Abstract
The lungs of extremely low gestational age neonates (ELGANs) are deficient in pulmonary surfactant and are incapable of efficient gas exchange necessary for successful transition from a hypoxic intrauterine environment to ambient air. To improve gas exchange and survival, ELGANs often receive supplemental oxygen with mechanical ventilation which disrupts normal lung developmental processes, including microvascular maturation and alveolarization. Factors that regulate these developmental processes include vascular endothelial growth factor and matrix metalloproteinases, both of which are influenced by generation of oxygen byproducts, or reactive oxygen species (ROS). ELGANs are also deficient in antioxidants necessary to scavenge excessive ROS. Thus, the accumulation of ROS in the preterm lungs exposed to prolonged hyperoxia, results in inflammation and development of bronchopulmonary dysplasia (BPD), a form of chronic lung disease (CLD). Despite advances in neonatal care, BPD/CLD remains a major cause of neonatal morbidity and mortality. The underlying mechanisms are not completely understood, and the benefits of current therapeutic interventions are limited. The association between ROS and biomarkers of microvascular maturation and alveolarization, as well as antioxidant therapies in the setting of hyperoxia-induced neonatal lung injury are reviewed in this article.
Keywords: Antioxidants, Bronchopulmonary dysplasia, Chronic lung disease, Matrix metalloproteinases, Reactive oxygen species
1. INTRODUCTION
Extremely low gestational age neonates (ELGANs, < 28 weeks gestation and/or birth weight < 1250 grams) are usually intubated at birth and mechanically ventilated for respiratory support and treatment of respiratory distress syndrome (RDS) [1]. Although advances in respiratory care and management have led to the increased survival of ELGANs, approximately one-third of them will develop bronchopulmonary dysplasia (BPD), the most common form of chronic lung disease (CLD) that occurs in 45–68% of infants < 29 weeks gestation [2]. BPD/CLD is characterized by prolonged need for oxygen therapy, increased partial pressure of carbon dioxide (PCO2), and abnormal lung compliance and the known risk factors include preterm birth, respiratory failure, oxygen toxicity, and barotrauma [3], with devastating complications such as pulmonary hypertension occurring in as many as 17–24% [4] and rising to 59% in periviable infants, < 25 weeks [5]. Pathological characteristics include disrupted microvascular maturation and poor alveolarization secondary to lung inflammation, mechanical injury, and oxygen toxicity, leading to alveolar oversimplification and inadequate blood-air interface [6–8]. Many factors regulate normal and pathological angiogenesis and alveolarization, including, but not limited to, vascular endothelial growth factor (VEGF) involved in microvascular maturation, matrix metalloproteinases responsible for alveolarization, extracellular matrix (ECM) and lung basement membrane proteolysis and remodeling, and antioxidants responsible for scavenging of reactive oxygen species (ROS), all of which are deficient in ELGANs. While several reviews on BPD have been published, this review focuses on, and highlights, the important role of ROS in the development of BPD, and examines the association between VEGF, ECM degrading enzymes or matrix metalloproteinases (MMPs), and ROS in its pathogenesis. It also discusses alternative treatments focusing mainly on antioxidant defenses for ROS scavenging and their likely therapeutic or preventive role in alleviating lung toxicities.
2. PERINATAL LUNG DEVELOPMENT
At about 22–25 days post conception, lung bud arises from the embryonic foregut. This is followed by subsequent branching of the bronchial tree [9]. Five major stages are identified that may overlap since lung structures develop simultaneously. These are embryonic, pseudo-glandular, canalicular, saccular, and alveolar stages [10, 11]. Between 16 and 28 weeks, lung growth is dominated by bronchial development, particularly, formation of alveolar ducts and terminal sacs. By around 24 weeks, these terminal sacs become abundant. The epithelium thins and Type 1 and II pneumocytes form. During this canalicular period, the network of blood vessels come much closer to the terminal saccules or primitive alveoli allowing for efficient respiration and gas exchange, despite bulk alveolarization occurring in the postnatal period [12]. Lung septation and alveolarization begin around 32–36 weeks gestation and are tightly coupled with vascular growth and branching [11], a complex process of endothelial cell differentiation and proliferation (vasculogenesis), formation and branching of new blood vessels (angiogenesis), and smooth muscle cell migration (arteriogenesis). The final stages of vascular development are characterized by increasing surface areas with thinning of alveolar walls surrounded by pulmonary capillaries allowing postnatal gas exchange. Alveolar and vascular growth occur side by side involving delicately choreographed processes of cell proliferation, differentiation, migration, and apoptosis. Complex signaling pathways regulate these processes which are disrupted by preterm birth and exposure to supplemental oxygen.
The lungs of ELGANs born at < 28 weeks gestation are in the canalicular and saccular or early alveolar stages of development and are deficient of pulmonary surfactant, which normally matures in utero at much lower oxygen levels of 20–30 mm Hg necessary to promote branching morphogenesis, angiogenesis, and ECM deposition. They are incapable of efficient gas exchange, as the rate of transfer of gas across the lung is directly proportional to the surface area and inversely proportional to the diffusion distance between blood and air, according to Fick’s Law of diffusion [4]. To improve gas exchange, ELGANs often receive supplemental oxygen with mechanical ventilation. Hyperoxia results in the production of harmful oxygen byproducts or ROS, and disrupts normal lung developmental processes including microvascular maturation and alveolarization [13]. Due to immature antioxidant systems to scavenge ROS, their accumulation in the lungs causes inflammation, lung tissue damage, and CLD/BPD [14]. While the etiology of CLD/BPD is multifactorial, overwhelming evidence suggests that oxidative stress and excessive ROS with inadequate antioxidant systems play a key role in its development and severity [15–17].
3. OVERVIEW OF ROS
ROS are abundant in nature because they may be formed endogenously or exogenously between cells. The unique molecular configuration of oxygen allows it to accept free electrons from normal oxidative metabolism [18] leading to the production of superoxide anion (O2˙−), hydroxyl radical (OH˙), and hydrogen peroxide (H2O2), of which the mitochondria and peroxisomes are major sources. About 90% of cellular oxygen uptake is due to mitochondrial respiration and 1–2% of the oxygen consumed is transformed into ROS [19, 20]. Uncoupling of the electron transport chain found in the mitochondria generates ROS and is the major producer [21, 22]. A good amount of ROS may also come from cytoplasmic and endoplasmic reticulum-bound enzyme systems and the plasma membrane [23, 24]. Generation of ROS is affected by the availability of oxygen, the redox state of the mitochondrial complexes, and mitochondrial membrane potential [25]. Multi-enzyme systems can also account for the production of ROS. These systems include flavoproteins that produce H2O2, cytochrome P450 monooxygenase system that produces superoxide, xanthine oxidoreductase that produces both superoxide and H2O2, and nitric oxide synthase that produces superoxide and nitric oxide (NO) [26–29]. NADPH oxidases (NOX 1–3), dual oxidases 1 and 2, and NOX 4 produce O2˙− endogenously [30, 31]. Other systems mainly, involved in inflammatory process, cyclooxygenase and lipoxygenase pathways in arachidonic acid metabolism can also generate ROS that contribute to the evolution of lung disease [32]. Superoxide anion reacts rapidly with nitric oxide producing peroxynitrite (ONOO−) [33, 34]. Lipid peroxidation is initiated once peroxynitrite or hydroxyl radical reacts with membrane lipids forming more complex radicals [35]. ROS are also important regulators of nitric oxide bioavailability, affecting airway and vascular reactivity [30]. ROS directly damage cellular proteins, lipids, and nucleic acids via protein oxidation and nitrosylation, lipid peroxidation, and oxidation of nucleic acids [36, 37]. These injuries can lead to impairment in enzymatic functions adversely affecting growth factors [38]. Lipid peroxidation results in the activation of sphingomyelinase releasing ceramide from cellular phospholipids, triggering apoptosis [39]. Breakage in DNA strands after oxidation of nucleic acids leads to necrosis and maladaptive apoptosis [40]. H2O2, a ubiquitous ROS [41, 42] permeates cell membranes through water channels [43–45], may act as a secondary messenger modulating cellular signaling [46]. H2O2 activates multiple signal transduction pathways, facilitating the actions of growth factors, cytokines, chemokines, and calcium signaling [47, 48]. During blood transfusion for anemia of prematurity, iron will react with H2O2forming hydroxyl radical that can injure cellular components [49, 50] leading to defective signaling pathways [51].
4. ROS AND PRETERM LUNGS
The premature lungs are highly susceptible to oxidative lung injury. Although necessary and essential for their survival, exposure of the immature lungs to oxygen leads to accumulation of ROS, including superoxide anion, the first ROS responder. Superoxide is very unstable and is rapidly converted to the more stable H2O2, and O2 by mitochondrial superoxide dismutase (MnSOD) in the mitochondrial matrix and copper, zinc SOD (CuZnSOD) in the cytosol. SOD is the primary ROS-detoxifying enzyme in the cell [52]. Complete disposal of H2O2requires the action of catalase, predominantly located in peroxisomes, and glutathione peroxidase in the mitochondria and cytosol [53]. Accumulation of H2O2 can lead to the formation of the highly reactive hydroxyl radical in the presence of ferrous iron via the Fenton reaction [54]. The hydroxyl radical oxidizes mitochondrial components such as lipids, proteins, and DNA via the self-propagating lipid peroxidation. Since mitochondrion lacks catalase, it relies on the reduced form of glutathione (GSH, about 10–20% of which is present in the mitochondria, the remainder is in the cytosol) to effectively scavenge H2O2. We and others have shown deficient antioxidant status in preterm infants [55, 56] and neonatal animal exposed to hyperoxia [57], suggesting an ROS/antioxidant imbalance that may lead to oxidative distress. Furthermore, ELGANs are often supplemented with iron for anemia. However, their low levels of plasma transferrin, the major iron-binding protein, predispose them to a higher risk for lipid peroxidation [58–62]. In the immature lungs, supraphysiological levels of oxygen and ROS result in a complex inflammatory reaction that is associated with accumulation of various cells (neutrophils and alveolar macrophages), and inflammatory mediators which result in increased microvascular permeability and lung damage [57]. Furthermore, exposure to high concentrations of oxygen during lung development results in alterations in capillary density [63], endothelial cell destruction [64], pulmonary inflammation [65], and inhibition of the process of alveolarization [66]. The immaturity of the lungs and inability of respiratory control result in apnea of prematurity and intermittent hypoxia (IH) events triggered by cessation of respiratory neural output [9, 67]. An IH event is usually defined as a decline in SaO2 by 5% lasting < 3 minutes in duration [67]. Re-oxygenation which occurs in hyperoxia or normoxia (IHR) reestablishes blood flow through damaged vessels, resulting in reperfusion injury. ROS are induced in both IH and IHR and may contribute to inflammation and cell death. Fluctuations in SaO2 together with the underdeveloped antioxidant-scavenging mechanisms add to the complexity of CLD/BPD.
5. ROS AND VEGF
At any stage in pulmonary development, ROS may interfere with the well-coordinated processes influencing signaling pathways that regulate pulmonary vascular development which involves angiogenesis (sprouting of new vessels from existing ones required for central vessels) and vasculogenesis (formation of capillaries from endothelial cells). Using the baboon BPD model, Coalson et al. [68–70] demonstrated that the premature lungs exposed to hyperoxia and mechanical ventilation developed bronchial and bronchiolar epithelial lesions, decreased alveolarization, and interstitial and peribronchiolar fibrosis. Poor alveolarization and disrupted microvascular maturation involve altered angiogenic factors such as VEGF, ECM and lung basement membrane proteolysis by MMPs, and mitochondrial dysfunction [71–76]. VEGF is a potent mitogen and inducer of endothelial cell growth and proliferation [77]. Its expression is high in distal airway epithelial cells during differentiation of human fetal lungs [78]. VEGF is highly induced by hypoxia via the highly conserved transcription factor, hypoxia-inducible factor (HIF)-1α, which regulates over 100 genes involved in proliferation and angiogenesis. Studies have shown that hyperoxia decreases neonatal lung VEGF protein and mRNAs [79]. Reduced lung VEGF mRNA and protein expression as well as decreased receptor Flt-1 were associated with characteristic patterns of alveolar simplification and dysmorphic microvasculature in the lungs of infants dying with BPD [80]. Premature infants who died with severe RDS had lower lung VEGF than survivors, and infants with BPD had lower tracheal VEGF [81–83]. We have shown similar findings in lungs from premature baboons delivered at 125 or 140 days gestation who received 100% oxygen with mechanical ventilation [84], and in preterm infants with BPD [85]. During recovery from hyperoxia, VEGF expression is increased in alveolar epithelial cells suggesting a role for VEGF in microvascular repair process [86]. Elevations in VEGF during hyperoxia reperfusion injury may involve mitochondrial ROS activation of HIF [50, 87].
6. ROS AND MATRIX METALLOPROTEINASES
Lung alveolarization, or the increase in gas-exchange surface area is characterized by the formation of alveoli and subdivision of sacculi [88]. During this stage, secondary septa are formed in the saccule to create alveoli [12, 89]. Secondary septation occurs in parallel with microvascular maturation, the transition from a double-layered to single-layered capillary network [90]. This process involves MMPs, which regulate the structural changes associated with alveolarization and microvascular maturation [91].
MMPs are enzymes that degrade type IV collagen, the major constituent of ECM and lung basement membranes necessary for mechanical and functional properties of lung tissue. MMPs belong to a family of zinc endopeptidases that are collectively capable of degrading essentially all ECM components, and as such, play a major role in inflammation, tissue remodeling, angiogenesis, migration, and invasion [92–94]. MMPs are regulated by cytokines, chemokines, growth factors, and tissue inhibitors of metalloproteinases (TIMPs 1–4) which form a high affinity complex in a 1:1 ratio [95]. A balance between MMPs and TIMPs is responsible for maintenance of normal ECM architecture, whereas an imbalance is believed to result in parenchymal destruction in lung diseases such as CLD complicating pulmonary fibrosis and asthma [96, 97]. MMPs are classified into: (1) collagenases (MMP-1, MMP-8, MMP-13); (2) gelatinases A (MMP-2) and B (MMP-9); (3) stromelysins (MMP-3, MMP-10, MMP-11, MMP-12); and (4) membrane-type MMPs [98, 99]. MMPs and TIMPs are produced ubiquitously in the body. However, in the lungs MMPs are produced by structural cells such as fibroblasts, endothelial, and epithelial cells, as well as alveolar macrophages [97, 100]. Overproduction of MMPs during alveolar remodeling can contribute to inflammatory lung disease. There is evidence that MMPs are involved in neonatal lung injury secondary to hyperoxia and mechanical ventilation in infants [101–107] and animal models [108–110], including studies in our laboratory [111]. From those studies, MMPs are highly involved in arrested alveolarization and abnormal arterial remodeling, which are the hallmarks of BPD. These previous reports also demonstrate a strong association between MMPs, ROS, and oxidative lung injury.
7. ROS AND ANTIOXIDANT DEFENSES
A balanced redox state determines the vulnerability of an organism to oxidative stress. Exposure to oxidative stress has potential deleterious side effects, and in some cases, can be lethal. The lungs are particularly susceptible because, of all the organs in the body, it is the most exposed to a higher partial oxygen pressure [112]. However, homeostasis is maintained by efficient antioxidant defense systems that keep oxygen toxicity in check. Mitochondrion is not only the main generator of ROS, but also the main organelle for antioxidant ROS scavenging. Similar systems are found in the cytosol and peroxisomes. Antioxidants can be intracellular and extracellular or enzymatic and non-enzymatic [113]. They may also be categorized as primary (preventing ROS formation), secondary (scavenging ROS), and tertiary (removing or repairing oxidatively modified molecules) [114]. The major detoxifying antioxidant is SOD enzymes [115]. Copper and zinc-containing SOD (CuZnSOD or SOD-1) is found in the cytoplasm [116]. Manganese-containing SOD (MnSOD or SOD-2) is found in the mitochondria [117, 118], and extracellular SOD (ECSOD or SOD-3), as the name indicates, is present extracellularly [119]. These three forms allow the dismutation of superoxide into oxygen (O2) and H2O2 [120]. Cytoplasmic and peroxisomal catalase breaks H2O2 into water and O2 and peroxiredoxins reduce it [121]. Catalase works best with high concentrations of H2O2 [122, 123]. Reductases and peroxidases that detoxify ROS and lipid peroxides belong to the thioredoxin and glutathione systems [124]. Two forms of glutathione peroxidase (GPx) have been identified in mitochondria [125, 126]. Peroxidases clear up to 90% of H2O2 produced in the mitochondria [127, 128]. Together these enzymes have been shown to have protective roles in lung diseases [129]. Multiple cell culture models suggested that overexpression of antioxidants prevents ROS-induced injury [130]. Other forms of free radical scavengers are small molecular weight compounds of the non-enzymatic type present endogenously or obtained through diet. GSH, cysteinyl tripeptide, uric acid, ascorbic acid, and tocopherols/tocotrienols are some of these compounds involved in protection from pulmonary diseases in neonatal and adult populations [131–135]. Recent literature highlights the importance of maintaining a delicate balance between ROS production in oxidative stress and scavenging. Too much or faulty scavenging may lead to overexpression of SOD that could lead to excessive H2O2production and may have adverse rather than beneficial effects [136]. Studies in our laboratory have shown that the use of MnTBAP, an SOD mimetic that scavenges superoxide and peroxynitrite, can suppress oxygen-induced inflammatory MMP-9 levels in lungs of neonatal rats exposed to IH [137], while the use of anti-VEGF drugs causes pulmonary hemorrhage concurrent with elevations in MMP-2 and MMP-9 [138].
8. THERAPEUTIC CONSIDERATIONS
Despite the promising role of antioxidant therapy in CLD in ELGANs, clinical trials have only yielded limited success in treatment [139]. It is postulated that once the disease process has progressed to a critical point, antioxidant therapy may already be ineffective. In early stages, however, it may still have potential roles in prevention. Low doses of dexamethasone, which has anti-inflammatory and antioxidant properties, were beneficial in infants who remain ventilator dependent after 1–2 weeks [140]. However, studies done in our laboratory showed that a low-dose dexamethasone alters the imbalance in lung MMPs and TIMPs which may lead to further lung injury [141]. In lamb models of persistent pulmonary hypertension (PPHN), intratracheal antioxidant administration combined with NO inhalation is shown to be more effective than NO inhaled alone [142, 143]. Recombinant human SOD also reduces peroxynitrite-mediated protein nitration mitigating cell injuries [144]. Nosocomial infection is a predictor of BPD [145]. Exposure of lung epithelial cells to hyperoxia impairs phagocytosis and bacterial clearance with increased IL-8 production, and overexpression of SOD has beneficial effects [146, 147]. Data analysis has pointed to the critical role of SOD in preventing hyperoxia-induced lung injury and the preservation of the alveolar architecture which suggests a potential role for antioxidant therapy in CLD. Scavenging of ROS may also interfere with normal signaling pathways in early lung development and oxidative stress may be localized only in some areas, limiting the efficacy of some antioxidants [148]. Administration of early high doses of antioxidant vitamins had no beneficial effects to curtail pulmonary responses to hypoxia in the baboon BPD model [149]. Multicenter trials using high-dose vitamin A in premature infants showed a 7% significant reduction of BPD, but long-term follow-up of treated subjects did not show long-term benefits [150, 151]. Vitamin C is thought to contribute to the regeneration of membrane bound alpha-tocopherol in preterm infants [152]. Vitamin E is relatively deficient in preterm infants, but clinical trials failed to demonstrate significant benefits [153, 154], and instead it may increase the risk of necrotizing enterocolitis (NEC) and sepsis [155]. Because iron is important in the production of ROS [54], studies showed that iron chelators effectively prevented ROS-induced lung injury [156]. N-Acetylcysteine (NAC) is a precursor of GSH. However, clinical trials comparing NAC against placebo in ventilated extremely low birth weight infants did not improve survival or decrease BPD at 36 weeks corrected age or improved pulmonary function at term [157, 158]. More recently, the use of omega-3 polyunsaturated fatty acids (ω-3 PUFAs) has been proposed for the prevention of ROS-induced lung injury. Maternal ω-3 PUFA supplementation appears to protect newborn rats from hyperoxia-induced lung injury [159, 160]. However, clinical trials show conflicting results. Preterm infants who received parenteral ω-3 PUFAs had a lower incidence of BPD [161, 162], but a large, randomized multicenter trial showed no benefit for reducing BPD [163]. Further longitudinal outcome studies need to be undertaken to determine the efficacy of ROS scavenging and its use in treating BPD. For example, studies implementing synergistic pharmacological strategies to prevent ROS production, scavenge, and decrease ROS that have already been produced due to oxygen therapy, and at the same time, excrete and metabolize other toxic oxidative products such as H2O2, should be conducted in appropriate animal models.
9. CONCLUSION
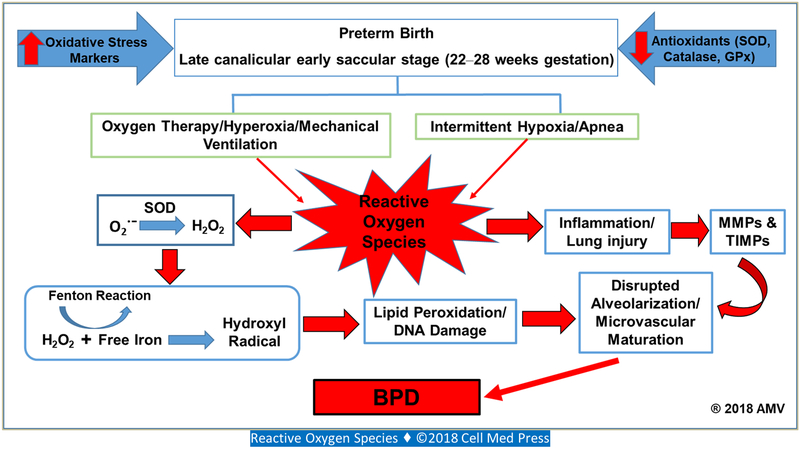
Injuries to cells by oxidative stress is brought about by the arrest in the normal process of proliferation and differentiation. Damage to DNA, protein, and lipids in cell membranes, and disruption of regulatory pathways further amplify the initial injury. The balance between ROS generation and scavenging is crucial for maintaining homeostasis and to the survival of the organism (Figure 1). The inadequate antioxidant system, the early stage of lung development in ELGANs, and their exposure to a hyperoxic environment after birth leads to the ontogeny of their lung disease. BPD/CLD is characterized by alveolar hypoplasia, and in some cases, with accompanying increased in pulmonary vascular resistance making it more challenging to maintain adequate ventilation and oxygenation. Prolonged exposure to oxygen and mechanical ventilation exacerbates oxidative stress and inflammatory injury to the premature lungs, leading to later fibrosis. Studies are underway to exploit the potential of antioxidant therapy to enhance the natural ROS-scavenging mechanisms in this population, in order to decrease morbidities and improve their outcomes. Preliminary data, however, have yielded inconclusive reports. This may suggest that the timing for therapeutic intervention and the specificity of the antioxidant to its target may be key to the success of this novel approach.
FIGURE 1. Graphic representation showing only the relationship between reactive oxygen species, matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs), and bronchopulmonary dysplasia (BPD).
Preterm infants who are at the highest risk for development of BPD are born in the late canalicular/early saccular stage of lung development. At this stage, the incidence of BPD is as high as 68%, and is characterized by alveolar oversimplification, disrupted alveolarization, reduced secondary septation, and aberrant microvascular maturation, all of which are associated with oxidative distress, inflammation, and MMPs and TIMPs. Preterm infants also have immature antioxidant systems. Superoxide anion (O2˙−) produced from exposure to high levels of oxygen, undergoes dismutation to hydrogen peroxide (H2O2) and O2. Deficiency of antioxidants such as catalase and glutathione peroxidase (GPx), scavengers of H2O2, will lead to accumulation of H2O2. Preterm infants are often supplemented with iron, and excess free iron due to deficient iron-binding capacity leads to its reaction with H2O2 via the Fenton reaction resulting in the formation of the hydroxyl radical, the highly reactive, self-propagating, and extremely powerful oxidant, that reacts with most organic molecules causing lipid peroxidation and DNA damage.
ACKNOWLEDGMENTS
The work from the authors’ laboratory described in this manuscript was supported in part by the Eunice Kennedy Shriver National Institute of Child Health & Human Development grant (1U54HD071594). The authors declare no conflicts of interest.
ABBREVIATIONS ∣
- BPD
bronchopulmonary dysplasia
- CLD
chronic lung disease
- ECM
extracellular matrix
- ELGAN
extremely low gestational age neonate
- GPx
glutathione peroxidase
- GSH
reduced form of glutathione
- IH
intermittent hypoxia
- MMP
matrix metalloproteinase
- NAC
N-acetylcysteine
- NOX
NADPH oxidase
- PUFA
polyunsaturated fatty acid
- RDS
respiratory distress syndrome
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TIMP
inhibitor of metalloproteinase
- VEGF
vascular endothelial growth factor
REFERENCES
- 1.Northway WH Jr., Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med 1967; 276(7):357–68. doi: 10.1056/NEJM196702162760701. [DOI] [PubMed] [Google Scholar]
- 2.Northway WH Jr. Bronchopulmonary dysplasia: then and now. Arch Dis Child 1990; 65(10 Spec No): 1076–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010; 126(3):443–56. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bui CB, Pang MA, Sehgal A, Theda C, Lao JC, Berger PJ, et al. Pulmonary hypertension associated with bronchopulmonary dysplasia in preterm infants. J Reprod Immunol 2017; 124:21–9. doi: 10.1016/j.jri.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Khemani E, McElhinney DB, Rhein L, Andrade O, Lacro RV, Thomas KC, et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics 2007; 120(6): 1260–9. doi: 10.1542/peds.2007-0971. [DOI] [PubMed] [Google Scholar]
- 6.Jobe AJ. The new BPD: an arrest of lung development. Pediatr Res 1999; 46(6):641–3. [DOI] [PubMed] [Google Scholar]
- 7.Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol 1998; 29(7):710–7. [DOI] [PubMed] [Google Scholar]
- 8.Coalson JJ, Winter V, deLemos RA. Decreased alveolarization in baboon survivors with bronchopulmonary dysplasia. Am J Respir Crit Care Med 1995; 152(2):640–6. doi: 10.1164/ajrccm.152.2.7633720. [DOI] [PubMed] [Google Scholar]
- 9.Hislop A. Developmental biology of the pulmonary circulation. Paediatr Respir Rev 2005; 6(1):35–43. doi: 10.1016/j.prrv.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Kajekar R. Environmental factors and developmental outcomes in the lung. Pharmacol Ther 2007; 114(2): 129–45. doi: 10.1016/j.pharmthera.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Schittny JC. Development of the lung. Cell Tissue Res 2017; 367(3):427–44. doi: 10.1007/s00441-016-2545-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burri PH. Structural aspects of postnatal lung development: alveolar formation and growth. Biol Neonate 2006; 89(4):313–22. doi: 10.1159/000092868. [DOI] [PubMed] [Google Scholar]
- 13.Gien J, Kinsella JP. Pathogenesis and treatment of bronchopulmonary dysplasia. Curr Opin Pediatr 2011; 23(3):305–13. doi: 10.1097/MOP.0b013e328346577f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhandari A, Bhandari V. Biomarkers in bronchopulmonary dysplasia. Paediatr Respir Rev 2013; 14(3): 173–9. doi: 10.1016/j.prrv.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Joung KE, Kim HS, Lee J, Shim GH, Choi CW, Kim EK, et al. Correlation of urinary inflammatory and oxidative stress markers in very low birth weight infants with subsequent development of bronchopulmonary dysplasia. Free Radio Res 2011; 45(9):1024–32. doi: 10.3109/10715762.2011.588229. [DOI] [PubMed] [Google Scholar]
- 16.Saugstad OD. Bronchopulmonary dysplasia: oxidative stress and antioxidants. Semin Neonatol 2003; 8(1):39–49. [DOI] [PubMed] [Google Scholar]
- 17.Ozsurekci Y, Aykac K. Oxidative stress related diseases in newborns. Oxid Med Cell Longev 2016; 2016:2768365. doi: 10.1155/2016/2768365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Auten RL. The role of oxygen in health and disease-a series of reviews. Pediatric Research 2009; 66:121–7. [DOI] [PubMed] [Google Scholar]
- 19.Papa S. Mitochondrial oxidative phosphorylation changes in the life span: molecular aspects and physiopathological implications. Biochim Biophys Acta 1996; 1276(2):87–105. [DOI] [PubMed] [Google Scholar]
- 20.Boveris A, Oshino N, Chance B. The cellular production of hydrogen peroxide. Biochem J 1972; 128(3):617–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gruber J, Schaffer S, Halliwell B. The mitochondrial free radical theory of ageing: where do we stand? Front Biosci 2008; 13:6554–79. [DOI] [PubMed] [Google Scholar]
- 22.Kang J, Pervaiz S. Mitochondria: redox metabolism and dysfunction. Biochem Res Int 2012; 2012:896751. doi: 10.1155/2012/896751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davydov DR. Microsomal monooxygenase in apoptosis: another target for cytochrome c signaling? Trends Biochem Sci 2001; 26(3): 155–60. [DOI] [PubMed] [Google Scholar]
- 24.Sumimoto H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J 2008; 275(13):3249–77. doi: 10.1111/j.1742-4658.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- 25.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J 2009; 417(1):1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SY, Kim TB, Moon KA, Kim TJ, Shin D, Cho YS, et al. Regulation of pro-inflammatory responses by lipoxygenases via intracellular reactive oxygen species in vitro and in vivo. Exp Mol Med 2008; 40(4):461–76. doi: 10.3858/emm.2008.40.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen CA, Lin CH, Druhan LJ, Wang TY, Chen YR, Zweier JL. Superoxide induces endothelial nitric-oxide synthase protein thiyl radical formation, a novel mechanism regulating eNOS function and coupling. J Biol Chem 2011; 286(33):29098–107. doi: 10.1074/jbc.M111.240127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grobe AC, Wells SM, Benavidez E, Oishi P, Azakie A, Fineman JR, et al. Increased oxidative stress in lambs with increased pulmonary blood flow and pulmonary hypertension: role of NADPH oxidase and endothelial NO synthase. Am J Physiol Lung Cell Mol Physiol 2006; 290(6):L1069–77. doi: 10.1152/ajplung.00408.2005. [DOI] [PubMed] [Google Scholar]
- 29.Jiang W, Couroucli XI, Wang L, Barrios R, Moorthy B. Augmented oxygen-mediated transcriptional activation of cytochrome P450 (CYP)1A expression and increased susceptibilities to hyperoxic lung injury in transgenic mice carrying the human CYP1A1 or mouse 1A2 promoter in vivo. Biochem Biophys Res Commun 2011; 407(1)79–85. doi: 10.1016/j.bbrc.2011.02.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic Biol Med 2007; 43(3):332–47. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Vliet A. NADPH oxidases in lung biology and pathology: host defense enzymes, and more. Free Radio Biol Med 2008; 44(6):938–55. doi: 10.1016/j.freeradbiomed.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ivanov I, Saam J, Kuhn H, Holzhutter HG. Dual role of oxygen during lipoxygenase reactions. FEBS J 2005; 272(10):2523–35. doi: 10.1111/j.1742-4658.2005.04673.x. [DOI] [PubMed] [Google Scholar]
- 33.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 2007; 87(1):315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 2008; 88(4): 1243–76. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA 1990; 87(4):1620–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janssen LJ, Catalli A, Helli P. The pulmonary biology of isoprostanes. Antioxid Redox Signal 2005; 7(1–2):244–55. doi: 10.1089/ars.2005.7.244. [DOI] [PubMed] [Google Scholar]
- 37.Janssen LJ. Isoprostanes and lung vascular pathology. Am J Respir Cell Mol Biol 2008; 39(4):383–9. doi: 10.1165/rcmb.2008-0109TR. [DOI] [PubMed] [Google Scholar]
- 38.Stadtman ER, Levine RL. Protein oxidation. Ann N Y Acad Sci 2000; 899:191–208. [DOI] [PubMed] [Google Scholar]
- 39.Fruhwirth GO, Hermetter A. Mediation of apoptosis by oxidized phospholipids. Subcell Biochem 2008; 49:351–67. doi: 10.1007/978-1-4020-8831-5_13. [DOI] [PubMed] [Google Scholar]
- 40.Auten RL, Whorton MH, Nicholas Mason S. Blocking neutrophil influx reduces DNA damage in hyperoxia-exposed newborn rat lung. Am J Respir Cell Mol Biol 2002; 26(4):391–7. doi: 10.1165/ajrcmb.26.4.4708. [DOI] [PubMed] [Google Scholar]
- 41.Bulkley GB. Free radical-mediated reperfusion injury: a selective review. Br J Cancer suppl 1987; 8:66–73. [PMC free article] [PubMed] [Google Scholar]
- 42.Zamocky M, Furtmuller PG, Obinger C. Evolution of catalases from bacteria to humans. Antioxid Redox Signal 2008; 10(9): 1527–48. doi: 10.1089/ars.2008.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bienert GP, Chaumont F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim Biophys Acta 2014; 1840(5): 1596–604. doi: 10.1016/j.bbagen.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 44.HenzIer T, Steudle E. Transport and metabolic degradation of hydrogen peroxide in Chara corallina: model calculations and measurements with the pressure probe suggest transport of H2O2 across water channels. J Exp Bot 2000; 51(353):2053–66. [DOI] [PubMed] [Google Scholar]
- 45.Bienert GP, Schjoerring JK, Jahn TP. Membrane transport of hydrogen peroxide. Biochim Biophys Acta 2006; 1758(8):994–1003. doi: 10.1016/j.bbamem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 46.Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol 2012; 298:229–317. doi: 10.1016/B978-0-12-394309-5.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parinandi NL, Kleinberg MA, Usatyuk PV, Cummings RJ, Pennathur A, Cardounel AJ, et al. Hyperoxia-induced NAD(P)H oxidase activation and regulation by MAP kinases in human lung endothelial cells. Am J Physiol Lung Cell Mol Physiol 2003; 284(1):L26–38. doi: 10.1152/ajplung.00123.2002. [DOI] [PubMed] [Google Scholar]
- 48.Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci USA 2003; 100(5):2432–7. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mari M, Morales A, Colell A, Garcia-Ruiz C, Fernandez-Checa JC. Mitochondrial glutathione, a key survival antioxidant. Antioxid Redox Signal 2009; 11(11):2685–700. doi: 10.1089/ARS.2009.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qutub AA, Popel AS. Reactive oxygen species regulate hypoxia-inducible factor 1alpha differentially in cancer and ischemia. Mol Cell Biol 2008; 28(16):5106–19. doi: 10.1128/MCB.00060-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vercellotti GM, Severson SP, Duane P, Moldow CF. Hydrogen peroxide alters signal transduction in human endothelial cells. J Lab Clin Med 1991; 117(1): 15–24. [PubMed] [Google Scholar]
- 52.Fridovich I. Superoxide dismutases. An adaptation to a paramagnetic gas. J Biol Chem 1989; 264(14)7761–4. [PubMed] [Google Scholar]
- 53.Holley AK, St Clair DK. Watching the watcher: regulation of p53 by mitochondria. Future Oncol 2009; 5(1):117–30. doi: 10.2217/14796694.5.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winterbourn CC. Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicol Lett 195; 82–83:969–74. [DOI] [PubMed] [Google Scholar]
- 55.Inayat M, Bany-Mohammed F, Valencia A, Tay C, Jacinto J, Aranda JV, et al. Antioxidants and biomarkers of oxidative stress in preterm infants with symptomatic patent ductus arteriosus. Am J Perinatol 2015; 32(9):895–904. doi: 10.1055/S-0035-1544948. [DOI] [PubMed] [Google Scholar]
- 56.Lee YS, Chou YH. Antioxidant profiles in full term and preterm neonates. Chang Gung Med J 2005; 28(12):846–51. [PubMed] [Google Scholar]
- 57.Patel A, Lakshminrusimha S, Ryan RM, Swartz DD, Wang H, Wynn KA, et al. Exposure to supplemental oxygen downregulates antioxidant enzymes and increases pulmonary arterial contractility in premature lambs. Neonatology 2009; 96(3):182–92. doi: 10.1159/000211667. [DOI] [PubMed] [Google Scholar]
- 58.Hirano K, Morinobu T, Kim H, Hiroi M, Ban R, Ogawa S, et al. Blood transfusion increases radical promoting non-transferrin bound iron in preterm infants. Arch Dis Child Fetal Neonatal Ed 2001; 84(3): F188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Makela E, Takala TI, Suominen P, Matomaki J, Salmi TT, Rajamaki A, et al. Hematological parameters in preterm infants from birth to 16 weeks of age with reference to iron balance. Clin Chem Lab Med 2008; 46(4):551–7. doi: 10.1515/CCLM.2008.109. [DOI] [PubMed] [Google Scholar]
- 60.Pitkanen OM, Hallman M, Andersson SM. Correlation of free oxygen radical-induced lipid peroxidation with outcome in very low birth weight infants. J Pediatr 1990; 116(5)760–4. [DOI] [PubMed] [Google Scholar]
- 61.Halliwell B, Gutteridge JM. Biologically relevant metal ion-dependent hydroxyl radical generation: an update. FEBS Lett 1992; 307(1): 108–12. [DOI] [PubMed] [Google Scholar]
- 62.Freeman BA, Crapo JD. Biology of disease: free radicals and tissue injury. Lab Invest 1982; 47(5):412–26. [PubMed] [Google Scholar]
- 63.Fracica PJ, Knapp MJ, Piantadosi CA, Takeda K, Fulkerson WJ, Coleman RE, et al. Responses of baboons to prolonged hyperoxia: physiology and qualitative pathology. J Appl Physiol (1985) 1991; 71(6):2352–62. doi: 10.1152/jappl.1991.71.6.2352. [DOI] [PubMed] [Google Scholar]
- 64.Warner BB, Stuart LA, Papes RA, Wispe JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol 1998; 275(1 Pt 1):L110–7. [DOI] [PubMed] [Google Scholar]
- 65.Manji JS, O’Kelly CJ, Leung WI, Olson DM. Timing of hyperoxic exposure during alveolarization influences damage mediated by leukotrienes. Am J Physiol Lung Cell Mol Physiol 2001; 281(4):L799–806. doi: 10.1152/ajplung.2001.281.4.L799. [DOI] [PubMed] [Google Scholar]
- 66.Martin RJ, Wang K, Koroglu O, Di Fiore J, Kc P. Intermittent hypoxic episodes in preterm infants: do they matter? Neonatology 2011; 100(3):303–10. doi: 10.1159/000329922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martin RJ, Di Fiore JM, Walsh MC. Hypoxic Episodes in bronchopulmonary dysplasia. Clin Perinatol 2015; 42(4):825–38. doi: 10.1016/j.clp.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Coalson JJ. Pathology of new bronchopulmonary dysplasia. Semin Neonatol 2003; 8(1):73–81. [DOI] [PubMed] [Google Scholar]
- 69.Coalson JJ, Winter VT, Gerstmann DR, Idell S, King RJ, Delemos RA. Pathophysiologic, morphometric, and biochemical studies of the premature baboon with bronchopulmonary dysplasia. Am Rev Respir Dis 1992; 145(4 Pt 1):872–81. doi: 10.1164/ajrccm/145.4_Pt_1.872. [DOI] [PubMed] [Google Scholar]
- 70.Coalson JJ, Kuehl TJ, Escobedo MB, Hilliard JL, Smith F, Meredith K, et al. A baboon model of bronchopulmonary dysplasia. II. Pathologic features. Exp Mol Pathol 1982; 37(3):335–50. [DOI] [PubMed] [Google Scholar]
- 71.Abman SH. Impaired vascular endothelial growth factor signaling in the pathogenesis of neonatal pulmonary vascular disease. Adv Exp Med Biol 2010; 661:323–35. doi: 10.1007/978-1-60761-500-2_21. [DOI] [PubMed] [Google Scholar]
- 72.Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, et al. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol 2000; 279(3):L600–7. doi: 10.1152/ajplung.2000.279.3.L600. [DOI] [PubMed] [Google Scholar]
- 73.Thebaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F, et al. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation 2005; 112(16):2477–86. doi: 10.1161/CIRCULATIONAHA.105.541524. [DOI] [PubMed] [Google Scholar]
- 74.Lee C, An J, Kim JH, Kim ES, Kim SH, Cho YK, et al. Low levels of tissue inhibitor of metalloproteinase-2 at birth may be associated with subsequent development of bronchopulmonary dysplasia in preterm infants. Korean J Pediatr 2015; 58(11):415–20. doi: 10.3345/kjp.2015.58.11.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vento G, Tirone C, Lulli P, Capoluongo E, Ameglio F, Lozzi S, et al. Bronchoalveolar lavage fluid peptidomics suggests a possible matrix metalloproteinase-3 role in bronchopulmonary dysplasia. Intensive Care Med 2009; 35(12):2115–24. doi: 10.1007/s00134-009-1646-6. [DOI] [PubMed] [Google Scholar]
- 76.Ratner V, Sosunov SA, Niatsetskaya ZV, Utkina-Sosunova IV, Ten VS. Mechanical ventilation causes pulmonary mitochondrial dysfunction and delayed alveolarization in neonatal mice. Am J Respir Cell Mol Biol 2013; 49(6):943–50. doi: 10.1165/rcmb.2012-0172OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ferrara N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol Cell Physiol 2001; 280(6):C1358–66. doi: 10.1152/ajpcell.2001.280.6.C1358. [DOI] [PubMed] [Google Scholar]
- 78.Acarregui MJ, Penisten ST, Goss KL, Ramirez K, Snyder JM. Vascular endothelial growth factor gene expression in human fetal lung in vitro. Am J Respir Cell Mol Biol 1999; 20(1): 14–23. doi: 10.1165/ajrcmb.20.1.3251. [DOI] [PubMed] [Google Scholar]
- 79.Maniscalco WM, Watkins RH, D’Angio CT, Ryan RM. Hyperoxic injury decreases alveolar epithelial cell expression of vascular endothelial growth factor (VEGF) in neonatal rabbit lung. Am J Respir Cell Mol Biol 1997; 16(5):557–67. doi: 10.1165/ajrcmb.16.5.9160838. [DOI] [PubMed] [Google Scholar]
- 80.Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001; 164(10 Pt 1): 1971–80. doi: 10.1164/ajrccm.164.10.2101140. [DOI] [PubMed] [Google Scholar]
- 81.Lassus P, Turanlahti M, Heikkila P, Andersson LC, Nupponen I, Sarnesto A, et al. Pulmonary vascular endothelial growth factor and Flt-1 in fetuses, in acute and chronic lung disease, and in persistent pulmonary hypertension of the newborn. Am J Respir Crit Care Med 2001; 164(10 Pt 1): 1981–7. doi: 10.1164/ajrccm.164.10.2012036. [DOI] [PubMed] [Google Scholar]
- 82.D’Angio CT, Maniscalco WM. The role of vascular growth factors in hyperoxia-induced injury to the developing lung. Front Biosci 2002; 7:d1609–23. [DOI] [PubMed] [Google Scholar]
- 83.Ambalavanan N, Novak ZE. Peptide growth factors in tracheal aspirates of mechanically ventilated preterm neonates. Pediatr Res 2003; 53(2):240–4. doi: 10.1203/01.PDR.0000047656.17766.39. [DOI] [PubMed] [Google Scholar]
- 84.Tambunting F, Beharry KD, Waltzman J, Modanlou HD. Impaired lung vascular endothelial growth factor in extremely premature baboons developing bronchopulmonary dysplasia/chronic lung disease. J Investig Med 2005; 53(5):253–62. doi: 10.2310/6650.2005.53508. [DOI] [PubMed] [Google Scholar]
- 85.Hasan J, Beharry KD, Valencia AM, Strauss A, Modanlou HD. Soluble vascular endothelial growth factor receptor 1 in tracheal aspirate fluid of preterm neonates at birth may be predictive of bronchopulmonary dysplasia/chronic lung disease. Pediatrics 2009; 123(6):1541–7. doi: 10.1542/peds.2008-1670. [DOI] [PubMed] [Google Scholar]
- 86.Maniscalco WM, Watkins RH, Finkelstein JN, Campbell MH. Vascular endothelial growth factor mRNA increases in alveolar epithelial cells during recovery from oxygen injury. Am J Respir Cell Mol Biol 1995; 13(4):377–86. doi: 10.1165/ajrcmb.13.4.7546767. [DOI] [PubMed] [Google Scholar]
- 87.Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci USA 1998; 95(20): 11715–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tschanz SA, Salm LA, Roth-Kleiner M, Barre SF, Burri PH, Schittny JC. Rat lungs show a biphasic formation of new alveoli during postnatal development. J Appl Physiol (1985) 2014; 117(1):89–95. doi: 10.1152/japplphysiol.01355.2013. [DOI] [PubMed] [Google Scholar]
- 89.Burri PH. The postnatal growth of the rat lung. 3. Morphology. Anat Rec 1974; 180(1):77–98. doi: 10.1002/ar.1091800109. [DOI] [PubMed] [Google Scholar]
- 90.Burri PH. Lung development and pulmonary angiogenesis In: Lung Development (Gaultier C, Bourbon J, Post M). University Press, Oxford, UK: 1999, pp. 122–51. [Google Scholar]
- 91.Fukuda Y, Ishizaki M, Kudoh S, Kitaichi M, Yamanaka N. Localization of matrix metalloproteinases-1, -2, and -9 and tissue inhibitor of metalloproteinase-2 in interstitial lung diseases. Lab Invest 1998; 78(6):687–98. [PubMed] [Google Scholar]
- 92.Massova I, Kotra LP, Fridman R, Mobashery S. Matrix metalloproteinases: structures, evolution, and diversification. FASEB J 1998; 12(12): 1075–95. [PubMed] [Google Scholar]
- 93.Woessner JF Jr. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J 1991; 5(8):2145–54. [PubMed] [Google Scholar]
- 94.Matrisian LM. The matrix-degrading metalloproteinases. Bioessays 1992; 14(7):455–63. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- 95.Woessner JF Jr. The family of matrix metalloproteinases. Ann N Y Acad Sci 1994; 732:11–21. [DOI] [PubMed] [Google Scholar]
- 96.Sweet DG, McMahon KJ, Curley AE, O’Connor CM, Halliday HL. Type I collagenases in bronchoalveolar lavage fluid from preterm babies at risk of developing chronic lung disease. Arch Dis Child Fetal Neonatal Ed 2001; 84(3):F168–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carney DE, McCann UG, Schiller HJ, Gatto LA, Steinberg J, Picone AL, et al. Metalloproteinase inhibition prevents acute respiratory distress syndrome. J Surg Res 2001; 99(2):245–52. doi: 10.1006/jsre.2001.6180. [DOI] [PubMed] [Google Scholar]
- 98.Matrisian LM. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet 1990; 6(4): 121–5. [DOI] [PubMed] [Google Scholar]
- 99.Birkedal-Hansen H. Proteolytic remodeling of extracellular matrix. Curr Opin Cell Biol 1995; 7(5)728–35. [DOI] [PubMed] [Google Scholar]
- 100.Vliagoftis H, Schwingshackl A, Milne CD, Duszyk M, Hollenberg MD, Wallace JL, et al. Proteinase-activated receptor-2-mediated matrix metalloproteinase-9 release from airway epithelial cells. J Allergy Clin Immunol 2000; 106(3):537–45. doi: 10.1067/mai.2000.109058. [DOI] [PubMed] [Google Scholar]
- 101.Shapiro SD, Kobayashi DK, Welgus HG. Identification of TIMP-2 in human alveolar macrophages: regulation of biosynthesis is opposite to that of metalloproteinases and TIMP-1. J Biol Chem 1992; 267(20): 13890–4. [PubMed] [Google Scholar]
- 102.Cederqvist K, Sorsa T, Tervahartiala T, Maisi P, Reunanen K, Lassus P, et al. Matrix metalloproteinases-2, -8, and -9 and TIMP-2 in tracheal aspirates from preterm infants with respiratory distress. Pediatrics 2001; 108(3):686–92. [DOI] [PubMed] [Google Scholar]
- 103.Danan C, Jarreau PH, Franco ML, Dassieu G, Grillon C, Abd Alsamad I, et al. Gelatinase activities in the airways of premature infants and development of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 2002; 283(5):L1086–93. doi: 10.1152/ajplung.00066.2002. [DOI] [PubMed] [Google Scholar]
- 104.Ekekezie II, Thibeault DW, Simon SD, Norberg M, Merrill JD, Ballard RA, et al. Low levels of tissue inhibitors of metalloproteinases with a high matrix metalloproteinase-9/tissue inhibitor of metalloproteinase-1 ratio are present in tracheal aspirate fluids of infants who develop chronic lung disease. Pediatrics 2004; 113(6): 1709–14. [DOI] [PubMed] [Google Scholar]
- 105.Davies PL, Spiller OB, Beeton ML, Maxwell NC, Remold-O’Donnell E, Kotecha S. Relationship of proteinases and proteinase inhibitors with microbial presence in chronic lung disease of prematurity. Thorax 2010; 65(3):246–51. doi: 10.1136/thx.2009.116061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schulz CG, Sawicki G, Lemke RP, Roeten BM, Schulz R, Cheung PY. MMP-2 and MMP-9 and their tissue inhibitors in the plasma of preterm and term neonates. Pediatr Res 2004; 55(5)794–801. doi: 10.1203/01.PDR.0000120683.68630.FB. [DOI] [PubMed] [Google Scholar]
- 107.Schock BC, Sweet DG, Ennis M, Warner JA, Young IS, Halliday HL. Oxidative stress and increased type-IV collagenase levels in bronchoalveolar lavage fluid from newborn babies. Pediatr Res 2001; 50(1):29–33. doi: 10.1203/00006450-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 108.Pardo A, Selman M, Ridge K, Barrios R, Sznajder JI. Increased expression of gelatinases and collagenase in rat lungs exposed to 100% oxygen. Am J Respir Crit Care Med 1996; 154(4 Pt 1): 1067–75. doi: 10.1164/ajrccm.154.4.8887609. [DOI] [PubMed] [Google Scholar]
- 109.Pardo A, Barrios R, Maldonado V, Melendez J, Perez J, Ruiz V, et al. Gelatinases A and B are up-regulated in rat lungs by subacute hyperoxia: pathogenetic implications. Am J Pathol 1998; 153(3):833–44. doi: 10.1016/S0002-9440(10)65625-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lukkarinen H, Hogmalm A, Lappalainen U, Bry K. Matrix metalloproteinase-9 deficiency worsens lung injury in a model of bronchopulmonary dysplasia. Am J Respir Cell Mol Biol 2009; 41 (1):59–68. doi: 10.1165/rcmb.2008-0179OC. [DOI] [PubMed] [Google Scholar]
- 111.Tambunting F, Beharry KD, Hartleroad J, Waltzman J, Stavitsky Y, Modanlou HD. Increased lung matrix metalloproteinase-9 levels in extremely premature baboons with bronchopulmonary dysplasia. Pediatr Pulmonol 2005; 39(1):5–14. doi: 10.1002/ppul.20135. [DOI] [PubMed] [Google Scholar]
- 112.Comhair SA, Erzurum SC. Antioxidant responses to oxidant-mediated lung diseases. Am J Physiol Lung Cell Mol Physiol 2002; 283(2):L246–55. doi: 10.1152/ajplung.00491.2001. [DOI] [PubMed] [Google Scholar]
- 113.Villegas L, Stidham T, Nozik-Grayck E. Oxidative stress and therapeutic development in lung diseases. J Pulm Respir Med 2014; 4(4). doi: 10.4172/2161-105X.1000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gutteridge JM, Halliwell B. Free radicals and antioxidants in the year 2000: a historical look to the future. Ann N Y Acad Sci 2000; 899:136–47. [DOI] [PubMed] [Google Scholar]
- 115.McCord JM. Superoxide radical: controversies, contradictions, and paradoxes. Proc Soc Exp Biol Med 1995; 209(2): 112–7. [DOI] [PubMed] [Google Scholar]
- 116.Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radio Biol Med 2002; 33(3):337–49. [DOI] [PubMed] [Google Scholar]
- 117.Weisiger RA, Fridovich I. Mitochondrial superoxide simutase: site of synthesis and intramitochondrial localization. J Biol Chem 1973; 248(13):4793–6. [PubMed] [Google Scholar]
- 118.Okado-Matsumoto A, Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu,Zn-SOD in mitochondria. J Biol Chem 2001; 276(42):38388–93. doi: 10.1074/jbc.M105395200. [DOI] [PubMed] [Google Scholar]
- 119.Hjalmarsson K, Marklund SL, Engstrom A, Edlund T. Isolation and sequence of complementary DNA encoding human extracellular superoxide dismutase. Proc Natl Acad Sci USA 1987; 84(18):6340–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ho YS, Xiong Y, Ma W, Spector A, Ho DS. Mice lacking catalase develop normally but show differential sensitivity to oxidant tissue injury. J Biol Chem 2004; 279(31):32804–12. doi: 10.1074/jbc.M404800200. [DOI] [PubMed] [Google Scholar]
- 121.Kelly FJ. Glutathione: in defense of the lung. Food Chem Toxicol 1999; 37:963–6. [DOI] [PubMed] [Google Scholar]
- 122.Cantin AM, North SL, Hubbard RC, Crystal RG. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol (1985) 1987; 63(1):152–7. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- 123.Manevich Y, Fisher AB. Peroxiredoxin 6, a 1-Cys peroxiredoxin, functions in antioxidant defense and lung phospholipid metabolism. Free Radic Biol Med 2005; 38(11): 1422–32. doi: 10.1016/j.freeradbiomed.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 124.Maiorino M, Scapin M, Ursini F, Biasolo M, Bosello V, Flohe L. Distinct promoters determine alternative transcription of gpx-4 into phospholipid-hydroperoxide glutathione peroxidase variants. J Biol Chem 2003; 278(36):34286–90. doi: 10.1074/jbc.M305327200. [DOI] [PubMed] [Google Scholar]
- 125.Esworthy RS, Ho YS, Chu FF. The Gpx1 gene encodes mitochondrial glutathione peroxidase in the mouse liver. Arch Biochem Biophys 1997; 340(1):59–63. doi: 10.1006/abbi.1997.9901. [DOI] [PubMed] [Google Scholar]
- 126.Cox AG, Winterbourn CC, Hampton MB. Measuring the redox state of cellular peroxiredoxins by immunoblotting. Methods Enzymol 2010; 474:51–66. doi: 10.1016/S0076-6879(10)74004-0. [DOI] [PubMed] [Google Scholar]
- 127.Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol 2008; 4(5):278–86. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 128.Ahmed MN, Suliman HB, Folz RJ, Nozik-Grayck E, Golson ML, Mason SN, et al. Extracellular superoxide dismutase protects lung development in hyperoxia-exposed newborn mice. Am J Respir Crit Care Med 2003; 167(3):400–5. doi: 10.1164/rccm.200202-108OC. [DOI] [PubMed] [Google Scholar]
- 129.Ilizarov AM, Koo HC, Kazzaz JA, Mantell LL, Li Y, Bhapat R, et al. Overexpression of manganese superoxide dismutase protects lung epithelial cells against oxidant injury. Am J Respir Cell Mol Biol 2001; 24(4):436–41. doi: 10.1165/ajrcmb.24.4.4240. [DOI] [PubMed] [Google Scholar]
- 130.Schachter M. Uric acid and hypertension. Curr Pharm Des 2005; 11(32):4139–43. [DOI] [PubMed] [Google Scholar]
- 131.Sanders KA, Huecksteadt T, Xu P, Sturrock AB, Hoidal JR. Regulation of oxidant production in acute lung injury. Chest 1999; 116(1 Suppl):56S–61S. [DOI] [PubMed] [Google Scholar]
- 132.Virtamo J. Vitamins and lung cancer. Proc Nutr Soc 1999; 58(2):329–33. [DOI] [PubMed] [Google Scholar]
- 133.Tsiligianni IG, van der Molen T. A systematic review of the role of vitamin insufficiencies and supplementation in COPD. Respir Res 2010; 11:171. doi: 10.1186/1465-9921-11-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shamseer L, Adams D, Brown N, Johnson JA, Vohra S. Antioxidant micronutrients for lung disease in cystic fibrosis. Cochrane Database Syst Rev 2010; (12):CD007020. doi: 10.1002/14651858.CD007020.pub2. [DOI] [PubMed] [Google Scholar]
- 135.Hemila H. Vitamin E supplementation and respiratory infections in older people. J Am Geriatr Soc 2007; 55(8):1311–3; author reply 3–4. doi: 10.1111/j.1532-5415.2007.01263.x. [DOI] [PubMed] [Google Scholar]
- 136.Hemila H, Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst Rev 2007; (1):CD005532. doi: 10.1002/14651858.CD005532.pub2. [DOI] [PubMed] [Google Scholar]
- 137.Chang M, Bany-Mohammed F, Kenney MC, Beharry KD. Effects of a superoxide dismutase mimetic on biomarkers of lung angiogenesis and alveolarization during hyperoxia with intermittent hypoxia. Am J Transl Res 2013; 5(6):594–607. [PMC free article] [PubMed] [Google Scholar]
- 138.Valencia AM, Cai CL, Tan J, Duggan TJ, Valencia GB, Aranda JV, et al. Intravitreal bevacizumab alters type IV collagenases and exacerbates arrested alveologenesis in the neonatal rat lungs. Exp Lung Res 2017; 43(3):120–33. doi: 10.1080/01902148.2017.1306897. [DOI] [PubMed] [Google Scholar]
- 139.Davis JM, Parad RB, Michele T, Allred E, Price A, Rosenfeld W, et al. Pulmonary outcome at 1-year corrected age in premature infants treated at birth with recombinant human CuZn superoxide dismutase. Pediatrics 2003; 111 (3):469–76. [DOI] [PubMed] [Google Scholar]
- 140.Jefferies AL. Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Paediatr Child Health 2012; 17(10):573–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Valencia AM, Beharry KD, Ang JG, Devarajan K, Van Woerkom R, Abrantes M, et al. Early postnatal dexamethasone influences matrix metalloproteinase-2 and -9, and their tissue inhibitors in the developing rat lung. Pediatr Pulmonol 2003; 35(6):456–62. doi: 10.1002/ppul.10293. [DOI] [PubMed] [Google Scholar]
- 142.Wedgwood S, Lakshminrusimha S, Farrow KN, Czech L, Gugino SF, Soares F, et al. Apocynin improves oxygenation and increases eNOS in persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol 2012; 302(6):L616–26. doi: 10.1152/ajplung.00064.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Farrow KN, Lakshminrusimha S, Reda WJ, Wedgwood S, Czech L, Gugino SF, et al. Superoxide dismutase restores eNOS expression and function in resistance pulmonary arteries from neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2008; 295(6):L979–87. doi: 10.1152/ajplung.90238.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lakshminrusimha S, Russell JA, Wedgwood S, Gugino SF, Kazzaz JA, Davis JM, et al. Superoxide dismutase improves oxygenation and reduces oxidation in neonatal pulmonary hypertension. Am J Respir Crit Care Med 2006; 174(12): 1370–7. doi: 10.1164/rccm.200605-676OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bancalari E. Changes in the pathogenesis and prevention of chronic lung disease of prematurity. Am J Perinatol 2001; 18(1):1–9. [DOI] [PubMed] [Google Scholar]
- 146.Walti H, Nicolas-Robin A, Assous MV, Polla BS, Bachelet M, Davis JM. Effects of exogenous surfactant and recombinant human copper-zinc superoxide dismutase on oxygen-dependent antimicrobial defenses. Biol Neonate 2002; 82(2):96–102. doi: 10.1159/000063095. [DOI] [PubMed] [Google Scholar]
- 147.Arita Y, Joseph A, Koo HC, Li Y, Palaia TA, Davis JM, et al. Superoxide dismutase moderates basal and induced bacterial adherence and interleukin-8 expression in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 2004; 287(6):L1199–206. doi: 10.1152/ajplung.00457.2003. [DOI] [PubMed] [Google Scholar]
- 148.Arita Y, Kazzaz JA, Joseph A, Koo HC, Li Y, Davis JM. Antioxidants improve antibacterial function in hyperoxia-exposed macrophages. Free Radio Biol Med 2007; 42(10): 1517–23. doi: 10.1016/j.freeradbiomed.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Berger TM, Frei B, Rifai N, Avery ME, Suh J, Yoder BA, et al. Early high dose antioxidant vitamins do not prevent bronchopulmonary dysplasia in premature baboons exposed to prolonged hyperoxia: a pilot study. Pediatr Res 1998; 43(6)719–26. doi: 10.1203/00006450-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 150.Tyson JE, Wright LL, Oh W, Kennedy KA, Mele L, Ehrenkranz RA, et al. Vitamin A supplementation for extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N Engl J Med 1999; 340(25): 1962–8. doi: 10.1056/NEJM199906243402505. [DOI] [PubMed] [Google Scholar]
- 151.Kennedy KA, Stoll BJ, Ehrenkranz RA, Oh W, Wright LL, Stevenson DK, et al. Vitamin A to prevent bronchopulmonary dysplasia in very-low-birth-weight infants: has the dose been too low? The NICHD Neonatal Research Network. Early Hum Dev 1997; 49(1): 19–31. [DOI] [PubMed] [Google Scholar]
- 152.Berger TM, Frei B. Pro- or antioxidant activity of vitamin C in preterm infants? Arch Dis Child Fetal Neonatal Ed 1995; 72(3): F211–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Saldanha RL, Cepeda EE, Poland RL. The effect of vitamin E prophylaxis on the incidence and severity of bronchopulmonary dysplasia. J Pediatr 1982; 101 (1):89–93. [DOI] [PubMed] [Google Scholar]
- 154.Watts JL, Milner R, Zipursky A, Paes B, Ling E, Gill G, et al. Failure of supplementation with vitamin E to prevent bronchopulmonary dysplasia in infants less than 1,500 g birth weight. Eur Respir J 1991; 4(2):188–90. [PubMed] [Google Scholar]
- 155.Johnson L, Bowen FW Jr., Abbasi S, Herrmann N, Weston M, Sacks L, et al. Relationship of prolonged pharmacologic serum levels of vitamin E to incidence of sepsis and necrotizing enterocolitis in infants with birth weight 1,500 grams or less. Pediatrics 1985; 75(4):619–38. [PubMed] [Google Scholar]
- 156.Wong CM, Preston IR, Hill NS, Suzuki YJ. Iron chelation inhibits the development of pulmonary vascular remodeling. Free Radio Biol Med 2012; 53(9): 1738–47. doi: 10.1016/j.freeradbiomed.2012.08.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ahola T, Lapatto R, Raivio KO, Selander B, Stigson L, Jonsson B, et al. N-Acetylcysteine does not prevent bronchopulmonary dysplasia in immature infants: a randomized controlled trial. J Pediatr 2003; 143(6)713–9. doi: 10.1067/S0022-3476(03)00419-0. [DOI] [PubMed] [Google Scholar]
- 158.Sandberg K, Fellman V, Stigson L, Thiringer K, Hjalmarson O. N-Acetylcysteine administration during the first week of life does not improve lung function in extremely low birth weight infants. Biol Neonate 2004; 86(4):275–9. doi: 10.1159/000080089. [DOI] [PubMed] [Google Scholar]
- 159.Sharma D, Nkembi AS, Aubry E, Houeijeh A, Butruille L, Houfflin-Debarge V, et al. Maternal PUFA omega-3 supplementation prevents neonatal lung injuries induced by hyperoxia in newborn rats. Int J Mol Sci 2015; 16(9):22081–93. doi: 10.3390/ijms160922081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhong Y, Catheline D, Houeijeh A, Sharma D, Du L, Besengez C, et al. Maternal omega-3 PUFA supplementation prevents hyperoxia-induced pulmonary hypertension in the offspring. Am J Physiol Lung Cell Mol Physiol 2018; 315(1):L116–L32. doi: 10.1152/ajplung.00527.2017. [DOI] [PubMed] [Google Scholar]
- 161.Skouroliakou M, Konstantinou D, Agakidis C, Delikou N, Koutri K, Antoniadi M, et al. Cholestasis, bronchopulmonary dysplasia, and lipid profile in preterm infants receiving MCT/omega-3-PUFA-containing or soybean-based lipid emulsions. Nutr Clin Pract 2012; 27(6):817–24. doi: 10.1177/0884533612454547. [DOI] [PubMed] [Google Scholar]
- 162.Zhang P, Lavoie PM, Lacaze-Masmonteil T, Rhainds M, Marc I. Omega-3 long-chain polyunsaturated fatty acids for extremely preterm infants: a systematic review. Pediatrics 2014; 134(1): 120–34. doi: 10.1542/peds.2014-0459. [DOI] [PubMed] [Google Scholar]
- 163.Collins CT, Makrides M, McPhee AJ, Sullivan TR, Davis PG, Thio M, et al. Docosahexaenoic acid and bronchopulmonary dysplasia in preterm infants. N Engl J Med 2017; 376(13):1245–55. doi: 10.1056/NEJMoa1611942. [DOI] [PubMed] [Google Scholar]