Abstract
Osteoarticular tuberculosis (OATB) is a rare form of tuberculosis (TB) whose incidence rose significantly nowadays especially in the underdeveloped countries. The main risk factors predisposing to this new challenge for the medical system are the Human Immunodeficiency Virus (HIV) epidemic, the migration from TB endemic areas and the development of drug and multidrug-resistant strains of Mycobacterium tuberculosis (Mt). The disease affects both genders and any age group although the distribution depending on gender is controversial and that depending on age has a bimodal pattern. In most cases the initial focus is elsewhere in the organism and the most frequent pathway of dissemination is lympho-haematogenous. The clinical picture includes local symptoms as pain, tenderness and limitation of motion, with some particularities depending on the segment of the osteoarticular system involved, sometimes accompanying systemic symptoms specific for TB and other specific clinical signs as cold abscesses and sinuses. The radiographic features are not specific, CT demonstrates abnormalities earlier than plain radiography and MRI is superior to plain radiographs in showing the extent of extraskeletal involvement. Both CT and MRI can be used in patient follow-up to evaluate responses to therapy. TBhas been reported in all bones of the body, the various sites including the spine (most often involved) and extraspinal sites (arthritis, osteomyelitis and tenosynovitis and bursitis). Two basic types of disease patterns could be present: the granular type (most often in adults) and the caseous exudative type (most often in children) one of which being predominant. The algorithm of diagnosis includes several steps of which detection of Mt is the gold standard. The actual treatment is primarily medical, consisting of antituberculosis chemotherapy (ATT), surgical interventions being warranted only for selected cases. It is essential that clinicians know and refresh their knowledge about manifestations of OATB.
Keywords: extrapulmonary tuberculosis, osteoarticular system, bones, joints
Introduction
Osteoarticular tuberculosis (OATB) defines any inflammatory process determined by Mycobacterium tuberculosis (Mt) localized in bones, joins or both structures. Tuberculosis (TB) still represents one of the major causes of skeletal infection in many parts of the world [1,2].
TB, and its bone and joint involvement too, is produced by members of a group of closely related bacterial species named the Mycobacterium tuberculosis complex (MTBC) all of them being obligate pathogens. Moreover, due to new molecular diagnostic markers identified using the complete genome sequencing of MTBC by polymerase chain reaction (PCR)-based typing methods, the Mt spinal involvement, better known also as Pott’s spine, is one of the oldest diseases known to mankind [3,4,5].
There are several terms used in the literature to name the disease: TB of the osteoarticular system; bone and joints TB; skeletal TB; musculoskeletal TB. The last two terms are used by some authors [6] to name only the extra spinal TB lesions.
Most authors consider that bone and joint tuberculous disease is always secondary to a primary or reactivated focus of infection [7]. However, there are authors [8] that are reporting isolated patients with osteoarticular involvement, apparently healthy, without personal of family history of TB, cases that could be considered as primary skeletal involvement.
OATB is also a part who does not represent a large proportion of extra pulmonary involvement (EPTB) of Mt [9,10]. Many studies placed the bone and joint TB lesions on the third position in the frequency hierarchy of extra pulmonary sites of TB [11,12]. However, there are studies on restricted geographic areas [13] in which skeletal TB lesions were found as the most common site of extra pulmonary involvement.
The incidence of OATB among the extra pulmonary sites varied both in time and depending on the socioeconomic status of different geographic areas. Thus, whereas in the past skeletal involvement accounted for only 10-18% of all extra pulmonary cases, incidence that remained almost the same (10-15%) in the last years in developed countries, nowadays OATB incidence raised up to 20% and even to 35% of all extra pulmonary cases mainly in underdeveloped countries [9,11,13,14,15,16,17,18,19,20,21,22,23,24,25,26].
Incidence. Predisposing factors
OATB is a rare form of TB. The limits of the incidence variation range among all TB cases are 1-6% [9,11,14,19,20,21,22,23,25,26,27,28,29,30,31,32].
This variation is observed both in time and in different geographical and social economic areas and is the consequence of a wide range of factors that predispose to or raise the risk of TB appearance in general and of osteoarticular involvement in particular.
These factors can be grouped depending on their area of intervention in three categories: general factors, factors encountered mostly in developed countries/areas and factors encountered mostly in underdeveloped countries/areas. They are summarized in Table 1 [1,2,8,18,24,33,34,35,36,37,38].
Table 1.
The predisposing/risk factors of TB and OATB
| Predisposing/Risk Factor | Type of Risk Factor | Prevalence* | ||
| A | B | C | ||
| Growing number of people with suppression of the immune system | - Immunosuppressive diseases | Yes | ||
| - Immunosuppressive therapies | Yes | |||
| - HIV positive patients/AIDS | Yes | |||
| Development of drug and multidrug-resistant strains of Mt | Yes | |||
| Increasing exposure of healthcare workers | Yes | |||
| Individual factors | - Women | Yes | ||
| - Repeated pregnancies and Lactation | Yes | |||
| - Blacks | Yes | |||
| - Alcohol abuse | Yes | |||
| - Drug abuse | Yes | |||
| Individual Diseases | - Diabetes mellitus | Yes | ||
| - Chronic renal failure | Yes | |||
| - Chronic obstructive disease | Yes | |||
| - Liver cirrhosis | Yes | |||
| - Lymphoproliferative disorders | Yes | |||
| Aging population | - Debilitated with other diseases | Yes | ||
| - Growing number | Yes | |||
| Declining public health interest in TB control | Yes | |||
| Immigration from countries/areas with high TB prevalence/TB endemic | Yes | |||
| Socioeconomic factors | - Poverty | Yes | ||
| - Homelessness | Yes | |||
| - Malnutrition mainly of protein | Yes | |||
| - Poor sanitation | Yes | |||
| - Overcrowded housing | Yes | |||
| HIV = human immunodeficiency virus; AIDS = acquired immunodeficiency syndrome; * A = General factor; B = Specific mostly for Developed countries/areas; C = Specific mostly for Underdeveloped countries/areas | ||||
The appearance and permanent increase of HIV epidemic influenced significantly the incidence and the prevalence of all forms of TB irrespective of geographical area or socioeconomic status. Moreover, this influence was reciprocal. Thus, on one hand, in the USA for instance, 10% of EPTB cases occur in HIV-infected patients and in some regions of Africa, up to one-third of adults with osteoarticular infections are HIV positive. On the other hand, TB is often the first manifestation of HIV infection [2,24,33,36,37,39,40].
The development of drug and multidrug-resistant strains of Mt is another major factor that influenced the alarming resurgence of TB in general and OATB in particular in both developed and developing countries.
Apart from the two above mentioned factors, the distinctive risk factors for developed countries are the growing number of elderly segment of population and the migration from TB endemic areas whereas for the developing countries the socioeconomic factors are on the first place. However, an interesting aspect should be pointed out for the developed countries from Europe especially, namely a surprising declining public health interest in TB control [2].
Gender and Age
Data in the literature concerning the relationship of TB osteoarticular involvement and the patients’ gender are controversial: whereas some authors are reporting a predominance of bone and joint lesions in women [10,11,19,41] others found the balance tilted in favor of men [6,12,42,43]. However, the spinal involvement, which is by far the most commonly affected site of the musculoskeletal system is encountered with equal frequency in both genders [34].
Bone and joint TB is encountered in any age group [1,35]. However, the dispersion in relation to age groups of OATB has a bimodal pattern: whereas in developed countries/areas the bone and joint lesions are encountered in the elderly (people older than 55 years), in the under developed countries/areas the lesions are more common in children and younger adults (around 30 years of age) [5,19,40,44,45,46,47,48]. Though, it should be stressed that skeletal TB in children started to be significant in the developed world [6].
Pathogenesis
The initial TB lesion is either primary or reactivated dormant visceral focus of infection. This could be a pulmonary lesion or an infection of the genitourinary (kidney) digestive (liver) or lymph nodes (mediastinal, mesenteric or cervical groups) systems [7,34,48,49,50,51,52,53,54,55,56,57].
The pathways through which TB spreads from the initial outbreak to osteoarticular structures are, in order of frequency, the following [7,15,43,45,54,58,59,60,61,62,63]:
- Blood vessels pathway-the most frequently encountered. It is paucibacillary.
- Lymphatic pathway-less commonly encountered.
- Direct spread from a contiguous lesion.
- Rare paths:
- Direct inoculation of the Mt into the site
- Bone and joints accidental or operative trauma
The routes and the frequency they are used in the dissemination of the TB infection to the musculoskeletal system structures is summarized in Table 2.
Table 2.
Pathways of Osteoarticular involvement by Mt
| Site | Pathway | |||
| Hematogenous | Lymphatic | Local spread | Direct inoculation | |
| Spinal | Yes | Less commonly | Rare | |
| Joints (Arthritis) | Yes | Less commonly | Yes | |
| Bone (Osteomyelitis) | Yes | Yes | ||
| Tenosynovitis/Bursitis | Yes | Yes | ||
| Myositis | Extremely rare | Yes | Yes | |
In the spine involvement, spread occurs either via the arterial or the venous route as a result of back flow. The most common route of spread to the vertebral body is the Batson's prevertebral venous plexus (a valve-less system that allows free flow of blood in both directions depending upon the pressure generated by the intraabdominal and intrathoracic cavities. The hematogenous route is represented by a rich vascular plexus consisting of an arterial arcade placed in the subchondral region of each vertebra that derives from anterior and posterior spinal arteries which facilitates Mt spread in the dense vasculature of cancellous bone of the vertebral bodies paradiskal regions [5,34].
The vascular pathway for the joints is represented by the subsynovial vessels [32].
Apart from the hematogenous pathway, the bones and joints could contaminate each other through bidirectional local spread. Usually the process starts in the growth plates of bones which have a better blood supply, and then spreads transphyseally into the joint spaces either from epiphyseal (more common in adults) or metaphyseal (more common in children) lesions [32,64,65].
The synovial and muscular involvement is most often the result of local spread from adjacent lymph nodes, bone, or articular TB lesions, the hematogenous or lymphatic spread without a previous bone involvement being extremely rare [32]. However, myositis could be caused by the use of a contaminated syringe or medical device [61]. Primary bursitis due to hematogenous spread is rare [66].
Clinical picture
TB infection of the bones and joints has some general features: it is chronic, slowly progressive and destructive, often resulting in walking difficulties and disability [10]. In joint involvement, the clinical picture is more flagrant and obvious in adults than in children [18].
The clinical manifestations are divided in two main groups: constitutional symptoms (accompanying systemic symptoms) and local symptoms.
Accompanying systemic symptoms
Accompanying systemic symptoms are present in approximately 20-30% of cases of OATB. The classical constitutional features indicating the presence of an active TB process are: malaise, low-grade fevers, evening rise in temperature, night sweats, weight loss, anorexia, generalized body aches, and fatigue [5,59,67,68].
Local symptoms
The most common local symptoms of OATB regardless the involved tissue/tissues are: pain, tenderness and limitation of motion [67]. However, there are some particularities of the local clinical manifestations depending on the segment of the osteoarticular system involved. These individual features are summarized in Table 3 [52,68].
Table 3.
The Side specific clinical complains
| Clinical Manifestation | Osteoarticular System Segment | ||||||||
| Bones | Joints-Synovial and Articular Involvement (Stages*) | Muscles | |||||||
| I | II | III | IV | V | |||||
| Insidious Onset | Yes | Yes | Yes | ||||||
| Local Tenderness/ Pain | Yes | Yes | Yes** | ||||||
| Local Warmth | Yes | Yes | |||||||
| Swelling/ Effusion | Yes | Yes | Yes | Yes | |||||
| Stiffness | Yes | ||||||||
| Limitation of motion | 25% | 25-50% | 75% | 75% Subluxation/ Dislocation | Ankylosis | ||||
| Lesion associated (bones/joints) | Yes | No | Erosion/Lysis (one/more) Diminution of Joint space | Subpericondral cyst Loss of Joint space | Joint Distruction | Yes | |||
| Legend: *= Tuli Classification [52] Stage I-Synovitis; Stage II-Early arthritis; Stages III and IV-Advanced Arthritis; Stage V-Ankylosis; **= “Night cries” that wake the patient from sleep | |||||||||
Other specific clinical signs
Other specific clinical signs include: lymphadenopathy, commonly encountered, formation of cold abscesses and sinus drainage, the latter being present in one third of cases [5,67,68].
Cold abscesses are characterized by lack of pain and other signs of inflammation and sinuses could be sometimes the only presentation, being often misdiagnosed as pyogenic infection or diabetic foot [5,69].
Clinical expression of a tuberculous sinus include: soft tissue fluctuation, bluish discoloration at the periphery, undermined edges, sero-sanguinous discharge, matted draining lymph nodes, and fixation to bone [67].
Sinuses are common and abscess formation may occur in osteomyelitis. On the other hand, sinus formation is rare in tenosynovitis [32,68]. Particular features of spine TB
Pain is the most frequent symptom, its intensity varying from constant mild dull aching to severe disabling. Spasm of the surrounding muscles could be severe.
Progression of kyphosis is most common in children with multiple levels of involvement in the thoracic spine and the deformity of the spine is more severe with how the diagnosis is delayed in the younger age group [5,18,34,53,54,68,70,71].
Findings in patients with tuberculous spinal disease are summarized in Table 4.
Table 4.
The clinical complains in Spine TB
| Clinical Manifestation | Spine Involvement | ||||
| General | Cervical | Thoracic | Lumbar | Sacral | |
| Thoracolumbar | |||||
| Involvement | 50% of all OATB | Uncommon | Most frequently | Less frequently | Uncommon |
| Most frequently | |||||
| Pain typically localized to the site of involvement | Yes | Yes | back pain | back pain | back pain |
| Local tenderness | Yes | Yes | Yes | Yes | Yes |
| Weakness | Yes | Yes | Yes | Yes | |
| Truncal rigidity/stiffness due to spasm of the surrounding muscles | Yes | Yes Torticolis | Yes | Yes | |
| Painful restricted joint movements in all directions | Yes | Yes | Yes | Yes | Yes |
| Kyphotic deformity | Gibbus | Gibbus | Gibbus | Sometimes | |
| Neurologic signs/deficit | Common | Numbness of the upper and lower extremities Progresses to Tetraplegia | Lower-extremity symptoms Progress to paraplegia | Uncommon Numbness | Numbness |
| Cold abscess | Yes | Hoarseness, Stridor, Dysphagia | Yes | Yes | |
| Lymphadenopathy | Common | ||||
| Sinuses | Common | ||||
Neurologic deterioration may occur in both the active and healed stages of the disease, the reported incidence of neurological deficits varying from 23 to 76%. The neurological symptoms are the consequence of the compression of the spinal cord or its roots, the level of spinal cord involvement determining the extent of neurological manifestations. For instance, patients with cauda equina compression due to lumbar and sacral vertebral damage could present cauda-equina syndrome, characterized by decreased or absent reflexes among the affected muscle groups (in contrast to the hyperreflexia seen with spinal cord compression) along with bladder involvement [5,54,72].
The causes of neurologic compromise during the active phase are inflammatory edema, extradural compression from posterior extension of an abscess (pus, caseous material, granulation tissue, sequestrae), or an internal gibbus following collapse or malalignment of the involved vertebrae [73]. Rarely, the neurologic compromise could be expressed as a “spinal tumor syndrome” without bony changes as a result of tubercular granulomas presence in extradural, intradural, or intramedullary locations [67].
Neurologic compromise in “healed disease” (meaning more than 2 years after disease onset) could be determined by: spinal stenosis, direct compression from an internal gibbus deformity, and constriction by peridural fibrosis [73].
Abscess formation is common. Abscesses may migrate posteriorly into the spinal canal, anterior underneath the anterior longitudinal ligament, as well as into neighboring visceral structures. In cervical region, large abscesses can determine hoarseness, stridor, and dysphagia. Abscesses appearing below the diaphragm, typically migrate along the psoas sheath and exit via sinuses in the groin or buttock region [53,54,68].
In most cases the spinal TB lesion is insidious in onset and slow in progression and only rarely there is an acute manifestation [5,53]
Clinical and radiographic presentation of skeletal TB in patients from endemic areas differs from that of individuals from non-endemic areas. Thus, in endemic areas patients present a higher incidence of multifocal skeletal involvement, periosteal reaction, bone sclerosis, and severe bone destruction. In turn, in non-endemic areas patients are older, with a debilitating underlying disease and usually present solitary, osteolytic lesions that involve the axial skeleton, thoracolumbar vertebral bodies, and hips [74].
Imaging investigation
Plain Radiographs
There are no specific radiographic features that are pathognomonic of TB of bones or joints and, in the advanced stages, mimics other osteoarticular lesions.
The most common radiographic figures are summarized in Table 5 [18,32,52,54,75,76,77].
Table 5.
Main radiographic findings in OATB
| Radiological Fetures | Osteoarticular System Segment | |||||
| Bones | Joints-Synovial and Articular Involvement (Stages*) | |||||
| I | II | III | IV | V | ||
| Soft tissue Swelling | Yes | Yes | Yes | |||
| Osteopenia/Osteoporosis | Yes | Yes | Yes | |||
| Periosteal reaction | Minimal | |||||
| Osteolysis | Yes | |||||
| Subchondral erosions | Yes | |||||
| Diminution in joint space | Yes | Yes | ||||
| Cysts | Yes | |||||
| Significant loss of joint space | Yes | |||||
| Joint destruction | Yes | |||||
| Sclerosis | Less | |||||
| Sequestration | Uncommon | |||||
| New bone formation | Yes | |||||
| Calcifications | In the distended bursae | |||||
| Ankylosis | Yes | |||||
| Legend: *= Tuli Classification [52] Stage I-Synovitis; Stage II-Early arthritis; Stages III and IV-Advanced Arthritis; Stage V-Ankylosis | ||||||
Tuberculous osteomyelitis may mimic a variety of conditions on plain radiographs. The most common presentation of osseous involvement is a solitary lytic lesion, usually with a sclerotic rim. Sometimes, lesions may cross the physis and new bone formation may be observed subperiosteally [52,54,68,78,79,80].
The classical triad of radiologic characteristics of TB tenosynovitis and arthritis, also known as the "Phemister triad", is: juxta-articular osteoporosis, peripheral osseous erosion and gradual narrowing of the intra-articular space though this can be mimicked in rheumatoid arthritis and fungal disease [81,82,83].
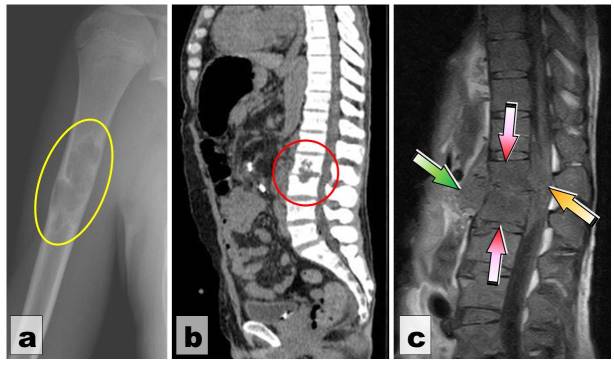
Radiographic changes in the joint are absent or non-specific in the early stages of disease. Subchondral erosions involve both sides of the joint and cross the epiphysis in more than one-third of affected children. Cysts in bones appear adjacent to a joint ( Fig.1a) [18,84,85].
Figure 1.
Imaging investigations. (a) Arm radiography: Solitary multilocular lytic lesion with a sclerotic rim (yellow oval); (b) Profile Spine CT-STIR1: L2-L3 “mirror” caries with intervertebral disk evanescence (red circle); (c) MRI of the lumbar spine T1-w sagittal plane: Erosive changes involving the inferior aspect of L1 and superior part of L2 with collapse of the intervertebral space (red arrows) and paravertebral abscess involving the spinal canal (yellow arrow) and the prevertebral soft tissues (green arrow)
Depending on the site of the vertebral lesion and the frequency of appearance, the spine lesions were systematized by Garg et al [5] as shown in Table 6.
Table 6.
Main radiographic findings in Spine TB (modified after Garg et al [5])
| Type of involvement | Radiological appearances | ||
| Vertebra | Intervertebral disk/space | ||
| Typical | Paradiskal | Adjacent margins of two consecutive vertebrae involved. | The intervening disk space reduced |
| Central | Central portion of a single vertebra involved | Proximal and distal disk spaces intact | |
| Anterior marginal | Destructive lesion in one of the anterior margins of the body of a vertebra | Minimally involving the disk space but sparing the vertebrae on either side | |
| Atypicsl | Skipped lesions | Circumferentially involvement of two noncontiguous vertebral levels without destruction of the adjacent vertebral bodies | No destruction of intervertebral disks |
| Posterior | Posterior arch involved without involvement of vertebral body | ||
| Synovial | Synovial membrane of atlanto-axial and atlanto-occipital joints | ||
For the spine, radiographic features suggestive of TB are [18,54,68,86]:
- Multiple levels of involvement (more than two levels)
- Multicentric involvement
- Involvement of the vertebral body with rarefaction of the vertebral end plates
- Relative preservation of the intervertebral disc/disc space but with increasing loss of disc height
- Variable degrees of osseous destruction with late fusion or bone collapse
- New-bone formation
- Subligamentous spread
- Larger sized paravertebral soft-tissue abscesses, often with calcifications and rim enhancement around
Chest radiographs may show evidence of pulmonary disease in half of patients with OATB, but active pulmonary disease is present in less than 20% of patients (6.9-29% of cases) [19,40,87,88].
Computed tomography (CT)
CT demonstrates abnormalities earlier than plain radiography and is best because allows the evaluation of the osseous or joint involvement degree [5,54].
Thus, CT is useful for the detection, visualization and evaluation of:
- The degree of bone destruction
- Sequestrum formation (although rare)
- The presence or absence of periosteal reaction
- Epidural lesions containing bone fragments
- Extension in the surrounding soft tissue, calcifications, sclerosis
- Soft tissue abscesses
- Calcifications within the cold abscess [5,32,85, 89,90,91,92].
For the spine, CT is particularly of the greatest value because visualizes:
- The disko-vertebral lesions and paravertebral abscesses (Fig.1b)
- The delineation of encroachment of the spinal canal by posterior extension of inflammatory tissue, bone or disk material [93,94].
Finally, CT is particularly useful for guiding fine needle aspiration or biopsy to provide material for histopathological examination, PCR-based assay for mycobacterial DNA, and culture [32].
Magnetic resonance imaging (MRI)
Magnetic resonance imaging (with gadolinium enhancement) is the modality of choice for early detection of joint TB even its early findings are nonspecific and may demonstrate intraosseous involvement earlier than the other imaging modalities in osteomyelitis. MRI is also more sensitive than x-ray and more specific than CT in the diagnosis of spinal TB because it can differentiate between granulation tissue and abscess, identify soft-tissue masses and assess the degree of bone destruction. However, bone anatomy and abnormalities, including calcifications and sequestra, are better seen on CT scanning. [5,32,54,95,96]. For joint lesions, MRI can detect: joint effusion, marrow edema, and sometimes abnormalities within the articular cartilage and subchondral bone during the stage of arthritis [97,98].
In tenosynovitis, the investigation helps to delineate the precise extent of soft tissue involvement and the associated lesions of neighboring bones and joints [32].
In bursitis, the examination reveals: bursal uniform distension, multiple small abscesses in the bursa or the presence of caseous necrosis and fibrotic material within the fluid-filled bursa (the latter on T2W images) [99,100].
For spinal TB, MRI offers more comprehensive information about:
- The extent of involvement/degree of destruction of the vertebral bodies and intervertebral disk (vertebral collapse, and spinal deformities)
- The location and size of paravertebral and/or epidural cold abscesses (Fig.1c)
- Rapid determination of the mechanism for neurologic involvement
- The presence of spinal cord pathology (impingement or compression, intradural /intramedullary disease, myelomalacia) [5,54,85,89,90,91,92].
Ultrasound examination(USG)
USG is the primary investigation to confirm the diagnosis of tenosynovitis and to reveal the degree and extent of tendon and tendon sheath involvement. It is also helpful, as we already mentioned above, for guiding fine needle aspiration or biopsy to provide material for other types of biological examination, especially in myositis and bursitis [32,101].
Morphology
Location
Tuberculosis has been reported in all bones of the body [102,103].
The various sites of Osteoarticular system include [16,18,48,66,68,78,83,94,104]:
- Spine-most often involved-50%
- Extraspinal sites-represent together the rest of 50%, being individually less common:
- Joints (TB arthropathy)-60% of all extraspinal sites
- Bones (TB osteomyelitis including the unusual TB dactylitis-metacarpal or phalanx)-38% of all extraspinal sites
- Tendon sheath and Bursae (TB tenosynovitis and bursitis)-2% or less of all extraspinal sites
In the spine, the most affected segment is the thoracic region with less than 50%, followed by the lumbar region and cervical region, gathering together these three regions 80% of all cases. In the rest, the lesions involve more than one region, the combinations being (in the decreasing order of frequency) the thoraco-lumbar region, the cervico-dorsal region and the lumbo-sacral region ( Fig.2) [34].
Figure 2.
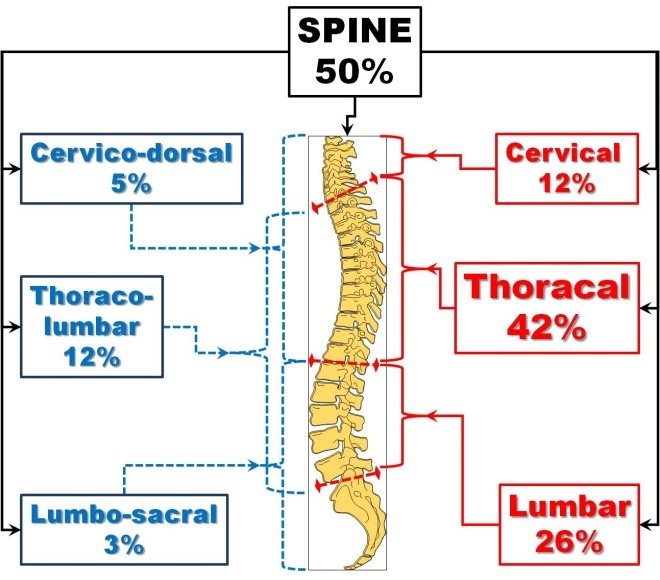
Spine Involvment
Arthritis. TB arthritis occurs most frequently at weight bearing joints such as the hip (1 red) and knee (2 red) which account for 15% to 30% and 10% to 20% of non-spinal cases of skeletal TB, respectively. They are followed by, in order of frequency, the sacroiliac (3 red), shoulder (4 red), elbow (5 red), and ankle joints (6 red) ( Fig.3 red marks) [16,18,20,32,48,105,106].
Figure 3.
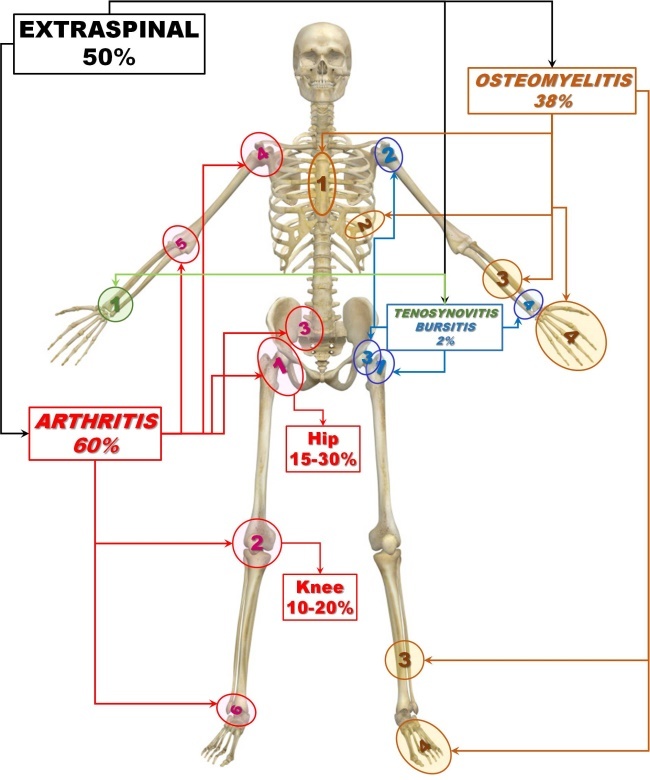
Extraspinal sites
Osteomyelitis. Extraspinal bone lesions caused by TB represent according to some authors less than 5% of cases of OATB [54,68].
TB lesions could be found in manubrium sterni, sternum isolated (1 brown), spinous processes, odontoid process, spine of the scapula, skull, pelvis, long bones of the extremities (3 brown) and the small bones of the hands and feet (metacarpus, metatarsus, and phalanges-4 brown)). The bones of the hands are more affected than the bones of the feet. The ribs are also frequently involved (2 brown) (Fig.3 brown marks) [32,34,107,108].
Tenosynovitis and Bursitis. The most frequently involved tendon sheaths are the flexor tendon sheaths of the dominant hand and the most affected bursae are the trochanteric, subacromial, subgluteal, and radioulnar wrist bursae (Fig.3 green marks) [24,54,66,109]
Myositis. The cases of TB pyomyositis are rare. Any muscle may be involved but the reported cases were mentioning the muscles of the upper and lower extremities as well as of the chest and abdominal wall [110,111,112,113].
TB arthropathy is characteristically monoarticular. However, oligoarticular or polyarticular joint disease could be encountered in around 10% of patients (5-15%) only occasionally with small joint involvement, and more often in those who are immunosuppressed [7,8,20,65,114].
The bone lesions are also usually solitary but multifocal bone involvement may be rarely seen (accounting for 10% of patients with skeletal TB and 1%-3% of all TB cases), with the lesions at different stages of development due either to hematogenous spread, with bacilli being seeded at different times or to a patient with suppressed immune response [30,32,57,115]
During the time, unusual forms of skeletal TB were reported such as:
- Multiple cystic TB (one or more large, oval areas of rarefaction, children) [116,117,118].
- Disseminated skeletal TB (multiple osseous and/or articular sites, in compromised host) [100,119,120,121].
- Closed multiple diaphysitis (swelling in forearms and legs in compromised children) [100].
- Spina ventosa, a spindle shaped expansion with multiple layers of subperiostial new bone, occurring in the short tubular bones of the hands and feet [68].
Morphological changes
The conflict between Mt and the hosting tissue (bone, synovial structures, cartilage, muscle, soft tissue) is evolving in several steps:
- Ingestion of Mt by mononuclear cells at the site of deposition
- Transformation of mononuclear cells into epithelioid cells and their coalescence into giant Langhans cells
- Tubercle formation by ring-shaped arrangement of lymphocytes around a group of epithelioid cells
- Caseation development within the center of the tubercle.
- Cold abscess formation by intensification of the host inflammatory response, resulting in exudation and liquefaction. The cold abscess is composed of serum, leukocytes, caseation, bone debris, and bacilli [68].
There could be several end results if this conflict such as:
- Resolution with minimal or no morbidity
- Healed disease with residual deformity
- Walled off lesions with calcification of caseous tissue
- A low-grade chronic granular lesion
- Local or miliary spread of the disease that may result in death [52].
Two basic types of disease patterns could be present:
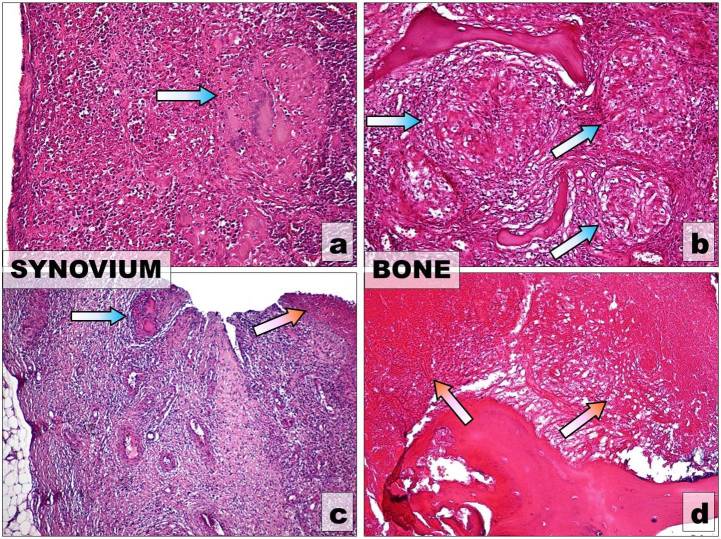
- Thegranular type (Fig.4 a and b)-occurs most often in adults. Is characterized by:
Figure 4.
Main histological patterns of OATB. UP: Granular type-Giant Langhans cell granulomas (blue arrows) placed (a) in the synovium (b) in the cancellous bone, H-E stain, x10; DOWN Caseous necrosis (red arrows) (a) in the synovium x4 (b) in the compact bone, x10, H-E stain
- More insidious and less destructive than the caseous exudative type
- Abscess formation less common.
- The caseous exudative type (Fig.4 c and d)-occurs most often in children. Is characterized by:
- Bone destruction
- Local swelling
- Abscess formation
- Sinus formation
- Constitutional symptoms.
Because host-parasite interactions in TB are dynamic, with mixed patterns and transitions, one of the two patterns may predominate either in osseous or synovial involvement [62,122,123].
Arthritis. Articular disease often starts as a synovitis, with joint effusion, followed by formation of granulation tissue that typically accompanies synovial proliferation (pannus). Then the pannus begins to erode and destroy the cartilage, determines further demineralization and marginal erosions of the periarticular bones and eventually results in destruction of the articular surfaces of the joint [105,124,125,126,127]. If the disease has been inactive for a long time, new bone formation could be expected [128].
Osteomyelitis. Extraspinal TB osteomyelitis presents as a solitary cold abscess (lytic lesion with a sclerotic rim), with swelling and only mild erythema and pain, and may be misdiagnosed as a tumor [24,68].
In the long bones, the lesions begin and develop most often in the epiphysis, more precisely in the metaphysis and causes tubercle formation in the marrow, with secondary infection of the trabeculae. In rare cases, the diaphysis may also be affected. The lesion may also penetrate the physis or extend into an adjacent joint [32,59,68].
The active phase of tuberculous osteomyelitis is characterized by bone destruction without sequestra and with minimal new bone formation [129].
Tenosynovitis. In general, the morphologic changes are nonspecific tendon and synovial thickening predominate, with relatively little synovial sheath effusion [32]. Jaovisidha et al [66] described three stages of TB tenosynovitis:
- The hygromatous stage-characterized by the presence of fluid inside the tendon sheath without associated sheath thickening.
- The serofibrinous stage-characterized by thickening of the flexor tendons and synovium, with multiple tiny nodules within the synovial fluid, corresponding to the rice bodies previously reported in the literature.
- The fungoid stage-characterized by a soft tissue mass involving the tendon and tendon sheath.
Bursitis. The bursa inflammation is expressing like a cold abscess or draining fistula that does not respond to conventional antibiotic therapy [24].
Bursa could be uniformly distended by caseous necrosis and fibrotic material, with calcifications in the wall or multiple small abscesses in the bursa could be seen [99,100].
Myositis. Abscess formation is the rule in all cases of pyomyositis. Associated cellulitis and osteoarticular involvement may also be present [110, 130].
Spine. Typical bone lesion for spine TB is destruction of the anterior region of vertebral bodies with subsequent collapse of the spine [105,126].
Two distinct patterns of vertebral TB are described [57]:
- The first is Spondylitis without disc involvement , which is exceedingly more common. Multilevel vertebral body involvement could occur in this form as skip lesions.
- The second pattern is Destruction of two or more contiguous vertebrae associated with late-onset disc infection , which results in intervertebral space narrowing due to disc herniation into the collapsed vertebral body; this is regarded as an atypical radiologic feature of vertebral TB.
In children, TB of the spine generally involves the osseous tissue of the vertebrae and not the cartilaginous growth plate [18].
Diagnosis
The algorithm of diagnosis in OATB includes several steps [8,26,53,131,132,133].
1. Epidemiological background-Pay attention to the patient’s country of origin and his history of prior known or possible TB contact.
2. Clinical examination-a high index of clinical suspicion given the indolent nature of tuberculous bone and joint disease and especially:
- In patients with no evidence of active chest disease (more than half of cases)
- In patients with HIV infection and relatively high CD4 counts and no other signs or symptoms of TB
3. Paraclinical examination
- Tuberculin Skin Test (TST)
- Enzyme-Linked Immunosorbent Assays (ELISA)
- Interferon Gamma Release Assays (IGRAs)
4. Imagistic examination
- Radiology
- Computed Tomography (CT) which can be helpful for:
- The detection of osseous or joint involvement/destruction, the presence or absence of periosteal reaction and soft tissue masses or calcifications, sclerosis, and soft tissue abscesses.
- Guidance of percutaneous biopsy or abscess drainage [32, 134].
- Magnetic Resonance Imaging (MRI)
- Useful in showing the extent of the disease, particularly in spinal lesions [135].
- The preferred technique to demonstrate early bone marrow changes in tuberculous osteomyelitis and arthritis, joint effusion, and cartilage destruction [32].
- Superior to plain radiographs in showing the extent of extraskeletal involvement, particularly in the case of compromise of the vertebral canal and the epidural space [74].
- Both CT and MRI can be used in patient follow-up to evaluate responses to therapy [135].
- USG-particularly useful for guiding fine needle aspiration or biopsy [32].
5. Detection of Mt by:
- Ziehl-Neelsen and immunohistochemistry stains on smears and histological slides
- Culture of Mt from bone/synovial/soft tissue/draining sinuses, synovial fluid although, in some cases, cultures may reveal colonizing bacteria or fungi that are erroneously assumed to be the causative pathogen.
- Polymerase Chain Reaction (PCR)
The material used for detection could be [132,133]:
- Synovial fluid or purulent infected material obtained by aspiration or fine needle aspiration biopsy
- Bone/synovial/soft tissue obtained by biopsy
The traditional criteria for diagnosing TB are:
- Chest radiology
- Detection of acid-fast bacilli by Ziehl-Neelsen stain on microscopy and culture [4,136,137,138].
Microscopy is the most rapid diagnostic tool but it is very insensitive, yielding only 10-30% of culture-positive samples [4].
Biopsy may be required to clear up diagnostic confusion, being the most definitive test for tuberculous arthritis. It must be performed in cases in which microbiologic tests give negative results, the demonstration of caseating granulomas on histological examination being of significant value [49,53,139].
Culture is the gold standard for the diagnosis of osseous TB is culture of mycobacteria from bone tissue or synovial fluid but may take four weeks to obtain conclusive results even with enhanced culture systems [4,53].
Newer methods of diagnosis, especially polymerase chain reaction (PCR) on obtained joint tissue biopsies, appears promising in the early diagnosis of tuberculous arthritis [140].
Drug susceptibility testing of isolates is essential. In this respect, the Xpert MTB/RIF assay is an automated nucleic acid amplification test that can simultaneously identify Mt and rifampin resistance; it has been shown to be fast and accurate in diagnosing musculoskeletal TB in children and adults [132,133,141].
Tuberculosis of spine in childhood
A special mention about spine TB in children should be made because in this group of age the deformity is "dynamic in continuum" and could lead either to correction or deterioration therefore needing active surveillance till the entire growth potential is completed [142].
The progression of the spine deformities implies three overlapping phases:
- Phase I-Active phase
- Phase II-Growth phase
- Phase III-Late phase
There is an increased collapse for each vertebral loss and may increase or decrease during the growth phase in children as opposed to the adults where the collapse is proportionate to the extent of destruction and stops with consolidation of the lesion [142].
Rajasekaran (1999) [143] defined a "Spine at risk" concept, described by four radiological signs which offer reliable prediction of progression of the deformity and are of inestimable assistance for identifying "children at risk" for severe deformity. These are:
- Facet joint dislocation which triggers disaster in childhood lesion
- Retropulsion sign
- Lateral translation
- Topplling Over sign.
He identified also the risk factors for severe increase of deformity, namely:
- Patients less than 10 years of age at the onset of the disease
- An initial kyphosis angle of more than 30 degrees;
- Vertebral body loss of greater than 1.5
- Involvement of more than 3 vertebral bodies;
- Presence of "spine at risk" signs in radiographs;
- Global involvement of the vertebrae and
- Children who have partial or no fusion during adolescent growth spurt.
Based on these observations, five main types of progression were finally defined during the Growth phase (Table 7) [144].
Table 7.
Main types of Spine TB progression through Phase II (modified after Rajasekaran-2013)
| Type | Subtype | Description | |
| Type I | Type Ia | Progression of the deformity throughout the growth phase continuously after Phase I | |
| Type Ib | A spurt of progression after a delay period of 3–6 years Progression showed the highest increase in deformity although the increase of deformity occurs | ||
| Type II | Type IIa | Progression shows beneficial effects during growth phase with a decrease in the deformity after the healed stage. | Immediately after Phase1 Maximum decrease of the deformity |
| Type IIb | After a delay period of 3–6 years | ||
| Type III | Progression with minimal destruction (No any major changes in the deformity during the active or the healed phases) | ||
Differential diagnosis
The differential diagnosis of skeletal TB includes:
- Subacute or chronic infections due to pathogens or diseases such as (depending upon epidemiologic factors):
- Staphylococcus aureus osteomyelitis
- Brucellosis
- Melioidosis
- Actinomycosis
- Candidiasis
- Histoplasmosis
- Metastatic malignancy (especially multifocal bone involvement)
For spine TB, the following differential diagnosis should be considered:
- Degenerative disc and facet joint disease
- Vertebral body collapse due to osteopenia (due to a variety of causes such as osteoporosis and chronic corticosteroid therapy)
- Spondyloarthropathy, spondylitis
- Pyogenic spinal infection
- Brucellar spondylitis
- Sarcoidosis
- Malignancy-(metastases, multiple myeloma, lymphoma)
Treatment
Before the advent of chemotherapy in 1940s, the only treatments available for TB were surgical interventions as well as a general improvement in an individual’s immunity through empirical methods as rest, good nutrition, sunlight, fresh air and hygiene [106,128,142,145].
Nowadays, the treatment of OATB is primarily medical, consisting of antituberculosis chemotherapy (ATT). Surgical interventions are only an adjunct to appropriate ATT, being warranted only for selected cases [18]. Adequate nutritional support is however essential, as in all forms of TB [53].
The goals of treatment are:
- Infection containment and eradication
- Pain relief
- Preservation and Restoration of bone and joint function [146].
Antimicrobial therapy
The treatment is based on antituberculous therapy (ATT). The selection of drugs is generally the same as that for pulmonary TB (isoniazid, rifampin, pyrazinamide, and streptomycin or ethambutol). The drug regimen depends on whether or not the patient has HIV infection or drug-resistant TB [7,62,147].
Successful medical treatment of TB requires a minimum of three drugs to which the Mt is susceptible, and at least one of these drugs must be bactericidal [18]. The optimal duration of therapy for OATB treatment is uncertain however many authors recommend at least 9 to 12 months for bone and joint involvement, especially for patients on regimens that do not include rifampin and/or for patients with extensive or advanced disease, particularly if it is difficult to assess the response to therapy [147,148,149,150,151].
The duration of therapy has wide variations depending on the site of the oasteoarticular system involved, such as:
- 6 months in sacral TB,
- 12-18 months in various spinal sites,
- 12-18 months in TB of craniovertebral junction
- 14-18 months in sternoclavicular joint involvement
- 12-20 months in TB affecting the talus
- 12 months in TB of metacarpals and phalanges [71, 152,153,154,155,156].
Multi-drug-resistant TB should be suspected if osteoarticular disease activity shows no signs of improvement after 4-6 months of uninterrupted therapy [53].
ATT gives good results with low morbidity and mortality, approximately 90% to 95% of patients healing without sequelae if treated at an early stage [7,157]. However, even chemotherapy can effectively treat the disease at any stage, the ultimate functional result depends upon the degree of tissue damage at the start of the treatment [54].
Auxiliary methods
In arthritis, Splints may be used for a short time to relieve acute symptoms and for a long time in specific cases of TB of the joints to prevent deformities of the infected extremities [158, 159]. In spine TB, various types of spinal support in the form of collars, braces and corsets, may need to be used [53].
Surgery
Extraspinal TB
The role of surgery in treatment of extraspinal TB is still somewhat controversial, varying throughout different regions of the world both in terms of indications for surgery and the specific procedures recommended.
As a general rule, surgery is reserved for specific indications, most often to establish the diagnosis or to treat complications of the disease process. Thus, surgery is recommended for approximately 5% of uncomplicated cases, and 60% of those with neurologic deficits [68,160].
Tuli [52] proposed a pattern of the natural history of TB arthritis progresses through five consecutive steps defining for each stage the therapeutic strategy too. This treatment algorithm is summarized in Table 8. ATT is mandatory for any of the five stages, surgery being reserved only for the advanced stages.
Table 8.
The therapeutic algorithm for TB joint involvement proposed by Tuli [52]
| Arthritis Stage | ATT | Auxiliary Treatment | Surgical Treatment |
| Stage I (Synovitis) | YES | 1) Rest | |
| 2) ROM | |||
| 3) Splinting | |||
| Stage II (early arthritis) | YES | 1) Rest | 1) Synovectomy |
| 2) ROM | |||
| 3) Splinting | |||
| Stage III (advanced arthritis) | YES | 2) Osteotomy | |
| 3) Arthrodesis | |||
| 4) Arthroplasty | |||
| Stage IV (advanced arthritis) | YES | 2) Osteotomy | |
| 3) Arthrodesis | |||
| 4) Arthroplasty | |||
| Stage V (Ankylosis) | YES | 2) Osteotomy | |
| 3) Arthrodesis | |||
| 4) Arthroplasty | |||
| Legend: ATT= Antituberculous treatment; ROM= Range of Motion exercises | |||
The main indications for surgery are:
- Biopsy
- Patient unresponsive after 4 to 5 months of ATT
- Severe joint cartilage destruction
- Joint deformity
- Large abscesses
- Patients with neurological manifestations
- Patients where healing gave a painful joint ankylosis
- Multiple drug resistance [26,47].
The range of surgical procedures that could be carried out includes:
- Incision and drainage of abscesses
- Synovectomy
- Excisional arthroplasty for the hip or the elbow
- Arthrodesis for the ankle, the wrist, or the knee
- Joint replacement if the disease has remained inactive for more than 10 years [7,24,147,158,161].
Spine TB
In spinal TB too, the treatment is primarily medical, with anti TB drug regimens. The surgical treatment is reserved for only two main situations:
- patients with neurological complication not responding to medical treatment
- some children with TB of the spine [34,142].
The major aim of treatment is to prevent paraplegia. Besides this, decompression, stabilization, and deformity correction are the main specific goals of the surgical interventions [34, 54].
Indications for surgery have to be individualized and are synthesized in Table 9.
Table 9.
Indications of surgery in spinal tuberculosis
| Type of Patient | Indication | Type of Indication | |||
| Without neurological complications | For biopsy, to establish a diagnosis in case of uncertainty (inability to obtain adequate material for culture by other means | R “Middle path” | |||
| Failure to respond to ATT | Evidence of ongoing infection | ||||
| Progressive bone destruction | |||||
| Failure to respond to conservative therapy | |||||
| Prevention of severe kyphosis in young children with extensive dorsal lesions | |||||
| Mechanical reasons | Destruction of two or more vertebrae | A “Middle path” | |||
| Involvement of the Posterior elements/Circumferential disease | |||||
| Spinal instability caused by destruction or collapse | |||||
| Progression of spinal instability despite ATT | |||||
| Deformity is likely | |||||
| Kyphosis/deformity > 40° at presentation | |||||
| Progression of kyphosis/ deformity despite ATT | |||||
| Large Abscess Paraspinal/Paravertebral | Increasing in size despite medical treatment | A | |||
| Respiratory obstruction developed | |||||
| Chest wall cold abscess | A | ||||
| With neurological complications | New, Worsening/Progressive and Severe/Advanced Neural complications/deficit | “Middle path” | |||
| Lack of improvement/recovery of Neural complications despite ATT | |||||
| Persistent Pain/Spasm | A demonstrable mechanical blockInstability because the lack of fusion | R“Middle path” | |||
| Nerve root compression | |||||
| Neurological deficits in patients for whom prolonged bed rest may lead to other problems | R | ||||
| Paraplegia of rapid onset/severe paraplegia | |||||
| Late-onset paraplegia | |||||
| Painful paraplegia in elderly patients | |||||
| Neural arch disease | |||||
| Spinal tumor syndrome (epidural spinal tuberculoma without osseous involvement) | |||||
| Recurrence | |||||
| Legend: ATT = Antituberculous treatment; A=Absolute indication; R=Relative indication | |||||
For acute situations, some authors defined absolute and relative indications that are mentioned in the table [5,18,54,162,163,164,165,166,167,168,169,170,171]. The specific indications of the “middle path”, popularized by Tuli in India in the 70s are also highlighted [148].The main strategies of approach in the surgical treatment of spinal TB are the following [54]:
- Chemotherapy alone (no capability for spinal surgery)
- “Middle path” (surgery for specific indications) [148]
- Routine decompression/debridement and bone grafting
Recommendations for surgical treatment are based upon:
- The location of involvement (anterior, posterior, circumferential)
- The risk or presence of kyphotic deformity
- The neurologic status
- The status of the disease (active or healed)
- The experience of the surgeon
- The resources available locally [54].
The operative approach of the spine could be anterior or posterior. The anterior approach is usually recommended and used and can allow:
- Abscesses evacuation
- Excision of all avascular material
- Safe anterior decompression of the spinal cord
The posterior approach is rarely indicated, ie in two circumstances:
- When posterior spinal elements are more involved than the anterior ones
- When both the anterior and the posterior elements are involved and posterior stabilization is needed before anterior decompression and arthrodesis is performed [172].
Outcome
The results of prolonged ATT treatment completed when necessary with surgery gives, in most patients, encouraging results. The few cases of osteoarticular multidrug-resistant TB have a good outcome too with second-line anti-TB drugs combined with surgery [24,172].
Conclusion
It is essential that clinicians know and update their knowledge on the manifestations of OATB so they can recognize and diagnose this curable disease before definitive surgery is practiced. The surgery should be limited to the diagnosis or treatment of life threatening complications.
Acknowledgments
Illustrations of histological and imaging aspects belong to OATB cases hospitalized in Pediatric Surgery and Orthopaedics and Traumatology departments of the Emergency County Hospital of Craiova, Romania.
References
- 1.Vallejo JG, Ong LT, Starke JR. Tuberculous osteomyelitis of the long bones in children. Pediatr Infect Dis. 1995;14(6):542–546. doi: 10.1097/00006454-199506000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Raviglione M C, Snider D E, Kochi A. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA. 1995;273(3):220–226. [PubMed] [Google Scholar]
- 3.Taylor GM, Murphy E, Hopkins R, Rutland P, Chistov Y. First report of Mycobacterium bovis DNA in human remains from the Iron Age. Microbiology. 2007;153(4):1243–1249. doi: 10.1099/mic.0.2006/002154-0. [DOI] [PubMed] [Google Scholar]
- 4.Donoghue HD. Human tuberculosis e an ancient disease, as elucidated by ancient microbial biomolecules. Microbes and Infection. 2009;11((14-15)):1156–1162. doi: 10.1016/j.micinf.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Garg RK, Somvanshi DS. Spinal tuberculosis: A review. J Spinal Cord Med. 2011;34(5):440–454. doi: 10.1179/2045772311Y.0000000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hosalkar HS, Agrawal N, Reddy S, Sehgal K, Fox EJ, Hill RA. Skeletal tuberculosis in children in the Western world: 18 new cases with a review of the literature. J Child Orthop. 2009;3(4):319–324. doi: 10.1007/s11832-009-0184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tuli SM. General principles of osteoarticular tuberculosis. Clin Orthop Relat Res. 2002;398:11–19. doi: 10.1097/00003086-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Vergara-Amador E, Galván-Villamarin F y Piña-Quintero M. Primary osteoarticular tuberculosis: the reappearance of a forgotten pathology. Rev. salud pública. 2007;9(3):465–470. doi: 10.1590/s0124-00642007000300015. [DOI] [PubMed] [Google Scholar]
- 9.Malaviya AN, Kotwal PP. Arthritis associated with tuberculosis. Best Pract Res Clin Rheumatol. 2003;17(2):319–343. doi: 10.1016/s1521-6942(02)00126-2. [DOI] [PubMed] [Google Scholar]
- 10.Lesić AR, Pešut DP, Marković-Denić L, Maksimović J, Cobeljić G, Milošević I, Atkinson HD, Bumbaširević M. The challenge of osteo-articular tuberculosis in the twenty-first century: a 15-year population-based study. Int J Tuberc Lung Dis. 2010;14(9):1181–1186. [PubMed] [Google Scholar]
- 11.Yoon HJ, Song YG, Park WI, Chol JP, Chanh KH, Kim JM. Clinical manifestations and diagnosis of extrapulmonary tuberculosis. Yonsei Med J. 2004;45:453–461. doi: 10.3349/ymj.2004.45.3.453. [DOI] [PubMed] [Google Scholar]
- 12.Wares F, R Balasubramanian, A Mohan, Sharma SK. Extrapulmonary Tuberculosis: Management & Control. In: Agarwal SP, Chauhan LS, et al., editors. Tuberculosis Control in India, Directorate General of Health Services/Ministry of Health and Family Welfare. India: Elsevier; 2005. pp. 95–114. [Google Scholar]
- 13.Lin YS, Huang YC, Chang LY, Lin TY, Wong KS. Clinical characteristics of tuberculosis in children in the north of Taiwan. J Microbiol Immunol Infect. 2005;38(1):41–46. [PubMed] [Google Scholar]
- 14.Petersdorf RG, Adams RD, Braunwald E, Isselbacher KJ, Martin JB, Wilson JD, et al., editors. Harrison’s principles of internal medicine. New York: McGraw Hill; 1983. [Google Scholar]
- 15.Muradali D, Gold WL, Vellend H, Becker E. Multifocal osteoarticular tuberculosis: report of four cases and review of its management. Clin Infect Dis. 1993;17(2):204–209. [PubMed] [Google Scholar]
- 16.Grosskopf I, Ben David A, Charach G, Hochman I, Pitlik S. Bone and joint tuberculosis—a 10-year review. Isr J Med Sci. 1994;30(4):278–283. [PubMed] [Google Scholar]
- 17.Mandell GL, Bennett JE, Dolin R. Mycobacterium tuberculosis. In: Mandell GL, Bennett JE, Dolin R, et al., editors. Principles and Practice of Infectious Diseases. Philadelphia: Churchill Livingstone; 1995. pp. 2231–2243. [Google Scholar]
- 18.Watts HG, Lifeso RM. Tuberculosis of bones and joints. J Bone Joint Surg Am. 1996;78(2):288–298. doi: 10.2106/00004623-199602000-00019. [DOI] [PubMed] [Google Scholar]
- 19.Jutte PC, Louenhout-Royackers JH, Borgdorf MW, Horn JR. Increase of bone and joint tuberculosis in the Netherlands. J Bone Joint Surg. 2004;86(6):901–904. doi: 10.1302/0301-620x.86b6.14844. [DOI] [PubMed] [Google Scholar]
- 20.Shah BA, Splain S. Multifocal osteoarticular tuberculosis. Orthopedics. 2005;28(3):329–332. doi: 10.3928/0147-7447-20050301-22. [DOI] [PubMed] [Google Scholar]
- 21.Sandher DS, Al‑Jibury M, Paton RW and Ormerod LP. Bone and joint tuberculosis: Cases in Blackburn between 1988 and 2005. J Bone Joint Surg Br. 2007;89(10):1379–1381. doi: 10.1302/0301-620X.89B10.18943. [DOI] [PubMed] [Google Scholar]
- 22.Hong L, Wu JG, Ding JG, Wang XY, Zheng MH, Fu RQ, Li WB, Peng WX, He WF, Sun QF. Multifocal skeletal tuberculosis: Experience in diagnosis and treatment. Med Mal Infect. 2010;40(1):6–11. doi: 10.1016/j.medmal.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Ali R, Jalil A, Qureshi A. Extra spinal osteoarticular tuberculosis: a case series of 66 patients from a tertiary care hospital in Karachi. J Pak Med Assoc. 2012;62(12):1344–1348. [PubMed] [Google Scholar]
- 24.Pigrau-Serrallach C, and Rodríguez-Pardo D. Bone and joint tuberculosis. Eur Spine J. 2013;22(Suppl 4):556–566. doi: 10.1007/s00586-012-2331-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houston A A, Macallan DC. Extrapulmonary tuberculosis. Medicine. 2014;42(1):18–22. [Google Scholar]
- 26.Chen SC SC, Chen KT. Updated Diagnosis and Management of Osteoarticular Tuberculosis. J Emerg Med Trauma Surg Care. 2014;1:002–002. [Google Scholar]
- 27.Garrido G, Gomez-Reino JJ, Fernandez-Dapica P, Palenque E, Prieto S. A Review of Peripheral Tuberculous Arthritis. Sem Arthritis Reum. 1988;18(2):142–149. doi: 10.1016/0049-0172(88)90007-8. [DOI] [PubMed] [Google Scholar]
- 28.Al-Saleh S, Al-Arfaj A, Naddaf H, Haddad Q, Memish Z. Tuberculous arthritis: a review of 27 cases. Ann Saudi Med. 1998;18:368–369. doi: 10.5144/0256-4947.1998.368. [DOI] [PubMed] [Google Scholar]
- 29.González-Gay MA, García-Porrúa C, Cereijo MJ, Rivas MJ, Ibanez D, Mayo J. The clinical spectrum of osteoarticular tuberculosis in non-human immunodeficiency virus patients in a defined area of northwestern Spain (1988–97) Clin Exp Rheumatol. 1999;17(6):663–669. [PubMed] [Google Scholar]
- 30.Morris BS, Varma R, Garg A, Awasthi M, Maheshwari M. Multifocal musculoskeletal tuberculosis in children: appearances on computed tomography. Skeletal Radiol. 2002;31(1):1–8. doi: 10.1007/s00256-001-0439-y. [DOI] [PubMed] [Google Scholar]
- 31.Ruiz G, Rodrigues JG, Giierri ML, Gonzalez A. Osteoarticular tuberculosis in a general hospital during the last decade. Clin Microbiol Infect. 2003;9(9):919–923. doi: 10.1046/j.1469-0691.2003.00671.x. [DOI] [PubMed] [Google Scholar]
- 32.Vanhoenacker FM, Sanghvi DA, and De Backer AI. Imaging features of extraaxial musculoskeletal tuberculosis. Indian J Radiol Imaging. 2009;19(3):176–186. doi: 10.4103/0971-3026.54873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lupatkin H, Brau N, Flomenbergh P, Simberkoff MS. Tuberculous abscesses in patients with AIDS. Clin Infect Dis. 1992;14(5):1040–1044. doi: 10.1093/clinids/14.5.1040. [DOI] [PubMed] [Google Scholar]
- 34.Sankaran B. Tuberculosis of Bones & Joints. Ind J Tub. 1993;40:109–118. [Google Scholar]
- 35.Wilcox WD, Laufer S. Tuberculosis in adolescents. A case commentary. Clin Pediat. 1994;33(5):258–262. doi: 10.1177/000992289403300501. [DOI] [PubMed] [Google Scholar]
- 36.Jaber B, Gleckman R. Tuberculous pancreatic abscess as an initial AIDS-defining disorder in a patient infected with the human immunodeficiency virus: Case report and review. Clin Infect Dis. 1995;20(4):890–894. doi: 10.1093/clinids/20.4.890. [DOI] [PubMed] [Google Scholar]
- 37.Moore SL, Rafii M. Imaging of musculoskeletal and spinal tuberculosis. Radiol Clin North Am. 2001;39(2):329–342. doi: 10.1016/s0033-8389(05)70280-3. [DOI] [PubMed] [Google Scholar]
- 38.Yang Z, Kong Y, Wilson F, Foxman B, Fowler AH, Marrs CF, Cave MD, Bates JH. Identification of risk factors for extrapulmonary tuberculosis. Clinic Infect Dis. 2004;38(2):199–205. doi: 10.1086/380644. [DOI] [PubMed] [Google Scholar]
- 39.Biviji A, Paiement G, Steinbach L. Musculoskeletal manifestations of human immunodeficiency virus infection. J Am Acad Orthop Surg. 2002;10(5):312–320. doi: 10.5435/00124635-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Peto HM, Pratt RH, Harrington TA, Lobue PA, Armstrong LR. Epidemiology of extrapulmonary tuberculosis in the United States, 1993-2006. Clin Infect Dis. 2009;49(9):1350–1357. doi: 10.1086/605559. [DOI] [PubMed] [Google Scholar]
- 41.Muangchan C, Nilganuwong S. The study of clinical manifestation of osteoarticular tuberculosis in Siriraj Hospital, Thailand. J Med Assoc Thai. 2009;92(Suppl 2):S101–S109. [PubMed] [Google Scholar]
- 42.Enache SD, Pleasea IE, Anusca D, Zaharia B, Pop OT. Osteoarticular tuberculosis-a ten years case review. Rom J Morphol Embryol. 2005;46(1):67–72. [PubMed] [Google Scholar]
- 43.Talbot JC, Bismil Q, Saralaya D, Newton DAG, Frizzel RM, and Shaw DL. Musculoskeletal tuberculosis in Bradford-a 6-year review. Ann R Coll Surg Engl. 2007;89(4):405–409. doi: 10.1308/003588407X183328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davies P, Humpries MJ, Byfield SP, Nunn AJ, Darbyshire JH, Citron KM, Fox W. Bone and Joint tuberculosis. A survey of notifications in England and Wales. J Bone Joint Surg B. 1984;66(3):326–330. doi: 10.1302/0301-620X.66B3.6427232. [DOI] [PubMed] [Google Scholar]
- 45.Kritski A, de Melo FAF. Tuberculosis in adults. . In: Palomino JC, Leao SC, Ritacco V, et al., editors. Tuberculosis 2007. From basic science to patient care. 1. 2007. www.TuberculosisTextbook.com . [Google Scholar]
- 46.Gunal S, Yang Z, Agarwal M, Koroglu M, Arici ZK, Durmaz R. Demographic and microbial characteristics of extrapulmonary tuberculosis cases diagnosed in Malatya, Turkey, 2001–2007. BMC Public Health. 2011;11:154–161. doi: 10.1186/1471-2458-11-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alaya Z, Osman W, Naouar N, Ben Ayèche ML, Bouajina E. Osteoarticular Tuberculosis: Clinical and Therapeutic Feature. MOJ Orthop Rheumatol. 2016;4(5):00149–00149. [Google Scholar]
- 48.Gogia KK, Gupta S. Osteoarticular Tuberculosis-A Study Associated with Socio Demographic Factors. Ann Int Med Den Res. 2016;2(6):12–17. [Google Scholar]
- 49.Rotrosen D. Infectious arthritis. In: Wilson JD, Braunwald E, Isselbacher KJ, et al., editors. Harrison's Principles of Internal Medicine. 12. New York: McGraw-Hill; 1991. pp. 544–548. [Google Scholar]
- 50.Peh WC, Cheung KM. Progressive shoulder arthropathy. Ann Rheum Dis. 1995;54(3):168–173. doi: 10.1136/ard.54.3.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boachie-Adjei O, Squillante RG. Tuberculosis of the spine. Orthop Clin North Am. 1996;27(1):95–103. [PubMed] [Google Scholar]
- 52.Tuli SM, et al., editors. Tuberculosis of the Skeletal System. 3. New Delhi: JayPee Brothers Medical Publishers Ltd; 2004. Tuberculous osteomyelitis; pp. 174–183. [Google Scholar]
- 53.Mousa HA-L. Bones and Joints Tuberculosis. Bahrain Medical Bulletin . 2007;29(1):1–9. [Google Scholar]
- 54.Shrestha OP, Sitoula P, Hosalkar HS, Banskota AK, Spiegel DA. Bone and Joint Tuberculosis. University of Pennsylvania Orthopaedic Journal. 2010;20:23–28. [Google Scholar]
- 55.Schirmer P, Renault CA, Holodniy M. Is spinal tuberculosis contagious? Int J Infect Dis. 2010;14(8):e659–e666. doi: 10.1016/j.ijid.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 56.Arathi N, Ahmad F, and Huda N. Osteoarticular Tuberculosis-A Three Years’ Retrospective Study. J Clin Diagn Res. 2013;7(10):2189–2192. doi: 10.7860/JCDR/2013/6859.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haghighatkhah H, Jafroodi Y, Taheri M S, Pourghorban R, Dehkordy AS. Multifocal Skeletal Tuberculosis Mimicking Langerhans Cell Histiocytosis in a Child: a Case Report With a Long-Term Follow-Up. Iran Red Crescent Med J. 2015;17(12):e19942–e19942. doi: 10.5812/ircmj.19942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heycock JB, Noble TC. Four cases of syringe transmitted tuberculosis. Tubercle. 1961;42(1):25–27. doi: 10.1016/s0041-3879(61)80014-7. [DOI] [PubMed] [Google Scholar]
- 59.Wright T, Sundaram M, McDonald D. Radiologic case study: tuberculous osteomyelitis and arthritis. Orthopedics. 1996;19(8):699–702. doi: 10.3928/0147-7447-19960801-19. [DOI] [PubMed] [Google Scholar]
- 60.Abdelwahab IF, Kenan S, Hermann G, Klein MJ. Tuberculous gluteal abscess without bone involvement. Skeletal Radiol. 1998;27(1):36–39. doi: 10.1007/s002560050333. [DOI] [PubMed] [Google Scholar]
- 61.Dhillon MS, Tuli SM. Osteoarticular tuberculosis of the foot and ankle. Foot Ankle Int. 2001;22(8):679–686. doi: 10.1177/107110070102200812. [DOI] [PubMed] [Google Scholar]
- 62.Sharma SK, Mohan A. Extrapulmonary tuberculosis. Indian J Med Res . 2004;120(4):316–353. [PubMed] [Google Scholar]
- 63.Pleșea E, Enache D. Morfopatologia Tuberculozei Extrapulmonare. Editura Medicală Universitară Craiova. 2008:139–139. [Google Scholar]
- 64.Iseman MD, et al., editors. A clinician's guide to tuberculosis. Philadelphia: Williams & Wilkins;; 2000. pp. 162–170. [Google Scholar]
- 65.De Vuyst D, Vanhoenacker F, Gielen J, Bernaerts A, De Schepper AM. Imaging features of musculoskeletal tuberculosis. Eur Radiol. 2003;13(8):1809–1819. doi: 10.1007/s00330-002-1609-6. [DOI] [PubMed] [Google Scholar]
- 66.Jaovisidha S1, Chen C, Ryu KN, Siriwongpairat P, Pekanan P, Sartoris DJ, Resnick D. Tuberculous tenosynovitis and bursitis: Imaging findings in 21 cases. Radiology. 1996;201(2):507–513. doi: 10.1148/radiology.201.2.8888250. [DOI] [PubMed] [Google Scholar]
- 67.Fanning A. Tuberculosis: 6. Extrapulmonary disease. CMAJ. 1999;160(11):1597–1603. [PMC free article] [PubMed] [Google Scholar]
- 68.Spiegel DA, Singh GK, Banskota A. Tuberculosis of the Musculoskeletal System. Techniques in Orthopaedics. 2005;20(2):167–178. [Google Scholar]
- 69.Mousa HA. Tuberculosis of bones and joints: diagnostic approaches. Int Orthop. 1998;22(4):245–246. doi: 10.1007/s002640050251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pun WK, Chow SP, Luk KD, Cheng CL, Hsu LC, Leong JC. Tuberculosis of the lumbosacral junction. Long-term follow-up of 26 cases. J Bone Joint Surg Br. 1990;72(4):675–678. doi: 10.1302/0301-620X.72B4.2143192. [DOI] [PubMed] [Google Scholar]
- 71.Moon MS, Moon YW, Moon JL, Kim SS, Sun DH. Conservative treatment of tuberculosis of the lumbar and lumbosacral spine. Clin Orthop Rel Res. 2002;398:40–49. doi: 10.1097/00003086-200205000-00007. [DOI] [PubMed] [Google Scholar]
- 72.Kotil K, Alan MS, Bilge T. Medical management of Pott disease in the thoracic and lumbar spine: a prospective clinical study. J Neurosurg Spine. 2007;6(3):222–228. doi: 10.3171/spi.2007.6.3.222. [DOI] [PubMed] [Google Scholar]
- 73.Hodgson AR, Skinsnes OK, Leong CY. The pathogenesis of Pott’s paraplegia. J Bone Joint Surg. 1967;49(6):1147–1156. [PubMed] [Google Scholar]
- 74.Alvarez S, McCabe WR. Extrapulmonary tuberculosis revisited: a review of experience at Boston City and other hospitals. Medicine. 1984;63(1):25–55. [PubMed] [Google Scholar]
- 75.Peh WC, Cheung KM. Progressive shoulder arthropathy. Ann Rheum Dis. 1995;54(3):168–173. doi: 10.1136/ard.54.3.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Teo HE, Peh WC. Skeletal tuberculosis in children. Pediatr Radiol. 2004;34(11):853–860. doi: 10.1007/s00247-004-1223-7. [DOI] [PubMed] [Google Scholar]
- 77.Thimmaiah VT, and Deepashree. Unusual Presentation of Tuberculosis of Elbow Joint: A Case Report. RRJMHS. 2013;2(4):17–20. [Google Scholar]
- 78.Martini M, Adjrad A, Bouddjemaa A. Tuberculous osteomyelitis. A review of 125 cases. Int Orthop. 1986;10(2):201–207. doi: 10.1007/BF00266209. [DOI] [PubMed] [Google Scholar]
- 79.Wang MN, Chen WM, Lee KS, Chin LS, Lo WH. Tuberculous osteomyelitis in young children. J Pediatr Orthop. 1999;19(2):151–155. doi: 10.1097/00004694-199903000-00003. [DOI] [PubMed] [Google Scholar]
- 80.Huang CH. Extra-articular tuberculous osteomyelitis. A report of 11 cases. Int Orthop. 1996;20(3):169–171. doi: 10.1007/s002640050056. [DOI] [PubMed] [Google Scholar]
- 81.Yao DC, Sartoris DJ. Musculoskeletal tuberculosis. Radiol Clin North Am. 1995;33(4):679–705. [PubMed] [Google Scholar]
- 82.World Health Organization. Treatment of Tuberculosis: Guidelines. 4. Geneva: 2010. [PubMed] [Google Scholar]
- 83.Narang S. Tuberculosis of the enthuses. Int Orthop. 2012;36:2373–2378. doi: 10.1007/s00264-012-1657-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hoffman EB, Crosier JH and Cremin BJ. Imaging in children with spinal tuberculosis. A comparison of radiography, computed tomography and magnetic resonance imaging. J Bone and Joint Surg. 1993;75(2):233–239. doi: 10.1302/0301-620X.75B2.8444943. [DOI] [PubMed] [Google Scholar]
- 85.Upadhyay SS, Saji MJ, Sell P, Yau ACM. The effect of age on the change in deformity after radical resection and anterior arthrodesis for tuberculosis of the spine. J Bone and Joint Surg. 1994;76(5):701–708. doi: 10.2106/00004623-199405000-00011. [DOI] [PubMed] [Google Scholar]
- 86.Griffith JF, Kumta SM, Leung PC, Cheng JC, Chow LT, Metreweli C. Imaging of musculoskeletal tuberculosis: a new look at an old disease. Clin Orthop Rel Res. 2002;398:32–39. doi: 10.1097/00003086-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 87.Reider HL, Snider DE Jr, Cauthen GM. Extrapulmonary tuberculosis in the United States. Am Rev Respir Dis. 1990;141(2):347–351. doi: 10.1164/ajrccm/141.2.347. [DOI] [PubMed] [Google Scholar]
- 88.Kramer N, Rosenstein ED. Rheumatologic manifestations of tuberculosis. Bull Rheum Dis. 1997;46(3):5–8. [PubMed] [Google Scholar]
- 89.Buchelt M, Lack W, Kutschera HP, Katterschafka T, Kiss H, Schneider B, Kotz R. Comparison of tuberculous and pyogenic spondylitis. An analysis of 122 cases. Clin Orthop Rel Res. 1993;296:192–199. [PubMed] [Google Scholar]
- 90.Moorthy S, Prabhu NK. Spectrum of MR imaging findings in spinal tuberculosis. AJR. 2002;179(4):979–983. doi: 10.2214/ajr.179.4.1790979. [DOI] [PubMed] [Google Scholar]
- 91.Joseffer SS, Cooper PR. Modern imaging of spinal tuberculosis. Journal of Neurosurgery. 2005;2(2):145–150. doi: 10.3171/spi.2005.2.2.0145. [DOI] [PubMed] [Google Scholar]
- 92.Chang MC, Wu HT, Lee CH, Liu CL, Chen TH. Tuberculous spondylitis and pyogenic spondylitis: comparative magnetic resonance imaging features. Spine. 2006;31(7):782–788. doi: 10.1097/01.brs.0000206385.11684.d5. [DOI] [PubMed] [Google Scholar]
- 93.Jain R, Sawhney S, Berry M. Computed tomography of tuberculosis: patterns of bone destruction. Clin Radiol. 1993;47(3):196–199. doi: 10.1016/s0009-9260(05)81162-6. [DOI] [PubMed] [Google Scholar]
- 94.Ridley N, Shaikh MI, Remedios D, Mitchell R. Radiology of skeletal tuberculosis. Orthopedics. 1998;21(11):1213–1220. doi: 10.3928/0147-7447-19981101-12. [DOI] [PubMed] [Google Scholar]
- 95.Bell GR1, Stearns KL, Bonutti PM, Boumphrey FR. MRI diagnosis of tuberculous vertebral osteomyelitis. Spine. 1990;15(6):462–465. doi: 10.1097/00007632-199006000-00006. [DOI] [PubMed] [Google Scholar]
- 96.Kim NH, Lee HM, Suh JH. Magnetic resonance imaging for the diagnosis of tuberculous spondylitis. Spine. 1994;19:2451–2455. doi: 10.1097/00007632-199411000-00016. [DOI] [PubMed] [Google Scholar]
- 97.Suh JS, Lee JD, Cho JH, Kim MJ, Han DY, Cho NH. MR imaging of tuberculous arthritis: clinical and experimental studies. J Magn Reson Imaging. 1996;6(1):185–189. doi: 10.1002/jmri.1880060133. [DOI] [PubMed] [Google Scholar]
- 98.Leigh Moore S, Rafii M. Advanced imaging of tuberculosis arthritis. Semin Musculoskelet Radiol. 2003;7(2):143–153. doi: 10.1055/s-2003-41348. [DOI] [PubMed] [Google Scholar]
- 99.Soler R, Rodriguez E, Rumuinan C, Santos M. MRI of musculoskeletal extraspinal tuberculosis. J Comput Assist Tomogr. 2001;25(2):177–183. doi: 10.1097/00004728-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 100.Babhulkar S, Pande S. Tuberculosis of the hip. Clin Orthop Relat Res. 2002;398:93–99. doi: 10.1097/00003086-200205000-00013. [DOI] [PubMed] [Google Scholar]
- 101.Verettas D, Kazakos C, Tilkeridis C, Dermon A, Petrou H, Galanis V. Polymerase chain reaction for the detection of Mycobacterium tuberculosis in synovial fluid, tissue samples, bone marrow aspirate and peripheral blood. Acta Orthop Belg. 2003;69(5):396–399. [PubMed] [Google Scholar]
- 102.Bloch AB, Rieder HL, Kelly GD, Cauthen GM, Hayden CH, Snider DE. The epidemiology of tuberculosis in the United States. Sem Respir Infect. 1989;4(3):157–170. [PubMed] [Google Scholar]
- 103.Hsu SH, Sun JS, Chen IH, Liu TK. Reappraisal of skeletal tuberculosis: role of radiological imaging. Formosan Med Assn. 1993;92(1):34–41. [PubMed] [Google Scholar]
- 104.Hugosson C, Nyman RS, Brismar J, Larsson SG, Lindahl S, Lundstedt C. Imaging of tuberculosis: V, Peripheral osteoarticular and soft-tissue tuberculosis. Acta Radiol. 1996;37(4):512–516. doi: 10.1177/02841851960373P216. [DOI] [PubMed] [Google Scholar]
- 105.Steinbock RT, et al., editors. Paleopathological diagnosis and interpretation: Bone diseases in ancient human populations. Springfield, Illinois, USA: Charles C Thomas; 1976. [Google Scholar]
- 106.Roberts CA, Buikstra J, et al., editors. The Bioarchaeology of Tuberculosis: A Global View on a Reemerging Disease. Springfield, Illinois, USA: University Press of Florida; 2003. [Google Scholar]
- 107.Workeabeba A, Betel A, Kebede M, Tinsae A. Tuberculous Dactylitis: An Uncommon Presentation of Skeletal Tuberculosis. Ethiop J Health Sci. 2016;26(3):301–303. doi: 10.4314/ejhs.v26i3.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zahraa J, Johnson D, Lim-Dunham JE, Herold BC. Unusual features of osteoarticular tuberculosis in children. J Pediatr. 1996;129(4):597–602. doi: 10.1016/s0022-3476(96)70126-9. [DOI] [PubMed] [Google Scholar]
- 109.Chafetz N, Genant HK, Hoaglund FT. Ischiogluteal tuberculous bursitis with progressive bony destruction. J Can Assoc Radiol. 1982;33:119–120. [PubMed] [Google Scholar]
- 110.Trikha V, Gupta V. Isolated tuberculous abscess in biceps brachii muscle of a young male. J Infect. 2002;44(4):265–266. doi: 10.1053/jinf.2002.0986. [DOI] [PubMed] [Google Scholar]
- 111.Morris BS, Maheshwari M, Chalwa A. Chest wall tuberculosis: A review of CT appearances. Br J Radiol. 2004;77(917):449–457. doi: 10.1259/bjr/82634045. [DOI] [PubMed] [Google Scholar]
- 112.Trikha V, Varshney MK, Rastogi S. Isolated tuberculosis of the vastus lateralis muscle: A case report. Scand J Infect Dis. 2006;38(4):304–306. doi: 10.1080/00365540500353267. [DOI] [PubMed] [Google Scholar]
- 113.Batra S, Ab Naell M, Barwick C, Kanvinde R. Tuberculous pyomyositis of the tigh masguerading as malignancy with concomitant tuberculous flexor tenosynovitis and dactylitis of the hand. Singapore Med J. 2007;48(11):1042–1046. [PubMed] [Google Scholar]
- 114.Abdulaziz S, Almoallim H, Ibrahim A, Samannodi M, Shabrawishi M, Meeralam Y, Abdulmajeed G, Banjar G, Qutub W, Dowaikh H. Poncet's disease (reactive arthritis associated with tuberculosis): retrospective case series and review of literature. Clin Rheumatol. 2012;31(10):1521–1528. doi: 10.1007/s10067-012-2042-0. [DOI] [PubMed] [Google Scholar]
- 115.Teo HE, Peh WC. Skeletal tuberculosis in children. Pediatr Radiol. 2004;34(11):853–860. doi: 10.1007/s00247-004-1223-7. [DOI] [PubMed] [Google Scholar]
- 116.Hsieh CK, Miltner LJ, Chang CP. Tuberculosis of the shaft of the large long bones of the extremities. J Bone Joint Surg. 1934;16(3):545–563. [Google Scholar]
- 117.Komins C. Multiple cystic tuberculosis: a review and revised nomenclature. Br J Rad. 1952;25(289):1–8. doi: 10.1259/0007-1285-25-289-1. [DOI] [PubMed] [Google Scholar]
- 118.Shannon FB, Moore M, Houkom JA, Waecker NJ Jr. Multifocal cystic tuberculosis of bone. J Bone Joint Surg. 1990;72(7):1089–1092. [PubMed] [Google Scholar]
- 119.Aggarwal AN, Dhammi IK, Jain AK. Multifocal skeletal tuberculosis. Trop Doct. 2001;31(4):219–220. doi: 10.1177/004947550103100415. [DOI] [PubMed] [Google Scholar]
- 120.Kumar K, Saxena MBL. Multifocal osteoarticular tuberculosis. Int Orthop. 1988;12(2):135–138. doi: 10.1007/BF00266978. [DOI] [PubMed] [Google Scholar]
- 121.O’Connor BT, Steel WM, Sanders R. Disseminated bone tuberculosis. J Bone Joint Surg. 1970;52:537–542. [PubMed] [Google Scholar]
- 122.Lenaerts A, Barry CE 3rd, Dartois V. Heterogeneity in tuberculosis pathology, microenvironments and therapeutic responses. Immunol Rev. 2015;264(1):288–307. doi: 10.1111/imr.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tuli SM, et al., editors. Tuberculosis of the Skeletal System. New Delhi: Jaypee Brothers Medical Publishers; 2016. [Google Scholar]
- 124.Davidson PT, Horowitz I. Skeletal tuberculosis: a review with patient presentations and discussion. Am J Med. 1970;48(1):77–84. doi: 10.1016/0002-9343(70)90101-4. [DOI] [PubMed] [Google Scholar]
- 125.Furia JP, Box GG, Lintner DM. Tuberculous arthritis of the knee presenting as a meniscal tear. Am J Orthop (Belle Mead NJ) 1996;25(2):138–142. [PubMed] [Google Scholar]
- 126.Ortner DJ, et al., editors. Identification of pathological conditions in human skeletal remains. USA: Elsevier; 2003. [Google Scholar]
- 127.Ponce de León D1, Acevedo-Vásquez E, Sánchez-Torres A, Cucho M, Alfaro J, Perich R, Pastor C, Harrison J, Sánchez-Schwartz C. Attenuated response to purified protein derivative in patients with rheumatoid arthritis: study in a population with a high prevalence of tuberculosis. Ann Rheum Dis. 2005;64(9):1360–1361. doi: 10.1136/ard.2004.029041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kaplan CJ. Conservative Therapy in Skeletal Tuberculosis: An Appraisal Based on Experience in South Africa. Tubercle. 1959;40(5):355–368. doi: 10.1016/s0041-3879(59)80135-5. [DOI] [PubMed] [Google Scholar]
- 129.Kahn DS, Pritzker KPH. The pathophysiology of bone infection. Clin Orthop. 1973;96:12–19. [PubMed] [Google Scholar]
- 130.Kim JY, Park YH, Choi KH, Park SH, Lee HY. MRI of tuberculous pyomyositis. J Comput Assist Tomogr. 1999;23(3):454–457. doi: 10.1097/00004728-199905000-00023. [DOI] [PubMed] [Google Scholar]
- 131.López M, Barros E, Uribe A, Toro A, López J. Perfiles epidemiológico y clínico de la tuberculosis osteoarticular, estudio observacional en el Hospital Universitario San Vicente de Paul de Medellín 1994-2004. Iatreia. 2003;18(3):279–288. [Google Scholar]
- 132.Versfeld GA, Solomon A. A diagnostic approach to tuberculosis of bones and joints. J Bone Joint Surg Br. 1982;64(4):446–449. doi: 10.1302/0301-620X.64B4.7096418. [DOI] [PubMed] [Google Scholar]
- 133.Mondal A. Cytological diagnosis of vertebral tuberculosis with fine-needle aspiration biopsy. J Bone Joint Surg Am. 1994;76(2):181–184. doi: 10.2106/00004623-199402000-00003. [DOI] [PubMed] [Google Scholar]
- 134.Yilmaz MH, Kantarci F, Mihmanli I, Kanberoglu K. Multifocal Skeletal Tuberculosis. South Med J. 2004;97(8):785–787. doi: 10.1097/00007611-200408000-00023. [DOI] [PubMed] [Google Scholar]
- 135.Thrush A, Enzmann D. MR imaging of infectious spondylitis. Am J Neuroradiol. 1990;11(6):1171–1180. [PMC free article] [PubMed] [Google Scholar]
- 136.American Thoracic Society and the Centers for Disease Control and Prevention. Diagnostic Standards and Classification of Tuberculosis in Adults and Children. . Am J Respir Crit Care Med. 2000;161(4 Pt 1):1376–1395. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 137.Merino P, Candel FJ, Gestoso I, Baos E, Picazo J. Microbiological diagnosis of spinal tuberculosis. Int Orthop. 2012;36(2):233–238. doi: 10.1007/s00264-011-1461-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Colmenero JD1, Ruiz-Mesa JD, Sanjuan-Jimenez R, Sobrino B, Morata P. Establishing the diagnosis of tuberculous vertebral osteomyelitis. Eur Spine J. 2013;22(Suppl 4):579–586. doi: 10.1007/s00586-012-2348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dass B, Puet TA, Watanakunakorn C. Tuberculosis of the spine (Pott's disease) presenting as 'compression fractures. Spinal Cord. 2002;40(11):604–608. doi: 10.1038/sj.sc.3101365. [DOI] [PubMed] [Google Scholar]
- 140.Titov AG, Vyshnevskaya EB, Mazurenko SI, Santavirta S, Konttinen YT. Use of polymerase chain reaction to diagnose tuberculous arthritis from joint tissues and synovial fluid. Arch Pathol Lab Med. 2004;128(2):205–209. doi: 10.5858/2004-128-205-UOPCRT. [DOI] [PubMed] [Google Scholar]
- 141.Held M, Laubscher M, Mears S, Dix-Peek S, Workman L, Zar H, Dunn R. Diagnostic Accuracy of the Xpert MTB/RIF Assay for Extrapulmonary Tuberculosis in Children With Musculoskeletal Infections. Pediatr Infect Dis J. 2016;35(11):1165–1168. doi: 10.1097/INF.0000000000001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shanmugasundaram TK. Bone and joint tuberculosis-Guidelines for management. Indian J Orthop. 2005;39(3):195–198. [Google Scholar]
- 143.Rajasekaran S, et al., editors. A longitudinal study on the progress of deformity in children with spinal tuberculosis. Chennai: Dr. MGR Medical University; 1999. [Google Scholar]
- 144.Rajasekaran S. Natural history of Pott's kyphosis. Eur Spine J. 2013;22(Suppl 4):634–640. doi: 10.1007/s00586-012-2336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Herzog H. History of tuberculosis. Respiration. 1998;65(1):5–15. doi: 10.1159/000029220. [DOI] [PubMed] [Google Scholar]
- 146.Tay BK, Deckey J, Hu SS. Spinal infections. J Am Acad Orthop Surg. 2002;10(3):188–197. doi: 10.5435/00124635-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 147.Sequeira W, Co H, Block JA. Osteoarticular tuberculosis: current diagnosis and treatment. Am J Ther. 2000;7(6):393–398. [PubMed] [Google Scholar]
- 148.Tuli SM. Result of treatment of spinal tuberculosis by the “middle path” regime. J Bone Joint Surg. 1975;57(1):13–23. [PubMed] [Google Scholar]
- 149.Jain AK. Treatment of tuberculosis of the spine with neurologic complications. Clin Orthop Rel Res. 2002;398:75–84. doi: 10.1097/00003086-200205000-00011. [DOI] [PubMed] [Google Scholar]
- 150.Chandir S, Hussain H, Salahuddin N, Amir M, Ali F, Lotia I, Khan AJ. Extrapulmonary Tuberculosis: A retrospective review of 194 cases at a tertiary care hospital in Karachi, Pakistan. J Pak Med Assoc. 2010;60(2):105–108. [PubMed] [Google Scholar]
- 151.Agarwal A, Qureshi NA, Khan SA, Kumar P, Samaiya S. Tuberculosis of the foot and ankle in children. J Orthopedic Sur. 2011;19(2):213–217. doi: 10.1177/230949901101900217. [DOI] [PubMed] [Google Scholar]
- 152.Dhillon MS, Gupta RK, Bahadur R, Nagi ON. Tuberculosis of the sternoclavicular joints. Acta Orthop Scand. 2001;72(5):514–517. doi: 10.1080/000164701753532862. [DOI] [PubMed] [Google Scholar]
- 153.Anand A, Sood LK. Isolated tuberculosis of talus without ankle and subtalar joint Involvement. Med J Malaysia. 2002;57(3):371–373. [PubMed] [Google Scholar]
- 154.Behari S, Nayak SR, Bhargava V, Banerji D, Chhabra DK, Jain VK. Craniocervical tuberculosis: Protocol of surgical management. Neurosurgery. 2003;52(1):72–81. [PubMed] [Google Scholar]
- 155.Wellons JC, Zomorodi AR, Villaviciencio AT, Woods CW, Lawson WT, Eastwood JD. Sacral tuberculosis: A case report and review of the literature. Surg Neurol. 2004;61(2):136–141. doi: 10.1016/s0090-3019(03)00265-9. [DOI] [PubMed] [Google Scholar]
- 156.Subasi M, Bukte Y, Kapukaya A, Gurkan F. Tuberculosis of the metacarpals and phalanges of the hand. Ann Plast Surg. 2004;53(5):469–472. doi: 10.1097/01.sap.0000130708.80606.6a. [DOI] [PubMed] [Google Scholar]
- 157.Hodgson SP, Ormerod LP. Ten-year experience of bone and joint tuberculosis in Blackburn 1978-1987. J R Coll Surg Edinb. 1990;35(4):259–262. [PubMed] [Google Scholar]
- 158.Al-Qattan MM, Al-Namla A, Al-Thunayan A, Al-Omawi M. Tuberculosis of the hand. J Hand Surg Am. 2011;36(8):1413–1421. doi: 10.1016/j.jhsa.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 159.Chen SH, Lee CH, Wong T, Feng HS. Long-term retrospective analysis of surgical treatment for irretrievable tuberculosis of the ankle. Foot Ankle Int. 2013;34(3):372–337. doi: 10.1177/1071100712464955. [DOI] [PubMed] [Google Scholar]
- 160.Jutte PC, Van Loenhout-Rooyackers JH. Routine surgery in addition to chemotherapy for treating spinal tuberculosis. Cochrane Database Syst Rev. 2006;25(1):CD004532–CD004532. doi: 10.1002/14651858.CD004532.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lawn SD, Zumla AI. Tuberculosis. Lancet. 2011;378(9785):57–72. doi: 10.1016/S0140-6736(10)62173-3. [DOI] [PubMed] [Google Scholar]
- 162.A controlled trial of anterior spinal fusion and debridement in the surgical management of tuberculosis of the spine in patients on standard chemotherapy. A study in Hong Kong. Fourth Report of the Medical Research Council Working Party on Tuberculosis of the Spine. British J Surg. 1974;61(11):853–866. doi: 10.1002/bjs.1800611102. [DOI] [PubMed] [Google Scholar]
- 163. Medical Research Council Working Party. Tuberculosis of the Spine. J Bone and Joint Surg. 1978;60-B(2):163–177. doi: 10.1302/0301-620X.60B2.350883. [DOI] [PubMed] [Google Scholar]
- 164.Upadhyay SS, Sell P, Saji MJ, Sell B, Yau AC, Leong JC. 17-year prospective study of surgical management of spinal tuberculosis in children. Hong Kong operation compared with debridement surgery for short- and long-term outcome of deformity. Spine. 1993;18(2):1704–1711. doi: 10.1097/00007632-199309000-00020. [DOI] [PubMed] [Google Scholar]
- 165.Upadhyay SS, Sell P, Saji MJ, Sell B, Hsu LC. Surgical management of spinal tuberculosis in adults. Hong Kong operation compared with debridement surgery for short and long term outcome of deformity. Clin Orthop Relat Res. 1994;(302):173–182. [PubMed] [Google Scholar]
- 166.Upadhyay SS, Saji MJ, Sell P, Sell B, Hsu LCS. Spinal deformity after childhood surgery for tuberculosis of the spine. A comparison of radical surgery and debridement. J. Bone and Joint Surg. 1994;76(1):91–98. [PubMed] [Google Scholar]
- 167.Upadhyay SS, Saji MJ, Sell P, Sell B, Yau AC. Longitudinal changes in spinal deformity after anterior spinal surgery for tuberculosis of the spine in adults. A comparative analysis between radical and debridement surgery. Spine. 1994;19(5):542–549. doi: 10.1097/00007632-199403000-00009. [DOI] [PubMed] [Google Scholar]
- 168.Ho EK , Leong JC. Tuberculosis of the spine. In: Weinstein SL , et al., editors. The Pediatric Spine. Principles and Practice. New York: Raven Press; 1994. pp. 837–849. [Google Scholar]
- 169.Khoo LT, Mikawa K, Fessler RG. A surgical revisitation of Pott distemper of the spine. Spine J. 2003;3(2):130–145. doi: 10.1016/s1529-9430(02)00410-2. [DOI] [PubMed] [Google Scholar]
- 170.Nene A, Bhojraj S. Results of nonsurgical treatment of thoracic spinal tuberculosis in adults. Spine J. 2005;5(1):79–84. doi: 10.1016/j.spinee.2004.05.255. [DOI] [PubMed] [Google Scholar]
- 171.Kim YT, Han KN, Kang CH, Sung SW, Kim JH. Complete resection is mandatory for tubercular cold abscess of the chest wall. Ann Thorac Surg. 2008;85(1):273–277. doi: 10.1016/j.athoracsur.2007.08.046. [DOI] [PubMed] [Google Scholar]
- 172.Suárez-García I, Noguerado A. Drug treatment of multidrug-resistant osteoarticular tuberculosis: a systematic literature review. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2012;16(11):e774–e778. doi: 10.1016/j.ijid.2012.07.011. [DOI] [PubMed] [Google Scholar]