Abstract
Receptors for thyrotropin-releasing hormone (TRH) and thyrotropin (thyroid-stimulating hormone–TSH) are important regulators of the function of the TSH-producing cells of the anterior pituitary gland and the thyroid gland, respectively, and thereby play a central role in thyroid hormone homeostasis. Although the roles of TRH- and TSH-stimulated signaling in these endocrine glands are well understood, these receptors are expressed in other sites and their roles in these extraglandular tissues are less well known. Moreover, one of the two subtypes of TRH receptors (TRH-R2) and the single TSH receptor (TSHR) exhibit constitutive signaling activity and the roles of constitutive signaling by these receptors are poorly understood. One approach to studying constitutive signaling is to use inverse agonists. In this chapter, we will describe the experimental procedures used to measure constitutive signaling by TRH-R2 and TSHR and the effects of their specific inverse agonists.
1. Introduction
The hypothalamic–pituitary–thyroid axis plays the major role in regulating thyroid hormone homeostasis in the body. Thyrotropin-releasing hormone (TRH), which is a tripeptide, is secreted by the hypothalamus into a portal system that directs TRH to the pituitary gland where it can bind to its seven transmembrane-spanning receptors (7TMRs or G protein-coupled receptors—GPCRs). In most mammalian species, there are two subtypes of TRH receptors, subtype 1 (TRH-R1) that is found in the anterior pituitary gland and in extrapituitary tissues and subtype 2 (TRH-R2) that is found in extrapituitary tissues but not in the pituitary (see below); in humans, however, there is only a single TRH receptor that is homologous structurally to TRH-R1. Activation of TRH-R1 in the anterior pituitary leads to stimulation of the synthesis and secretion into the systemic circulation of thyrotropin (thyroid-stimulating hormone— TSH), which is an ~ 30 kDa heterodimeric glycoprotein, from where it binds to TSH receptors (TSHRs), which are 7TMRs, on thyroid hormone-producing cells (thyrocytes) of the thyroid gland. Activation of TSHRs on thyrocytes leads to stimulation of the synthesis and secretion into the general circulation of thyroid hormones. Thyroid hormones, in turn, regulate the biology of virtually every cell of the body. In a classical endocrine negative feedback loop, thyroid hormones bind to nuclear receptors in the hypothalamus and pituitary to inhibit synthesis of both TRH and TSH, respectively.
In addition to the above-noted difference in expression ofTRH-R1 and TRH-R2 in the pituitary gland, TRH-R1 and TRH-R2 are expressed in different sites within the rat brain and in different cells outside of the central nervous system (Sun et al., 2003). Although a number of effects of administration of TRH and its analogs to intact animals and to cells in culture have been observed, we think it is fair to state that the physiological roles of TRH receptors in extrapituitary tissues are still not understood. TRH-R1 and TRH-R2 exhibit very similar binding profiles for numerous TRH analogs and, therefore, most peptidic TRH analogs cannot distinguish between TRH-R1 and TRH-R2. However, some unnatural peptidic analogs (Monga et al., 2008) and a nonpeptidic, small molecule antagonist (Engel et al., 2008) that bind preferentially to TRH-R1 or TRH-R2 have been described and these ligands may help distinguish the unique physiological roles of each receptor. For this chapter, the most important distinction between TRH-R1 and TRH-R2 is that TRH-R2 is constitutively active whereas TRH-R1 is not. While homology modeling and related receptor mutagenesis studies have suggested a structural basis for its increased basal activity (Deflorian et al., 2008), the functional role of this constitutive signaling by TRH-R2 is not understood. Inverse agonists inhibit constitutive activity. Midazolam, a short-acting drug in the benzodiazepine class, is an inverse agonist for TRH-R2 that can be used in studies in vitro (Lu et al., 2004) but its depressant effects prevent it from being used in animal studies.
In addition to its expression on the surface of thyrocytes, TSHR is also found in extrathyroidal sites including bone, and orbital and peripheral adipose tissue (Neumann et al., 2009) but its physiologic or pathologic roles in these extrathyroidal tissues is not well understood. Moreover, as for TRH-R2, the role of constitutive signaling by TSHR has not been elucidated. TSH analogs, antibodies that bind to TSHR and, more recently, small molecule TSHR ligands have been used to study TSHR signaling (Neumann et al., 2009). Of note, several TSHR-binding antibodies have been shown to be inverse agonists (Chen et al., 2007; Moriyama et al., 2003; Sanders et al., 2008) and we have recently described a small molecule TSHR inverse agonist (Neumann et al., 2010). However, inverse agonists have not yet been used to delineate the role of TSHR constitutive signaling in animal studies.
2. TRH-R2 and Its Inverse Agonist Midazolam
With many 7TMRs, in particular, those coupled via Gs to adenylyl cyclase (see TSHR below), it is possible to measure constitutive signaling by measuring the production of the proximal second messenger molecules. In our experience, it has been more difficult to measure constitutive signaling by 7TMRs that couple via Gq/11 to phospholipase C. This is primarily due to the rapid turnover of second messenger inositol-1,4,5-trisphosphate. We were able to quantify constitutive signaling in a 7TMR expressed by the Kaposi’s sarcoma virus (human herpesvirus 8) by measuring the more stable inositol monophosphate, a metabolic breakdown product of inositol-1,4,5- trisphosphate (Arvanitakis et al., 1997). However, we could not measure constitutive TRH-R2 signaling by measuring inositol monophosphate production using cells prelabeled with radioactive inositol or an ELISA that measures unlabeled inositol monophosphate even though the degradation of inositol monophosphate was inhibited by LiCl. We, therefore, measured constitutive signaling of TRH-R2 using a reporter gene assay, which takes advantage of the amplification that occurs during signal transduction to gene transcription.
2.1. Use of protein kinase C-activated reporter genes in cells transfected to express TRH-R2
Over the years, we have used a number of transfection cocktails to coexpress TRH-R2 at controlled levels with several protein kinase C (PKC) reporter genes in a number of different adherent mammalian cell lines. We have had success with several of these combinations. Of note, although we have not made an exhaustive comparison between mouse and rat TRH-R2s, we have not found substantive differences in their signaling characteristics.
2.1.1. Required materials
Cells used: HEK 293 EM cells (Robbins and Horlick, 1998) were obtained from Dr R. A. Horlick because they are very adherent to tissue culture wells. We have also used HEK 293 and COS-1 cells that can be obtained from the American Type Culture Collection (ATCC).
Plasmids expressing mouse TRH-R1, TRH-R2, and mutants of the wild-type receptors that we constructed. DNA of high quality (endotoxin-free plasmid DNA, use of Plasmid Maxi kits) is recommended.
Many useful reporter plasmids for 7TMR signaling through PKC or other kinases are available within the PathDetect® In Vivo Signal Transduction Pathway cis- or trans-Reporting System from Agilent (Santa Clara, CA). For constitutive signaling by TRH-R2, we have used the AP-1-Luciferase cis-Reporting System (Cat # 219073).
Transfection cocktail: Many available transfection reagents will provide satisfactory results. We have used FuGene® 6 Transfection Reagent from Roche (Mannheim, Germany).
Growth medium: Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal bovine serum.
Ligand-binding assays: [3H][methyl-His]TRH ([3H]MeTRH) is available from Perkin Elmer (Waltham, MA) and [3H]TRH is available from American Radiolabeled Chemicals (St. Louis, MO).
Hank’s Balanced Salt Solution (HBSS) containing 10 mM HEPES, pH 7.4
Trypsin (0.05%)–EDTA (0.53 mM) solution
Phosphate buffered saline (PBS), pH 7.4
- Disposables
- Tissue culture—175-cm2 flasks, 24-well plates
- Test tubes—50, 15, 1.5 ml (Eppendorf)
- Pipettes
2.1.2. Measurement of constitutive signaling activity of TRH-R2
Most investigators (see other chapters) measure constitutive signaling by relating the agonist-independent signaling parameter to a measurement of the number of receptors when it is feasible to measure both in the same cell or cell population. Receptor number is usually measured by ligand or antireceptor antibody binding. Some investigators will make these measurements at a single level of receptor expression. This is sometimes unavoidable, for example, if one is measuring constitutive signaling of receptors expressed endogenously. However, most measurements of constitutive signaling are made using receptors expressed exogenously and we believe that showing a direct correlation between the level of constitutive signaling and the number of receptors is the most definitive way of estimating the level of constitutive signaling activity for any receptor. Moreover, if ligand or antibody binding cannot be used, it is possible to vary the level of receptor expression plasmid input to show constitutive activity although this will not permit definitive quantitative comparisons amongst different receptors.
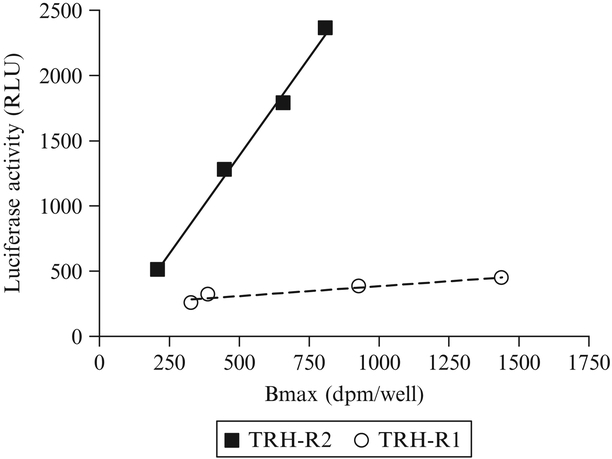
Constitutive activity of TRH-R2 was demonstrated definitively as a direct correlation between receptor number and luciferase reporter activity in cells transiently transfected with increasing amounts of receptor expression vector (Fig. 9.1). This finding allowed studies of TRH-R2 signaling to be performed in cells stably expressing TRH-R2. Because there is no cell line that expresses TRH-R2 endogenously, we have used HEK 293 EM cells stably transfected with mouse TRH-R2 for these studies.
Figure 9.1.
Constitutive signaling activities of TRH-R2 and TRH-R1 measured by reporter gene assay in HEK 293 EM cells. HEK 293 EM cells were transfected with increasing amounts of receptor expression plasmids and a single amount of reporter gene plasmid. After 24 h, luciferase activities (relative light units, RLU) and maximum specific ligand binding (receptor density, Bmax) were measured. The figure is adapted from Wang and Gershengorn (1999)
One day prior to transfection
Harvest HEK 293 EM cells from a 175-cm2 flask with 10 ml of Trypsin–EDTA, count cells, and seed 80,000 cells per well of a 24-well plate in 0.5 ml DMEM with 10% fetal bovine serum.
On the day of transfection
Prepare Fugene® 6 suspension: Add Fugene® 6 directly to DMEM without serum to a final volume of 100 μl/well and mix by vortexing for 1–3 s. The amount of Fugene® 6 required is calculated so that the ratio of Fugene® 6 to DNA (added in step 3) will be 3:1 (e.g., 3 μl Fugene® 6 per 1 μg DNA).
Incubate the suspension for 5 min at RT.
Prepare transfection cocktail: Add receptor plasmid DNA (0.01–0.2 μg) and 0.2 μg AP-1-Luciferase DNA to ~ 100 μl of Fugene® 6 suspension to achieve the desired 3:1 ratio. Mix thoroughly by vortexing for 1–3 s and incubate for 15–60 min at RT. If dose-dependent constitutive activity is measured (as in Fig. 9.1), add empty vector plasmid DNA to the wells with less than 0.2 μg receptor plasmid DNA to achieve a final total DNA concentration of 0.4 μg in all wells.
Add the transfection cocktail (100 μl/well) dropwise to the wells containing 0.5 ml DMEM with 10% fetal bovine serum, dispersing it evenly throughout the well.
Incubate in 5% CO2 at 37 °C.
After 48–72 h
- Measure luciferase activity
- Wash wells with 1 ml PBS three times.
- Add 0.5 ml lysis buffer (25 mM Gly-Gly, pH 7.8, 15 mM MgSO4, 4 mM EGTA, 1% Triton X-100) and incubate for 15 min at RT.
- Scrape cells, place in 1.5-ml tube and spin at maximum speed in Eppendorf centrifuge for 5 min in the cold.
- Decant supernatant into a fresh tube.
- Immediately prior to measuring luciferase activity add 0.1 ml sample to 0.5 ml assay reagent (25 mM Gly-Gly, pH 7.8, 15 mM MgSO4, 4 mM EGTA, 15 mM KH2PO4, 2 mM DTT, 2 mM ATP, 0.4 mM luciferin).
- Measure the light emitted from the reaction in a luminometer using an integration time of 10–30 s.
- Measure receptor density
- Wash wells with 0.5 ml HBSS three times.
- Add 0.25 ml HBSS containing 0.1–10 nM [3H]MeTRH or 1–10 nM [3H]TRH without or with 1000-fold excess unlabeled MeTRH or TRH. For some mutant receptors with lower binding affinities, it may be necessary to increase the concentration of [3H]MeTRH or [3H] TRH.
- Incubate for 1 h at 37 °C.
- Wash cell monolayers three times with 0.5 ml ice-cold HBSS.
- Solubilize the cells with 0.5 ml 0.4 N NaOH.
- Count 0.4 ml in a beta scintillation counter.
- Specific binding is calculated as the difference between total binding (3H-labeled ligand alone) minus nonspecific binding (3H-labeled ligand plus unlabeled ligand). Maximum binding (total receptor binding) is calculated from nonlinear regression analysis; we use the PRISM program (GraphPad Software, Inc., San Diego, CA). The number of TRH receptors is calculated using the specific radioactivity of the 3H-labeled ligand assuming a stoichiometry of ligand:receptor of 1:1.
Measurements of constitutive signaling activities in cells expressing increasing levels of TRH-R2 and TRH-R1 are illustrated in Fig. 9.1 (Wang and Gershengorn, 1999).
Midazolam is a benzodiazepine drug that is an inverse agonist at TRH receptors. In a parallel series of experiments to that illustrated in Fig. 9.1, we showed that midazolam inhibited constitutive signaling by TRH-R2 by 42% at 10 μM and by 74% at 50 μM (Wang and Gershengorn, 1999).
3. TSHR and Its Inverse Agonist NCGC00161856
In contrast to 7TMRs that couple via Gq/11 to phospholipase C where it is difficult to measure constitutive signaling by measuring proximal messenger molecules, it is relatively easy to measure the production of the second messenger cAMP for 7TMRs coupled via Gs to adenylyl cyclase. These studies are generally performed in the presence of the phosphodiesterase inhibitor isobutylmethylxanthine (IBMX), and the accumulated cAMP can be measured readily by a number of antibody-based assays. We routinely have used IBMX and measured cAMP by immunoassay. In addition, we occasionally have used reporter genes (see Section 2.1.1), in particular the PathDetect® AP-1 cis- and CREB trans-Reporting Systems.
To monitor TSHR constitutive signaling in a more physiologically relevant cell type, we also use primary cultures of human thyroid cells (thyrocytes) that express TSHRs endogenously. In thyrocytes, we measure cAMP production along with expression levels of mRNA transcripts for TSH-regulated genes by reverse transcription followed by quantitative real time polymerase chain reaction (qRT-PCR).
3.1. Use of immunoassays to measure cAMP in cells endogenously expressing TSHRs and in cells stably or transiently transfected to express TSHR
3.1.1. Required materials
-
Cells used: HEK 293 EM cells (Robbins and Horlick, 1998) were obtained from Dr R. A. Horlick because they are very adherent to tissue culture wells. As we described the method for transient transfection above (Section 2.1.1) and TSHR has been shown to exhibit constitutive signaling by showing a direct correlation between receptor number and agonist-independent cAMP production (Vassartet al., 1991), we will describe studies using HEK 293 EM cells stably expressing TSHR (HEK-TSHR cells). The same protocol can be used in cells transiently expressing human TSHR and mutants of TSHR and in primary cultures of human thyrocytes.
Human thyrocytes: Normal thyroid tissue was collected from patients undergoing total thyroidectomy for thyroid cancer at the National Institutes of Health Clinical Center. Patients provided informed consent on an Institutional Review Board-approved protocol and materials were received anonymously via approval of research activity through the Office of Human Subjects Research. The tissue was kept in HBSS on ice during transport. Monodispersed cells were obtained by mincing the tissue into small pieces, washing three times with ice-cold HBSS, and digesting with HBSS containing 3 mg/ml Collagenase Type IV (GIBCO, Carlsbad, CA) for 30 min or longer with constant shaking in a water bath at 37 °C. After centrifugation for 5 min at 160 × g, the supernatant was removed, the cells were washed with DMEM once and resuspended in 10 ml DMEM with 10% fetal bovine serum, plated in 10-cm tissue culture dishes and incubated at 37 °C in a humidified 5% CO2 incubator. After 24 h, the cells were rinsed with DMEM two times, fresh growth medium was added and the primary cultures of adherent thyrocytes formed a confluent monolayer within 5–7 days.
Measurement of cAMP: cAMP-Screen Direct® System from Applied Biosystems (Bedford, MA).
Growth medium: DMEM containing 10% fetal bovine serum.
Ligand-binding assays: 125I-TSH (bovine) from BRAHMS Aktien-gesellschaft (Hennigsdorf, Germany).
Antibody binding: Mouse antihuman TSHR antibody 2C11 was obtained from Serotec Ltd (Oxford, UK) and Alexa Fluor® 488-conjugated F(ab′)2 rabbit antimouse IgG antibody was from Molecular Probes (Invitrogen, Carlsbad, CA).
HBSS containing 10 mM HEPES, pH 7.4
Trypsin (0.05%)–EDTA (0.53 mM) solution
EGTA (1 mM)/EDTA (1 mM) buffer
PBS without Ca2+ or Mg2+
Fluorescence activated cell sorter (FACS) buffer (PBS with 0.1% bovine serum albumin and 0.1% sodium azide)
1% paraformaldehyde (PFA)
- Disposables
- Tissue culture—175-cm2 flasks, 24-well plates
- Test tubes—50, 15, 5, 1.5 ml (Eppendorf)
- Pipettes
3.1.2. Measurement of constitutive signaling activity of TSHR by cAMP production
One day prior to beginning the experiment:
Harvest HEK-TSHR cells from a 175-cm2 flask with 10 ml of Trypsin– EDTA, count cells and seed 220,000 cells per well of a 24-well plate in 0.5 ml DMEM with 10% fetal bovine serum. For thyrocytes, seed 80,000 cells per well.
On the day of the experiment:
Aspirate the growth medium and wash the cell monolayer three times with 0.5 ml HBSS at RT.
Incubate cells in 0.25 ml HBSS in 5% CO2 incubator at 37 °C for 30 min.
Aspirate the buffer and replace it with HBSS containing 1 mM IBMX and test substance(s) and incubate for desired times.
At the desired times, either add 0.25 ml lysis buffer (from the cAMP-Screen Direct® Kit) directly to the wells to measure total cAMP or remove the media from the cells by aspiration and add 0.25 ml lysis buffer to the wells to measure intracellular cAMP.
Measure cAMP using the chemiluminescent immunoassay system (Applied Biosystems cAMP-Screen Direct® System) according to the manufacturer’s instructions.
Measuring receptor density by ligand (125I-TSH) binding to HEK-TSHR cells
Wash wells with 0.5 ml HBSS three times.
Add 0.25 ml HBSS containing 0.2 g BSA and 2.5 g milk powder per 100 ml and ~ 60,000 cpm 125I-TSH without or with 1000-fold excess unlabeled bovine TSH. For some mutant receptors with lower affinities, it may be necessary to increase the concentration of 125I-TSH.
Incubate for 2 h at 4 °C.
Wash cell monolayers with 0.5 ml ice-cold HBSS three times.
Solubilize the cells with 0.5 ml 0.4 N NaOH.
Count 0.4 ml in a gamma counter.
Specific binding is calculated as the difference between total binding (125I-TSH alone) minus nonspecific binding (125I-TSH plus unlabeled TSH). As the apparent affinity of TSHR for 125I-TSH is relatively low, the binding assay is usually performed with a fixed concentration of 125I-TSH and various concentrations of unlabeled TSH. Maximum binding (total receptor binding) is calculated from nonlinear regression analysis; we use the PRISM program (GraphPad Software, Inc., San Diego, CA). The number of TSHRs is calculated using the various specific radioactivities (decreased by adding unlabeled TSH) of the 125I-TSH at each dose assuming a stoichiometry of ligand:receptor of 1:1.
Estimating relative TSHR expression using antihuman TSHR antibody
Wash cell monolayer with 0.5 ml PBS.
Add 0.5 ml EGTA/EDTA buffer and incubate for 5 min at RT.
Detach cells and spin at 1500 rpm for 2 min.
Discard supernatant and add 0.1 ml 5% donkey serum in FACS buffer and incubate for 10 min at 4 °C.
Wash with 2 ml FACS buffer and spin at 1500 rpm for 2 min.
Discard supernatant and add 0.1 ml anti-TSHR antibody 2C11 (final concentration 10 μg/ml) or 0.1 ml mouse IgG (final concentration 10 μg/ml).
Incubate for 1 h at 4 °C.
Wash twice with 2 ml FACS buffer.
Add 0.1 ml secondary antibody (Alexa Fluor® 488-conjugated F(ab′)2 anti-IgG).
Incubate in the dark for 30 min at 4 °C.
Wash twice with 2 ml FACS buffer at 4 °C.
Add 0.15–0.2 ml 1% PFA (depending on cell pellet size).
Measure fluorescence in a FACS analyzer. The TSHR-expressing cells are those whose fluorescence levels are greater than cells labeled with an “isotype control” mouse IgG.
3.2. Use of qRT-PCR to measure mRNAs for thyroglobulin (TG), thyroperoxidase (TPO), TSHR, sodium-iodide symporter (NIS), and Type 2 deiodinase (DIO2) in primary cultures of human thyrocytes endogenously expressing TSHRs
3.2.1. Required materials
Human thyrocytes: see Section 3.1.1b
Measurement of mRNA levels—primers and probes for TG, TPO, TSHR, NIS, DIO2, and GAPDH were obtained from Applied Biosystems (Foster City, CA).
Growth medium—DMEM containing 10% fetal bovine serum.
Trypsin (0.05%)—EDTA (0.53mM) solution
- Disposables
- Tissue culture—175-cm2 flasks, 24-well plates, 12-well plates
- Test tubes—50, 15, 5, 1.5 ml (Eppendorf)
- Pipettes
3.2.2. Measurement of constitutive signaling activity by effects on thyroid-selective genes
Three days prior to mRNA isolation
Harvest thyrocytes with Trypsin–EDTA and seed 60,000 cells per well of a 24-well plate or 100,000 cells per well of a 12-well plate in DMEM with 10% fetal bovine serum.
On the first day of the experiment (24 h after seeding of the cells)
Change medium to DMEM with 2% fetal bovine serum containing 1 mM IBMX without or with test factors (e.g., inverse agonist).
On the third day of the experiment (48 h after addition of test factors)
Aspirate buffer and extract total RNA using the RNeasy® Micro Kit from Qiagen (Valencia, CA).
Prepare cDNA using a High Capacity cDNA Archive Kit from Applied Biosystems (Foster City, CA).
qRT-PCR is performed in 0.025 ml reactions using cDNA prepared from ~ 100 ng of RNA and Universal Master Mix (Applied Biosystems, Foster City, CA). The mRNA level of each sample is normalized to GAPDH to correct for differences in cDNA input.
NCGC00161856 is an inverse agonist of TSHR (Neumann et al., 2010). Figure 9.2 illustrates the effects of NCGC00161856 on cAMP production (over 2 h) and on mRNA levels for TG, TPO, TSHR, NIS, and DIO2 after 48 h exposure in primary cultures of human thyrocytes. The inverse agonist reduces basal cAMP levels in a dose-dependent fashion. Interestingly, the transcript analyses suggest that basal signaling by TSHR plays an important role in maintaining expression levels of several key thyroid-specific genes. By reducing basal signaling, the inverse agonist NCGC00161856 decreases expression of these key genes.
Figure 9.2.
Effect of inverse agonist NCGC00161856 (NCGC) on constitutive signaling by TSHR measured as cAMP production and mRNA levels for TG, TPO, TSHR, NIS, and DIO2 in primary cultures of human thyrocytes. (A) Thyrocytes were incubated in HBSS with 1 mM IBMX and without (control) or with increasing concentrations of NCGC for 2 h. The cAMP production measured without 1 mM IBMX was subtracted from all samples. (B) Thyrocytes were incubated in DMEM containing 2% fetal bovine serum and 1 mM IBMX without or with 30 μM NCGC for 48 h. This figure is from Neumann et al. (2010)
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
REFERENCES
- Arvanitakis L, Geras-Raaka E, Varma A, Gershengorn MC, and Cesarman E (1997). Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature 385, 347–350. [DOI] [PubMed] [Google Scholar]
- Chen CR, McLachlan SM, and Rapoport B (2007). Suppression of thyrotropin receptor constitutive activity by a monoclonal antibody with inverse agonist activity. Endocrinology 148, 2375–2382. [DOI] [PubMed] [Google Scholar]
- Deflorian F, Engel S, Colson A-O, Raaka BM, Gershengorn MC, and Costanzi S (2008). Understanding the structural and functional differences between mouse thyrotropin-releasing hormone receptors 1 and 2. Proteins 71, 783–794. [DOI] [PubMed] [Google Scholar]
- Engel S, Skoumbourdis AP, Childress J, Neumann S, Deschamps JR, Thomas CJ, Colson AO, Costanzi S, and Gershengorn MC (2008). A virtual screen for diverse ligands: Discovery of selective G protein-coupled receptor antagonists. J. Am. Chem. Soc 130, 5115–5123. [DOI] [PubMed] [Google Scholar]
- Lu X, Huang W, Worthington S, Drabik P, Osman R, and Gershengorn MC (2004). A model of inverse agonist action at thyrotropin-releasing hormone receptor type 1: Role of a conserved tryptophan in helix 6. Mol. Pharmacol 66, 1192–1200. [DOI] [PubMed] [Google Scholar]
- Monga V, Meena CL, Kaur N, and Jain R (2008). Chemistry and biology of thyrotropin-releasing hormone (TRH) and its analogs. Curr. Med. Chem 15, 2718–2733. [DOI] [PubMed] [Google Scholar]
- Moriyama K, Okuda J, Saijo M, Hattori Y, Kanamoto N, Hataya Y, Matsuda F, Mori T, Nakao K, and Akamizu T (2003). Recombinant monoclonal thyrotropin-stimulation blocking antibody (TSBAb) established from peripheral lymphocytes of a hypothyroid patient with primary myxedema. J. Endocrinol. Invest 26, 1076–1080. [DOI] [PubMed] [Google Scholar]
- Neumann S, Raaka BM, and Gershengorn MC (2009). Human TSH receptor ligands as pharmacological probes with potential clinical application. Expert Rev. Endocrinol. Metab 4, 669–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S, Huang W, Eliseeva E, Titus S, Thomas CJ, and Gershengorn MC (2010). A small molecule inverse agonist for the human TSH receptor. Endocrinology 151, 3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins AK, and Horlick RA (1998). Macrophage scavenger receptor confers an adherent phenotype to cells in culture. Biotechniques 25, 240–244. [DOI] [PubMed] [Google Scholar]
- Sanders J, Evans M, Betterle C, Sanders P, Bhardwaja A, Young S, Roberts E, Wilmot J, Richards T, Kiddie A, Small K, Platt H, et al. (2008). A human monoclonal autoantibody to the thyrotropin receptor with thyroid-stimulating blocking activity. Thyroid 18, 735–746. [DOI] [PubMed] [Google Scholar]
- Sun Y, Lu X, and Gershengorn MC (2003). Thyrotropin-releasing hormone receptors—Similarities and differences. J. Mol. Endocrinol 30, 87–97. [DOI] [PubMed] [Google Scholar]
- Vassart G, Parmentier M, Libert F, and Dumont J (1991). Molecular genetics of the thyrotropin receptor. Trends Endocrinol. Metab 2, 151–156. [DOI] [PubMed] [Google Scholar]
- Wang W, and Gershengorn MC (1999). Rat TRH receptor type 2 exhibits higher basal signaling activity than TRH receptor type 1. Endocrinology 140, 4916–4919. [DOI] [PubMed] [Google Scholar]