted to have a memory problem, the most direct treatment would be to train the memory domain only. However, the effectiveness of this kind of training is in doubt [37]. The effects of training are often short-lived, and the improvement does not translate to daily functions. This phenomenon may be explained by the theory of the disconnection syndrome. The impairment of cognition is due to the connectivity problem rather than solely due to the loss of function of the individual brain areas responsible for that cognitive function. If this is really the case, the aim of cognitive training should be to enhance brain connectivity instead of training up individual cognitive domains. Such connectivity training may have longer and better effects and could likely be generalised to improvement in daily functioning. Further study is needed to demonstrate this conceptual idea.
Limitations of study
There were a few limitations of this study. First, the sample size was relatively small and may result in under-power of the current study to detect the difference between the groups. Besides, the pattern difference in correlation between neuropsychological performance and the cortical thickness between MCI and HC groups was mainly descriptive instead of the direct statistical result in the current study. Further study with larger sample size would be needed in order to perform the direct statistical test for comparing the correlation between two groups because a significant amount of multiple comparisons would be involved. Another limitation of our study is the significant difference in education level and age between the MCI group and the HC group; the participants in the MCI group were older and had lower education levels. Comparison of the two groups and correlational analysis were performed with education and age as co-variates to minimise the effect of a baseline difference between the two groups. At last, we had not done the familywise correction for the cortical thickness comparison, which may increase the chance of the false positive result in the current study.
Conclusions
Our findings suggest that the MCI group had significant thinning over the right temporal, left anterior cingulate and left lateral occipital regions compared with the HC group. Cortical thinning in the temporal region was associated with the global cognition change in participants with MCI and may be useful to predict the progression of MCI to AD. The different pattern between the MCI group and the HC group in the correlation of cortical thickness to neuropsychological performance may be explained by the hypothesis of MCI as a disconnection syndrome. Further imaging studies such as resting state and diffusion tensor imaging are warranted to investigate the alteration in functional and structural connectivity in subjects with MCI. Treatment for cognitive impairment should be directed to the enhancement of brain connectivity in view of the role that a disconnection problem plays in cognitive decline.
Acknowledgements
The authors thank internal research grant from the Education University of Hong Kong for their support in providing funding. We would also like to thank Dr Corine Wong for the expert advice on the statistical part of current study.
References
- [1].Prince MJ, Wimo A, Guerchet MM, Ali GC, Wu YT, Prina M. World Alzheimer Report 2015. - The Global Impact of Dementia: An analysis of prevalence, incidence, cost and trends. London: Alzheimer's Disease International; 2015. [Google Scholar]
- [2].Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST (2001). Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology, 56:1133–1142. [DOI] [PubMed] [Google Scholar]
- [3].Schindler SE, Jasielec MS, Weng H, Hassenstab JJ, Grober E, McCue LM, et al. (2017). Neuropsychological measures that detect early impairment and decline in preclinical Alzheimer disease. Neurobiol Aging, 56:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lerch JP, Evans AC (2005). Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage, 24:163–173. [DOI] [PubMed] [Google Scholar]
- [5].Ries ML, Carlsson CM, Rowley HA, Sager MA, Gleason CE, Asthana S, et al. (2008). Magnetic resonance imaging characterization of brain structure and function in mild cognitive impairment: a review. J Am Geriatr Soc, 56:920–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhao H, Li X, Wu W, Li Z, Qian L, Li S, et al. (2015). Atrophic Patterns of the Frontal-Subcortical Circuits in Patients with Mild Cognitive Impairment and Alzheimer's Disease. PLoS One, 10:e0130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Li C, Wang J, Gui L, Zheng J, Liu C, Du H (2011). Alterations of whole-brain cortical area and thickness in mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis, 27:281–290. [DOI] [PubMed] [Google Scholar]
- [8].Querbes O, Aubry F, Pariente J, Lotterie JA, Demonet JF, Duret V, et al. (2009). Early diagnosis of Alzheimer's disease using cortical thickness: impact of cognitive reserve. Brain, 132:2036–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pettigrew C, Soldan A, Zhu Y, Wang MC, Moghekar A, Brown T, et al. (2016). Cortical thickness in relation to clinical symptom onset in preclinical AD. Neuroimage Clin, 12:116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lezak MD. Neuropsychological assessment. USA: Oxford University Press; 2004. [Google Scholar]
- [11].Dickerson BC, Fenstermacher E, Salat DH, Wolk DA, Maguire RP, Desikan R, et al. (2008). Detection of cortical thickness correlates of cognitive performance: Reliability across MRI scan sessions, scanners, and field strengths. Neuroimage, 39:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Walhovd KB, Fjell AM, Dale AM, McEvoy LK, Brewer J, Karow DS, et al. (2010). Multi-modal imaging predicts memory performance in normal aging and cognitive decline. Neurobiol Aging, 31:1107–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sanchez-Benavides G, Gomez-Anson B, Quintana M, Vives Y, Manero RM, Sainz A, et al. (2010). Problem-solving abilities and frontal lobe cortical thickness in healthy aging and mild cognitive impairment. J Int Neuropsychol Soc, 16:836–845. [DOI] [PubMed] [Google Scholar]
- [14].Chiu HF, Lee H, Chung W, Kwong P (1994). Reliability and validity of the Cantonese version of mini-mental state examination-a preliminary study. Hong Kong Journal of Psychiatry, 4:25–28. [Google Scholar]
- [15].Folstein MF, Folstein SE, McHugh PR (1975). "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res, 12:189–198. [DOI] [PubMed] [Google Scholar]
- [16].Morris JC (1993). The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology, 43:2412–2414. [DOI] [PubMed] [Google Scholar]
- [17].Chu LW, Chiu KC, Hui SL, Yu GK, Tsui WJ, Lee PW (2000). The reliability and validity of the Alzheimer's Disease Assessment Scale Cognitive Subscale (ADAS-Cog) among the elderly Chinese in Hong Kong. Ann Acad Med Singapore, 29:474–485. [PubMed] [Google Scholar]
- [18].Rosen WG, Mohs RC, Davis KL (1984). A new rating scale for Alzheimer's disease. Am J Psychiatry, 141:1356–1364. [DOI] [PubMed] [Google Scholar]
- [19].Wechsler D. Wechsler abbreviated intelligence scale. San Antonio: The Psychological Corporation; 1999. [Google Scholar]
- [20].Chiu HF, Chan CK, Lam LC, Ng KO, Li SW, Wong M, et al. (1997). The modified Fuld Verbal Fluency Test: a validation study in Hong Kong. J Gerontol B Psychol Sci Soc Sci, 52:P247–250. [DOI] [PubMed] [Google Scholar]
- [21].Lam LC, Ho P, Lui VW, Tam CW (2006). Reduced semantic fluency as an additional screening tool for subjects with questionable dementia. Dement Geriatr Cogn Disord, 22:159–164. [DOI] [PubMed] [Google Scholar]
- [22].Lam LC, Tam CW, Leung GT, Lui VW, Fung AW, Chiu HF, et al. (2010). Combined clinical and cognitive criteria to identify mild cognitive impairment in a southern Chinese community. Alzheimer Dis Assoc Disord, 24:343–347. [DOI] [PubMed] [Google Scholar]
- [23].Dale AM, Sereno MI (1993). Improved Localizadon of Cortical Activity by Combining EEG and MEG with MRI Cortical Surface Reconstruction: A Linear Approach. J Cogn Neurosci, 5:162–176. [DOI] [PubMed] [Google Scholar]
- [24].Dale AM, Fischl B, Sereno MI (1999). Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage, 9:179–194. [DOI] [PubMed] [Google Scholar]
- [25].Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 31:968–980. [DOI] [PubMed] [Google Scholar]
- [26].Julkunen V, Niskanen E, Koikkalainen J, Herukka SK, Pihlajamaki M, Hallikainen M, et al. (2010). Differences in cortical thickness in healthy controls, subjects with mild cognitive impairment, and Alzheimer's disease patients: a longitudinal study. J Alzheimers Dis, 21:1141–1151. [DOI] [PubMed] [Google Scholar]
- [27].Singh V, Chertkow H, Lerch JP, Evans AC, Dorr AE, Kabani NJ (2006). Spatial patterns of cortical thinning in mild cognitive impairment and Alzheimer's disease. Brain, 129:2885–2893. [DOI] [PubMed] [Google Scholar]
- [28].Sluimer JD, van der Flier WM, Karas GB, van Schijndel R, Barnes J, Boyes RG, et al. (2009). Accelerating regional atrophy rates in the progression from normal aging to Alzheimer's disease. Eur Radiol, 19:2826–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gomez C, Stam CJ, Hornero R, Fernandez A, Maestu F (2009). Disturbed beta band functional connectivity in patients with mild cognitive impairment: an MEG study. IEEE Trans Biomed Eng, 56:1683–1690. [DOI] [PubMed] [Google Scholar]
- [30].Koenig T, Prichep L, Dierks T, Hubl D, Wahlund LO, John ER, et al. (2005). Decreased EEG synchronization in Alzheimer's disease and mild cognitive impairment. Neurobiol Aging, 26:165–171. [DOI] [PubMed] [Google Scholar]
- [31].Wang J, Zuo X, Dai Z, Xia M, Zhao Z, Zhao X, et al. (2013). Disrupted functional brain connectome in individuals at risk for Alzheimer's disease. Biol Psychiatry, 73:472–481. [DOI] [PubMed] [Google Scholar]
- [32].Liang P, Li Z, Deshpande G, Wang Z, Hu X, Li K (2014). Altered causal connectivity of resting state brain networks in amnesic MCI. PLoS One, 9:e88476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Das SR, Pluta J, Mancuso L, Kliot D, Orozco S, Dickerson BC, et al. (2013). Increased functional connectivity within medial temporal lobe in mild cognitive impairment. Hippocampus, 23:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lacalle-Aurioles M, Navas-Sanchez FJ, Aleman-Gomez Y, Olazaran J, Guzman-De-Villoria JA, Cruz-Orduna I, et al. (2016). The Disconnection Hypothesis in Alzheimer's Disease Studied Through Multimodal Magnetic Resonance Imaging: Structural, Perfusion, and Diffusion Tensor Imaging. J Alzheimers Dis, 50:1051–1064. [DOI] [PubMed] [Google Scholar]
- [35].Chee MW, Chen KH, Zheng H, Chan KP, Isaac V, Sim SK, et al. (2009). Cognitive function and brain structure correlations in healthy elderly East Asians. Neuroimage, 46:257–269. [DOI] [PubMed] [Google Scholar]
- [36].Iacono D, O'Brien R, Resnick SM, Zonderman AB, Pletnikova O, Rudow G, et al. (2008). Neuronal hypertrophy in asymptomatic Alzheimer disease. J Neuropathol Exp Neurol, 67:578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bahar-Fuchs A, Clare L, Woods B (2013). Cognitive training and cognitive rehabilitation for mild to moderate Alzheimer's disease and vascular dementia. Cochrane Database Syst Rev:Cd003260. [DOI] [PMC free article] [PubMed] [Google Scholar]


