Abstract
While separated by large expanses of dry terrain unsuitable for aquatic biota, aridland waters possess high biodiversity. How aquatic micrometazoans disperse to, and colonize, these isolated ephemeral habitats are not well understood. We used a multi-faceted approach including wind tunnel and rehydration experiments, and next-generation sequencing to assess potential movement of diapausing propagules of aquatic invertebrates by anemochory across regional scales (102–105 km). Wind tunnel experiments using dry playa sediments with added micrometazoan propagules demonstrated that after entrainment by saltation and downwind transport were subsequently recoverable as viable animals when rehydrated. Further, rehydration of fallen natural dust yielded micrometazoans, including rotifers, gastrotrichs, microcrustaceans, and nematodes. Using conserved DNA primers, we identified >3,300 eukaryotic Operational Taxonomic Units (excluding fungi) in the dust including some taxa found in rehydration experiments. Thus, we provide strong evidence that anemochory can disperse micrometazoans among isolated, ephemeral ecosystems in North American deserts and likely elsewhere.
Keywords: anemochory, desert, dispersal, dust, next-generation sequencing, sediment rehydration
Introduction
Desert aquatic habitats differ from temperate systems in many respects; most obvious is a limited hydroperiod (Williams, 2006). Indeed ability to withstand prolonged dryness with large variations in temperature and ultraviolet radiation is critical for the biota of these habitats (Jocque et al. 2010). Yet desert aquatic life is adapted to this duality: flourishing when basins are filled, but withstanding inevitable drought of uncertain duration. Persistence is accomplished through life history adaptations including production of small, drought-resistant stages that quickly re-animate and develop rapidly when suitable conditions return (Brock et al. 2003, Walsh et al. 2014). These stages also can facilitate dispersal (Vanschoenwinkel et al., 2008).
In summarizing the roles of major ecological processes responsible for the distribution of biota, McGill (2010) noted that for dispersal, data at intermediate spatial scales are lacking (~102 to 105 m). This holds true for our understanding of aquatic biota movement among hydrologically disconnected landscapes. Intermediate scales are particularly important in drylands where regional monsoons govern timing and duration of rehydration of temporary waterbodies (Scuderi et al., 2010). These rains fill basins, which upon drying render the land into an expanse of polygonal shaped mud cracks or saline crusts (Fig. 1). Winds blowing across these landforms entrain dust (silt-and clay-sized grains, diameter <50 µm) and sand (diameter >50 µm) (Feld et al. 2010). Researchers have estimated that on a global, annual basis >1,000 Tg of soil is emitted from the ground into the atmosphere as dust, with approximately one-third derived from ephemeral aquatic systems (Ginoux et al., 2012). Along with dust, resting stages of micrometazoans can be transported; however, it is unclear whether this is strictly a local phenomenon or whether such transport occurs across hundreds of kilometers.
Fig. 1.
A model of aeolian dispersal of micrometazoan propagules and subsequent community development in aridland aquatic systems. Dispersal of dust and propagules (Brown dots) begins with physical processes (Brown arrows) that act as filters that sort all particles: (1) Sources (of particulate materials), (2) Anemochory (wind dispersal). For propagules, additional filters (Blue) govern potential for colonization: (3) ability enter into a desiccated state (propagule production), (4) resistance of propagules to stresses associated with drying and prolonged drought, and (5) resilience, the ability of the propagules to hatch depending on edaphic conditions. While life history adaptations of new arrivals or those already present (Dashed lines) may sanction persistence and hatching, continuance is not assured. Community development and propagule replenishment are biological processes. Collectively these processes work in concert shaping the α, β, and γ–diversity of active aquatic communities. Terms used describing aeolian and biotic processes are given in Supplementary Document 4.
The ‘Everything is everywhere’ hypothesis posits that most aquatic microbiota (~1–2 mm) are cosmopolitan because they have small propagules that disperse easily by anemo-, hydro-, and zoochory (Fontaneto, 2011, Viana et al. 2013). Research supports this view for bacteria (Yamaguchi et al., 2012), fungi and protists (Barberan et al. 2015), and phytoplankton (Incagnone et al. 2015), but little is known regarding micrometazoans (Fontaneto, 2011). Thus we know a good deal about the dispersal of small aquatic biota at local and global scales, including both temperate and cold habitats (Havel and Shurin 2004, Fontaneto et al. 2006, Nkem et al. 2006). We also recognize the importance of biotic networks (i.e., co-dispersal via zoochory) that enhance dispersal (Tesson et al. 2016). If propagule stages are small enough and sufficiently resistant, they should be able to disperse with sediments during storm events, and survive (Finlay 2002).
In contrast, the extent to which dispersal mechanisms operate on regional scales in dryland systems is relatively unknown. In the US desert southwest hydrochory is restricted to endorheic flows or to rivers that carry biota through aridlands, potentially dispersing it into local floodplain basins during times of exceptional floods (Kobayashi et al. 2015). While the potential for zoochory among aridland basins is clear, it is primarily limited to wet seasons (Sánchez et al. 2012, Viana et al. 2013, Valls et al. 2017). In aridlands, aquatic system isolation and stochastic rain events followed by prolonged dryness and subsequent wind dispersal may be important forces shaping species distribution, extirpation, interrupted gene flow, ecological specialization, speciation, and endemism (Ricklefs, 2008, Collins et al., 2014, Hubert et al. 2015).
Previous research has established anemochory of aquatic micrometazoans on local scales (≤10s of kilometers): i.e., branchiopod (Graham and Wirth 2008) and Artemia cysts (Parekh et al. 2014) were recovered from wind tunnel sediments, and dispersing stages of >15 invertebrate taxa were captured in an isolated rocky outcrop (Vanschoenwinkel et al. 2009). Further, Vanschoenwinkel et al. (2011) documented zooplankton dispersal ≤140 km downwind across those outcrops. Important factors in anemochory include wind speed and direction (Horváth et al. 2016), as well as propagule morphology and sediment grain size (Pinceel et al. 2016). Mesocosm experiments also indirectly support local dispersal (Cáceres and Soluk 2002, Cohen and Shurin 2003). Numerous studies have documented that biological material may be wind-dispersed over distances >1000 km: e.g., bacteria and fungi (Barberan et al. 2015), and pollen (Grewling et al. 2016). While these studies have examined components of propagule dispersal, ours is the first to investigate whether aquatic micrometazoans can become entrained in regional-scale, aeolian events, be transported hundreds of kilometers, and remain viable.
As depicted in our conceptual model (Fig 1), we propose that geophysical processes of aeolian transport in deserts operate to disperse micrometazoans and biological processes determine community structure (Field et al. 2010, Heino et al. 2015). Our model can be described as follows. Regional water sources contain propagules of locally defined, but regionally diverse species assemblages (Sources). This γ-diversity is then filtered by dispersal through wind corridors that entrain dormant propagules along with dust (Anemochory). Propagules move varying distances based on size, density, and other properties that affect their aerodynamics, as well as the vagaries of the wind (Jenkins et al. 2007). Thus we should expect to find that distance decay plays a role in dispersal of micrometazoans and that geographic features of the land can influence dispersal rates (Incagnone et al. 2015). Propagules landing in suitable habitats may emerge from dormancy during wet phases (Biotic processes) (Pinceel et al. 2013) and depending on community dynamics may become established (De Meester et al. 2002).
Scattered across the drylands of southwestern USA and northern Mexico are countless, highly disconnected, ephemeral aquatic habitats that collectively form a hotspot of biodiversity (Olson and Dinerstein 2002). This area also often experiences large, regional-scale dust storms that preferentially emanate from these habitats when they are dry (Lee et al. 2009, Baddock et al. 2011). Our research question was: Can micrometazoans be dispersed via wind over regional scales (102–105 km) and survive transport to colonize distant aquatic habitats? Thus, we examined five elements necessary for aeolian transport. Each of which should contribute to the dispersal of micrometazoans in aridland aquatic ecosystems as described in our conceptual model (Fig 1). (1) To determine possible sources of dust landing at a Chihuahuan Desert collecting site on the campus of The University of Texas at El Paso (UTEP), we back-calculated trajectories of 13 dust-bearing, wind events (Sources). (2) To assess whether the size of falling sediments overlapped sizes of micrometazoan propagules, we analyzed the particle size distribution of the dust falling from those events (Anemochory). (3) To determine whether these propagules are entrainable during dust storms, we conducted wind tunnel experiments that mimic dust emissions from the sediment-propagule banks of dry playas (Sources; Anemochory). (4) To investigate propagule viability after transport, we conducted rehydration experiments for wind tunnel-entrained dust and fallen dust in the Chihuahuan desert (Hatching). (5) To more fully characterize the taxonomic distribution of propagules from selected windstorms, we conducted next-generation sequencing (NGS) on fallen dust. This method captures taxa that do not respond to hatching cues provided in rehydration experiments.
Methods
Collection and Processing of Dust
Falling dust was collected from windstorms (2002–2016) using one of the following types of passive collection traps: marble dust collectors (MDCO), modified Wilson and Cooke (MWAC) samplers, and Big Spring Number Eight (BSNE) samplers (Goossens & Offer 2000). Samples were collected using MDCOs from two sites in El Paso County, TX: 10 samples from UTEP Biology Building Rooftop (B), and two from Hueco Tanks State Park and Historic Site (HT) (Rivas et al. 2018; Supplemental Document S1, Table S1). Additional samples from the arid southwest USA were collected: one from Jornada Basin LTER (LJ), NM, and two from White Sands Missile Range (WSMR), NM (using MWAC) and two from Yellow Lake playa (YL), TX (using BSNE). Samples were collected November through May, when regional-scale dust storms prevail (Novlan et al. 2007). We measured particle size by laser diffraction (Sperazza et al. 2004) using ~0.3 g of each sample with a Malvern Mastersizer 2000 (Malvern Instruments, UK). Particle size was also determined for sediments captured in each section of the wind tunnel (0.5 g). The dry method was used to preserve the initial particle size of transported sediment.
Wind Trajectories
Using the NOAA HYSPLIT model (Stein et al. 2015) we determined potential sources of transported materials using back-calculated flow trajectories of wind events for dustfall samples collected at UTEP. This method determines origins and transport pathways of air masses based on the latitude and longitude coordinates of starting or ending points. All parameters were set to the default settings. These included a total run time of 24 hr (encompassing the duration of the dust event), 24 trajectories (generating a mean trajectory), and a height of 500 m above ground level (representing dust arriving at the receptor site in the atmospheric boundary layer, from where it could fall out into the collector).
Wind Tunnel Experiments
To test entrainment of playa invertebrate diapausing stages (five common freshwater species: Eulimnadia texana, Triops longicaudatus, Streptocephalus sp., Daphnia magna, Brachionus calyciflorus; two representative brackish water species: Artemia salina, B. plicatilis) by saltation (energetic sandblasting; the dominant method of dust emission (Shao, 2008)) aeolian transport was simulated in a laboratory, suction-type wind tunnel at the USDA-ARS Big Spring Field Station (Van Pelt et al. 2009). Details of experiments are provided in Supplemental Document S2, Table S2. Successful transport of propagules in these simulations was demonstrated by counting propagules deposited in each of three downwind sections of the wind tunnel. These are a transfer section (dispersal of a few meters), a settling chamber (10–100s of meters), and the filter section (up to 106 meters). Subsamples of sediment deposited in each section were rehydrated in an appropriate medium (see Rehydration Experiments: Wind Tunnel, below) and monitored for hatching. Number of propagules recovered in each section was calculated as sum of hatchlings plus any unhatched propagules remaining after rehydration.
Rehydration Experiments
Subsamples of dust from (a) dust collectors and (b) wind tunnel experiments were rehydrated as follows.
(a) Collectors: A total of 47 samples were rehydrated from dust collected from UTEP Biology Building Rooftop (B), Hueco Tanks State Park and Historic Site (HT), White Sands Missile Range (WSMR), Yellow Lake (YL), and Jornada Basin LTER (LJ). Subsamples (1–3 g) were rehydrated in 250 mL of sterile MBL medium (Stemberger, 1981). They were incubated at 25°C (2 subsamples) and 12°C (1 subsample) under a 12:12 (Light:Dark) photocycle. Additional details are given the Supplemental Document S1. They were examined under a dissecting scope for emerging invertebrates daily until no new taxa were found for three successive observations, and finally after one additional month. Micrometazoans were identified, photographed, and preserved as vouchers (Supplemental Document S1, Fig. S1).
(b) Wind tunnel: Sieved wind tunnel sediments were divided into subsamples: three from the transfer section, five from the settling chamber, and one from the filter section. About 2.0 g of sediment were rehydrated (3–5 subsamples) in 75 mL of an appropriate medium (Supplemental Document S2). All cultures were maintained at room temperature, under constant illumination, and observed daily for 3 weeks. The filter section represented a single collection point, but the sample was divided into two subsamples for rehydration. As a control, five subsamples of 3.0 g of abrader sand was rehydrated with MBL and incubated under the same conditions.
NGS and Community Analysis
Total DNA was extracted from dust samples (n=17; ~0.25 g each) using a PowerSoil kit (MoBio, Carlsbad, CA) following the manufacturer’s protocol. DNA was submitted to MRDNA labs (Shallowater, TX) for 18S tag-encoded FLX-Titanium amplicon pyrosequencing using SSU_F04//SSU_R22 primer sets. Replicate samples of two dust events (UTEP [BD14: Biology Building Rooftop, Dec 2014; HT [HTMy14: Hueco Tanks State Park and Historic Site, May 2014]) were sequenced, giving a total of 19.
We analyzed sequencing reads using QIIME (v.1.9.1; Caporaso et al. 2010) and clustered at 97% sequence identity to delineate operational taxonomic units (OTUs). OTUs were then taxonomically assigned using BLAST (Altschul et al. 1990) against the Silva v.128 reference (Yilmaz et al. 2014). Fungal sequences were excluded in downstream analysis. Abundance barcharts based on remaining OTU assignments were constructed to illustrate patterns of diversity among samples. We also conducted Principal Coordinate Analysis (PCoA) to assess similarity of OTUs. (Additional details are given in the Supplemental Document S3).
Results
Mean particle size of sediment samples for each dust event ranged from: Hueco Tanks (HT), 100–112 µm; UTEP (B), 81–295 µm (10–35% of collected material represented dust <50 µm; Fig. 2; Supplemental Document S1, Table S1); White Sands Missile Range (WSMR), 178–200 µm; and Yellow Lake (YL), 6–12 µm. The substantially smaller particle size at Yellow Lake (YL) represented aggregates of clay and fine silt-sized, lacustrine mud particles loosely bound by salts. Note that the size range of micrometazoan propagules falls within the size range of dispersing dust we collected (Fig. 2).
Fig. 2.
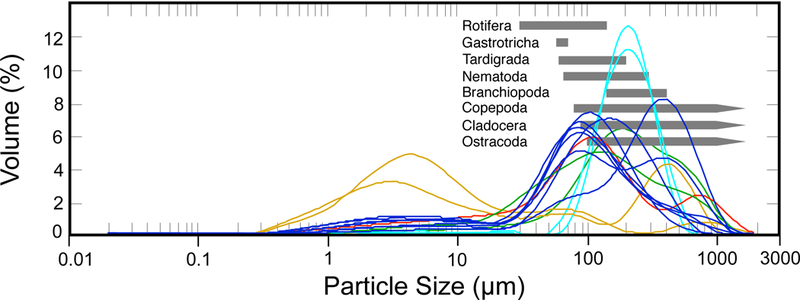
Relative volume proportion of fallen dust particles collected during this study, with size ranges of micrometazoan propagules. COLORED LINES: Particle size of transported windblown dust collected in our preliminary studies. (Each line represents the grain size distribution for a unique dust event. Dark blue: UTEP Biology Roof Top (BRT); light blue: White Sands Missile Range (WS), green: Jornada Basin LTER; red: Hueco Tanks State Park and Historic Site (HT);HT gold: Yellow Lake playa) SHADED BARS: Size range of propagules for these taxa. While there is diversity in the distribution of particle size in these events, note that the bulk of the material being transported usually falls within a range of ~40 to 600 µm. Note that the sizes of propagule of these aquatic invertebrates (grey bars) overlap with the particle size of transported windblown dust and sand collected as part of this study (dark blue curves).
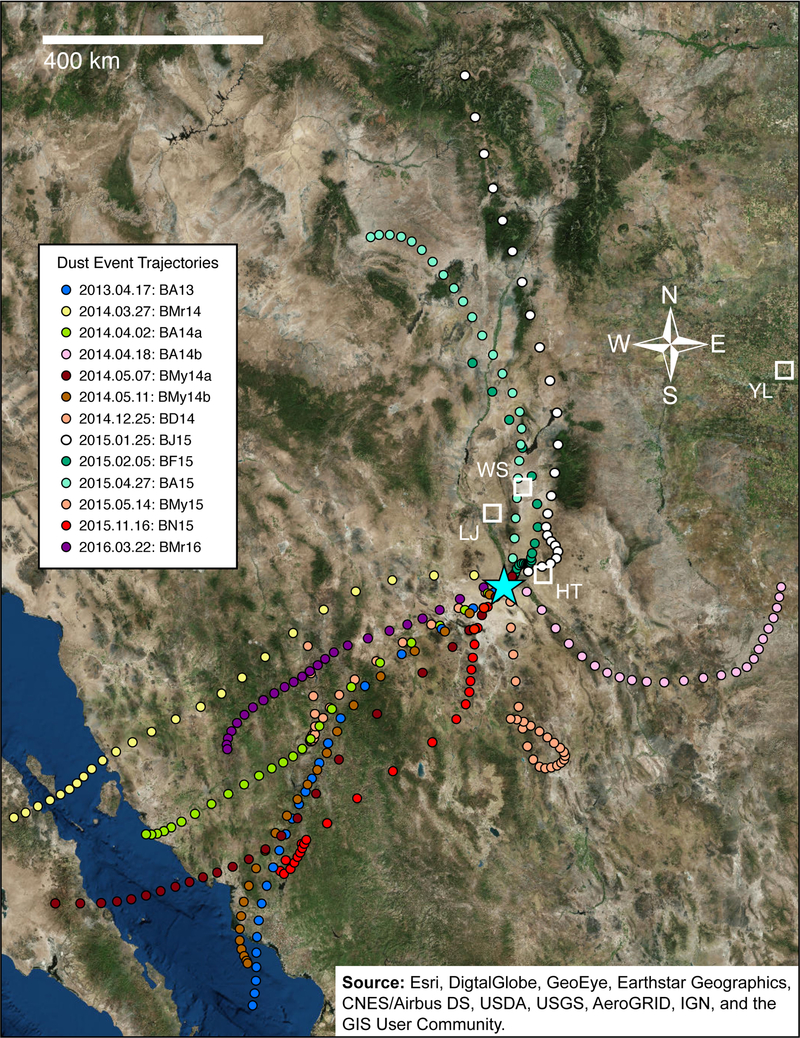
NOAA HYSPLIT back-trajectories for samples collected at UTEP, demonstrated that dust was transported from multiple directions, predominately from the Southwest (n=7), with two from the South, one from the East and three from the North (Fig. 3). All of these windstorms crossed extensive regions of the Chihuahuan Desert encompassing ephemeral aquatic systems located in the Sierra Madre Occidental and the Rio Grande valley.
Fig. 3.
HYSPLIT, 24-hr back trajectories of 13 regional dust events arriving in El Paso, TX at UTEP (blue star) during the period of April 2013 – March 2016. Reference codes after the dates in the insert key refer to our collection codes for dust recorded in the Supplemental Document S1, Table S1. [ArcGIS Desktop: Release 10.4 Redlands, CA: Environmental Systems Research Institute.]
Wind Tunnel Experiments
As expected mean particle size decreased in the three downstream sections of the wind tunnel: transfer section, settling chamber, and filter section (462 (SD= ±6.3%), 184 (SD= ±5.5%), and 52 (SD= ±5.2%) µm, respectively). Rehydration of sediments from each section indicated that propagules were transported throughout (Supplemental Document S2, Table S2) and that some individuals of all taxa were viable. For example, in the first experiment >18,000 fairy shrimp propagules of the original ~300,000 were recovered in the transfer section, of which ~18% of were viable. In the settling chamber almost 19,000 fairy shrimp propagules were recovered, of which ~38% were viable. Finally in the filter section >6,000 propagules were recovered and 0.6% were viable. Rehydration of the abrader sand yielded no organisms.
Dustfall Rehydration Experiments
Rehydrations yielded representatives of several broad taxonomic groups: algae (all, except BD14–2), ciliates (Hueco Tanks State Park and Historic Site (HT), UTEP Biology Building Rooftop (B), White Sands Missile Range (WS), Yellow Lake (YL)), gastrotrichs (UTEP Biology Building Rooftop (B)), nematodes (UTEP Biology Building Rooftop (B), Hueco Tanks State Park and Historic Site (HT)), ostracods (Yellow Lake (YL)), fairy shrimp (Yellow Lake (YL)), and monogonont rotifers Cephalodella sterea (UTEP Biology Building Rooftop (B)), Proales cf. similis (Yellow Lake (YL)), Ptygura beauchampi (UTEP Biology Building Rooftop (B)) and bdelloid rotifers Adineta vaga, Philodina tranquilla (UTEP Biology Building Rooftop (B) & Hueco Tanks State Park and Historic Site (HT)). Bdelloid rotifers occurred in 21% of rehydrated samples, while monogonont rotifers and nematodes were found in 6%, and gastrotrichs, ostracods, and fairy shrimp were found in 2%.
NGS and Community Analysis
Eukaryotic-specific primers (SSU) recovered 34,086 reads and 3,327 OTUs after fungi were removed (Supplemental Document S3). As seen in the barchart, sequencing replicates had similar taxonomic assemblages but at different proportions (Fig. 4A: HTMy13–1, 13–2 [Hueco Tanks May 2013, replicates 1 & 2]; BD14–1, 14–2 [UTEP Biology Building Rooftop, December 2014, replicates 1 &2]). This is also evident in the Hueco Tanks (HTMY13) sequencing replicates shown in the PCoA plot (Fig. 4B). Typically, and as seen here, abundant taxa mask the diversity of the rare forms. For instance, BMy14b (UTEP Biology Building Rooftop, May 2014) has 54 metazoan OTUs, ~82% of reads were ciliates. The assigned taxonomy identified rotifers, nematodes, ostracods, and gastrotrichs; rehydration experiments yield a similar suite of taxa (Table 1). PCoA plots show differences in taxonomic composition among sampling locations, with BD14–2 (UTEP Biology Building Rooftop, Dec 2014, replicate 2) having a unique taxonomic composition (Fig. 4B; Supplemental Document S3, Fig. S1–4). Focusing on the UTEP samples, taxonomic assemblages can be differentiated by wind direction. Species assemblages in dust collected from wind events originating from the southwest can be isolated from those originating from other directions by a plane across the three axes.
Fig. 4.
Next-generation sequencing of wind-transported sediment samples. Panel A is the abundance barchart representing proportions of Operational Taxonomic Units (OTUs) at a Silva’s (V.128) level 5 taxonomic cluster (containing taxonomic groups between subkingdom and species levels of classification) with all fungi removed. In the abundance barchart, Columns 1–3: Hueco Tanks State Park and Historic Site (HT), El Paso Co. TX, multi-month deposition samples (1,2 are duplicate sequencing runs); Columns 4–14: UTEP Biology Building Rooftop (B), designation is MonthYear; Columns: 8,9 duplicate samples demonstrating repeatability (BD14–1, BD14–2); Column 15: Jornada Basin LTER (LJ), Doña Co., NM; Columns 16–17: White Sands Missile Range (WS) Otero/Doña Ana Co., NM; Column 18–19: Yellow Lake Playa (YL), Lubbock Co., TX. Panel B is the corresponding PCoA plot. The legend for the abundance chart is available in Supplemental Document S3. Note that not all samples are represented in PCoA plots because clustering is not accurate at low read numbers. Blue spheres are dust samples collected other than at UTEP, orange spheres represent samples collected during wind events coming from the south, green from the southwest, and red from the north. Samples that arrived at the UTEP collectors from the SW (except BD14–2) are delineated from the others by the green lines drawn across planes in the plot.
Table 1.
Specific metazoan taxa that were found in both the environmental sequencing (OTUs and [reads]) and during rehydration (R) of dust events. Full results are recorded in the Supplemental Document S3, Table S4.
| Collector site Sample | Micrometazoans | ||||
|---|---|---|---|---|---|
| Gastrotricha | Nematoda | Ostracoda | Anostraca | Rotifera | |
| Hueco Tanks | |||||
| HTMy13–1 | — | 3 [14] | — | — | R |
| HTMy13–2 | — | 11 [62] | — | — | R |
| HTF14 | — | R | — | — | — |
| UTEP | |||||
| BA13 | — | 1 [6]; R | 3 [13] | — | R |
| BMr14 | — | — | — | — | R |
| BMy14b | R | 6 [7]; R | — | — | R |
| BD14–1 | — | 1 [1] | — | — | — |
| BA15 | — | R | — | — | — |
| BMy15 | — | 6 [16] | — | — | — |
| BN15 | — | 3 [3]; R | — | — | 7 [34]; R |
| BMr16 | — | 2 [3] | — | — | 3 [5]; R |
| Jornada | |||||
| LJMr14 | — | 5 [49] | — | — | 1 [1] |
| White Sands | |||||
| WSJ10a | 1 [4] | 2 [5] | — | — | — |
| WSJ10b | — | 4 [6] | — | — | — |
| Yellow Lake | |||||
| YLMr02 | — | — | R | — | — |
| YLMy03 | — | 2 [7] | 20 [1349] | R | R |
Discussion
We confirm that propagules of micrometazoans are entrained during Chihuahuan desert wind events and that anemochory provides a mechanism for their dispersal over regional scales. In addition, some propagules retain viability through the entire dispersal process. Our conclusions are based on a unique approach that weds geological and biological methods. These techniques can be used to further explore community assembly in ephemeral aquatic systems.
The modeled back trajectories show that wind events in the Chihuahuan Desert deposited material in downwind collectors at UTEP. These sediments crossed dust-emitting ephemeral aquatic systems, including playas such as the Paleolake Palomas Basin, Mexico (Baddock et al. 2016) and Lake Lucero (White Sands, New Mexico) (White et al. 2015), centered 79 and 112 km away from UTEP, respectively (Fig. 3). Other regional dust sources traversed by the incoming winds include ephemeral riverbeds, sand sheets and dunes, alluvial systems, and agricultural lands (Rivera Rivera et al. 2010, Baddock et al. 2011, Horváth et al. 2016). Easterly winds infrequently bring dust to the Chihuahuan Desert from the Great Plains, including particles derived from playas such as Yellow Lake (Sweeney et al. 2016). These trajectories match previously identified dust flow pathways into El Paso (Novlan et al. 2007).
As sediment particles move during wind events, saltation, bombardment, and collisions of large particles loft smaller sediment grains, as well as dormant stages of aquatic invertebrates into the atmosphere (Fig. 1: Physical processes). We know that factors such as propagule size, density, morphology, original habitat, and wind influence transport distance. In our work assessing regional transport, the size range of micrometazoan propagules coincided with that of windfallen dust and sand, both for rotifers (~50–200 μm) and crustaceans, such as Notostraca (~400 μm), Anostraca (~270–380 μm), and Spinicaudata (~200 μm) (Thiéry and Gasc 1991) (Fig. 2). Moreover, Ricci et al. (2003) asserted that bdelloid rotifers adhere to sand grains as they enter anhydrobiosis and others have shown that these taxa can be transported during local wind events (Havel and Shurin 2004, Vanschoenwinkel et al. 2009).
Wind tunnel experiments have long been used to study many aspects of dust emission and aeolian transport (Anderson et al. 1991), but no attempts to use them for documentation of anemochory of invertebrate propagules have been made until recently. Pinceel et al. (2016) demonstrated dispersal using a highly simplified wind tunnel analog, determining the threshold velocity of emission and that wind dispersal is highly correlated to propagule morphology and size. Our wind tunnel experiments more accurately recreated the mechanics of dust emission, including bombardment by saltating sands onto polygon-shaped, soil samples representative of natural playa surfaces. Collection of sediments and propagules from downstream points represented long distance dispersal (Supplemental Documents S2). However, even if established that micrometazoan propagules are transported with dust, it remained to be shown that they retained viability throughout the highly turbulent, energetic process of saltation (Shao 2008).
Our wind tunnel experiments demonstrate emission of viable propagules (Supplemental Documents S2). While some propagules were deposited in the transfer section of the wind tunnel, representing dispersal of only a few meters, the greatest numbers of propagules were always present in the settling chamber. Propagules transported to this section suggest dispersal of 10s to 100s of m downwind. However, a sizeable number of propagules were present in the filter section, suggesting potential dispersal of up to ~106 m. The exception was the absence of Daphnia ephippia in the filter section. It is possible that the unique shape of an ephippium does not permit long distance dispersal. Moreover, the dispersing propagules from all seven taxa showed viability after transport (Supplemental Documents S2). Our wind tunnel results show a decline in number of propagules dispersed in air from the settling chamber to the filter section (farthest downstream). This decline shows that not all propagules dispersal equally well.
Resurrection ecology has demonstrated that viable micrometazoan propagules can be recovered from lake sediments. We used this technique to demonstrate the potential viability of propagules found in falling dust. However similar to species recovery from sediments, we posited that micrometazoans recovered from dust are a small subset of the regional pool.
Our results confirm high taxonomic diversity of micrometazoans transported through regional dust events. While not confirming viability, NGS better captures taxonomic diversity of environmental samples (Santoferrara et al. 2016). We found hundreds of micrometazoan OTUs in fallen dust. The five samples with highest diversity (excluding fungi) were collected from the UTEP site (29–54 OTUs; Supplemental Document S3, Table S3). Of these, several were assigned to taxa that were found in rehydration experiments and/or used as propagules in the wind tunnel experiments (Table 1). Because different portions of the samples were used, it is not surprising that there is incomplete correspondence between taxa recovered by the two methods. Dust is emitted from a variety of sources and winds mix it as it crosses different landforms. Moreover, routes and speeds of storms vary, giving each event a unique signature of entrained materials.
Although we have filled in some gaps in our understanding of regional dispersal of aquatic micrometazoans by wind, there are several remaining knowledge gaps. Chief among these are the following. (1) Regional differences and inter-annual variation in aeolian transport of aquatic propagules. (2) Detailed examination of emission and dispersal dynamics, and survival of dispersing propagules. (3) The role of propagule aerodynamics and morphology in emission and transport by wind. (4) Traits that determine survival during deflation, transport, and colonization. (5) How soils and landform characteristics influence deflation of propagules during windstorms. While much remains to be known, here we demonstrated that micrometazoan propagules remain viable after entrainment and dispersal over long distances. Thus anemochory performs an important role in regional dispersal and potential colonization of some micrometazoans in dryland aquatic habitats.
Supplementary Material
Significance statement.
Ephemeral desert aquatic systems are known for their diverse biota including bacteria, algae, protists, fungi, and micrometazoans. When these systems dry, many organisms survive as resting stages (propagules), which may hatch when water returns to the habitat. Alternatively, propagules have the potential to disperse. It is unclear whether propagules of micrometazoans can disperse by wind across regional scales (≥100s km) to potentially colonize hydrologically disconnected basins. Our study provides the first evidence that micrometazoan propagules remain viable after being emitted by saltation (energetic sandblasting) into the air — the initiation mechanism of desert dust storms — and that regional-scale windstorms aid in their dispersal (anemochory) to new habitats. Accordingly, anemochory likely plays an important role in regional dispersal and potential colonization of aquatic invertebrates in drylands.
Acknowledgements
We thank the reviewers for their help in improving the manuscript. Staff at Hueco Tanks State Park and Historic Site (permits 2013, 2014, 2015–03) facilitated sampling. WSMR samples were collected under Desert Southwest Cooperative Ecosystem Services Unit Cooperative Agreement DACA87–05-H-0018 with the U.S. Department of Defense. John Stout (Yellow Lake) and Nicholas Webb and Magda Galloza (Jornada LTER) kindly provided samples. Funding was provided by NSF DEB 1257068 and 1257116, 2G12MD007592 from NIH, NIMHD Bioinformatics Core, NOAA Office of Education Educational Partnership Program with Minority Serving Institutions Cooperative Agreement #NA11SEC4810003, and UTEP’s Interdisciplinary Research program. Statements contained within the article are not the opinions of the funding agency or the U.S. government, but are the author’s opinions. The U.S. Department of Agriculture is an equal opportunity employer and provider. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. We thank Chris Anderson, Maite Martin, and Amanda Ostwald for their assistance in the laboratory.
Footnotes
Data Availability Statement: All the data used to analyze the data for this paper are available at the UTEP Bioinformatics Data Repository at http://datarepo.bioinformatics.utep.edu/getdata?acc=ACIEJDV41U1ZN5I
References
- 1.Altschul SF, Gish W, Miller W, Myers EW, and Lipman DJ. 1990. Basic local alignment search tool. Journal of Molecular Biology 215: 403–410. [DOI] [PubMed] [Google Scholar]
- 2.Anderson RS, Sørensen M, and Willetts BB. 1991. A review of recent progress in our understanding of aeolian sediment transport. Acta Mechanica Supplementum 1: 1–19. [Google Scholar]
- 3.Baddock MC, Gill TE, Bullard JE, Acosta MD, and Rivera Rivera NI. 2011. Geomorphology of the Chihuahuan Desert based on potential dust emissions. Journal of Maps 7: 249–259. [Google Scholar]
- 4.Baddock MC, Ginoux P, Bullard JE, and Gill TE. 2016. Do MODIS-defined dust sources have a geomorphological signature? Geophysical Research Letters 43: 2606–2613. [Google Scholar]
- 5.Barberan A, Ladau J, Leff JW, Pollard KS, Menninger HL, Dunn RR, and Fierer N. 2015. Continental-scale distributions of dust-associated bacteria and fungi. PNAS 112: 5756–5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brock MA, Nielsen DL, Shiel RJ, Green JD & Langley JD 2003. Drought and aquatic community resilience: the role of eggs and seeds in sediments of temporary wetlands. Freshwater Biology 48:1207–1218. [Google Scholar]
- 7.Cáceres CE, and Soluk DA. 2002. Blowing in the wind: a field test of overland dispersal and colonization by aquatic invertebrates. Oecologia 131: 402–408. [DOI] [PubMed] [Google Scholar]
- 8.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, Mcdonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, and Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nature Methods 7: 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen GM, and Shurin JB. 2003. Scale-dependence and mechanisms of dispersal in freshwater zooplankton. Oikos 103: 603–617. [Google Scholar]
- 10.Collins SL, Belnap J, Grimm NB, Rudgers JA, Dahm CN, D’Odorico P, Litvak M, Natvig DO, Peters DC, Pockman WT, Sinsabaugh RL, and Wolf BO. 2014. A multiscale, hierarchical model of pulse dynamics in arid-land ecosystems. Annual Review of Ecology, Evolution, and Systematics 45: 397–419. [Google Scholar]
- 11.De Meester L, Gómez A, Okamura B, and Schwenk K. 2002. The Monopolization Hypothesis and the dispersal–gene flow paradox in aquatic organisms. Acta Oecologia 23: 121–125. [Google Scholar]
- 12.Field JP, Belnap J, Breshears DD, Neff JC, Okin GS, Whicker JJ, Painter TH, Ravi S, Reheis MC, and Reynolds RL 2010. The ecology of dust. Frontiers in Ecology and the Environment 8: 423–430. [Google Scholar]
- 13.Finlay BJ 2002. Global dispersal of free-living microbial eukaryotic species. Science 296: 1061–1063. [DOI] [PubMed] [Google Scholar]
- 14.Fontaneto D 2011. Biogeography of microscopic organisms Cambridge University Press, Cambridge. [Google Scholar]
- 15.Fontaneto D, Ficetola GF, Ambrosini R, and Ricci C. 2006. Patterns of diversity in microscopic animals: are they comparable to those in protists or in larger animals? Global Ecology and Biogeography 15: 153–162. [Google Scholar]
- 16.Ginoux P, Prospero JM, Gill TE, Hsu NC, and Zhao M. 2012. Global-scale attribution of anthropogenic and natural dust sources and their emission rates based on MODIS Deep Blue aerosol products. Reviews of Geophysics 50: RG3005. [Google Scholar]
- 17.Goossens D and Offer YZ. 2000. Wind tunnel and field calibration of six aeolian dust samplers. Atmospheric Environment 34: 1043–1057. [Google Scholar]
- 18.Graham TB, and Wirth D. 2008. Dispersal of large branchiopod cysts: potential movement by wind from potholes on the Colorado Plateau. Hydrobiologia 600: 17–27. [Google Scholar]
- 19.Grewling ŁP Bogawski D. Jenerowicz, Czarnecka-Operacz M, Šikoparija B, Skjøth CA, and Smith M. 2016. Mesoscale atmospheric transport of ragweed pollen allergens from infected to uninfected areas. International Journal of Biometeorology 60: 1493–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Havel JE, and Shurin JB. 2004. Mechanisms, effects, and scales of dispersal in freshwater zooplankton. Limnology and Oceanography 49: 1229–1238. [Google Scholar]
- 21.Heino J, Melo AS, Siqueira T, Soininen J, Valanko S, and Bini LM. 2015. Metacommunity organisation, spatial extent and dispersal in aquatic systems: patterns, processes and prospects. Freshwater Biology 60: 845–869. [Google Scholar]
- 22.Horváth Z, Vad CF, and Ptacnik R. 2016. Wind dispersal results in a gradient of dispersal limitation and environmental match among discrete aquatic habitats. Ecography 39: 726–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hubert N, Calcagno V, Etienne RS, and Mouquet N. 2015. Metacommunity speciation models and their implications for diversification theory. Ecological Letters 18: 864–681. [DOI] [PubMed] [Google Scholar]
- 24.Incagnone G, Marrone F, Barone R, Robba L, and Naselli-Flores L. 2015. How do freshwater organisms cross the “dry ocean”? A review on passive dispersal and colonization processes with a special focus on temporary ponds. Hydrobiologia 750: 103–123. [Google Scholar]
- 25.Jenkins DG, Brescacin CR, Duxbury CV, Elliott JA, Evans JA, Grablow KR, Hillegass M, Lyon BN, Metzger GA, Olandese ML, Pepe D, Silvers GA, Suresch HN, Thompson TN, Trexler CM, Williams GE, Williams NC, and Williams SE. 2007. Does size matter for dispersal distance? Global Ecology and Biogeography 16: 415–425. [Google Scholar]
- 26.Jocque M, Vanschoenwinkel B & Brendonck L 2010. Freshwater rock pools: a review of habitat characteristics, faunal diversity and conservation value. Freshwater Biology 55: 1587–1602. [Google Scholar]
- 27.Kobayashi T, Ralph TJ, Ryder DS, Hunter SJ, Shiel RJ, and Segers H. 2015. Spatial dissimilarities in plankton structure and function during flood pulses in a semi-arid floodplain wetland system. Hydrobiologia 747: 19–31. [Google Scholar]
- 28.Lee JA, Gill T, Mulligan KR, Dominguez Acosta M, and Perez AE. 2009. Land use/land cover and point sources of the 15 December 2003 dust storm in southwestern North America. Geomorphology, 2009 105: 18–27. [Google Scholar]
- 29.McGill BJ 2010. Matters of scale. Science 328: 575–576. [DOI] [PubMed] [Google Scholar]
- 30.Nkem JN, Wall DH, Virginia RA, Barrett JE, Broos EJ, Porazinska DL, and Adams BJ. 2006. Wind dispersal of soil invertebrates in the McMurdo Dry Valleys, Antarctica. Polar Biology 29: 346–352. [Google Scholar]
- 31.Novlan DJ, Hardiman M, and Gill TE. 2007. A synoptic climatology of blowing dust events in El Paso, Texas from 1932–2005. 16th Conference on Applied Climatology, American Meteorological Society J3. 12: 1–13. [Google Scholar]
- 32.Olson DM, and Dinerstein E. 2002. The Global 200: priority ecoregions for global conservation. Annals of the Missouri Botanical Garden 89: 199–224. [Google Scholar]
- 33.Parekh PA, Paetkau MJ, and Gosselin LA. 2014. Historical frequency of wind dispersal events and role of topography in the dispersal of anostracan cysts in a semi-arid environment. Hydrobiologia 740: 51–59. [Google Scholar]
- 34.Pinceel T, Brendonck L, and Vanschoenwinkel B. 2016. Propagule size and shape may promote local wind dispersal in freshwater zooplankton-a wind tunnel experiment. Limnology and Oceanography 61: 122–131. [Google Scholar]
- 35.Pinceel T, Vanschoenwinkel B, Uten J, and Brendonck L. 2013. Mechanistic and evolutionary aspects of light-induced dormancy termination in a temporary pond crustacean. Freshwater Science 32: 517–524. [Google Scholar]
- 36.Ricci C, Melone G, Santo N, and Caprioli M. 2003. Morphological response of a bdelloid rotifer to desiccation. Journal of Morphology 257: 246–253. [DOI] [PubMed] [Google Scholar]
- 37.Ricklefs RE 2008. Disintegration of the ecological community. American Naturalist 172: 741–750. [DOI] [PubMed] [Google Scholar]
- 38.Rivas JA Jr., Mohl J, Van Pelt RS, Leung M-Y, Wallace RL, Gill TE, Walsh EJ 2018. Data for evidence for regional aeolian transport of freshwater micrometazoans in arid regions. UTEP Bioinformatics Data Repository http://datarepo.bioinformatics.utep.edu/getdata?acc=ACIEJDV41U1ZN5I [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rivera Rivera NI, Gill TE, Bleiweiss MP, and Hand JL. 2010. Source characteristics of hazardous Chihuahuan Desert dust outbreaks. Atmospheric Environment 44: 2457–2468. [Google Scholar]
- 40.Sánchez MI, Hortas F, Figuerola J, and Green AJ. 2012. Comparing the potential for dispersal via waterbirds of a native and an invasive brine shrimp. Freshwater Biology 57: 1896–1903. [Google Scholar]
- 41.Santoferrara LF, Grattepanche J-D, Katz LA, and McManus GB. 2016. Patterns and processes in microbial biogeography: do molecules and morphologies give the same answers? The ISME Journal 10: 1779–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scuderi LA, Laudadio CK, and Fawcett PJ. 2010. Monitoring playa lake inundation in the western United States: Modern analogues to late-Holocene lake level change. Quaternary Research 73: 48–58. [Google Scholar]
- 43.Shao Y 2008. Physics and modelling of wind erosion Springer. [Google Scholar]
- 44.Sperazza M, Moore JN, and Hendrix MS. 2004. High-resolution particle size analysis of naturally occurring very fine-grained sediment through laser diffractometry. Journal of Sedimentary Research 74: 736–743. [Google Scholar]
- 45.Stein AF, Draxler RR, Rolph GD, Stunder BJB, Cohen MD, and Ngan F. 2015. NOAA’s HYSPLIT atmospheric transport and dispersion modeling system. Bulletin of the American Meteorological Society 96: 2059–2077. [Google Scholar]
- 46.Stemberger RS 1981. A general approach to the culture of planktonic rotifers. Canadian Journal of Fisheries and Aquatic Sciences 38: 721–724. [Google Scholar]
- 47.Sweeney MR, Zlotnik VA, Joeckel RM, and Stout JE. 2016. Geomorphic and hydrologic controls of dust emissions during drought from Yellow Lake playa, West Texas, USA. Journal of Arid Environments 133: 37–46. [Google Scholar]
- 48.Tesson SVM, Okamura B, Dudaniec RY, Vyverman W, Löndahl J, Rushing C, Valentini A, and Green AJ. 2015. Integrating microorganism and macroorganism dispersal: modes, techniques and challenges with particular focus on co-dispersal. Écoscience 22: 109–124. [Google Scholar]
- 49.Thiéry A, and Gasc C. 1991. Resting eggs of Anostraca, Notostraca and Spinicaudata (Crustacea, Branchiopoda) occurring in France: Identification and taxonomical value. Hydrobiologia 212: 245–259. [Google Scholar]
- 50.Valls L, Castillo-Escrivà A, Barrera L, Gómez E, Gil-Delgado JA, Mesquita-Joanes F, and Armengol X. 2017. Differential endozoochory of aquatic invertebrates by two duck species in shallow lakes. Acta Oecologica 80: 39–46. [Google Scholar]
- 51.Van Pelt RS, Peters P, and Visser S. 2009. Laboratory wind tunnel testing of three commonly used saltation impact sensors. Aeolian Research 1: 55–62. [Google Scholar]
- 52.Vanschoenwinkel B, Gielen S, Seaman M, and Brendonck L. 2008. Any way the wind blows -frequent wind dispersal drives species sorting in ephemeral aquatic communities. Oikos 117: 125–134. [Google Scholar]
- 53.Vanschoenwinkel B, Gielen S, Seaman M, and Brendonck L. 2009. Wind mediated dispersal of freshwater invertebrates in a rock pool metacommunity: differences in dispersal capacities and modes. Hydrobiologia 635: 363–372. [Google Scholar]
- 54.Vanschoenwinkel B, Mergeay J, Pinceel T, Waterkeyn A, Vandewaerde H, Seaman M, and Brendonck L. 2011. Long distance dispersal of zooplankton endemic to isolated mountaintops-an example of an ecological process operating on an evolutionary time scale. PloS one 6: e26730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Viana DS, Santamaría L, Michot TC, and Figuerola J. 2013. Migratory strategies of waterbirds shape the continental-scale dispersal of aquatic organisms. Ecography 36: 430–438. [Google Scholar]
- 56.Yamaguchi N, Ichijo T, Sakotani A, Baba T, and Nasu M. 2012. Global dispersion of bacterial cells on Asian dust. Scientific Reports 2: 525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walsh EJ, Smith HA, and Wallace RL. 2014. Rotifers of temporary waters. International Review of Hydrobiology 99: 3–19. [Google Scholar]
- 58.White WH, Hyslop NP, Trzepla K, Yatkin S, Rarig RS, Gill TE, and Jin L. 2015. Regional transport of a chemically distinctive dust: Gypsum from White Sands, New Mexico (USA). Aeolian Research 16: 1–10. [Google Scholar]
- 59.Williams DD 2006. The Biology of Temporary Waters Oxford University Press. [Google Scholar]
- 60.Yilmaz P, Parfrey LW, Yarza P, Gerken J, Pruesse E, Quast C, Schweer T, Peplies J, Ludwig W, and Glockner FO. 2014. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Research 42: D643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.