Summary
Background
Plasma is integral to haemostatic resuscitation after injury, but the timing of administration remains controversial. Anticipating approval of lyophilised plasma by the US Food and Drug Administration, the US Department of Defense funded trials of prehospital plasma resuscitation. We investigated use of prehospital plasma during rapid ground rescue of patients with haemorrhagic shock before arrival at an urban level 1 trauma centre.
Methods
The Control of Major Bleeding After Trauma Trial was a pragmatic, randomised, single-centre trial done at the Denver Health Medical Center (DHMC), which houses the paramedic division for Denver city. Consecutive trauma patients in haemorrhagic shock (defined as systolic blood pressure [SBP] ≤70 mm Hg or 71–90 mm Hg plus heart rate ≥108 beats per min) were assessed for eligibility at the scene of the injury by trained paramedics. Eligible patients were randomly assigned to receive plasma or normal saline (control). Randomisation was achieved by preloading all ambulances with sealed coolers at the start of each shift. Coolers were randomly assigned to groups 1:1 in blocks of 20 according to a schedule generated by the research coordinators. If the coolers contained two units of frozen plasma, they were defrosted in the ambulance and the infusion started. If the coolers contained a dummy load of frozen water, this indicated allocation to the control group and saline was infused. The primary endpoint was mortality within 28 days of injury. Analyses were done in the as-treated population and by intention to treat. This trial is registered with ClinicalTrials.gov, number NCT01838863.
Findings
From April 1, 2014, to March 31, 2017, paramedics randomly assigned 144 patients to study groups. The as-treated analysis included 125 eligible patients, 65 received plasma and 60 received saline. Median age was 33 years (IQR 25–47) and median New Injury Severity Score was 27 (10–38). 70 (56%) patients required blood transfusions within 6 h of injury. The groups were similar at baseline and had similar transport times (plasma group median 19 min [IQR 16–23] vs control 16 min [14–22]). The groups did not differ in mortality at 28 days (15% in the plasma group vs 10% in the control group, p=0∙37). In the intention-to-treat analysis, we saw no significant differences between the groups in safety outcomes and adverse events. Due to the consistent lack of differences in the analyses, the study was stopped for futility after 144 of 150 planned enrolments.
Interpretation
During rapid ground rescue to an urban level 1 trauma centre, use of prehospital plasma was not associated with survival benefit. Blood products might be beneficial in settings with longer transport times, but the financial burden would not be justified in an urban environment with short distances to mature trauma centres.
Introduction
For more than 50 years, impaired coagulation has been associated with severe injury, and crystalloid resuscitation has been the standard.1 In civilian settings, the first preemptive plasma resuscitation after injury was proposed in the late 1970s in Denver, CO, USA.2 The rationale was that coagulopathy would be lessened and progression to the “bloody vicious cycle”, in which coagulopathy coupled with acidosis and hypothermia (called the lethal triad) result in uncontrolled bleeding, would be prevented.3 Benefits of early plasma resuscitation, however, were not highlighted until the military reported increased survival with high ratios of plasma to red blood cells in US combat support hospitals in Iraq in 2003 and 2005.4 This experience prompted several retrospective civilian studies5,6 followed by a multicentre prospective study that seemed to indicate a survival benefit with early plasma admin istration.7 The retrospective studies, though, were plagued by survivor bias (ie, patients had to survive long enough to receive plasma). Indeed randomised clinical trials have shown no survival benefit.8,9 A 2016 systematic review concluded that, although transfusion of blood products before reaching hospital is a plausible therapeutic approach, the evidence at the time was of poor quality, did not show outcome improve ments, and recommended assessment in randomised controlled trials.10
Laboratory data suggest that plasma has benefits beyond the coagulation system, including restoration of endothelial glycocalyx11 and reduction of intestinal permeability,12 metabolic derangements,13 and hyper-fibrinolysis.14 Prehospital plasma infusion has been shown to be feasible during helicopter transport,15 but the logistics of storage and thawing in fast ground transport have been challenging.1,16 Anticipation of approval of the lyophilised plasma by the US Food and Drug Administration (FDA) prompted the US Department of Defense to fund several randomised controlled trials across the USA to produce robust evidence on early plasma resuscitation. One, the Control of Major Bleeding After Trauma Trial (COMBAT) assessed use of prehospital plasma during short ground transportation to an urban trauma centre and is reported here. Another trial, the multicentre Prehospital Air Medical Plasma Trial (NCT01818427) is testing prehospital plasma during helicopter transport for the treatment of haemorrhagic shock.
In the COMBAT trial we investigated whether plasma-first resuscitation affected trauma-induced coagulopathy and adverse outcomes after injury in patients with haemorrhagic shock. We tested the hypothesis that mortality would be lower among patients who received plasma before arrival at a level 1 trauma facility than among those who received standard care with normal saline.
Methods
Study design and participants
COMBAT was a pragmatic, randomised, placebo-controlled, clinical trial based at the Denver Health Medical Center (DHMC), Denver, CO, USA, which is a level 1 trauma centre (tertiary-care facility capable of providing total injury care that meets the minimum requirement for annual volume of severely injured patients, and has in-house trauma surgeons available 24 h and prompt availability of specialists [eg, in orthopaedic surgery, neuro surgery, anaesthesiology, emergency medicine, radiol ogy, internal medicine, plastic surgery, oromaxilo-facial care], referral resource for nearby regions, leadership in prevention, public education, continuing education of the trauma teams, and quality assessment, teaching, and research programmes). The centre is verified by the American College of Surgeons and state certified by Colorado, and affiliated with the University of Colorado Denver. The study design has been described previously.16 Eligible patients were injured adults (age >18 years), with systolic blood pressure (SBP) 70 mm Hg or lower or 71–90 mm Hg and heart rate 108 beats per min thought to be due to acute blood loss. These criteria were based on a similarly structured trial.17 SBP less than 90 mm Hg is widely used to define trauma severity at the scene of an injury because it is strongly predictive for injury severity.18 Exclusion criteria were prisoner status, known pregnancy, isolated gunshot to the head, asystole or cardiopulmonary resuscitation before randomisation, known objection to blood products, opt-out bracelets or necklaces, or family objection to the patient’s enrolment.
Owing to the pragmatic character of the trial and rapid enrolment and randomisation, the study was exempted from needing written informed consent by the local insti tutional review board. The study was done according to FDA Investigational New Drug regulations (application 15216) and monitored by the Department of Defense Human Research Protection Office. The community consultation and public disclosure processes have been described previously,19 and the full protocol for the study is available upon request. Patients or next of kin were informed about enrolment at the earliest opportunity and could discontinue participation at any time. An independent data and safety monitoring board (DSMB) oversaw the trial and reviewed all suspected adverse events and interim analyses.
Randomisation and masking
The 33 ambulances based at DHMC were loaded with prepackaged coolers at the start of each shift and all were fitted with equipment for quick thawing of plasma. Plasma and dummy (frozen water) loads for the coolers were randomly assigned 1:1 in blocks of 20 according to a schedule generated by the research coordinators. These were delivered to the DHMC Paramedic Division in sealed aluminium cassettes by study staff not involved in enrolment or data analysis, to mask allocation. Eligibility was assessed at the injury scene by the responding paramedics. Once determined, a field blood sample was drawn before any treatment was given. Assignments were determined by the contents of the coolers, which contained either two units of AB plasma (universal donor, ~250 mL each) or frozen water in a plastic bag. If the cooler contained plasma, it was thawed and admini stered immediately. If the cooler contained water, paramedics gave patients normal saline (0·9%) per the standard of care. We used frozen water to avoid the burden of defrosting and the risk of exposing patients to cold saline. Further masking of the care team by making the appearance of plasma and saline similar was not possible because the FDA does not permit colorants in intravenous solutions.16 We had initially planned that patients would receive similar volumes of plasma and saline (<800 mL) to maintain equipoise in total prehospital resuscitation volume. However, this approach was not feasible and did not meet the standard of care. Infusion of normal saline, therefore, was based on haemodynamic need. All other prehospital treatments in the plasma and control groups were given per standard protocols. All hospital treatments were guided by institution resuscitation protocols.
Procedures
All AB fresh-frozen plasma units were drawn via plasmapheresis and frozen within 24 h. We used plasma frozen within 24 h of collection in this study because of its wide clinical availability in US trauma hospitals. It has slightly lower concentrations of coagulation factors than plasma fresh frozen within 8 h of collection, but the two products are generally used interchangeably. In line with the 2014 American Association of Blood Banks standards for blood banks and transfusion services,20 donors were men, never-pregnant women, or women who if ever pregnant had tested negative for HLA antibodies. All transfusions adhered to a strict thromboelastography-based protocol.21 Resuscitation, surgical interventions, and outcomes were reviewed by an in-house review panel and the DSMB.
All patients were monitored closely for any clinical changes potentially associated with transfusions. Febrile and hypotension transfusion reactions are difficult to identify in this group of patients because many are hypothermic and rewarmed in areas such as trauma bays, operating rooms, and the surgical intensive care unit, while being resuscitated from haemorrhage-related hypotension.
Plasma was stored and defrosted with our innovative field plasma system.16 Briefly, a contained-circulation, plasma warming device (Plasmatherm, Barkey, Leopolds hoehe, Germany) that was adapted for vehicular use defrosted plasma stored in special bags in less than 3 min. The bags were designed to increase the ratio of surface area to volume and were frozen under com pression to produce thin, flat units. These units were extremely fragile and required transport in padded, rigid metal canisters. At the beginning of each shift, paramedics loaded one of the newly designed insulated coolers, which were capable of storing the plasma units at the mandated temperature of less than 18°C for at least 28 h. The details of these complex systems created and implemented for COMBAT have been published.16
Blood sampling and coagulation tests
Blood samples were collected in anticoagulated tubes containing sodium citrate and lithium heparin, at the scene of the injury (before any treatment) and in hospital, immediately on arrival and at 2, 4, 6, 12, and 24 h after injury. Rapid thromboelastography was done with a TEG 5000 Thrombelastograph (Haemonetics, Stoughton, MA, USA) on samples collected in anticoagulated tubes containing sodium citrate and lithium heparin, activated with tissue factor and kaolin immediately before testing. A team of on-site trained professional research assistants was available at all times to perform thromboelastography within min utes of sample collection. Thromboelastography indices obtained were G index, activated clotting time (ACT), angle, maximum amplitude (MA), and percentage of lysis 30 min after MA (LY30).
Outcomes and variables
The primary outcome was mortality within 28 days after injury. We assessed a composite secondary outcome of multiple organ failure (MOF, according to the Denver MOF Score22), death, or both, by day 28 (deemed present if the patient died, developed MOF within 28 days of injury, or both, and deemed absent if the patient survived and did not develop MOF), indicators of trauma-induced coagulopathy (thromboelastography G index and international normalised ratio [INR]), and shock (base deficit and lactate concentration). For patients discharged before day 28, professional research assistants verified outcomes by contacting the patient by telephone.
Exploratory outcomes included time from injury to need for first red blood cell transfusion (defined as the time to when the attending trauma surgeon judged the patient met the institution’s clinical and laboratory criteria for transfusion of red blood cells) as this outcome has high military importance. Other exploratory outcomes were thromboelastography indices, number of ventilation-free days, number of intensive-care-free days, and development of MOF. Safety-related outcomes included acute lung injury (defined with the Berlin definition23 as arterial partial pressure of oxygen/fractional concentration of oxygen in inspired air ≤300 mm Hg × 0∙83 to adjust for the altitude of Denver >48 h after injury) within 28 days and possible transfusion-related acute lung injury (ie, partial pressure of oxygen/fractional concentration of oxygen in inspired air ≤300 mm Hg within 6 h of blood transfusions without other attributable causes). Of note, among these injured patients, even if acute lung injury occurred temporally close to blood transfusion, it could have been due to several other potential causes, (eg, haemorrhage, trauma, shock, ischaemia and reperfusion, and massive trans fusion24), which made a firm diagnosis of transfusion-related acute lung injury impossible. Any cases of suspected transfusion-related acute lung injury were adjudicated by the blood bank and the treatment team.
A binary variable was used to denote control or plasma group assignment. Comorbidities were defined as in the Acute Physiology and Chronic Health Evaluation II.25 Injury severity in specific body regions was defined with the Abbreviated Injury Scale. Overall severity was measured by the New Injury Severity Score, which is the sum of the squares of the three highest Abbreviated Injury Scale scores, regardless of body region.26
Statistical analysis
Our power calculations accounted for two interim analyses and a final analysis, equally distributed throughout the trial. To estimate sample size, we assumed that the control group would have a mean INR of 1∙5 (SD 1∙0) and a mean G index on thromboelastography of 4∙9 dynes/cm2 (SD 2∙3), based on historical data. We calculated in PASS (version 14) that 150 patients (75 in each group) would provide 80% power to detect minimum differences between groups of 0∙5 in INR and 1∙2 dynes/cm2 in G index with 20% attrition. With these parameters, and assuming 25% mortality, per our institution’s historical data21 and similar trials,27 the study was powered to detect 19 percentage points difference in mortality (ie, from 25% to 6%).
Categorical variables were expressed as frequency (%) and compared with the χ2 test or Fisher’s exact test. Continuous variables were reported as median (IQR) or mean (SD) and compared with Wilcoxon’s rank-sum test. We created Kaplan-Meier curves to compare survival, with Wilcoxon’s test (privileges early differences) and the log-rank test (privileges late differences). Statistical test assumpt ions were carefully assessed, and none was significantly violated. We assessed effect size by calculating rel ative risks with 95% CIs for categorical variables and median difference (Hodges–Lehmann estimation) for continuous variables.
We assessed the effectiveness of randomisation by comparing demographic characteristics, injury mechanisms, New Injury Severity Score, proportion of patients with traumatic brain injury (defined as Abbreviated Injury Scale score for head injury ≥3), physiological derangement (systolic blood pressure, heart rate), and coagulation indicators (thrombo elastography values and INR) at the scene of injury. In accordance with CONSORT guidelines, no p values were reported for baseline comparisons.
We did an intention-to-treat (ITT) safety assessment to allow unbiased assessment of the risk associated with randomisation assignment, and an as-treated analysis to assess the effects of the intervention on the proposed outcomes. The ITT safety analysis included all patients deemed eligible by emergency medical response personnel at the scene of the injury and for whom a cooler was opened, regardless of which product they received, whether they were later deter mined to be ineligible due to non-traumatic injury or study exclusion criteria, or whether they refused to continue to participate when approached by the research team after intervention. The institutional review board allowed us limited access to information on safety outcomes (death and infectious and non-infectious complications) for patients who withdrew from the study. The as-treated analysis included eligible patients for whom we obtained consent to continue participation and had full access to data, and who were assessed by the treatment they actually received. Pragmatic trials, especially those in emergency care settings, rely heavily on this type of analysis and, therefore, we present these results before those by ITT.
We did an additional unplanned safety analysis (with significance set at the same level as the other analyses) to investigate further mortality differences between groups. This safety analysis included all randomised patients grouped by treatment received (as treated) in addition to the ITT safety analyses described above.
Analyses were done with SAS version 9.4. All tests were two tailed, and overall trial significance was set at p<0·05 for all outcomes (primary, secondary, and exploratory). Because interim analyses might inflate the overall trial significance, to ensure the overall level of p<0·05 was maintained, we used the O’Brien-Fleming spending function to adjust the significance level of each interim analysis and the final analysis so that in the latter significance was set at p<0∙0379 for all outcomes. For safety-related outcomes, the DSMB used p values only as guidance but was not constrained by them, being free to determine potential for harm based on other criteria. Details are available on request.
We analysed temporal trends in outcomes collected at sequential times after injury with mixed linear models (continuous variables) to account for the repeated correlated data. These models accommodate missing values in the temporal sequences without losing other observations contributed by the individual. Because values were not missing at random (ie, they were missed because of high and low disease severity), multiple imputation approaches were unsuitable. Therefore, we applied methods that used all observations contributed by each patient and did not just assess those with complete data. An interaction term between time and study group tested the hypothesis that assignment to a particular study group modified the temporal trends for endpoints in the first 6 h after injury. The thromboelastography values ACT, angle, MA, and G index, all coagulation factors and INR did not deviate sub stantially from normal distribution and we analysed them with the linear mixed models. LY30 was significantly skewed and, therefore, we used a Box-Cox power transformation (λ=0·25), which succeeded in approximating normality. This trial is registered with ClinicalTrials.gov, number NCT01838863.
Role of the funding source
The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
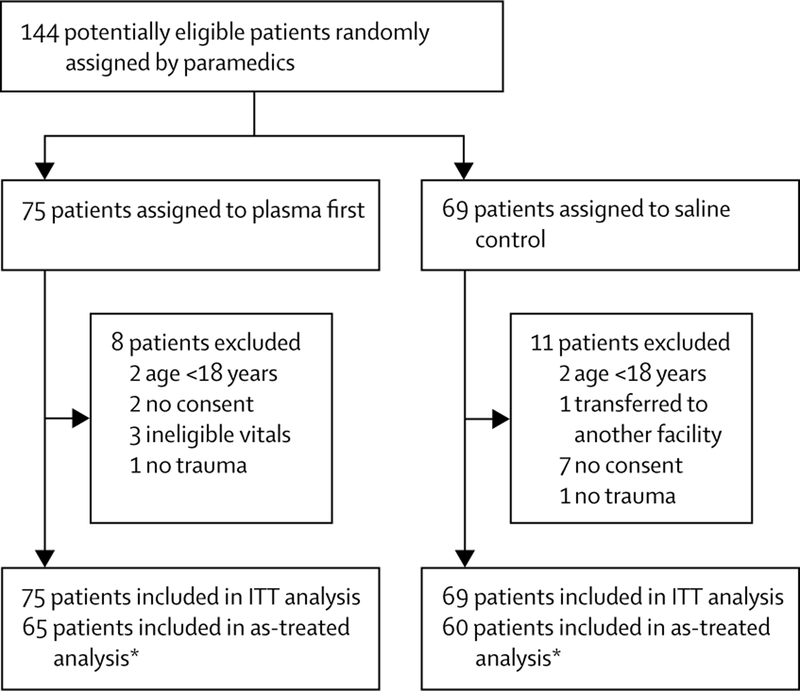
From April 1, 2014, to March 31, 2017, 144 patients were randomly assigned to the study groups by paramedics (figure). The as-treated analyses involved 125 patients (65 in the plasma group and 60 in the control group). The main reasons for exclusion were age younger than 18 years and no consent (figure). The median time from injury to arrival at hospital was 28 min (IQR 22–34) for the plasma group and 24 min (19–31) for the control group, and for transport time (scene to hospital arrival) was 19 min (16–23) for patients in the plasma group and 16 min (14–22) among controls (p=0∙04). Time from injury to transfusion of first plasma unit was 59 min in the control group (IQR 40–115) and 24 min (20–31) in the plasma group (p<0·0001).
Randomisation resulted in two similar groups, with a slight predominance of shock and hyperfibrinolysis at the scene of injury in the plasma group (table 1). With the exception of coagulation factor VII, concentrations of coagulation factors in the plasma group were slightly lower than in the control group. Patients were gener ally young, just over half had severe injuries (53∙0% New Injury Severity Score >25) and many patients were in shock, indicated by low systolic blood pressure (62% ≤70 mm Hg; table 1). 62 (50%) patients had blunt injuries (23 [37%] from motor vehicle crashes, 17 (27%) from automobile-pedestrian accidents, 10 [16%] from motor cycle crashes, five [8%] from falls, and seven [11%] from other causes). 28 (22%) of 125 patients had traumatic brain injuries and 16 (13%) died.
Table 1:
Baseline characteristics
| Plasma group (n=65) | Control group (n=60) | |
|---|---|---|
| Demographics | ||
| Age (years) | 33·0 (25·0–51·0) | 32·5 (25·5–42·0) |
| Body-mass index (kg/m2) | 27·1 (23·9–30·5) | 26·1(23·2–29·5) |
| Sex | ||
| Men | 52 (80%) | 51 (85%) |
| Women | 13 (20%) | 9 (15%) |
| Clinical characteristics | ||
| Comorbidities | 10 (15%) | 8 (13%) |
| Blunt injury | 30 (46%) | 32 (53%) |
| New injury severity score | 27·0 (10·0–41·0) | 27·0 (11·5–36·0) |
| Score >25 | 33 (51%) | 34 (57%) |
| Abbreviated injury scale maximum score | ||
| Head and neck | 0 (0–2) | 0 (0–2·5) |
| Traumatic brain injury* | 13 (20%) | 15 (25%) |
| Chest | 1 (0–3) | 3 (0–3) |
| Abdomen and pelvis | 0 (0–3) | 0 (0–3) |
| Extremities | 1 (0–3) | 0 (0–2) |
| Physiology and shock (at scene of injury) | ||
| Worst heart rate (beats per min) | 110 (98–120) | 112 (100–120) |
| Lowest SBP (mm Hg) | 64 (50–80) | 70 (55–80) |
| SBP≤70mmHg | 44 (68%) | 33 (55%) |
| Lowest temperature (°C) | 36·0 (34·8–36·6) | 36·0 (35·1–37·0) |
| Lowest Glasgow coma scale score | 14 (7–15) | 14 (8–15) |
| Haemoglobin concentration (g/dL) |
15·1 (13·5–15·7) | 14·2 (13·1–16·0) |
| Platelet count (× 103 per μL) | 301 (250–357) | 274 (218–336) |
| Coagulation (at scene of injury) | ||
| INR | 1·1 (1·0–1·2) | 1·1 (1·0–1·1) |
| INR >1.3 | 2/36 (6%) | 2/29 (7%) |
| Rapid thromboelastography | ||
| G (dynes/cm2) | 8·2 (6·8–10·6) | 8·7 (6·9–10·2) |
| Activated clotting time (s) | 121 (113–128) | 121 (113–128) |
| Maximum amplitude (mm) | 62 (57·5–68·0) | 63·5 (58·0–67·0) |
| Angle (°) | 70·6 (66·9–75·6) | 70·6 (66·1–74·3) |
| Lysis | ||
| LY30(%) | 2·2 (1·0–3·7) | 1·8 (0·9–3·7) |
| Hypenibrinolysis (LY30 >3·0%) | 15/43 (35%) | 11/37 (30%) |
| Physiological lysis (LY30 0·9–3·0%) | 20/43 (46%) | 17/37 (46%) |
| Lysis shutdown (Ly30 <0·9%) | 8/43 (19%) | 9/37 (24%) |
| Coagulation factor (% activity) | ||
| Fibrinogen (mg/dL) | 253 (224–310) | 278 (250–331) |
| II | 95·0 (85·0–105·0) | 98·0 (85·0–105·0) |
| V | 85·0 (73·0–99·0) | 91·5 (77·0–100·0) |
| VII | 101·5 (84·0–127·0) | 89·5 (80·0–114·0) |
| VIII | 396·8 (273·0–476·0) | 411·8 (353·2–464·6) |
| IX | 157·0 (128·0–180·0) | 160·5 (148·0–174·0) |
| XI | 126·5 (106·0–153·0) | 141·5 (100·0–168·0) |
| XIII† | None abnormal | None abnormal |
Data are median (IQR) or n (%). SBP=systolic blood pressure. INR=international normalised ratio. LY30=percentage of lysis 30 min after maximum amplitude.
Abbreviated injury scale for head ≥3.
Measured with qualitative assay.
All 65 patients in the plasma group received two full plasma units: 21 (32%) received two units during transport; 24 (37%) received one unit during transport and the second unit in the emergency department; and 20 (31%) started the first plasma unit during transport but it was completed and followed by the second unit in the emergency department. Two patients assigned plasma received saline incorrectly because paramedics mistook the contents of the metal canister for the dummy load, which was discovered after arrival at hospital. These patients were included in the control group in the as-treated analyses. More patients in the plasma group died than in the control group, but not significantly so (table 2).
Table 2:
Outcomes
| Plasma group (n=65) | Control group (n=60) | Effect size (95% Cl)* | p value | |
|---|---|---|---|---|
| Clinical outcome | ||||
| Mortality at 28 days† | 10 (15%) | 6 (10%) | 1·54 (0·60 to 3·98) | 0·37 |
| Mortality at 24 h | 8 (12%) | 6 (10%) | 1·23 (0·45 to 3·34) | 0·68 |
| Acute lung injury within 28 days | 28 (43%) | 30 (50%) | 0·86 (0·59 to 1·26) | 0·44 |
| Multiple organ failure within 28 days (Denver score >3) | 4 (6%) | 1 (2%) | 3·69 (0·42 to 32·11) | 0·37 |
| Composite outcome (multiple organ failure or death) at 28 days‡ | 14 (21%) | 7 (12%) | 1·85 (0·80 to 4·26) | 0·14 |
| Ventilator-free days | 26 (11 to 28) | 26 (18 to 28) | 0 (−1·00 to 0) | 0·35 |
| Intensive-care-free days | 23 (7 to 26) | 24 (17 to 26) | 0 (−3·00 to 1·00) | 0·49 |
| Physiology and shock | ||||
| SBP on arrival (mm Hg) | 96 (80 to 110) | 90 (72 to 111) | 5·00 (−6·00 to 15·00) | 0·38 |
| Heart rate on arrival (bpm) | 105 (76 to 124) | 111 (92 to 128) | −6·00 (−17·00 to 4·00) | 0·23 |
| Haemoglobin concentration on arrival (g/dL) |
12·6 (11·3 to 14·7) | 13·5 (11·9 to 14·7) | −0·30 (−1·10 to 0·50) | 0·50 |
| Lowest haemoglobin concentration in 1–6 h (g/dL) | 11·3 (9·6 to 12·6) | 11·0 (9·1 to 12·8) | 0·20 (−0·70 to 1·00) | 0·67 |
| Haemoglobin concentration <70 g/L in 1–6 h | 3 (5%) | 2 (3%) | 0·41 (0·24 to 8·13) | 1·00 |
| Base deficit on arrival (mEq/L)‡ | 9·0 (5·5 to 13·0) | 8·8 (6·0 to 13·0) | 0 (−2·70 to 2·00) | 0·80 |
| Base deficit >10 mEq/L | 21/51 (41%) | 22/50 (44%) | 0·94 (0·59–1·47) | 0·77 |
| Lactic acid concentration on arrival (mg/dL)‡ | 5·5 (3·9 to 8·5) | 4·9 (3·2 to 7·0) | 0·60 (−0·60 to 1·80) | 0·30 |
| Coagulation (on arrival at hospital) | ||||
| INRon arriva† | 1·27 (1·11 to 1·40) | 1·15 (1·08 to 1·29) | 0·60 (−001 to 0·14) | 0·10 |
| INR>1·3 | 28/63 (44%) | 14/58 (24%) | 1·84 (1·08 to 3·14) | 0·02 |
| Rapid thromboelastography | ||||
| G (dynes/cm2)‡ | 7·7 (6·2 to 8·9) | 7·1 (5·4 to 9·7) | 0·30 (−0·90 to 1·40) | 0·66 |
| Activated clotting time (s) | 128 (113 to 136) | 121 (113 to 136) | 0 (−7·00 to 8·00) | 0·76 |
| Maximum amplitude (mm) | 60·5 (55·5 to 64·0) | 58·5 (52·0 to 66·0) | 100 (−2·50 to 4·50) | 0·67 |
| Angle (°) | 70·9 (66·1 to 76·1) | 69·3 (63·2 to 74·4) | 2·20 (−0·80 to 5·40) | 0·16 |
| LY30(%) | 1·3 (0·3 to 2·6) | 1·6 (0·7 to 3·1) | −0·20 (−0·90 to 0·30) | 0·32 |
| Hyperfibrinolysis (LY30 >3·0%) | 14/56 (23%) | 13/51 (25%) | 0·91 (0·47 to 1·78) | 0·78 |
| Physiological lysis (LY30 0·9–3·0%) | 25/56 (45%) | 23/51 (45%) | 0·99 (0·65 to 1·51) | 0·96 |
| Lysis shutdown (LY30 <0·9%) | 18/56 (32%) | 15/51 (29%) | 1·09 (0·62 to 1·93) | 0·76 |
| Coagulation factor on arrival at hospital(% activity) | ||||
| Fibrinogen on arrival (mg/dL) | 195·0 (157·0 to 275·0) | 222·0 (154·5 to 282·0) | −10·00 (−30·00 to 48·00) | 0·68 |
| II | 71·0 (57·0 to 88·0) | 79·0 (65·0 to 92·0) | −6·00 (−15·00 to 3·00) | 0·14 |
| V | 64·0 (41·0 to 83·0) | 69·0 (52·0 to 91·0) | −7·00 (−20·00 to 5·00 | 0·32 |
| VII | 72·0 (56·0 to 94·0) | 74·0 (52·0 to 94·0) | 3·00 (−8·00 to 13·00) | 0·61 |
| VIII | 283·4 (168·4 to 434·2) | 355·2 (279·0 to 462·6) | −71·70 (−148·00 to 2·00) | 0·06 |
| IX | 121·0 (87·0 to 142·0) | 135·0 (99·0 to 159·0) | −11·00 (−30·00 to 2·00 | 0·36 |
| XI | 81·0 (58·0 to 127·0) | 109·0 (72·0 to 135·0) | −14·00 (−35·00 to 8·00) | 0·21 |
| XIII§ | 0/47 | 2/41 (5%) | 0·18 (0·01 to 3·54) | 0·21 |
| Transfusions or fluids after injury | ||||
| Red blood cell units per 24 h | 2·0 (0 to 9·0) | 1·5 (0 to 9·0) | 0 (−1·0 to 0) | 0·89 |
| Red blood cell units needed within 24 h | 36 (55%) | 35 (58%) | 0·95 (0·70 to 1·29) | 0·74 |
| Massive transfusion (>10 units red blood cells) or death within 6 h | 15 (23%) | 12 (20%) | 115 (0·59 to 2·26) | 0·68 |
| Time from injury to first red blood cell unit (mm)‡ | 46·5 (32·0 to 55·5) | 37·0 (24·0 to 46·0) | 8·00 (0 to 16·00) | 0·05 |
| Time from emergency admission to first red blood cell unit (min)‡ | 16 (7 to 28) | 10 (4 to 18) | 5·0 (0 to 11·0) | 0·05 |
| Plasma units needed per 24 h|| | 0 (0 to 4·0) | 0 (0 to 3·0) | 0 (0 to 0) | 0·98 |
| Plasma needed within 24 h¶ | 29 (45%) | 26 (43%) | 1·03 (0·69 to 1·53) | 0·88 |
| Platelet units per 24 h | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) | 0·31 |
| Platelets needed within 24 h | 15 (23%) | 11 (18%) | 1·26 (0·63 to 2·52) | 0·51 |
| Cryoprecipitate units per 24 h | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) | 0·30 |
| Cryoprecipitate needed within 24 h | 8 (12%) | 4 (7%) | 1·85 (0·59 to 5·82) | 0·28 |
| Tranexamicacid needed within 6 h | 6 (9%) | 8 (13%) | 0·69 (0·26 to 1·88) | 0·47 |
| Factor VII per needed within 24 h | 1(2%) | 0 | 0·69 (0·30 to 1·56) | 1·00 |
| Normal saline in used the field (mL) | 150 (0 to 300) | 250 (100 to 500) | −100 (−200 to 0) | 0·02 |
Data are median (IQR) or n (%). Significance was set at p<0∙0379 in the final analyss, per the O’Brien-Fleming spending function, to maintain overall study significance at p<0∙05. SBP=systolic blood pressure. INR=international normalised ratio. LY30=percentage lysis 30 min after maximum amplitude. bpm=beats per minute.
For cells with 0 values, the relative risk was estimated by adding 0∙5 to each cell.
Primary endpoint.
Secondary endpoint.
Measured with a qualitative assay.
Excludes plasma given in field.
After 144 of 150 planned patients had been enrolled, the DSMB, the institutional review board, and FDA approved termination of the study for futility because outcomes had not differed in any of the interim analyses, indicating that no difference should be anticipated (as-treated: first analysis, plasma one death [5%] vs control two deaths [9%], p=1·00; second analysis, six [13%] vs five [12%], p=1∙00; and third analysis, ten [15%] vs six [10%], p=0∙37; ITT first analysis [n=144]: plasma two deaths [9%] vs control two deaths [7%], p=1∙00; second analysis, eight [16%] vs five [10%], p=0·55; and third analysis 12 [16%] vs six [9%], p=0∙19).
Coagulation factors, transfusion requirements and safety outcomes (acute lung injury, MOF, and other complications) were similar in the two groups, as were the median numbers of ventilation-free and intensive-care-free days (table 2). Of note, significantly more patients in the plasma group had INR values greater than 1·3 than controls (table 2). The time from injury to first red blood cell transfusion was longer in the plasma group than in the control, without increased mortality, although this difference was not significant when we compared Kaplan-Meier curves (Wilcoxon’s test p=0∙37, log-rank test p=0∙76). Control patients received, by design, more saline than plasma patients, but the volume of prehospital infusion was small (median 250 mL) for both groups probably because of short transport times.
We detected no significant interactions between treatment groups and temporal trends for thromboelastography ACT, angle, G index, MA, or LY30 or for INR and coagulation factors (data not shown).
The ITT safety analysis included all 144 randomised patients (figure). Mortality did not differ significantly between groups (plasma 12 (16%) vs control six (9%), p=0∙19). 23 infections (nine pneumonia, four pseudo-membranous colitis, three surgical-site infections, and seven others) were diagnosed in 14 (21%) patients in the plasma group and 20 (seven pneumonia, six urinary tract infections, two surgical-site infections, and five others) were diagnosed in 13 (19%) controls (p=0∙98). Thrombotic events did not differ significantly (p=0∙53): four patients in the plasma group had deep venous thrombosis (n=1), pulmonary embolisms (n=2), or pulmonary infarction (n=1) and two control patients developed deep venous thrombosis. Acute lung injury was diagnosed in 29 (39%) plasma patients compared with 29 (42%) control patients (p=0∙68). 21 (39%) control patients and 20 (27%) plasma recipients developed acute lung injury within 6 h of receiving a blood product (p=0∙62), but none was deemed to be attributable to transfusions by the treatment team and blood bank. No patients developed transfusion-related urticarial rashes or signs of anaphylaxis.
A second ITT safety analysis was done in 144 randomised patients (73 who received plasma and 69 originally assigned to saline plus the two patients who incorrectly received saline). 12 (16%) patients in the plasma group died compared with six (9%) in the control group (p=0·15). Secondary and exploratory outcomes and infectious and non-infectious complications in these groups did not differ (data not shown).
Discussion
In this randomised controlled trial of plasma resuscitation during ground transport of patients with presumed haemorrhagic shock, the intervention yielded no survival benefit compared with the standard of care. The logistical challenge of defrosting and trans-fusing plasma before arrival at hospital only delayed transportation by a median of 3 min. Of note, prehospital plasma was not associated with increased incidence of adverse events.
An important finding from this study was the rarity of coagulopathy before arrival at hospital based on INR and thromboelastography. Similarly to our study, previous studies have identified high INR within 10 min of hospital arrival in 25% of severely injured patients.28 We found that INR increased from the scene of injury to hospital arrival, paradoxically even more in the plasma group than in the control group. This finding was expected because plasma does not help to reduce INR to normal levels. Indeed, the INR values of plasma units can be greater than 1∙3 and might have minimal effect in correcting mildly raised INR values in recipients.29
The modern resuscitation approach for haemorrhagic shock involves limiting crystalloids before arrival at hospital and starting early haemostatic resuscitation with blood products at the time of arrival. Evidence generally supports early plasma transfusion after injury,6,7,9,30 but the optimum ratio of blood products is undefined9 and how early after injury plasma resuscitation is beneficial re mains unanswered. In most of our patients, plasma transfusions were started within 1 h of injury. Prehospital administration of plasma reduced this time to 30 min but did not improve clinical outcomes. In a study of helicopter transport, prehospital administration of plasma also showed no survival benefits, perhaps because of the small sample size.15 The effort needed to thaw and transfuse plasma in urban areas with short transport times to trauma centres might outweigh any benefits.
This study has some limitations. The scene of injury is often chaotic and sometimes perilous. Therefore, we adopted easily recognisable inclusion and exclusion criteria to assess eligibility. The inclusion criteria were based on historical trends at our institution and the Resuscitation Outcomes Consortium trials of traumatic hypovolaemic shock.27 Those trials and our study had almost identical rates of blood transfusions (60%). Thus, some of the hypotension seen before arrival at hospital might not have been due to haemorrhage. The difficulty in promptly and accurately identifying the patients at risk of needing transfusions might be a reason for the lack of effect we saw with plasma-first resuscitation. We are exploring alternatives to hypotension and tachycardia to identify patients at risk of blood transfusion. A challenge for future similar trials in prehospital settings will be to improve identi fication of the target population without burdening emergency care personnel or increasing transport times. Additionally, assignment to the control group might have freed paramedics from defrosting procedures, allowing them to focus on other tasks that could have resulted in improved outcomes in these patients. Another limitation is that it was done in an environment where plasma was available immediately upon hospital arrival, which limits the generalisability of our findings to rural locations, austere environments, or developing countries. One of the strengths of this study is its pragmatic nature; it was designed to minimise intrusion of study procedures into the health care provided. Thus, we did not introduce any new methods to diagnose or screen for haemorrhage, especially because methods available for point-of-care assessment in the USA do not yet have suitable precision.
Our findings indicate that plasma does not improve outcomes after injury when given within 30 min during rapid ground transportation to mature, level 1 trauma centres. Of note, though, no increases in adverse events were seen. Use of plasma first might have beneficial effects in austere environments with longer transport times, and further study is warranted. The advent of lyophilised plasma, with easy storage and reconstitution, will facilitate the logistics of such studies.
Figure: Trial profile.
ITT=intention-to-treat. *Two patients originally meant to receive plasma were incorrectly treated with saline and were analysed in the the saline group.
Research in context
Evidence before this study
Despite advances in civilian and military transport after trauma, survival of patients with severe bleeding has changed little over the past 50 years. One of the major causes of death is uncontrolled bleeding associated with trauma-induced coagulopathy, often attributed to depletion in clotting factors, uncontrollable fibrinolysis, or both. Treatment of trauma-induced coagulopathy with early plasma became widely used after the military 2003 and 2005 experiences in Iraq. Although some evidence supports plasma early in resuscitation after injury, the timing is controversial. Retrospective studies have shown substantial survivor bias (ie, patients had to survive long enough to receive plasma), and randomised controlled trials have found no survival benefit. In anticipation of US Food and Drug Administration (FDA) approval of lyophilised plasma, the US Department of Defense funded several trials in level 1 trauma centres across the USA to test plasma infusion in the prehospital phase of treatment. One, the COMBAT trial, was done in the context of rapid ground transportation in an urban area, and another, the multicentre PAMPer trial, assessed the use of prehospital plasma in the context of longer helicopter transportation. The COMBAT trial is reported here. In preparation for this trial, we searched MEDLINE, Embase, CENTRAL, WHO International Clinical Trials Registry, and ClinicalTrials.gov, without language restrictions, from inception until March 1, 2014. Searches were repeated during the trial, and results were compared with updated evidence at each interim analysis. The retrieved information was enriched with personal discussions between experts at international and regional scientific meetings and with the US Department of Defense and the FDA.
Added value of this study
COMBAT is to our knowledge the first rigorous randomised controlled trial testing prehospital plasma for control of haemorrhage after injury in the context of rapid ground transport to a mature urban trauma centre. Our findings showed no survival benefit when plasma was given within 30 min of injury, starting in the ambulance. In addition, trauma-induced coagulopathy and complications did not differ between groups. The short time to mechanical haemorrhage control and the immediate availability of plasma in the hospital might explain the absence of benefit with this approach.
Implications of all the available evidence
Complex systems to maximise use and minimise wastage of AB plasma units through thawing only what was needed for transfusion were developed for the COMBAT trial, and might be helpful for studies in different settings. A beneficial effect of prehospital plasma might manifest in austere environments with long transportation times. The ease of storage and reconstitution of lyophilised plasma might facilitate the logistics of such studies. At this time, however, there is no evidence to justify the risks, use of precious blood components, and the financial burden associated with prehospital plasma delivery in an urban environment where high-level trauma centres are close to the site of injury.
Acknowledgments
We thank all personnel in the Denver Health Paramedics Division for their dedication to this study and the wellbeing of their patients. We also thank the professional research assistants and the research team at the Trauma Research Center of the University of Colorado Denver for their dedication in the management and implementation of the study. We thank Victoria Bress for managing the trauma research centre operations. This work was supported by the US Department of Defense (USAMRAA, W81XWH-12-2-0028). Supplies for the viscoelastic tests were supplied at a discount by Haemonetics and Instrumentation Laboratory. Data storage was supported by the National Institutes of Health (NIH) National Center for Research Resources via the Colorado Clinical Translational Science Institute (UL1 RR025780). HBM, MPC, and TC were supported by grants from the NIH National Institute of General Medical Sciences(T32 GM008315 and P50 GM049222). The contents of this Article are the authors’ sole responsibility and do not necessarily represent official views of the US Department of Defense or NIH.
Funding US Department of Defense.
Footnotes
Declaration of interests
We declare no competing interests.
References
- 1.Moore EE, Chin TL, Chapman MC, et al. Plasma first in the field for postinjury hemorrhagic shock. Shock 2014; 41 (suppl 1): 35–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elerding SC, Aragon GE, Moore EE. Fatal hepatic hemorrhage after trauma. Am J Surg 1979; 138: 883–88. [DOI] [PubMed] [Google Scholar]
- 3.Kashuk JL, Moore EE, Millikan JS, Moore JB. Major abdominal vascular trauma—a unified approach. J Trauma 1982; 22: 672–79. [DOI] [PubMed] [Google Scholar]
- 4.Borgman MA, Spinella PC, Perkins JG, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma 2007; 63: 805–13. [DOI] [PubMed] [Google Scholar]
- 5.Spinella PC, Perkins JG, Grathwohl KW, et al. Effect of plasma and red blood cell transfusions on survival in patients with combat related traumatic injuries. J Trauma 2008; 64 (suppl): S69–77. [DOI] [PubMed] [Google Scholar]
- 6.Brown LM, Aro SO, Cohen MJ, et al. A high fresh frozen plasma: packed red blood cell transfusion ratio decreases mortality in all massively transfused trauma patients regardless of admission international normalized ratio. J Trauma 2011; 71 (suppl 3): S358–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holcomb JB, del Junco DJ, Fox EE, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg 2013; 148: 127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nascimento B, Callum J, Tien H, et al. Effect of a fixed-ratio (1:1:1) transfusion protocol versus laboratory-results–guided transfusion in patients with severe trauma: a randomized feasibility trial. Can Med Assoc J 2013; 185: E583–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 2015; 313: 471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith IM, James RH, Dretzke J, Midwinter MJ. Prehospital blood product resuscitation for trauma: a systematic review. Shock 2016; 46: 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kozar RA, Peng Z, Zhang R, et al. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth Analg 2011; 112: 1289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ban K, Peng Z, Pati S, Witkov RB, Park PW, Kozar RA. Plasma-mediated gut protection after hemorrhagic shock is lessened in Syndecan-1–/– mice. Shock 2015; 44: 452–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Alessandro A, Moore HB, Moore EE, et al. Plasma fixation reduces lactate acidosis, enhances redox homeostasis, amino acid and purine catabolism in a rat model of profound hemorrhagic shock. Shock 2016; 46: 173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore HB Moore EE, Morton AP, et al. Shock induced systemic hyperfibrinolysis is attenuated by plasma first resuscitation. J Trauma a Acute Care Surg 2015; 79: 897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holcomb JB, Swartz MD, DeSantis SM, et al. Multicenter observational prehospital resuscitation on helicopter study. J Trauma Acute Care Surg 2017; 83 (suppl 1): S83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chapman MP, Moore EE, Chin TL, et al. Combat: initial experience with a randomized clinical trial of plasma-based resuscitation in the field for traumatic hemorrhagic shock. Shock 2015; 44 (suppl 1): 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bulger EM, Jurkovich GJ, Nathens AB, et al. Hypertonic resuscitation of hypovolemic shock after blunt trauma: a randomized controlled trial. Arch Surg 2008; 143: 139–48. [DOI] [PubMed] [Google Scholar]
- 18.Sasser SM, Hunt RC, Faul M, et al. Guidelines for field triage of injured patients: recommendations of the National Expert Panel on Field Triage, 2011. MMWR Recomm Rep 2012; 61: 1–20. [PubMed] [Google Scholar]
- 19.Chin TL, Moore EE, Coors M, et al. Exploring ethical conflicts in emergency trauma research: the COMBAT study experience. Surgery 2015; 157: 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Association of Blood Banks. Standards for blood banks and transfusion services, 29th edn Bethesda, MD: American Association of Blood Banks, 2014: 7. [Google Scholar]
- 21.Gonzalez E, Moore EE, Moore HB, et al. Goal-directed viscoelastic-guided hemostatic resuscitation of trauma induced coagulopathy: a pragmatic randomized clinical trial. Ann Surg 2015; 236: 1051–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sauaia A, Moore EE, Johnson JL, Ciesla DJ, Biffl WL, Banerjee A. Validation of postinjury multiple organ failure scores. Shock 2009; 31: 438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012; 307: 2526–33. [DOI] [PubMed] [Google Scholar]
- 24.Kleinman S, Caulfield T, Chan P, et al. Toward an understanding of transfusion-related acute lung injury: statement of a consensus panel. Transfusion 2004; 44: 1774–89. [DOI] [PubMed] [Google Scholar]
- 25.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13: 818–29. [PubMed] [Google Scholar]
- 26.Osler T, Baker SP, Long W. A modification of the injury severity score that both improves accuracy and simplifies scoring. J Trauma 1997; 43: 922–25. [DOI] [PubMed] [Google Scholar]
- 27.Bulger EM, May S, Kerby JD, et al. Out-of-hospital hypertonic resuscitation after traumatic hypovolemic shock: a randomized, placebo controlled trial. Ann Surg 2011; 253: 431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brohi K, Cohen MJ, Ganter MT, et al. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma 2008; 64: 1211–17. [DOI] [PubMed] [Google Scholar]
- 29.Holland LL, Foster TM, Marlar RA, Brooks JP. Fresh frozen plasma is ineffective for correcting minimally elevated international normalized ratios. Transfusion 2005; 45: 1234–5. [DOI] [PubMed] [Google Scholar]
- 30.Holcomb JB, Donathan DP, Cotton BA, et al. Prehospital transfusion of plasma and red blood cells in trauma patients. Prehosp Emerg Care 2015; 19: 1–9. [DOI] [PubMed] [Google Scholar]