Abstract
Background/Objectives
Polyphenols are plant secondary metabolites with a large variability in their chemical structure and dietary occurrence that have been associated with some protective effects against several chronic diseases. To date, limited data exist on intake of polyphenols in populations. The current cross-sectional analysis aimed at estimating dietary intakes of all currently known individual polyphenols and total intake per class and subclass, and to identify their main food sources in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort.
Methods
Dietary data at baseline were collected using a standardized 24 h dietary recall software administered to 36,037 adult subjects. Dietary data were linked with Phenol-Explorer, a database with data on 502 individual polyphenols in 452 foods and data on polyphenol losses due to cooking and food processing.
Results
Mean total polyphenol intake was the highest in Aarhus-Denmark (1,786 mg/day in men and 1,626 mg/day in women) and the lowest in Greece (744 mg/day in men and 584 mg/day in women). When dividing the subjects into three regions, the highest intake of total polyphenols was observed in the UK health conscious group, followed by non-Mediterranean (non-MED) and MED countries. The main polyphenol contributors were phenolic acids (52.5-56.9%), except in men from MED countries and in the UK health conscious group where they were flavonoids (49.1-61.7%). Coffee, tea and fruits were the most important food sources of total polyphenols. A total of 437 different individual polyphenols were consumed, including 94 consumed at a level >1 mg/d. The most abundant ones were the caffeoylquinic acids and the proanthocyanidin oligomers and polymers.
Conclusion
This study describes the large number of dietary individual polyphenols consumed and the high variability of their intakes between European populations, particularly between MED and non-MED countries.
Keywords: polyphenols, dietary intake, food sources, EPIC
Introduction
Polyphenols are plant secondary metabolites widely distributed in plant-based foods, such as tea, coffee, wine, fruit, vegetables, whole-grain cereals, and cocoa [1]. Dietary polyphenols constitute a large family of ˃500 different compounds with highly diverse structures from simple molecules, such as phenolic acids, to large ones, such as proanthocyanidin polymers [1]. According to their chemical structure, polyphenols are divided into 4 main classes: flavonoids, phenolic acids, lignans, and stilbenes [2]. In foods, flavonoids, lignans and stilbenes are usually found as glycosides, whereas phenolic acids are most often present as esters with various polyols; some polyphenols such as flavanols are mainly present as aglycones (free forms) [1]. It is important to consider these structural variations as glycosylation/esterification greatly influences polyphenol absorption in the gut and bioavailability [3]. Over the last two decades, the literature on polyphenols has grown exponentially following the recognition of their antioxidant, anti-inflammatory, and anti-carcinogenic properties and more evidence for their potential beneficial effects upon health has accumulated [4]. However, epidemiological data on the relationship between polyphenol intake and the risk of chronic diseases and mortality is still limited, especially in prospective studies, and largely concerns flavonoids and lignans [2,5–7]. To calculate polyphenol intake, most of these studies have used the U.S. Department of Agriculture (USDA) databases on polyphenol contents in foods [8–10], which only contain data on flavonoids expressed as aglycones. The more recently published Phenol-Explorer database (www.phenol-explorer.eu) [11] is more comprehensive and gathers food composition data on all known polyphenols, either aglycones, glycosides or esters depending on how they are found in foods. Moreover, a new module of the Phenol-Explorer database contains data on the effects of cooking and food processing on polyphenol contents [12]. These retention factors permit to take into account the effects of food cooking and processing measurements when calculating polyphenol intake and to improve the reliability of such measurements [13].
Several descriptive papers on intakes of flavonoids and other polyphenols have been published using either the USDA databases [14–16], the Phenol-Explorer database [17–19] or custom databases [20]. The purpose of the present work is to describe intake of all currently known dietary polyphenols in several European countries using the more comprehensive Phenol-Explorer database and especially applying polyphenol-specific retention factors. The European Prospective Investigation into Cancer and Nutrition (EPIC) study offers a unique opportunity to estimate the intake of individual polyphenols, to identify their main food sources, and to compare these intakes between different European countries showing large variations in diets.
Material and Methods
Study population
The EPIC study is a large cohort study conducted in 10 European countries (Denmark, France, Germany, Greece, Italy, Norway, Spain, Sweden, The Netherlands and the UK) and aims at investigating the role of diet and environmental factors on the etiology of cancer and other chronic diseases [21,22]. Over half million participants were recruited mostly from the general population residing within defined geographical areas, with some exceptions: women of a health insurance company for teachers and school workers (France), women attending breast cancer screening (Utrecht-The Netherlands, and Florence-Italy), mainly blood donors (most centers in Italy and Spain) and a cohort consisting predominantly of vegetarians (the ‘health-conscious’ group in Oxford, UK) [22]. The initial 23 EPIC administrative centers were redefined into 27 geographical regions relevant to the analysis of dietary consumption patterns [23].
Data used in the present work were derived from the EPIC calibration study, in which a 24-hour dietary recall (24-HDR) was administered. The cohort comprises an 8% (n=36,994) random sample stratified by age, sex and center, and weighted for expected cancer cases in each stratum of the whole EPIC cohort [23]. After exclusion of 941 subjects who were aged younger than 35 years or older than 74 years because of low participation in these age categories, and 16 individuals due to incomplete dietary information, 36,037 participants were included in the present analysis. Approval for the study was obtained from ethical review boards of the International Agency for Research on Cancer (IARC) and from all local participating institutions. All participants provided written informed consent.
Dietary and lifestyle information
Dietary assessment was performed with a single 24-HDR using specialized software (EPIC-Soft) [24], and administered in a face-to-face interview, except in Norway, where it was obtained by telephone interview [25]. Data on age, as well as on body weight and height, were self-reported by the participants during the 24-HDR interview. Data on other lifestyle factors, including educational level, physical activity and smoking history, were collected at baseline through standardized questionnaires [23,26]. The mean time interval between completion of the baseline questionnaire measures and the 24-HDR interview varied by country, and ranged from the same day to 3 years later [23].
Food composition database on polyphenols
The Phenol-Explorer database provides data on 502 polyphenol compounds in 452 plant-based foods collected from 638 scientific peer-reviewed articles [11]. All animal foods that contain none or only traces of plant polyphenols were excluded.
Phenol-Explorer contains data on four main polyphenol classes: flavonoids, phenolic acids, stilbenes, and lignans, as well as on a number of miscellaneous minor polyphenols that include tyrosols, alkylphenols (mainly alkylresorcinols) and alkylmethoxyphenols among others, described in the Phenol-Explorer database and in this manuscript as “other polyphenols” [11]. Total polyphenol content was calculated as the sum of the contents of individual compounds expressed in mg/100g food fresh weight. The data used in the present study were mainly acquired by chromatography without previous hydrolysis of the food extracts except for a few polyphenols bound to the food matrix. Lignans in all foods, ellagic acid in walnuts, and hydroxycinnamic acids in cereals, legumes and olives were quantified by chromatography after previous basic or acid hydrolysis. Proanthocyanidin dimers were measured as individual compounds by chromatography without prior hydrolysis whereas other proanthocyanidin oligomers (trimers, 4-6 and 7-10 oligomers) and proanthocyanidin polymers were measured as mixtures by normal phase HPLC. Overall data on 463 individual polyphenols were used.
Some missing polyphenol content values for orange fruit and breakfast cereals were extrapolated from orange juice and wheat flour, respectively. The polyphenol content of different types of coffee, “American” or filtered diluted coffee and espresso, reported in the 24-HDR, were estimated by multiplying the polyphenol contents of “normal” filtered coffee from the Phenol-Explorer database by 0.4 and 2, respectively [27,28]. When the type of herbal tea was unspecified in the 24-HDRs, the polyphenolic composition of a mix of herbal teas was applied on a country by country basis. The effect of food cooking and processing was accounted for by applying polyphenol-specific retention factors from Phenol-Explorer [12] to 24-HDR foods wherever relevant. Phenol-Explorer contains data on 1,253 aggregated retention factors, including data on 161 polyphenols and 35 processes, such as domestic cooking, storage and industrial processing.
Statistical analyses
Dietary polyphenol intakes were estimated using general linear models and presented as means and standard errors (s.e.) stratified by sex and center, and adjusted for age and weighted by season and day of the week of the 24-HDR to control for different distributions of participants across seasons and 24-HDR days. The contribution of each polyphenol class, subclass and family to the total polyphenol intake was calculated as a percentage according to three ad hoc European regions: 1) Mediterranean (MED) countries: all centers in Greece, Spain, Italy and the south of France; 2) non-MED countries: all centers in the north-east and north-west of France, Germany, the Netherlands, UK general population, Denmark, Sweden and Norway; 3) UK health conscious group. The contribution of each food group to the intake of total and polyphenol classes by European region was also calculated as a percentage. Differences in polyphenol intakes stratified by European region were also compared using general linear models according to the categories of sex, age (35-44, 45-54, 55-64, and 64-74), BMI (<25, 25-30, and >30kg/m2), educational level (no formal education, primary school, technical/professional school, secondary school, university, or not specified), smoking status (never, former, current smoker, and not specified), and Cambridge physical activity index (inactive, moderately inactive, moderatively active active, and not specified). All these models were adjusted for sex, age (y), center, BMI (kg/m2) and energy intake, and weighted by season and day of 24-HDR. P values <0.05 (two-tailed) were considered significant. All analyses were conducted using the SPSS Statistics software (version 19.0; SPSS Inc.).
Results
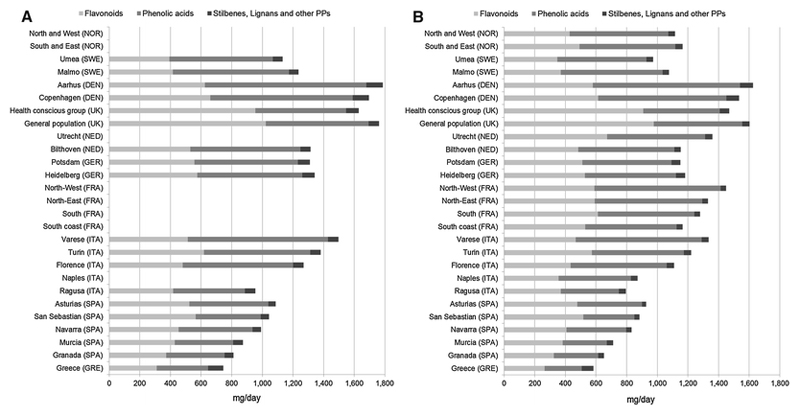
A south-to-north gradient in the daily mean intake of total polyphenols was observed among EPIC centers, in both men and women (Figure 1). The highest total polyphenol intake in both sexes was in Aarhus-Denmark (in men 1,786 mg/d and in women 1,626 mg/d); whereas the lowest intake was in Greece in both men (744 mg/d) and women (584 mg/d). Table 1 shows the mean intakes of total polyphenols and polyphenol classes adjusted for sex, age, BMI (except where stratified for these variables), center and energy intake, and weighted by season and weekday of the 24-HDR. The intake of phenolic acids, stilbenes and other polyphenols was higher in men than in women, whereas for flavonoids and lignans the opposite was observed. No differences were observed for total polyphenol intake between men and women. The intake of flavonoids, stilbenes, lignans and other polyphenols increased with age, whereas the opposite was seen for total polyphenols and phenolic acids for which a maximum intake was observed at the age of 45-54y. When comparing the intake by European region, the UK health conscious group (1,521 mg/d) had the highest intake of total polyphenols due to the higher intake of flavonoids. Non-MED countries (1,284 mg/d) showed a higher total polyphenol intake when compared to MED countries (1,011 mg/d), with phenolic acids as the main contributors. MED countries presented the highest intake of stilbenes compared to the other regions. Individuals with BMI<25, and/or a university degree, and/or current smokers showed the highest intakes of total polyphenols. No differences in total polyphenol intake were observed between groups showing different physical activity levels (Table 1).
Figure 1.
Adjusted daily polyphenol mean intake (mg/d), stratified by sex (A, men; B, women) and center ordered from north to south, adjusted for age and weighted by season and week day of dietary recalls. GRE, Greece; SPA, Spain; ITA, Italy, FRA, France, GER, Germany; NED, The Netherlands; DEN, Denmark; SWE, Sweden; NOR, Norway; UK, United Kingdom
Table 1.
Adjusteda mean daily intakes of total and polyphenol classes by socio-demographic and lifestyle factors in the EPIC study
| Stratification variable | N | Polyphenols (mg/d) | Flavonoids (mg/d) | Phenolic acids (mg/d) | Lignans (mg/d) | Stilbenes (mg/d) | Other polyphenols (mg/d) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | s.e. | P-value | Mean | s.e. | P-value | Mean | s.e. | P-value | Mean | s.e. | P-value | Mean | s.e. | P-value | Mean | s.e. | P-value | ||
| Sex | 0.16 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||||||||||
| Men | 13,028 | 1177 | 8 | 492 | 5 | 625 | 6 | 2.5 | 0.2 | 3.0 | 0.1 | 55 | 0.5 | ||||||
| Women | 23,009 | 1192 | 6 | 546 | 4 | 593 | 5 | 3.6 | 0.1 | 2.4 | 0.0 | 46 | 0.3 | ||||||
| Age | <0.001 | <0.001 | <0.001 | 0.002 | 0.009 | <0.001 | |||||||||||||
| 35-44 | 3,335 | 1167 | 14 | 490 | 8 | 623 | 11 | 2.8 | 0.3 | 2.4 | 0.1 | 48 | 0.8 | ||||||
| 45-54 | 12,595 | 1200 | 7 | 504 | 5 | 641 | 6 | 2.7 | 0.2 | 2.7 | 0.0 | 50 | 0.4 | ||||||
| 55-64 | 14,940 | 1187 | 7 | 531 | 4 | 598 | 6 | 3.3 | 0.1 | 2.8 | 0.0 | 52 | 0.4 | ||||||
| 65-74 | 5,167 | 1148 | 12 | 541 | 7 | 548 | 9 | 3.5 | 0.2 | 2.8 | 0.1 | 52 | 0.7 | ||||||
| European region | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||||||||||
| Mediterranean countries | 11,285 | 1011 | 7 | 449 | 5 | 503 | 6 | 3.6 | 0.1 | 3.1 | 0.1 | 53 | 0.4 | ||||||
| Non-Mediterranean countries | 24,443 | 1284 | 5 | 522 | 3 | 700 | 4 | 2.3 | 0.1 | 2.1 | 0.0 | 57 | 0.3 | ||||||
| UK health conscious group | 309 | 1521 | 44 | 909 | 27 | 538 | 34 | 9.1 | 0.9 | 1.4 | 0.3 | 63 | 2.6 | ||||||
| Body mass index (kg/m2) | 0.002 | <0.001 | <0.001 | 0.043 | 0.036 | <0.001 | |||||||||||||
| <25 | 16,854 | 1194 | 7 | 542 | 4 | 594 | 6 | 3.3 | 0.1 | 2.7 | 0.0 | 52 | 0.4 | ||||||
| 25 to 30 | 13,766 | 1186 | 7 | 510 | 4 | 620 | 6 | 2.8 | 0.1 | 2.7 | 0.0 | 50 | 0.4 | ||||||
| >30 | 5,417 | 1150 | 11 | 471 | 7 | 625 | 8 | 3.1 | 0.2 | 2.5 | 0.1 | 49 | 0.6 | ||||||
| Educational level | <0.001 | <0.001 | 0.029 | 0.036 | <0.001 | 0.011 | |||||||||||||
| None | 1,709 | 1124 | 21 | 488 | 13 | 578 | 17 | 3.3 | 0.4 | 2.7 | 0.1 | 52 | 1.2 | ||||||
| Primary completed | 10,469 | 1134 | 8 | 457 | 5 | 621 | 7 | 2.7 | 0.2 | 2.3 | 0.1 | 50 | 0.5 | ||||||
| Technical/professional | 8,038 | 1168 | 10 | 511 | 6 | 602 | 8 | 2.9 | 0.2 | 2.7 | 0.1 | 50 | 0.5 | ||||||
| Secondary school | 7,152 | 1205 | 10 | 543 | 6 | 607 | 8 | 3.2 | 0.2 | 2.8 | 0.1 | 50 | 0.5 | ||||||
| University degree | 8,155 | 1275 | 9 | 598 | 6 | 618 | 7 | 3.4 | 0.2 | 3.2 | 0.1 | 52 | 0.5 | ||||||
| Smoking status | <0.001 | <0.001 | <0.001 | 0.08 | <0.001 | <0.001 | |||||||||||||
| Never smoker | 17,483 | 1137 | 7 | 532 | 4 | 549 | 5 | 3.2 | 0.1 | 2.4 | 0.0 | 50 | 0.5 | ||||||
| Former smoker | 10,288 | 1200 | 8 | 537 | 5 | 605 | 6 | 3.0 | 0.2 | 3.0 | 0.1 | 52 | 0.4 | ||||||
| Current Smoker | 7,726 | 1265 | 9 | 470 | 6 | 739 | 7 | 2.8 | 0.2 | 3.0 | 0.1 | 50 | 0.5 | ||||||
| Physical activity | 0.32 | <0.001 | 0.14 | 0.05 | 0.002 | 0.001 | |||||||||||||
| Inactive | 7,463 | 1173 | 9 | 506 | 6 | 611 | 7 | 3.5 | 0.2 | 2.6 | 0.1 | 49 | 0.5 | ||||||
| Moderately inactive | 11,969 | 1187 | 8 | 522 | 5 | 608 | 6 | 3.4 | 0.2 | 2.8 | 0.0 | 51 | 0.4 | ||||||
| Moderately active | 8,400 | 1187 | 9 | 534 | 6 | 596 | 7 | 2.8 | 0.2 | 2.9 | 0.1 | 51 | 0.5 | ||||||
| Active | 6,380 | 1198 | 10 | 549 | 6 | 591 | 8 | 3.0 | 0.2 | 2.7 | 0.1 | 53 | 0.6 | ||||||
Means and standard error (s.e.) were computed using general linear models adjusted for sex, age, center, energy intake and body mass index (as appropriate) and weighted by season and weekday of recall. P value is for differences in means.
Phenolic acids were the main contributors to the total polyphenol intake in non-MED countries (57% and 53% in men and women, respectively) and in women from MED countries (54%) (Table 2). Flavonoids were the second most abundant contributors in these groups (38-44%). In contrast, in the UK health conscious group and in men from MED countries, flavonoids were the largest contributor to total polyphenol intake ranging from 49 to 62%, followed by phenolic acids (34-44%). Stilbenes and lignans accounted for <0.7% of total polyphenol intake with levels of intake not exceeding 3.1 and 9.1 mg/day, respectively, in any of the EPIC regions.
Table 2.
Number of individual polyphenols and contribution of classes and subclasses of total polyphenols, and the top three most consumed polyphenols for each polyphenol class and subclass by sex and European region in the EPIC studya
| Polyphenol classes and subclasses | MED countries (%) | non-MED countries (%) | UK Health conscious group (%) | Number of individual polyphenols | Top 3 most consumed individual polyphenols | |||
|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | |||
| Total polyphenols | 100 | 100 | 100 | 100 | 100 | 100 | 437 | 5-Caffeoylquinic acid, 4-caffeoylquinic acid, 3-caffeoylquinic acid |
| Flavonoids | 49.1 | 40.9 | 37.5 | 43.5 | 58.2 | 61.7 | 234 | Proanthocyanidin polymers, proanthocyanidin 4-6 oligomers, proanthocyanidin 7-10 oligomers |
| Anthocyanins | 5.4 | 4.3 | 3.2 | 3.7 | 2.2 | 2.9 | 65 | Cyanidin 3-O-rutinoside, malvidin 3-O-glucoside, delphinidin 3-O-rutinoside |
| Chalcones | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 2 | Xanthohumol, butein |
| Dihydrochalcones | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 4 | Phloridzin, phloretin 2'-O-xylosyl-glucoside, 3-hydroxyphloretin 2'-O-glucoside |
| Dihydroflavonols | 0.8 | 0.2 | 0.2 | 0.2 | 0.1 | 0.1 | 2 | Dihydromyricetin 3-O-rhamnoside, dihydroquercetin 3-O-rhamnoside |
| Flavanols | 34.1 | 28.7 | 27.7 | 32.0 | 43.5 | 47.8 | 25 | Proanthocyanidin polymers, proanthocyanidin 4-6 oligomers, proanthocyanidin 7-10 oligomers |
| Flavan-3-ol monomers | 29.1 | 21.5 | 16.6 | 18.1 | 21.7 | 22.3 | 10 | (+)-Gallocatechin, (-)-epicatechin, (-)-epigallocatechin 3-O-gallate |
| Proanthocyanidins | 9.1 | 5.7 | 4.7 | 5.3 | 5.6 | 6.4 | 11 | Proanthocyanidin polymers, proanthocyanidin 4-6 oligomers, proanthocyanidin 7-10 oligomers |
| Theaflavins | 24.8 | 19.2 | 14.4 | 15.6 | 18.3 | 18.8 | 4 | Theaflavin 3'-O-gallate, theaflavin 3,3'-O-digallate, theaflavin |
| Flavanones | 3.3 | 3.0 | 2.6 | 3.3 | 4.0 | 3.0 | 17 | Hesperidin, narirutin, didymin |
| Flavones | 1.5 | 1.5 | 0.7 | 0.9 | 1.2 | 1.0 | 38 | Apigenin 6,8-di-C-glucoside, apigenin 6,8-C-galactoside-C-arabinoside, apigenin 6,8-C-arabinoside-C-glucoside |
| Flavonols | 3.7 | 2.9 | 2.8 | 3.2 | 4.8 | 5.1 | 68 | Quercetin 3-O-rutinoside, quercetin 3-O-glucoside, quercetin 3,4'-O-diglucoside |
| Isoflavonoids | 0.0 | 0.1 | 0.1 | 0.1 | 2.2 | 1.6 | 13 | Genistin, 6'-O-malonylgenistin, daidzin |
| Phenolic acids | 43.6 | 54.4 | 56.8 | 52.5 | 36.4 | 34.4 | 100 | 5-Caffeoylquinic acid, 4-caffeoylquinic acid, 3-caffeoylquinic acid |
| Hydroxybenzoic acids | 3.3 | 3.6 | 3.5 | 4.1 | 6.0 | 7.4 | 27 | 5-O-Galloylquinic acid, gallic acid, ellagic acid |
| Hydroxycinnamic acids | 40.2 | 50.7 | 53.3 | 48.4 | 30.4 | 27.0 | 68 | 5-Caffeoylquinic acid, 4-caffeoylquinic acid, 3-caffeoylquinic acid |
| Hydroxyphenylacetic acids | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 4 | 4-Hydroxyphenylacetic acid, homovanillic acid, 3,4-dihydroxyphenylacetic acid |
| Hydroxyphenylpropanoic acids | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1 | Dihydro-p-coumaric acid |
| Stilbenes | 0.5 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 7 | Piceatannol 3-O-glucoside, resveratrol 3-O-glucoside, d-viniferin |
| Lignans | 0.3 | 0.4 | 0.1 | 0.2 | 0.7 | 0.5 | 27 | Secoisolariciresinol, lariciresinol, sesamolin |
| Other polyphenols class | 6.4 | 4.2 | 5.3 | 3.6 | 4.7 | 3.4 | 69 | 5-Heneicosylresorcinol, 5-nonadecylresorcinol, 5-heptadecylresorcinol |
| Alkylmethoxyphenols | 0.2 | 0.2 | 0.3 | 0.2 | 0.2 | 0.1 | 3 | 4-Ethylguaiacol, 4-vinylguaiacol, 4-vinylsyringol |
| Alkylphenols | 2.1 | 1.6 | 4.0 | 2.5 | 3.7 | 2.6 | 14 | 5-Heneicosylresorcinol, 5-nonadecylresorcinol, 5-heptadecylresorcinol |
| Tyrosols | 3.6 | 1.9 | 0.4 | 0.4 | 0.5 | 0.4 | 14 | Tyrosol, 3,4-DHPEA-EDA, oleuropein-aglycone |
| Other polyphenols subclass | 0.6 | 0.5 | 0.7 | 0.5 | 0.3 | 0.2 | 38 | Pyrogallol, catechol, eugenol |
| Cucurminoids | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 3 | Curcumin, demethoxycurcumin, bisdemethoxycurcumin |
| Furanocoumarins | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 4 | Bergapten, isopimpinellin, xanthotoxin |
| Hydroxybenzaldehydes | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 5 | Syringaldehyde, protocatechuic aldehyde, p-anisaldehyde |
| Hydroxybenzoketones | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 2 | 2,3-Dihydroxy-1-guaiacylpropanone, 3-methoxyacetophenone |
| Hydroxycinnamaldehydes | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 2 | Ferulaldehyde, sinapaldehyde |
| Hydroxycoumarins | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 6 | 4-Hydroxycoumarin, esculin, esculetin |
| Hydroxyphenylpropenes | 0.1 | 0.0 | 0.3 | 0.2 | 0.0 | 0.0 | 5 | Eugenol, acetyl eugenol, anethole |
| Methoxyphenols | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1 | Guaiacol |
| Naphtoquinones | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1 | Juglone |
| Phenolic terpenes | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 3 | Carnosic acid, rosmanol, carnosol |
| Other polyphenols family | 0.4 | 0.4 | 0.3 | 0.3 | 0.2 | 0.1 | 6 | Pyrogallol, catechol, phlorin |
Values are percentages derived from general lineal models adjusted for sex, age and center and weighted by season and weekday of dietary recalls.
Regarding polyphenol subclasses, the two most important contributors to total polyphenol intake were hydroxycinnamic acids (ranging from 27% in women from the UK health conscious group to 53% in men from non-MED countries) and flavanols (ranging from 28% in men from non-MED countries to 48% in women from the UK health conscious group). They were followed by various subclasses: anthocyanidins, flavanones, flavonols, hydroxybenzoic acids, alkylphenols and tyrosols which each accounted for 2-6% of total polyphenol intake. Other subclasses were less remarkable, each one contributing less than 1% to total polyphenol intake in most European regions.
The main food sources of polyphenols according to European regions are presented in Table 3. In MED countries, coffee (36%), fruits (25%), and wine (10%) were the major dietary sources of polyphenols. In non-MED countries, coffee (41%), tea (17%), and fruits (13%) were the most important food sources. In the UK health conscious group, tea ranked first (41%), followed by coffee (21%), and fruits (9%).
Table 3.
Contributions of food groups and some specific foods to the intake of total polyphenols and polyphenol classes by European region in the EPIC studya
| polyphenols (%) |
Flavonoids (%) |
Phenolic acids (%) |
Lignans (%) |
Stilbense (%) |
Other polyphenols (%) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Food items | MED countries |
non-MED countries |
UK Health |
MED countries |
non-MED countries |
UK Health |
MED countries |
non-MED countries |
UK Health |
MED countries |
non-MED countries |
UK Health |
MED countries |
non-MED countries |
UK Health |
MED countries |
non-MED countries |
UK Health |
| Potatoes and other tubers | 1.0 | 1.3 | 0.9 | 0.0 | 0.0 | 0.0 | 1.9 | 2.4 | 2.6 | 0.5 | 1.0 | 0.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Vegetables | 4.6 | 1.7 | 1.5 | 4.7 | 1.5 | 0.6 | 5.0 | 1.9 | 2.5 | 11.2 | 9.8 | 9.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.2 |
| Leafy vegetables | 1.2 | 0.6 | 0.1 | 0.8 | 0.4 | 0.1 | 1.7 | 0.8 | 0.1 | 0.3 | 0.2 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Fruiting vegetables | 1.8 | 0.3 | 0.2 | 1.1 | 0.2 | 0.0 | 2.6 | 0.4 | 0.3 | 7.4 | 3.2 | 1.9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Root vegetables | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.2 | 0.2 | 0.4 | 0.4 | 0.9 | 0.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Cabbages | 0.1 | 0.1 | 0.2 | 0.0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.4 | 2.0 | 4.4 | 5.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Grain and pod vegetables | 0.4 | 0.1 | 0.2 | 0.7 | 0.0 | 0.0 | 0.1 | 0.2 | 0.6 | 0.3 | 0.3 | 0.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Onion, garlic | 0.8 | 0.3 | 0.3 | 1.7 | 0.7 | 0.4 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Stalk vegetables, sprouts | 0.1 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.5 | 0.5 | 0.2 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.1 |
| Mixed salad, mixed vegetables | 0.3 | 0.1 | 0.3 | 0.2 | 0.1 | 0.0 | 0.3 | 0.2 | 0.7 | 0.2 | 0.4 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Legumes | 3.4 | 0.8 | 4.0 | 6.9 | 1.9 | 4.4 | 0.8 | 0.1 | 0.5 | 2.8 | 0.6 | 1.2 | 0.2 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 |
| Fruits | 25.2 | 12.5 | 8.6 | 44.9 | 24.6 | 10.0 | 9.0 | 4.0 | 4.2 | 10.7 | 7.1 | 4.9 | 1.9 | 2.9 | 3.0 | 17.8 | 3.1 | 3.4 |
| Citrus fruit | 2.3 | 0.8 | 0.6 | 5.0 | 1.8 | 0.7 | 0.0 | 0.0 | 0.0 | 4.2 | 2.6 | 1.4 | 0.0 | 0.0 | 0.0 | 2.0 | 0.9 | 0.8 |
| Apple and pear | 11.7 | 6.4 | 3.9 | 22.2 | 13.0 | 4.9 | 3.8 | 1.8 | 1.7 | 1.9 | 1.2 | 0.8 | 0.0 | 0.0 | 0.0 | 0.6 | 0.2 | 0.1 |
| Grape | 2.1 | 0.8 | 0.9 | 4.6 | 1.8 | 1.1 | 0.1 | 0.0 | 0.1 | 0.3 | 0.2 | 0.2 | 1.3 | 1.0 | 1.3 | 0.0 | 0.0 | 0.0 |
| Stone fruits | 5.5 | 2.3 | 1.1 | 8.9 | 3.5 | 1.4 | 3.3 | 1.5 | 1.1 | 2.4 | 1.1 | 0.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Berries | 1.2 | 1.6 | 1.4 | 2.5 | 3.3 | 0.9 | 0.2 | 0.4 | 0.6 | 0.6 | 1.1 | 0.7 | 0.6 | 1.6 | 1.3 | 0.0 | 0.0 | 0.0 |
| Other fruits | 0.5 | 0.5 | 0.6 | 0.9 | 1.1 | 0.9 | 0.1 | 0.1 | 0.3 | 1.2 | 0.9 | 1.1 | 0.1 | 0.3 | 0.4 | 0.0 | 0.0 | 0.0 |
| Olives | 1.9 | 0.2 | 0.2 | 0.7 | 0.1 | 0.0 | 1.6 | 0.2 | 0.2 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 15.2 | 2.0 | 2.5 |
| Nuts | 1.5 | 0.3 | 0.6 | 0.6 | 0.3 | 0.1 | 2.5 | 0.3 | 0.7 | 0.2 | 0.2 | 0.1 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 |
| Seeds | 0.1 | 0.1 | 0.3 | 0.0 | 0.0 | 0.0 | 0.2 | 0.0 | 0.4 | 4.2 | 15.6 | 48.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Dairy products | 0.3 | 0.6 | 0.7 | 0.6 | 1.3 | 1.8 | 0.2 | 0.1 | 0.1 | 0.0 | 0.1 | 0.1 | 0.0 | 0.1 | 0.3 | 0.0 | 0.0 | 0.0 |
| Cereal products | 3.7 | 7.0 | 4.4 | 1.2 | 0.6 | 0.5 | 2.4 | 7.0 | 4.0 | 13.7 | 24.3 | 6.6 | 0.0 | 0.0 | 0.0 | 34.6 | 68.5 | 73.9 |
| Flour, flakes, starches, semolina and pastry | 0.2 | 0.1 | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.4 | 0.3 | 0.2 | 0.0 | 0.0 | 0.0 | 1.0 | 0.7 | 1.4 |
| Pasta, rice, other grains | 1.4 | 0.4 | 0.4 | 0.5 | 0.2 | 0.0 | 1.5 | 0.4 | 0.8 | 0.7 | 0.6 | 0.1 | 0.0 | 0.0 | 0.0 | 8.0 | 3.4 | 2.8 |
| Bread, crispbread, rusks and crackers | 1.8 | 6.0 | 1.4 | 0.4 | 0.2 | 0.0 | 0.6 | 6.1 | 1.1 | 12.5 | 21.9 | 4.8 | 0.0 | 0.0 | 0.0 | 23.5 | 60.4 | 26.7 |
| Breakfast cereals | 0.2 | 0.5 | 2.4 | 0.0 | 0.1 | 0.3 | 0.2 | 0.5 | 2.0 | 0.1 | 1.4 | 1.4 | 0.0 | 0.0 | 0.0 | 2.1 | 4.0 | 43.1 |
| Cakes, biscuits and sweets | 5.0 | 7.0 | 7.7 | 9.9 | 15.7 | 10.3 | 0.9 | 0.7 | 1.4 | 19.8 | 2.1 | 2.6 | 0.1 | 0.6 | 0.5 | 2.8 | 2.6 | 2.5 |
| Cakes and biscuits | 1.7 | 1.9 | 2.6 | 2.8 | 3.8 | 0.9 | 0.6 | 0.4 | 0.9 | 1.3 | 1.8 | 1.2 | 0.1 | 0.3 | 0.1 | 2.8 | 2.5 | 2.3 |
| Chocolate, candy bars, paste, confetti | 2.6 | 4.2 | 4.6 | 5.9 | 10.1 | 0.0 | 0.1 | 0.1 | 0.2 | 0.0 | 0.1 | 0.1 | 0.1 | 0.2 | 0.3 | 0.0 | 0.0 | 0.0 |
| Confectionary non-chocolate, candied fruits | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.8 | 0.0 | 0.0 | 0.2 | 18.5 | 0.0 | 1.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 |
| Ice cream, water ice | 0.5 | 0.6 | 0.4 | 1.1 | 1.4 | 2.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 |
| Sugar, honey, jam and syrup | 0.1 | 0.2 | 0.0 | 0.0 | 0.3 | 5.8 | 0.1 | 0.1 | 0.1 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Meat, fish and eggs | 0.0 | 0.1 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 1.0 | 0.3 |
| Fats and oils | 1.6 | 0.2 | 0.3 | 0.1 | 0.0 | 0.0 | 0.5 | 0.1 | 0.3 | 26.1 | 11.7 | 10.0 | 0.0 | 0.0 | 0.0 | 23.2 | 2.1 | 4.0 |
| Vegetable oils | 1.6 | 0.1 | 0.2 | 0.1 | 0.0 | 0.0 | 0.5 | 0.1 | 0.2 | 26.0 | 11.5 | 9.7 | 0.0 | 0.0 | 0.0 | 22.8 | 1.1 | 2.6 |
| Margarine and animal fats | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.3 | 0.0 | 0.0 | 0.0 | 0.5 | 1.0 | 1.5 |
| Non alcoholic beverages | 42.3 | 62.6 | 65.7 | 12.1 | 43.9 | 67.8 | 72.8 | 81.1 | 80.9 | 0.9 | 5.3 | 4.1 | 0.1 | 1.4 | 2.5 | 9.0 | 12.9 | 7.4 |
| Fruit and vegetable juices | 1.4 | 3.3 | 2.7 | 3.0 | 7.4 | 3.5 | 0.1 | 0.3 | 0.4 | 0.1 | 0.2 | 0.1 | 0.0 | 0.2 | 2.5 | 0.9 | 0.5 | 0.3 |
| Carbonated/soft/isotonic drinks | 0.1 | 0.7 | 0.4 | 0.2 | 1.7 | 0.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.0 | 0.0 | 1.2 | 0.1 | 0.0 | 0.0 | 0.0 |
| Coffee | 35.9 | 40.9 | 20.9 | 0.0 | 0.1 | 0.0 | 70.6 | 74.6 | 57.5 | 0.5 | 3.0 | 1.2 | 0.0 | 0.0 | 0.0 | 8.1 | 11.7 | 7.1 |
| Tea | 4.6 | 17.4 | 40.8 | 8.6 | 34.2 | 63.8 | 1.7 | 5.9 | 21.2 | 0.3 | 1.6 | 2.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Herbal tea | 0.3 | 0.3 | 0.9 | 0.3 | 0.5 | 0.1 | 0.4 | 0.2 | 1.7 | 0.1 | 0.3 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.7 | 0.0 |
| Alcoholic beverages | 10.4 | 5.1 | 3.7 | 18.2 | 9.0 | 3.9 | 3.3 | 1.9 | 2.0 | 3.9 | 4.3 | 2.3 | 97.2 | 94.4 | 93.2 | 7.6 | 6.2 | 5.1 |
| Wine | 10.2 | 4.6 | 3.5 | 18.1 | 8.6 | 3.8 | 3.2 | 1.4 | 1.7 | 3.7 | 2.9 | 1.7 | 96.9 | 92.3 | 92.3 | 7.3 | 4.6 | 4.3 |
| Beer, cider | 0.1 | 0.4 | 0.2 | 0.1 | 0.2 | 0.1 | 0.1 | 0.5 | 0.4 | 0.3 | 1.3 | 0.5 | 0.0 | 0.1 | 0.6 | 0.2 | 1.5 | 0.8 |
| Spirits and coktails | 0.0 | 0.1 | 0.0 | 0.1 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.3 | 2.0 | 0.4 | 0.0 | 0.1 | 0.0 |
| Condiments, spices and sauces | 0.6 | 0.3 | 0.5 | 0.4 | 0.4 | 0.4 | 0.3 | 0.1 | 0.2 | 5.5 | 16.5 | 8.7 | 0.4 | 0.2 | 0.2 | 3.9 | 1.5 | 2.1 |
| Other products | 0.3 | 0.5 | 1.0 | 0.4 | 0.7 | 0.2 | 0.1 | 0.2 | 0.3 | 0.4 | 1.3 | 0.9 | 0.0 | 0.2 | 0.1 | 0.8 | 2.0 | 1.0 |
Values are percentages derived from general linear models adjusted for sex, age and center and weighted by season and weekday of dietary recalls.
The main food sources of flavonoids were fruits in MED countries (45%), and tea in both non-MED countries (34 %) and the UK health conscious group (64%). Coffee was the most important dietary source of phenolic acids in all European regions (58-75%). The major food sources of lignans were region-related: vegetable oils (26%) in MED countries, cereal products (24%) in non-MED countries, and seeds (49%) in the UK health conscious group. Wine was clearly the most prominent food source of stilbenes accounting for >92% of intake in all regions.
A total of 437 individual polyphenols were consumed by the EPIC population (Table 2). Ninety-four polyphenols were consumed in a mean quantity of at least 1 mg/d, 118 polyphenols between 0.1 and 1 mg/d, 112 polyphenols between 0.01 and 0.1mg/d, and 113 polyphenols between >0 and 0.01mg/d (Supplemental table 1). Finally, 26 additional polyphenols documented in the food composition table were either not consumed or their intake could not be calculated since the consumption of their food sources was not reported in dietary recalls (Supplemental table 2). Mean intakes of the top 25 individual polyphenols are shown in Table 4 together with their three main food sources. Mean intake of all individual polyphenols are presented in Supplemental table 1. In men from MED countries and participants from the UK health conscious group, the top three most consumed polyphenols were 5-caffeoylquinic acid, proanthocyanidin polymers, and 4-caffeoylquinic acid, whereas for the other groups, they were 5-caffeoylquinic acid, 4-caffeoylquinic acid, and 3-caffeoylquinic acid.
Table 4.
Adjusteda mean intakes (mg/d) of top 25 most consumed individual polyphenols and their main food sources by European region in the EPIC study
| Polyphenol (mg/d) | Polyphenol subclass | ALL | MED countries | non-MED countries | UK health-conscious group | Top 3 main food sources | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | |||||||||||
| Mean | se | Mean | se | Mean | se | Mean | se | Mean | se | Mean | se | Mean | se | |||
| 5-Caffeoylquinic acid | Hydroxycinnamic acids | 195 | 1.1 | 149 | 2.8 | 178 | 4.1 | 231 | 1.7 | 201 | 1.2 | 159 | 11.5 | 134 | 9.2 | Coffee (78.2%), potatoes (7.3%), apples and pears (6.0%) |
| 4-Caffeoylquinic acid | Hydroxycinnamic acids | 133 | 0.9 | 83 | 2.1 | 113 | 3.4 | 166 | 1.4 | 142 | 1.0 | 102 | 9.9 | 84 | 7.5 | Coffee (97.5%), tea (1.5%), stone fruits (0.4%) |
| 3-Caffeoylquinic acid | Hydroxycinnamic acids | 122 | 0.8 | 83 | 2.0 | 109 | 3.0 | 147 | 1.3 | 129 | 0.9 | 88 | 8.6 | 75 | 6.8 | Coffee (92.4%), stone fruits (6.0%), tea (0.4%) |
| Proanthocyanidin polymers (>10 mers) | Flavanols | 78 | 0.6 | 103 | 2.6 | 74 | 1.4 | 75 | 1.4 | 74 | 0.8 | 133 | 16.6 | 118 | 11.4 | Apples and pears (24.6%), chocolate products (19.8%), legumes (11.4%) |
| Proanthocyanidin 4-6 oligomers | Flavanols | 49 | 0.4 | 60 | 1.2 | 46 | 0.8 | 48 | 0.8 | 48 | 0.5 | 64 | 6.5 | 66 | 7.0 | Apples and pears (33.1%), chocolate products (24.9%), wine (7.5%) |
| Ferulic acid | Hydroxycinnamic acids | 38 | 0.3 | 17 | 0.7 | 15 | 0.5 | 77 | 0.9 | 35 | 0.4 | 41 | 6.6 | 31 | 3.5 | Bread (66.2%), pasta and rice (9.7%), breakfast cereals (6.3%) |
| Proanthocyanidin 7-10 oligomers | Flavanols | 34 | 0.2 | 46 | 1.0 | 33 | 0.5 | 33 | 0.5 | 32 | 0.3 | 50 | 5.5 | 44 | 4.1 | Apples and pears (39.8%), chocolate products (17.5%), legumes (8.4%) |
| Hesperidin | Flavanones | 27 | 0.3 | 22 | 0.7 | 19 | 0.4 | 27 | 0.7 | 30 | 0.5 | 46 | 6.9 | 35 | 5.0 | Fruit juices (66.2%), citrus fruit (28.6%), carbonated/soft drinks (3.9%) |
| 5-Feruloylquinic acid | Hydroxycinnamic acids | 26 | 0.2 | 16 | 0.4 | 22 | 0.7 | 32 | 0.3 | 27 | 0.2 | 19 | 2.0 | 15 | 1.5 | Coffee (99.7%), root vegetables (0.2%), cream desserts (0.1%) |
| (+)-Gallocatechin | Flavanols | 24 | 0.3 | 3.4 | 0.2 | 10 | 0.3 | 27 | 0.6 | 32 | 0.4 | 73 | 9.0 | 81 | 6.0 | Tea (99.6%), wine (0.3%), fruiting vegetables (0.1%) |
| (-)-Epicatechin | Flavanols | 21 | 0.1 | 19 | 0.3 | 16 | 0.2 | 21 | 0.3 | 22 | 0.2 | 33 | 2.8 | 37 | 2.1 | Tea (35.9%), apples and pears (25.7%), wine (11.4%) |
| 5-O-Galloylquinic acid | Hydroxybenzoic acids | 20 | 0.2 | 2.8 | 0.2 | 8.8 | 0.3 | 23 | 0.5 | 27 | 0.4 | 60 | 7.5 | 67 | 5.0 | Tea (100%) |
| 4-Feruloylquinic acid | Hydroxycinnamic acids | 19 | 0.1 | 12 | 0.3 | 16 | 0.5 | 23 | 0.2 | 20 | 0.1 | 14 | 1.4 | 11 | 1.1 | Coffee (99.8%), root vegetables (0.1%), cream desserts (0.1%) |
| Proanthocyanidin trimers | Flavanols | 19 | 0.2 | 20 | 0.5 | 17 | 0.4 | 19 | 0.4 | 19 | 0.2 | 22 | 2.7 | 28 | 4.3 | Chocolate products (34.5%), apples and pears (25.3%), wine (7.9%) |
| (-)-Epigallocatechin 3-O-gallate | Flavanols | 18 | 0.2 | 2.8 | 0.2 | 7.8 | 0.3 | 21 | 0.4 | 24 | 0.3 | 48 | 5.9 | 53 | 3.9 | Tea (99.5%), herbal tea (0.5%) |
| Procyanidin dimer B2 | Flavanols | 18 | 0.1 | 21 | 0.3 | 15 | 0.2 | 18 | 0.2 | 18 | 0.1 | 26 | 2.1 | 27 | 1.5 | Apples and pears (45.3%), tea (24.1%), wine (15.3%) |
| (-)-Epicatechin 3-O-gallate | Flavanols | 15 | 0.1 | 3.4 | 0.1 | 6.8 | 0.2 | 17 | 0.3 | 20 | 0.2 | 43 | 4.6 | 46 | 3.1 | Tea (87.4%), herbal tea (8.8%), wine (2.8%) |
| Procyanidin dimer B1 | Flavanols | 14 | 0.1 | 17 | 0.4 | 14 | 0.3 | 13 | 0.2 | 15 | 0.1 | 23 | 2.4 | 26 | 1.7 | Tea (43.6%), wine (15.8%), stone fruits (14.4%) |
| (-)-Epigallocatechin | Flavanols | 14 | 0.1 | 2.4 | 0.1 | 6.2 | 0.2 | 17 | 0.3 | 19 | 0.2 | 38 | 4.6 | 42 | 3.1 | Tea (99.1%), wine (0.3%), herbal tea (0.2%) |
| (+)-Catechin | Flavanols | 14 | 0.1 | 17 | 0.3 | 10 | 0.1 | 14 | 0.2 | 14 | 0.1 | 23 | 2.0 | 24 | 1.3 | Tea (30.7%), wine (29.6%), apples and pears (7.7%) |
| Gallic acid | Hydroxybenzoic acids | 13 | 0.2 | 11 | 0.9 | 11 | 0.7 | 12 | 0.2 | 14 | 0.3 | 26 | 3.0 | 29 | 2.0 | Tea (60.4%), wine (16.3%), mixed fruits (12.8%) |
| Cyanidin 3-O-rutinoside | Anthocyanins | 11 | 0.4 | 12 | 0.8 | 17 | 1.1 | 7.4 | 0.6 | 12 | 0.5 | 5.2 | 1.9 | 8.8 | 2.1 | Stone fruits (64.5%), fruit juices (12.8%), carbonated/soft drinks (7.2%) |
| 5-Heneicosylresorcinol | Alkylyphenols | 10 | 0.1 | 8.4 | 0.2 | 5.6 | 0.1 | 15 | 0.1 | 10 | 0.1 | 22 | 2.1 | 14 | 1.0 | Bread (81.0%), pasta and rice (8.7%), breakfast cereals (7.5%) |
| 5-Nonadecylresorcinol | Alkylyphenols | 10 | 0.1 | 6.8 | 0.2 | 4.4 | 0.1 | 17 | 0.2 | 10 | 0.1 | 17 | 1.6 | 10 | 0.6 | Bread (88.6%), pasta and rice (5.1%), breakfast cereals (4.4%) |
| 3-Feruloylquinic acid | Hydroxycinnamic acids | 9.3 | 0.1 | 6.0 | 0.2 | 8.0 | 0.2 | 11 | 0.1 | 10 | 0.1 | 6.7 | 0.7 | 5.4 | 0.5 | Coffee (97.9%), stone fruits (1.4%), root vegetables (0.3%) |
Abbreviations: MED, Mediterranean.
Means and standard error (s.e.) were computed using general linear models adjusted for sex, age, and center and weighted by season and weekday of recall.
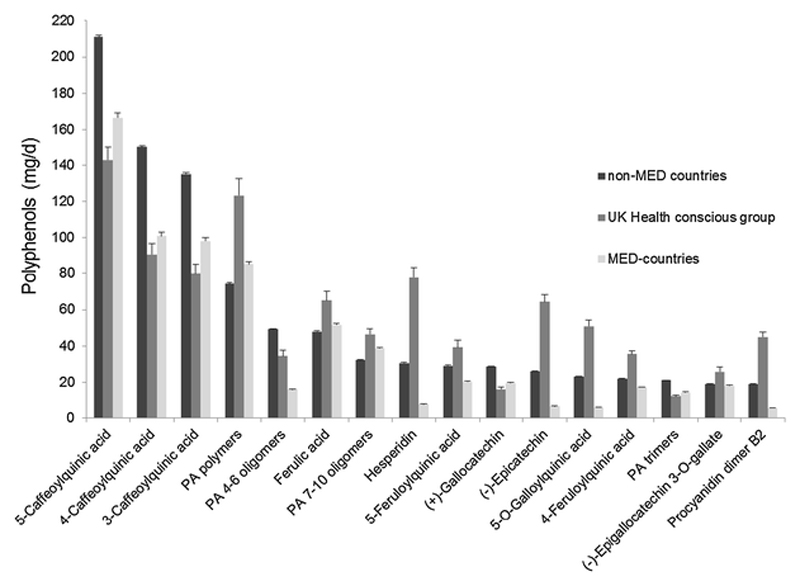
A large variability in both the mean intake and the ranking of the top ten most consumed polyphenols was observed between European regions (Figure 2). For example, 5-caffeoylquinic acid intake in men ranged from 149 mg/d from MED countries to 231 mg/d from non-MED countries (Table 4). However, the main food sources for each individual polyphenol by European region were very similar, and therefore, are listed for the EPIC study as a whole (Table 4 and Supplemental table 1). Coffee was the major food source (>78%) of caffeoyl- and feruloylquinic acids, whereas tea was the main food source (>99.5%) of (+)-gallocatechin, (-)-epigallocatechin, (-)-epigallocatechin 3-O-gallate and 5-O-galloylquinic acid. Apples and pears, and chocolate products were the main food sources of proanthocyanidin oligomers and polymers. Hesperidin was provided exclusively from citrus products (citrus fruit and juices). Bread and cereal products were the main sources of ferulic acid and alkylphenols (5-heneicosylresorcinol and 5-nonadecylresorcinol).
Figure 2.
Adjusted daily intake (mg/d), mean and standard error, of the top ten polyphenol of each European region adjusted for age, sex and center and weighted by season and weekday of dietary recall in the EPIC study.
Abbreviations: MED Mediterranean, PA proanthocyanidins
Discussion
In the current study, dietary intake of total polyphenols, polyphenol classes and individual polyphenols were estimated across ten European countries, using a standardized 24-HDR and the Phenol-Explorer database, allowing easy comparisons between groups of individuals and countries. Main food sources were also identified and the influence of some socio-demographic characteristics was assessed. Most previous descriptive studies on polyphenols used the USDA food composition data to document intake of approximately 50 polyphenol aglycones [14–16,20,29–31]. In the current study, intakes of 437 polyphenols, either glycosides, esters, or aglycones are described. This study follows one of our earlier studies in which intakes of 337 polyphenols in the French SUpplémentation en VItamines et Minéraux AntioXydants (SU.VI.MAX) cohort were reported [17].
In addition to the large number of polyphenols and diversity of countries considered, the present study is also the first one to take into account the effects of cooking and processing on polyphenol contents in foods by applying retention factors recently collected in the Phenol-Explorer database [12]. In our previous studies in EPIC, a common retention factor for each cooking method (boiled, fried, and microwaved) was eventually applied irrespective of the polyphenol compound and food considered [15,31]. This simple approach could only lead to a crude estimation of the effects of cooking on polyphenol intake. Even after applying retention factors, mean polyphenol intakes in our French EPIC centers (1,166-1,449 mg/d) were slightly higher than in the French SU.VI.MAX cohort (1,193 mg/d) [17], indicating that the use of retention factors does not seem to have high impact on the estimated total polyphenol intake. This can be explained by the fact that these retention factors mainly concern vegetables and legumes [12] which were not major contributors to polyphenol intake in the present or previous studies [14,15,17,20]. It is still important to highlight that polyphenol losses due to cooking and food processing can have a great impact on the intake of some individual polyphenols mainly present in vegetables, such as quercetin 3,4'-O-diglucoside that is only present in onions [1].
Using a single 24-HDR for each of the 36,037 subjects across 10 European countries in EPIC, 437 different individual polyphenols have been documented. Intakes of 347, 337 and 290 polyphenols have been reported utilizing food frequency questionnaires and the Phenol-Explorer database in the Polish arm of the HAPIEE (Health, Alcohol and Psychosocial factors In Eastern Europe) study [19], SU.VI.MAX study [17] and in the Spanish PREDIMED (PREvención con DIeta MEDiterránea) study [18], respectively. However, a similar number of individual polyphenols (around 100) was consumed at a level higher than 1 mg/d on average in these three studies [17,18]. Interestingly, although the number of most abundant polyphenols (>1 mg/d) is similar by region/cohort, there are considerable differences in the individual polyphenols included in that group. For example, oleuropein (tyrosol subclass) was highly consumed in men from MED countries (3.3 mg/d, rank 49), but not in men from non-MED countries (0.3 mg/d, rank 147). This is due to the fact that some polyphenols are exclusively present in a few characteristic foods (e.g. oleuropein is only found in olives and olive oil) and that their consumption largely varies according to regions. Another example is delphinidin 3-O-rutinoside (anthocyanidin subclass) only found in blackcurrants; its intake varied from 7.0 mg/d (rank 31) in men from non-MED countries to 0.04 mg/d (rank 245) in men from MED countries. This illustrates the need for further detailed analyses of polyphenols in foods not commonly consumed in Western countries and poorly described in the Phenol-Explorer or USDA databases, to document their intake across different geographical areas of the world.
Our results show a wide range of total polyphenol intake and a south-to-north geographical gradient. Mean intake was as much as three times higher in men from Aarhus-Denmark (1,786 mg/d) than in women from Greece (584 mg/d). When stratified by regions, total polyphenol intake in the non-MED countries was higher than that of the MED countries. This small gradient in total polyphenol intake was mainly due to a 1.5-fold higher intake of phenolic acids in northern EPIC cohorts. Hydroxycinnamic acids, and more specifically the 5-, 4-, and 3-caffeoylquinic acids, are by far the highest contributors to total polyphenol in non-MED countries due to the high coffee consumption in this region, which explains almost 90% of the phenolic acid intake as similarly observed in previous studies [17,20,30]. Intakes of phenolic acids in the current study are much lower than those reported in our previous study [30]. This is explained by the fact that in the present work, we took into account differences in polyphenol concentrations according to coffee types. People from non-MED countries usually drink a diluted type of filtered coffee, whereas in MED countries coffee is often consumed as espresso, a coffee 5-fold more concentrated than the diluted filtered one [28].
Flavonoid intake was slightly higher in non-MED countries compared to MED countries. In the UK health conscious group, flavonoid intake was almost twice that of the other two regions. This is mainly due to the high intake of flavan-3-ol monomers, with tea being the main source as described previously [32]. It is worth bearing in mind that, thearubigins were not included in the present study, since only crude analytical data are available [33]. However, if these data were considered, the contribution of flavonoids to total polyphenol intake in countries with a traditional tea culture would be significantly higher [33]. Conversely, in MED-countries the relatively high intake of flavonoids is due to proanthocyanidins, mainly coming from fruits [31,32]. Intakes of flavonoid sub-classes, that are particularly abundant in fruits and fruit products (anthocyanidins, flavanones, dihydrochalcones and dihydroflavonols), were higher in MED countries as observed earlier [34,35]. Isoflavonoid intake is very low in European [17,20,36] and other Western populations [14,16] in comparison to Asian populations [37], but the intake in the UK health conscious group was quite large, due to the high consumption of soya products in this mainly vegetarian cohort [36] and the use of flour fortified with soya protein in the United Kingdom [38].
Lignans are minor contributors to total polyphenol intake as reported earlier (<0.7%) [17,18,20]. We estimated the intake of 29 lignans, including those usually assessed in other cohort studies, such as lariciresinol, matairesinol, pinoresinol, and secoisolariciresinol [36,39]. One of the three most widely consumed lignans was sesamolin, a lignan not considered in previous studies on polyphenol intake. This highlights the importance of using the most comprehensive databases when evaluating the relationships between lignan/polyphenol intake and health outcomes.
Resveratrol is the most well-known stilbene and in the last decade, it has received much attention due to its ability to modulate the expression of sirtuin deacetylases, and to extend lifespan of model organisms [40]. However, the intake of stilbenes in the European populations is very low, and their intake does not exceed 3.1 mg/day in agreement with values previously published [17,18,41].
Other polyphenols beside the four classes described above, include alkylphenols, contributing from 1.6% of total polyphenols in women from MED countries to 4.0% in men from non-MED countries, where whole grain cereals are more widely consumed. They also include tyrosols (0.4-3.6% of total polyphenols) characteristic of foods more abundant in the diet of MED countries, such as olive oil, olives and wine [11]. Intakes of other polyphenols were almost negligible (<0.7% of total polyphenol).
The present study shows a number of strengths, in particular the large number of EPIC participants, the use of the Phenol-Explorer database as the most comprehensive food composition database for polyphenols, the uniform application of retention factors to take into account the effects of cooking and food processing on polyphenol contents in foods, and the use of a standardized 24-HDR in all participants, allowing to easily compare results across countries. However, our study has some limitations. Our results cannot be totally generalizable, since not all the EPIC cohorts are population-based [21,22]. Another limitation of this study is that each person contributed only one 24-HDR, hence variation in intakes cannot be evaluated at the individual level and it is less likely to reflect true usual individual diet. However, analyses were weighted by season and weekday of dietary recall and 24-HDR is a useful method to describe the average dietary intake of a group, particularly when estimated from a large number of subjects [42]. A relevant weakness is also the likely underestimation of total polyphenol intake, due to some missing or insufficient food composition data for polyphenols such as thearubigins [33] or proanthocyanidins which are not easily measured in foods as well as the lack of data on the content of polyphenol-rich additives in processed foods [2], and the omission of herb/plant supplements in the present analysis [42]. Another limitation that may result in inaccuracies in intake estimates for the major coffee polyphenols is linked to the lack of detailed data on polyphenol composition for various types of coffee drinks, according to brewing methods and cultivars (Arabica vs Robusta) [27,43]. A similar limitation exists for herbal teas, which were insufficiently documented in the 24-HDR and vary widely in polyphenol composition. The lack of food composition data in these polyphenol-rich foods, such as coffee and herbal tea was minimized estimating their composition as described in the methodology section.
In conclusion, this study provides the most detailed description of polyphenol intake in a large European multi-center study. It shows a large heterogeneity in both the nature of polyphenols and levels of intake across countries, particularly between MED and non-MED countries. The main food sources for individual polyphenols were similar between regions. However, the major food sources for total polyphenols and specific polyphenol subclasses may vary widely according to regions or countries and food preferences since many compounds show a discrete distribution in foods and can be specific of a food or group of foods consumed in particular regions and not in others. This can be particularly important for polyphenols which would show highly potent and specific biological properties. Lastly, we also showed that socio-demographic, anthropometric and lifestyle factors were associated with differential intake of polyphenols. These descriptive data provide a unique platform to further investigate the role of polyphenols in the prevention of diseases and in healthy aging in one of the largest European cohorts with detailed dietary data.
Supplementary Material
Financial Support
This study was supported by the Institut National du Cancer, Paris (INCa grants 2011-105) and the Wereld Kanker Onderzoek Fonds (WCRF NL 2012/604). The EPIC study was supported by the European Commission: Public Health and Consumer Protection Directorate 1993 to 2004, Research Directorate-General 2005; the French National Cancer Institute (L’Institut National du Cancer; INCA) (grant number 2009-139); Ligue contre le Cancer; the Institut Gustave Roussy; the Mutuelle Générale de l’Education Nationale; the Institut National de la Santé et de la Recherche Médicale (INSERM); the German Cancer Aid; the German Cancer Research Center (DKFZ); the German Federal Ministry of Education and Research; the Danish Cancer Society; The Danish Council for Strategic Research; Health Research Fund (FIS) of the Spanish Ministry of Health (RTICC (DR06/0020/0091); the participating regional governments from Asturias, Andalucía, Murcia, Navarra and Vasco Country and the Catalan Institute of Oncology of Spain; Cancer Research UK; Medical Research Council, UK; the Stroke Association, UK; British Heart Foundation; Department of Health, UK; Food Standards Agency, UK; the Wellcome Trust, UK; the Hellenic Health Foundation (Greece); Italian Association for Research on Cancer; Compagnia San Paolo, Italy; Dutch Ministry of Public Health, Welfare and Sports; Dutch Ministry of Health; Dutch Prevention Funds; LK Research Funds; Dutch ZON (Zorg Onderzoek Nederland); World Cancer Research Fund (WCRF); Statistics Netherlands (The Netherlands); Swedish Cancer Society; Swedish Scientific Council; Regional Government of Skane, The County Council of Västerbotten, Sweden; Nordforsk - Centre of Excellence programme; some authors are partners of ECNIS, a network of excellence of the 6th Framework Program of the European Commission.
Abbreviations
- 24-HDR
24-hours dietary recall
- EPIC
European Prospective Investigation into Cancer and Nutrition
- MED
Mediterranean
- s.e
standard error
- SU.VI.MAX
SUpplémentation en VItamines et Minéraux AntioXydants
- USDA
U.S. Department of Agriculture
Footnotes
Conflict of interest and Funding disclosure: Raul Zamora-Ros, Viktoria Knaze, Joseph A. Rothwell, Bertrand Hémon, Aurelie Moskal, Kim Overvad, Anne Tjønneland, Cecilie Kyrø, Guy Fagherazzi, Marie-Christine Boutron-Ruault, Marina Touillaud, Verena Katzke, Tilman Kühn, Heiner Boeing, Jana Förster, Antonia Trichopoulou, Elissavet Valanou, Eleni Peppa, Domenico Palli, Claudia Agnoli, Fulvio Ricceri, Rosario Tumino, Maria Santucci de Magistris, Petra H.M. Peeters, H. Bas Bueno-de-Mesquita, Dagrun Engeset, Guri Skeie, Anette Hjartåker, Virginia Menéndez, Antonio Agudo, Esther Molina-Montes, José María Huerta, Aurelio Barricarte, Pilar Amiano, Emily Sonestedt, Lena Maria Nilsson, Rikard Landberg, Timothy J. Key, Kay-Thee Khaw, Nicholas J. Wareham, Yunxia Lu, Nadia Slimani, Isabelle Romieu, Elio Riboli, Augustin Scalbert have no conflict of interest.
Conflict of Interest
The authors declare no conflict of interest.
Authors’ Contributions to Manuscript
RZ-R., IR and AS designed the research. RZ-R, VK, JAR, BH and AM performed the research; RZ-R performed the statistical analysis. RZ-R and AS wrote the paper. ER is the overall coordinator of the EPIC study. All authors contributed to recruitment, data collection/acquisition, follow-up and/or management of the EPIC cohort, as well as the interpretation of the present findings and approval of the final version for publication.
References
- 1.Perez-Jimenez J, Neveu V, Vos F, Scalbert A. Systematic analysis of the content of 502 polyphenols in 452 foods and beverages: an application of the phenol-explorer database. J Agric Food Chem. 2010;58:4959–69. doi: 10.1021/jf100128b. [DOI] [PubMed] [Google Scholar]
- 2.Zamora-Ros R, Touillaud M, Rothwell JA, Romieu I, Scalbert A. Measuring exposure to the polyphenol metabolome in observational epidemiologic studies: current tools and applications and their limits. Am J Clin Nutr. 2014;100:11–26. doi: 10.3945/ajcn.113.077743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–42S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 4.Vauzour D, Rodriguez-Mateos A, Corona G, Oruna-Concha MJ, Spencer JP. Polyphenols and human health: prevention of disease and mechanisms of action. Nutrients. 2010;2:1106–31. doi: 10.3390/nu2111106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Ouyang YY, Liu J, Zhao G. Flavonoid intake and risk of CVD: a systematic review and meta-analysis of prospective cohort studies. Br J Nutr. 2014;111:1–11. doi: 10.1017/S000711451300278X. [DOI] [PubMed] [Google Scholar]
- 6.Zamora-Ros R, Jimenez C, Cleries R, Agudo A, Sánchez MJ, Sánchez-Cantalejo E, Molina-Montes E, Navarro C, Chirlaque MD, Huerta JM, et al. Dietary flavonoid and lignan intake and mortality in a Spanish cohort. Epidemiology. 2013;24:726–33. doi: 10.1097/EDE.0b013e31829d5902. [DOI] [PubMed] [Google Scholar]
- 7.Zamora-Ros R, Forouhi NG, Sharp SJ, González CA, Buijsses B, Guevara M, van der Schouw YT, Amiano P, Boeing H, Bredsdorff L, et al. The association between dietary flavonoid and lignan intakes and incident type 2 diabetes in European population: the EPIC-InterAct study. Diabetes Care. 2013;36:3961–70. doi: 10.2337/dc13-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.U.S.Departament of Agriculture. USDA Database for the Proanthocyanidin Content of Selected Foods. Beltsville: MD: USDA; 2004. [Google Scholar]
- 9.U.S.Departament of Agriculture. USDA Database for the Flavonoid Content of Selected Foods. Beltsville: MD: USDA; 2007. [Google Scholar]
- 10.U.S.Departament of Agriculture. USDA Database for the Isoflavone Content of Selected Foods. Beltsville: MD: USDA; 2008. [Google Scholar]
- 11.Neveu V, Perez-Jimenez J, Vos F, Crespy F, du Chaffaut L, Mennen L, Knox C, Eisner R, Cruz J, Wishart D, et al. Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford) 2010;2010:bap024. doi: 10.1093/database/bap024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rothwell JA, Perez-Jimenez J, Neveu V, Medina-Remón A, M’hiri N, García-Lobato P, Manach C, Knox C, Eisner R, Wishart DS, et al. Phenol-Explorer 3.0: a major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database (Oxford) 2013:bat070. doi: 10.1093/database/bat070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rothwell JA, Medina-Remon A, Perez-Jimenez J, Neveu V, Knaze V, Slimani N, Scalbert A. Effects of food processing on polyphenol contents: A systematic analysis using phenol-explorer data. Mol Nutr Food Res. 2014 doi: 10.1002/mnfr.201400494. [DOI] [PubMed] [Google Scholar]
- 14.Chun OK, Chung SJ, Song WO. Estimated dietary flavonoid intake and major food sources of US adults. J Nutr. 2007;137:1244–52. doi: 10.1093/jn/137.5.1244. [DOI] [PubMed] [Google Scholar]
- 15.Zamora-Ros R, Knaze V, Lujan-Barroso L, Romieu I, Scalbert A, Slimani N, Hjartaker A, Engeset D, Skeie G, Overvad K, et al. Differences in dietary intakes, food sources, and determinants of total flavonoids between Mediterranean and non-Mediterranean countries participating in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br J Nutr. 2013;109:1498–507. doi: 10.1017/S0007114512003273. [DOI] [PubMed] [Google Scholar]
- 16.Johannot L, Somerset SM. Age-related variations in flavonoid intake and sources in the Australian population. Public Health Nutr. 2006;9:1045–54. doi: 10.1017/s1368980006009712. [DOI] [PubMed] [Google Scholar]
- 17.Perez-Jimenez J, Fezeu L, Touvier M, Arnault N, Manach C, Hercberg S, Galan P, Scalbert A. Dietary intake of 337 polyphenols in French adults. Am J Clin Nutr. 2011;93:1220–8. doi: 10.3945/ajcn.110.007096. [DOI] [PubMed] [Google Scholar]
- 18.Tresserra-Rimbau A, Medina-Remon A, Perez-Jimenez J, Martínez-González MA, Covas MI, Corella D, Salas-Salvadó J, Gómez-Gracia E, Lapetra J, Arós F, et al. Dietary intake and major food sources of polyphenols in a Spanish population at high cardiovascular risk: the PREDIMED study. Nutr Metab Cardiovasc Dis. 2013;23:953–9. doi: 10.1016/j.numecd.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Grosso G, Stepaniak U, Topor-Madry R, Szafraniec K, Pajak A. Estimated dietary intake and major food sources of polyphenols in the Polish arm of the HAPIEE study. Nutrition. 2014;30:1398–403. doi: 10.1016/j.nut.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ovaskainen ML, Torronen R, Koponen JM, Sinkko H, Hellström J, Reinivuo H, Mattila P. Dietary intake and major food sources of polyphenols in Finnish adults. J Nutr. 2008;138:562–6. doi: 10.1093/jn/138.3.562. [DOI] [PubMed] [Google Scholar]
- 21.Riboli E, Kaaks R. The EPIC Project: rationale and study design. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol. 1997;26(Suppl 1):S6–14. doi: 10.1093/ije/26.suppl_1.s6. [DOI] [PubMed] [Google Scholar]
- 22.Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, Charrondière UR, Hémon B, Casagrande C, Vignat J, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5:1113–24. doi: 10.1079/PHN2002394. [DOI] [PubMed] [Google Scholar]
- 23.Slimani N, Kaaks R, Ferrari P, Casagrande C, Clavel-Chapelon F, Lotze G, Kroke A, Trichopoulos D, Trichopoulou A, Lauria C, et al. European Prospective Investigation into Cancer and Nutrition (EPIC) calibration study: rationale, design and population characteristics. Public Health Nutr. 2002;5:1125–45. doi: 10.1079/PHN2002395. [DOI] [PubMed] [Google Scholar]
- 24.Slimani N, Ferrari P, Ocké M, Welch A, Boeing H, Liere M, Pala V, Amiano P, Lagiou A, Mattisson I, et al. Standardization of the 24-hour diet recall calibration method used in the european prospective investigation into cancer and nutrition (EPIC): general concepts and preliminary results. Eur J Clin Nutr. 2000;54:900–17. doi: 10.1038/sj.ejcn.1601107. [DOI] [PubMed] [Google Scholar]
- 25.Brustad M, Skeie G, Braaten T, Slimani N, Lund E. Comparison of telephone vs face-to-face interviews in the assessment of dietary intake by the 24 h recall EPIC SOFT program--the Norwegian calibration study. Eur J Clin Nutr. 2003;57:107–13. doi: 10.1038/sj.ejcn.1601498. [DOI] [PubMed] [Google Scholar]
- 26.Wareham NJ, Jakes RW, Rennie KL, Schuit J, Mitchell J, Hennings S, Day NE. Validity and repeatability of a simple index derived from the short physical activity questionnaire used in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutr. 2003;6:407–13. doi: 10.1079/PHN2002439. [DOI] [PubMed] [Google Scholar]
- 27.Parras P, Martínez-Tome M, Jiménez AM, Murcia MA. Antioxidant capacity of coffees of several origins brewed following three different procedures. Food Chem. 2007;102:582–92. [Google Scholar]
- 28.U.S.Departament of Agriculture. USDA National Nutrient Database for Standard Reference, Release 25. Washington, DC: US Department of Agriculture, Agricultural Research Service; 2012. [Google Scholar]
- 29.Dilis V, Trichopoulou A. Antioxidant intakes and food sources in Greek adults. J Nutr. 2010;140:1274–9. doi: 10.3945/jn.110.121848. [DOI] [PubMed] [Google Scholar]
- 30.Zamora-Ros R, Rothwell JA, Scalbert A, Knaze V, Romieu I, Slimani N, Fagherazzi G, Perquier F, Touillaud M, Molina-Montes E, et al. Dietary intakes and food sources of phenolic acids in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br J Nutr. 2013:1–12. doi: 10.1017/S0007114513000688. [DOI] [PubMed] [Google Scholar]
- 31.Zamora-Ros R, Andres-Lacueva C, Lamuela-Raventos RM, Berenguer T, Jakszyn P, Barricarte A, Ardanaz E, Amiano P, Dorronsoro M, Larrañaga N, et al. Estimation of dietary sources and flavonoid intake in a Spanish adult population (EPIC-Spain) J Am Diet Assoc. 2010;110:390–8. doi: 10.1016/j.jada.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 32.Knaze V, Zamora-Ros R, Lujan-Barroso L, Romieu I, Scalbert A, Slimani N, Riboli E, van Rossum CT, Bueno-de-Mesquita HB, Trichopoulou A, et al. Intake estimation of total and individual flavan-3-ols, proanthocyanidins and theaflavins, their food sources and determinants in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br J Nutr. 2012;108:1095–108. doi: 10.1017/S0007114511006386. [DOI] [PubMed] [Google Scholar]
- 33.Zamora-Ros R, Knaze V, Romieu I, Scalbert A, Slimani N, Clavel-Chapelon F, Touilaud M, Perquier F, Skeie G, Engeset D, et al. Impact of thearubigins on the estimation of total dietary flavonoids in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur J Clin Nutr. 2013;67:779–82. doi: 10.1038/ejcn.2013.89. [DOI] [PubMed] [Google Scholar]
- 34.Zamora-Ros R, Knaze V, Lujan-Barroso L, Slimani N, Romieu I, Touillaud M, Kaaks R, Teucher B, Mattiello A, Grioni S, et al. Estimation of the intake of anthocyanidins and their food sources in the European Prospective Investigation in to Cancer and Nutrition (EPIC) study. Br J Nutr. 2011;106:1090–9. doi: 10.1017/S0007114511001437. [DOI] [PubMed] [Google Scholar]
- 35.Zamora-Ros R, Knaze V, Lujan-Barroso L, Slimani N, Romieu I, Fedirko V, de Magistris MS, Ericson U, Amiano P, Trichopoulou A, et al. Estimated dietary intakes of flavonols, flavanones and flavones in the European Prospective Investigation into Cancer and Nutrition (EPIC) 24-h dietary recall cohort. Br J Nutr. 2011;106:1915–25. doi: 10.1017/S000711451100239X. [DOI] [PubMed] [Google Scholar]
- 36.Zamora-Ros R, Knaze V, Lujan-Barroso L, Kuhnle GG, Mulligan AA, Touillaud M, Slimani N, Romieu I, Powell N, Tumino R, et al. Dietary intakes and food sources of phytoestrogens in the European Prospective Investigation into Cancer and Nutrition (EPIC) 24-hour dietary recall cohort. Eur J Clin Nutr. 2012;66:932–41. doi: 10.1038/ejcn.2012.36. [DOI] [PubMed] [Google Scholar]
- 37.Chan SG, Ho SC, Kreiger N, Darlington G, So KF, Chong PY. Dietary sources and determinants of soy isoflavone intake among midlife Chinese Women in Hong Kong. J Nutr. 2007;137:2451–5. doi: 10.1093/jn/137.11.2451. [DOI] [PubMed] [Google Scholar]
- 38.Mulligan AA, Welch AA, McTaggart AA, Bhaniani A, Bingham SA. Intakes and sources of soya foods and isoflavones in a UK population cohort study (EPIC-Norfolk) Eur J Clin Nutr. 2007;61:248–54. doi: 10.1038/sj.ejcn.1602509. [DOI] [PubMed] [Google Scholar]
- 39.Peterson J, Dwyer J, Adlercreutz H, Scalbert A, Jacques P, McCullough ML. Dietary lignans: physiology and potential for cardiovascular disease risk reduction. Nutr Rev. 2010;68:571–603. doi: 10.1111/j.1753-4887.2010.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 41.Zamora-Ros R, Andres-Lacueva C, Lamuela-Raventos RM, Berenguer T, Jakszyn P, Martínez C, Sánchez MJ, Navarro C, Chirlaque MD, Tormo MJ, et al. Concentrations of resveratrol and derivatives in foods and estimation of dietary intake in a Spanish population: European Prospective Investigation into Cancer and Nutrition (EPIC)-Spain cohort. Br J Nutr. 2008;100:188–96. doi: 10.1017/S0007114507882997. [DOI] [PubMed] [Google Scholar]
- 42.Willet W. Nutritional Epidemiology. 2nd ed. New York: Oxford University Press; 1998. [Google Scholar]
- 43.Skeie G, Braaten T, Hjartaker A, Lentjes M, Amiano P, Jakszyn P, Pala V, Palanca A, Niekerk EM, Verhagen H, et al. Use of dietary supplements in the European Prospective Investigation into Cancer and Nutrition calibration study. Eur J Clin Nutr. 2009;63(Suppl 4):S226–S238. doi: 10.1038/ejcn.2009.83. [DOI] [PubMed] [Google Scholar]
- 44.Ludwig IA, Mena P, Calani L, Cid C, Del Rio D, Lean ME, Crozier A. Variations in caffeine and chlorogenic acid contents of coffees: what are we drinking? Food Funct. 2014;5:1718–26. doi: 10.1039/c4fo00290c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.