Abstract
Hypoxia resulting from reduced oxygen (O 2) levels in the arterial blood is sensed by the carotid body (CB) and triggers reflex stimulation of breathing and blood pressure to maintain homeostasis. Studies in the past five years provided novel insights into the roles of heme oxygenase-2 (HO-2), a carbon monoxide (CO)-producing enzyme, and NADH dehydrogenase Fe-S protein 2, a subunit of the mitochondrial complex I, in hypoxic sensing by the CB. HO-2 is expressed in type I cells, the primary O2-sensing cells of the CB, and binds to O 2 with low affinity. O 2-dependent CO production from HO-2 mediates hypoxic response of the CB by regulating H 2S generation. Mice lacking NDUFS2 show that complex I-generated reactive oxygen species acting on K + channels confer type I cell response to hypoxia. Whether these signaling pathways operate synergistically or independently remains to be studied.
Keywords: Carotid body, Heme-oxygenase, Gasotransmitter, NADH dehydrogenase Fe-S protein 2
Introduction
Systemic hypoxia, which arises from decreased oxygen (O 2) levels in the arterial blood, is a fundamental physiological stimulus. The duration of hypoxia can be acute, ranging from seconds to minutes, or chronic, lasting hours to days. Acute hypoxia evokes rapid changes in the cardiorespiratory systems to ensure optimal O 2 delivery to tissues. Cardiorespiratory responses to acute hypoxia are primarily reflexive in nature, initiated by sensory organs located in the carotid artery and aorta. Carotid bodies (CBs), which reside at the bifurcation of the common carotid arteries, are the major sensory organs for monitoring arterial blood O 2 levels 1. Although structures similar to CBs are seen at the aortic arch and in the abdominal arteries, much of the information on the mechanisms of hypoxic sensing has come from studies on the CB 1. Here, we present studies reported in the past five years on the roles for heme oxygenase-2 (HO-2), a carbon monoxide (CO)-synthesizing enzyme, and NDUFS2, a mitochondrial complex I subunit, in hypoxic sensing by the CB.
Physiology of hypoxic sensing by the carotid body
The CB receives sensory innervation from the carotid sinus nerve, whose cell bodies reside in the petrosal ganglion. Under basal conditions (arterial blood pO 2 of about 100 mmHg), sensory nerve discharge (that is, frequency of action potentials) is low. In response to even a modest decrease in arterial blood pO 2 from 100 to 80 mmHg, the sensory discharge increases and the response is fast, occurring within a few seconds after the onset of hypoxia 1. The increased sensory discharge is non-adapting and is maintained during the entire duration of hypoxia 2 or may progressively increase during sustained hypoxia, lasting several hours 3. The exquisite sensitivity and the speed of the response with little or no adaptation are the unique features of hypoxic sensing by the CB. The increased CB sensory nerve activity is relayed to brainstem neurons, leading to reflex stimulation of breathing and blood pressure (CB chemo reflex) 1.
The CB tissue is made of two major cell types: type I cells (also called glomus cells), which are of neuronal origin, and type II cells, which resemble glial cells of the nervous system. Type I cells along with the nearby sensory nerve ending function as a “sensory unit” 1. Stimulus response of breathing to graded hypoxia parallels the CB sensory nerve activity 1. Consequently, carotid sinus nerve activity is measured as an index of CB hypoxic sensing 1. Type I cell responses to acute hypoxia are measured by monitoring exocytosis and changes in [Ca 2+] i and K + channel conductance 1. Although type I cells respond to hypoxia with elevated [Ca 2+] i or K + channel inhibition (or both), they are not always reflected in the sensory nerve activity 4, 5, which is essential for evoking the physiologically important CB chemo reflex. Therefore, it is necessary to corroborate the cellular responses to hypoxia with the sensory nerve discharge for assessing the physiological relevance of CB hypoxic sensing.
Transduction mechanisms
The consensus is that hypoxia inhibits certain K + channels in type I cells and the resulting depolarization leads to Ca 2+-dependent release of neurotransmitter or neurotransmitters, which stimulate the nearby sensory nerve ending, leading to increased sensory discharge 1. The roles for K + channels and AMP kinase (AMPK) in CB hypoxic sensing have been discussed in detail elsewhere 1, 6, 7 and will not be presented in this commentary. The following section presents studies conducted in the past five years that have provided novel insights into the roles for HO-2 and mitochondrial complex subunit NDUFS2 in hypoxic sensing by the CB.
Heme oxygenase-2
Type I cells express HO-2-like immunoreactivity 8. HO-2 is remarkably sensitive to O 2 availability, and graded hypoxia progressively decreases CO production in the CB 9. Reduced CO production by hypoxia is also seen in HEK-293 cells with heterologous expression of HO-2 9, suggesting that HO-2 is inherently sensitive to O 2. HO-2 binds to O 2 with low affinity with an apparent K m of 65 ± 5 mmHg (about 80 µM). The O 2 sensitivity of HO-2 is due to Cys 265 and Cys 282 residues in the heme regulatory motif 9. Intact Cys 265 and Cys 282 residues lower the affinity of HO-2 for O 2 and thereby enable the enzyme to transduce changes in O 2 into changes in CO production. Substituting Cys 265 and Cys 282 with alanine allows the HO-2 to bind to O 2 with high affinity 9.
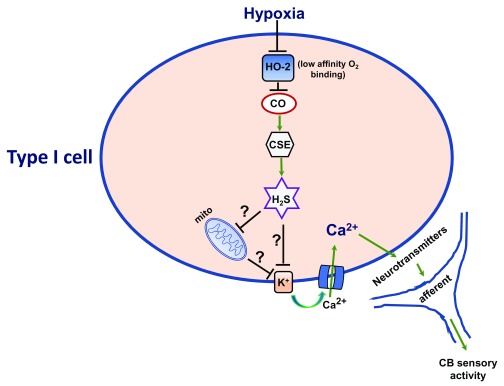
It was proposed, based on the findings that CO inhibits CB sensory nerve excitation by hypoxia 8– 10 and that hypoxia reduces CO production in a stimulus-dependent way 9, that low sensory nerve activity during normoxia is due to high CO levels inhibiting CB sensory nerve activity but that hypoxia, by reducing CO production, relieves the inhibition and thereby increases the sensory nerve discharge 8. Recent studies determined how CO inhibits CB sensory nerve activity under normoxia 9, 10. Type I cells also express cystathionine gamma-lyase (CSE), an enzyme catalyzing hydrogen sulfide (H 2S) production 11. H 2S is a potent stimulator of CB sensory nerve activity in rats, mice, rabbits, and cats 11– 13. During normoxia, H 2S levels are low and hypoxia increases H 2S levels in a stimulus-dependent manner 11. The increased H 2S production is not due to inherent O 2 sensitivity of CSE; rather, it is due to changes in CO production 9. High CO levels during normoxia inhibit CSE-derived H 2S generation through protein kinase G (PKG)-dependent phosphorylation of Ser 377 of CSE, and reduced CO generation during hypoxia relieves the inhibition of CSE, leading to increased H 2S generation in the CB 9. CSE inhibitors and CSE knockout mice exhibit impaired type I cell and sensory nerve and breathing response to hypoxia 11, 14. These findings suggest that low sensory discharge during normoxia is due to inhibition of H 2S generation by high levels of HO-2-derived CO but that the increased sensory nerve activity by hypoxia is due to relieving inhibition of H 2S synthesis by CO ( Figure 1).
Figure 1. Heme oxygenase-2 (HO-2) signaling in hypoxic sensing by the carotid body (CB).
Ca 2+, calcium channel; CO, carbon monoxide; CSE, cystathionine gamma-lyase; H 2S, hydrogen sulfide; K +, potassium channel; mito, mitochondria.
Genetic disruption of HO-2 increases baseline CB sensory nerve activity and elevates H 2S levels under normoxia, a phenotype similar to hypoxia 9. However, hypoxia further increased CB sensory activity and elevated H 2S levels in HO-2 null CBs, indicating the existence of a redundant hypoxic sensing mechanism (or mechanisms). This redundant hypoxic sensing was due to compensatory upregulation of neuronal nitric oxide synthase (nNOS) in type I cells of HO-2 null mice 9. nNOS, like HO-2, also binds to O 2 with low affinity, and nitric oxide (a product of nNOS), like CO, also inhibits CSE-derived H 2S through PKG signaling 9. Blockade of nNOS in HO-2 null mice renders CBs completely insensitive to hypoxia 9. These findings suggest that, in the absence of HO-2, nNOS contributes to CB hypoxic sensing by regulating CSE-derived H 2S production 9.
A recent study examined the role for HO-2–CO signaling in CB hypoxic sensing of three genetically distinct rat strains that are commonly used in experimental research 10. As compared with Sprague-Dawley rat CB, Brown-Norway (BN) rat CB showed markedly attenuated sensory nerve and type I cell responses to hypoxia, and this phenotype was associated with higher CO and lower H 2S levels in the glomus tissue. The elevated CO levels in the BN rat CB were due to high affinity of HO-2 to its substrate hemin 10. The attenuated CB response to hypoxia is associated with a blunted chemo reflex. The CB chemo reflex is essential for ventilatory adaptations to high-altitude hypoxia 15, 16. BN rats exposed to hypobaric hypoxia showed severe pulmonary edema 10, which is a sign of chronic mountain sickness. Treating BN rats with a HO inhibitor restored the hypoxic response of the CB and prevented pulmonary edema caused by hypobaric hypoxia 10.
In contrast to BN rat CBs, CBs of spontaneous hypertensive (SH) rats showed heightened sensitivity to hypoxia, and this phenotype is associated with low CO levels and high H 2S levels in the CB 10. The low CO levels in SH rat CBs were due to low hemin affinity of HO-2 10. Current evidence suggests that a hyperactive CB chemo reflex is a major contributor of hypertension in SH rats 17, 18. Systemic administration of a CSE inhibitor normalized CB sensory nerve and type I cell responses to hypoxia and reduced hypertension 10. Although chronic ablation of CB also reduced hypertension to the same level as seen with a CSE inhibitor, combined CB ablation and CSE inhibitor treatment had no further effect on blood pressure 10. These findings suggest that CO-regulated H 2S contributes to a hyperactive CB in SH rats. It has long been known that the CB chemo reflex exhibits substantial inter-individual variations in humans and experimental animals 19– 21. Studies on BN and SH rats indicate that inter-individual variations in chemo reflex are due in part to variations in HO-2–CO signaling in the CB.
A recent study suggests that HO-2–CO signaling in the CB also plays an important role in the pathology of sleep apnea (SA), which is a highly prevalent respiratory disease 22. SA is characterized by episodic cessation of breathing leading to chronic intermittent hypoxia (CIH). Patients with SA and CIH-exposed rodents exhibit a heightened CB chemo reflex, leading to chronic elevation of sympathetic nerve activity and hypertension 23– 26. Rodents exposed to CIH exhibit elevated reactive oxygen species (ROS) levels in the CB 27, 28. Peng et al. showed that ROS inhibit CO generation by HO-2 by acting on the Cys 265 residue in the heme regulatory motif, thereby increasing H 2S levels in the CB 22. Pharmacological or genetic blockade of CSE-derived H 2S prevents CIH-induced CB hyperactivity, sympathetic nerve excitation, and hypertension 22. Collectively, these studies suggest that disrupted HO-2–CO signaling in the CB leads to dire physiological consequences.
Although the studies described above suggest that CO-regulated H 2S is an important mediator of CB hypoxic sensing, a recent study questioned this possibility. Kim et al. 29 reported that inhibitors of H 2S synthesis had no effect on [Ca 2+] i and TASK K + channel responses of type I cells to anoxia (pO 2 of about 5 mmHg). Peng et al. 5 re-examined the role for CSE-derived H 2S in the CB sensory nerve and type I cell [Ca 2+] i responses to hypoxia (pO 2 of about 37 mmHg) and anoxia (pO 2 of about 5 mmHg). The authors found that hypoxia increased H 2S levels in the CB, stimulated sensory nerve activity, and elevated [Ca 2+] i in type I cells and all of these responses were blocked by a CSE inhibitor and in CSE knockout mice. In striking contrast, anoxia, though producing very low pO 2, had no effect on H 2S levels in the CB and produced only a weak CB sensory nerve excitation as compared with hypoxia. CB sensory and type I cell responses to anoxia were unaffected by CSE inhibitors and in CSE knockout mice. Moreover, anoxia (100% N 2) depressed breathing whereas hypoxia (12% O 2) stimulated breathing 5. CSE knockout mice showed an absence of breathing stimulation by hypoxia, whereas the depressed breathing by anoxia was unaffected in these mice 5. These findings suggest that hypoxia and anoxia are not the same stimuli for studying CB physiology and that HO-2–CO-regulated H 2S mediates CB response to “physiologically relevant” hypoxia but not anoxia.
NADH dehydrogenase Fe-S protein 2 (NDUFS2) mitochondrial complex I subunit
Mitochondrial electron transport chain (ETC) inhibitors mimic the effects of hypoxia on CB sensory nerve activity 1 and type I cells 30– 33. Mills and Jöbsis 34 reported that CBs express a putative cytochrome aa3 with two O 2 affinities: one with high and another with low affinity for O 2. Based on spectral analysis, subsequent studies suggested that CBs express a cytochrome, which is half-reduced at a pO 2 of 60 to 80 mmHg, and this cytochrome is not expressed in either the superior cervical or the nodose ganglion 35, 36. Acute hypoxia increased the NADH/NAD ratio and decreased mitochondrial membrane potential in type I cells, and these effects were not seen in other non-chemoreceptor tissues such as dorsal root ganglion 37. These studies led to the suggestion that mitochondrial ETC participates in CB hypoxic sensing.
Rotenone, an inhibitor of the mitochondrial complex I, selectively blocks the type I cell response to hypoxia 32. NDUFS2 is a component of the complex I, which binds to ubiquinone 38– 40. Recent studies examined the role for complex I in CB hypoxic sensing in mice with targeted deletion of Ndufs2 in tyrosine hydroxylase-positive (TH +) cells such as type I cells 41. Mice lacking NDUFS2 in TH + cells showed an absence of breathing stimulation by hypoxia and of hypoxia-evoked exocytosis and K + channel inhibition in type I cells. However, type I cell responses to severe hypercapnia (20% CO 2) were intact 41. Given that these mice have a deletion of NDUFS2 since birth, the lack of breathing response to hypoxia might be secondary to metabolic adaptations during development. Additional studies were performed on adult (2-month-old) mice with conditional knockout of Ndufs2 (ESR-NDUFS2 mice). Like the TH-NDUFS2 mice, mice with conditional knockdown of Ndufs2 showed an absence of stimulation of breathing as well as type I cell responses to hypoxia 41. The lack of cellular responses to hypoxia was associated with decreased complex I activity, complex I formation, and complex I-dependent O 2 consumption, whereas the functions of other mitochondrial complexes were intact. These studies suggest that NDUFS2 of the mitochondrial complex I contributes to hypoxic sensing by the CB.
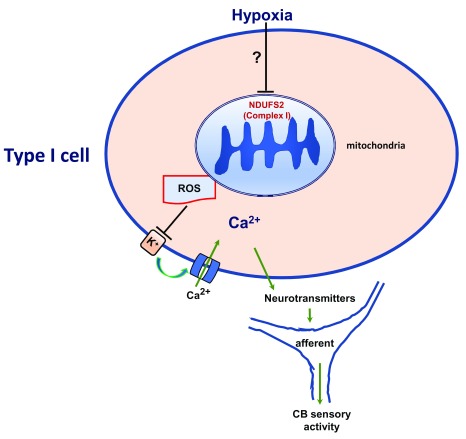
How might NDUFS2 confer hypoxic sensitivity on type I cells? Mitochondrial ETC-generated ROS have been implicated in pulmonary artery myocyte responses to acute hypoxia 42– 44. Acute hypoxia increased ROS in wild-type type I cell cytosol and mitochondrial intermembrane space, and these responses were attenuated in NDUFS2 null type I cells 41. Intracellular application of H 2O 2, like hypoxia, inhibited background K + currents in type I cells 41. These findings led to the suggestion that inhibition of NDUFS2 leads to an increase in ROS production, which in turn (by inhibiting K + currents) leads to depolarization of type I cells by hypoxia ( Figure 2).
Figure 2. NADH dehydrogenase Fe-S protein 2 (NDUFS2), a mitochondrial complex I subunit, signaling in hypoxic sensing by the carotid body (CB).
Ca 2+, calcium channel; K +, potassium channel; ROS, reactive oxygen species.
Summary and future directions
It has long been thought that an O 2 sensor or sensors in type I cells initiate hypoxic sensing in the CB 1, 45, 46. To be considered an O 2 sensor, a molecule should satisfy certain criteria, namely (a) its presence in type I cells, (b) low-affinity binding to O 2, (c) altered function by hypoxia should initiate signaling events leading to increased CB sensory nerve activity, and (d) loss of CB hypoxic sensing by disrupting its function. HO-2 satisfies the proposed criteria for an O 2 sensor in the CB and contributes to CB sensory excitation by regulating H 2S production through O 2-dependent CO production. However, further studies are needed to delineate the cellular mechanism(s) underlying CB activation by H 2S. H 2S donors, like hypoxia, depolarize 47 and inhibit K + channel conductance in type I cells 12, 48 and increase NADH auto-fluorescence in type I cells, an effect attributed to the inhibition of mitochondrial ETC 47. It is likely that H 2S mediates sensory nerve excitation by hypoxia by inhibiting mitochondrial ETC, thereby affecting K + conductance of type I cells ( Figure 1).
Studies with genetically engineered mice suggest that the inactivation of NDUFS2 is an important step for the type I cell response to hypoxia. However, it remains to be determined whether graded hypoxia inhibits NDUFS2 and establishes the affinity of O 2 for this enzyme. NDUFS2 is a ubiquitously expressed enzyme in the body. However, unlike many other tissues, CBs are highly sensitive to changes in O 2 levels. Consequently, the uniqueness of NDUFS2 signaling in the CB remains to be established.
Finally, whether HO-2 and NDUFS2 signaling operates independently or works in concert is not clear. The CB responds to a wide range of pO 2 values (about 80–20 mmHg). It was proposed that interactions between multiple signaling pathways working in concert as a “chemosome” enable the CB to sense a wide range of pO 2 values 45, 46. Given that high concentrations of H 2S can inhibit mitochondrial ETC 47, HO-2 and NDUFS2 signaling might work in concert as a “chemosome” for the full expression of the CB to a wide range of hypoxia, a possibility that remains to be investigated.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Plamena R Angelova, Department of Clinical and Movement Neurosciences, UCL Queen Square Institute of Neurology, London, UK
Prem Kumar, Institute of Clinical Sciences, College of Medical and Dental Sciences, University of Birmingham, Birmingham, UK
Norbert Weissmann, German Center for Lung Research (DZL), ECCPS, Justus Liebig University Giessen, Giessen, Germany
Funding Statement
Work in the authors’ laboratory is supported by National Institutes of Health grant P01-HL-90554.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 3 approved]
References
- 1. Kumar P, Prabhakar NR: Peripheral chemoreceptors: function and plasticity of the carotid body. Compr Physiol. 2012;2(1):141–219. 10.1002/cphy.c100069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kou YR, Ernsberger P, Cragg PA, et al. : Role of alpha 2-adrenergic receptors in the carotid body response to isocapnic hypoxia. Respir Physiol. 1991;83(3):353–364. 10.1016/0034-5687(91)90054-M [DOI] [PubMed] [Google Scholar]
- 3. Nielsen AM, Bisgard GE, Vidruk EH: Carotid chemoreceptor activity during acute and sustained hypoxia in goats. J Appl Physiol (1985). 1988;65(4):1796–1802. 10.1152/jappl.1988.65.4.1796 [DOI] [PubMed] [Google Scholar]
- 4. Donnelly DF: Chemoreceptor nerve excitation may not be proportional to catecholamine secretion. J Appl Physiol (1985). 1996;81(2):657–664. 10.1152/jappl.1996.81.2.657 [DOI] [PubMed] [Google Scholar]
- 5. Peng YJ, Makarenko VV, Gridina A, et al. : H 2S mediates carotid body response to hypoxia but not anoxia. Respir Physiol Neurobiol. 2019;259:75–85. 10.1016/j.resp.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peers C, Wyatt CN, Evans AM: Mechanisms for acute oxygen sensing in the carotid body. Respir Physiol Neurobiol. 2010;174(3):292–298. 10.1016/j.resp.2010.08.010 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. Rakoczy RJ, Wyatt CN: Acute oxygen sensing by the carotid body: a rattlebag of molecular mechanisms. J Physiol. 2018;596(15):2969–2976. 10.1113/JP274351 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 8. Prabhakar NR, Dinerman JL, Agani FH, et al. : Carbon monoxide: a role in carotid body chemoreception. Proc Natl Acad Sci U S A. 1995;92(6):1994–1997. 10.1073/pnas.92.6.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yuan G, Vasavda C, Peng YJ, et al. : Protein kinase G-regulated production of H 2S governs oxygen sensing. Sci Signal. 2015;8(373):ra37. 10.1126/scisignal.2005846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peng YJ, Makarenko VV, Nanduri J, et al. : Inherent variations in CO-H 2S-mediated carotid body O 2 sensing mediate hypertension and pulmonary edema. Proc Natl Acad Sci U S A. 2014;111(3):1174–1179. 10.1073/pnas.1322172111 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 11. Peng YJ, Nanduri J, Raghuraman G, et al. : H 2S mediates O 2 sensing in the carotid body. Proc Natl Acad Sci U S A. 2010;107(23):10719–10724. 10.1073/pnas.1005866107 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Li Q, Sun B, Wang X, et al. : A crucial role for hydrogen sulfide in oxygen sensing via modulating large conductance calcium-activated potassium channels. Antioxid Redox Signal. 2010;12(10):1179–1189. 10.1089/ars.2009.2926 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 13. Jiao Y, Li Q, Sun B, et al. : Hydrogen sulfide activates the carotid body chemoreceptors in cat, rabbit and rat ex vivo preparations. Respir Physiol Neurobiol. 2015;208:15–20. 10.1016/j.resp.2015.01.002 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Makarenko VV, Nanduri J, Raghuraman G, et al. : Endogenous H 2S is required for hypoxic sensing by carotid body glomus cells. Am J Physiol Cell Physiol. 2012;303(9):C916–923. 10.1152/ajpcell.00100.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dempsey JA, Powell FL, Bisgard GE, et al. : Role of chemoreception in cardiorespiratory acclimatization to, and deacclimatization from, hypoxia. J Appl Physiol (1985). 2014;116(7):858–66. 10.1152/japplphysiol.01126.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. Dempsey JA, Forster HV: Mediation of Ventilatory Adaptations. Physiol Rev. 1982;62(1):262–346. 10.1152/physrev.1982.62.1.262 [DOI] [PubMed] [Google Scholar]
- 17. Paton JF, Sobotka PA, Fudim M, et al. : The carotid body as a therapeutic target for the treatment of sympathetically mediated diseases. Hypertension. 2013;61(1):5–13. 10.1161/HYPERTENSIONAHA.111.00064 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Przybylski J: Do arterial chemoreceptors play a role in the pathogenesis of hypertension? Med Hypotheses. 1981;7(2):127–131. 10.1016/0306-9877(81)90109-2 [DOI] [PubMed] [Google Scholar]
- 19. Hodges MR, Forster HV, Papanek PE, et al. : Ventilatory phenotypes among four strains of adult rats. J Appl Physiol (1985). 2002;93(3):974–983. 10.1152/japplphysiol.00019.2002 [DOI] [PubMed] [Google Scholar]
- 20. Strohl KP, Thomas AJ, St Jean P, et al. : Ventilation and metabolism among rat strains. J Appl Physiol (1985). 1997;82(1):317–323. 10.1152/jappl.1997.82.1.317 [DOI] [PubMed] [Google Scholar]
- 21. Weil JV: Variation in human ventilatory control-genetic influence on the hypoxic ventilatory response. Respir Physiol Neurobiol. 2003;135(2–3):239–246. 10.1016/S1569-9048(03)00048-X [DOI] [PubMed] [Google Scholar]
- 22. Yuan G, Peng YJ, Khan SA, et al. : H 2S production by reactive oxygen species in the carotid body triggers hypertension in a rodent model of sleep apnea. Sci Signal. 2016;9(441):ra80. 10.1126/scisignal.aaf3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peng YJ, Yuan G, Khan S, et al. : Regulation of hypoxia-inducible factor-α isoforms and redox state by carotid body neural activity in rats. J Physiol. 2014;592(17):3841–3858. 10.1113/jphysiol.2014.273789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kara T, Narkiewicz K, Somers VK: Chemoreflexes--physiology and clinical implications. Acta Physiol Scand. 2003;177(3):377–384. 10.1046/j.1365-201X.2003.01083.x [DOI] [PubMed] [Google Scholar]
- 25. Narkiewicz K, van de Borne PJ, Pesek CA, et al. : Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation. 1999;99(9):1183–1189. 10.1161/01.CIR.99.9.1183 [DOI] [PubMed] [Google Scholar]
- 26. Lesske J, Fletcher EC, Bao G, et al. : Hypertension caused by chronic intermittent hypoxia--influence of chemoreceptors and sympathetic nervous system. J Hypertens. 1997;15(12 Pt 2):1593–1603. 10.1097/00004872-199715120-00060 [DOI] [PubMed] [Google Scholar]
- 27. Peng YJ, Overholt JL, Kline D, et al. : Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci U S A. 2003;100(17):10073–10078. 10.1073/pnas.1734109100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pawar A, Nanduri J, Yuan G, et al. : Reactive oxygen species-dependent endothelin signaling is required for augmented hypoxic sensory response of the neonatal carotid body by intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol. 2009;296(3):R735–742. 10.1152/ajpregu.90490.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim D, Kim I, Wang J, et al. : Hydrogen sulfide and hypoxia-induced changes in TASK (K 2P3/9) activity and intracellular Ca 2+ concentration in rat carotid body glomus cells. Respir Physiol Neurobiol. 2015;215:30–38. 10.1016/j.resp.2015.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Duchen MR, Biscoe TJ: Mitochondrial function in type I cells isolated from rabbit arterial chemoreceptors. J Physiol. 1992;450:13–31. 10.1113/jphysiol.1992.sp019114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Buckler KJ, Vaughan-Jones RD: Effects of mitochondrial uncouplers on intracellular calcium, pH and membrane potential in rat carotid body type I cells. J Physiol. 1998;513(Pt 3):819–833. 10.1111/j.1469-7793.1998.819ba.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ortega-Sáenz P, Pardal R, García-Fernandez M, et al. : Rotenone selectively occludes sensitivity to hypoxia in rat carotid body glomus cells. J Physiol. 2003;548(Pt 3):789–800. 10.1113/jphysiol.2003.039693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wyatt CN, Buckler KJ: The effect of mitochondrial inhibitors on membrane currents in isolated neonatal rat carotid body type I cells. J Physiol. 2004;556(Pt 1):175–191. 10.1113/jphysiol.2003.058131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mills E, Jöbsis FF: Simultaneous measurement of cytochrome a 3 reduction and chemoreceptor afferent activity in the carotid body. Nature. 1970;225(5238):1147–1149. 10.1038/2251147a0 [DOI] [PubMed] [Google Scholar]
- 35. Lahiri S, Ehleben W, Acker H: Chemoreceptor discharges and cytochrome redox changes of the rat carotid body: role of heme ligands. Proc Natl Acad Sci U S A. 1999;96(16):9427–9432. 10.1073/pnas.96.16.9427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Streller T, Huckstorf C, Pfeiffer C, et al. : Unusual cytochrome a 592 with low PO 2 affinity correlates as putative oxygen sensor with rat carotid body chemoreceptor discharge. FASEB J. 2002;16(10):1277–1279. 10.1096/fj.02-0166fje [DOI] [PubMed] [Google Scholar]
- 37. Duchen MR, Biscoe TJ: Relative mitochondrial membrane potential and [Ca2+]i in type I cells isolated from the rabbit carotid body. J Physiol. 1992;450:33–61. 10.1113/jphysiol.1992.sp019115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kashani-Poor N, Zwicker K, Kerscher S, et al. : A central functional role for the 49-kDa subunit within the catalytic core of mitochondrial complex I. J Biol Chem. 2001;276(26):24082–24087. 10.1074/jbc.M102296200 [DOI] [PubMed] [Google Scholar]
- 39. Baradaran R, Berrisford JM, Minhas GS, et al. : Crystal structure of the entire respiratory complex I. Nature. 2013;494(7438):443–448. 10.1038/nature11871 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 40. Rhein VF, Carroll J, Ding S, et al. : NDUFAF7 methylates arginine 85 in the NDUFS2 subunit of human complex I. J Biol Chem. 2013;288(46):33016–33026. 10.1074/jbc.M113.518803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fernández-Agüera MC, Gao L, González-Rodríguez P, et al. : Oxygen Sensing by Arterial Chemoreceptors Depends on Mitochondrial Complex I Signaling. Cell Metab. 2015;22(5):825–837. 10.1016/j.cmet.2015.09.004 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Archer SL, Huang J, Henry T, et al. : A redox-based O2 sensor in rat pulmonary vasculature. Circ Res. 1993;73(6):1100–1112. 10.1161/01.RES.73.6.1100 [DOI] [PubMed] [Google Scholar]
- 43. Leach RM, Hill HM, Snetkov VA, et al. : Divergent roles of glycolysis and the mitochondrial electron transport chain in hypoxic pulmonary vasoconstriction of the rat: identity of the hypoxic sensor. J Physiol. 2001;536(Pt 1):211–224. 10.1111/j.1469-7793.2001.00211.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Waypa GB, Chandel NS, Schumacker PT: Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ Res. 2001;88(12):1259–1266. 10.1161/hh1201.091960 [DOI] [PubMed] [Google Scholar]
- 45. Prabhakar NR: Sensing hypoxia: physiology, genetics and epigenetics. J Physiol. 2013;591(9):2245–2257. 10.1113/jphysiol.2012.247759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Prabhakar NR: O 2 sensing at the mammalian carotid body: why multiple O 2 sensors and multiple transmitters? Exp Physiol. 2006;91(1):17–23. 10.1113/expphysiol.2005.031922 [DOI] [PubMed] [Google Scholar]
- 47. Buckler KJ: Effects of exogenous hydrogen sulphide on calcium signalling, background (TASK) K channel activity and mitochondrial function in chemoreceptor cells. Pflugers Arch. 2012;463(5):743–754. 10.1007/s00424-012-1089-8 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Telezhkin V, Brazier SP, Cayzac SH, et al. : Mechanism of inhibition by hydrogen sulfide of native and recombinant BK Ca channels. Respir Physiol Neurobiol. 2010;172(3):169–178. 10.1016/j.resp.2010.05.016 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation