Abstract
CD44 seems to confer the needed conditions for malignant neoplasms to grow and progress. The present study aims to investigate the role of CD44 expression in pre-invasive and invasive squamous lesions of the skin. We investigated 89 cases of preinvasive and invasive cutaneous lesions, of which 28 corresponded to actinic keratosis (KIN- keratinocyte intraepithelial neoplasia) with varying degrees of severity and 61 cases of squamous cell carcinoma with variable degrees of differentiation. The statistical analysis of CD44 immunoexpression indicated significantly higher values for KIN I and II compared to KIN III, as well as for KIN lesions in comparison with squamous cell carcinomas. Similar results were observed in well differentiated carcinomas compared to moderate and poorly differentiated lesions. These observations suggest that CD44 expression plays a role in the progression of cutaneous squamous neoplasia.
Keywords: CD44, actinic keratosis, squamous cell carcinomas
Introduction
CD44 is a family of polymorphic membranous glycoproteins that mediates cell-cell and cell-extracellular matrix interactions. Certain isoform variants of CD44 (CD44v) appear to confer the necessary malignant properties for tumor cell growth and progression of cancer [1,2,3,4], being involved in invasion and metastasis mechanisms. CD44 is the major receptor for hyaluronan, which was considered an early marker of malignant transformation, a prerequisite for tumor progression [5,6].
At present, only limited information is available on the factors responsible for the onset and progression of cutaneous squamous cell carcinomas [7]. A promising candidate in this regard is CD44, but the knowledge about the distribution of this molecule in human skin and skin tumors is low. However, some studies have shown that CD44 influences the proliferation and differentiation of keratinocytes [7,8,9,10].
In addition, recent studies suggest that the CD44 phenotype in squamous cell carcinomas may be considered a candidate for stem cell therapy [11].
In the present study, we have planned to investigate the expression of CD44 in pre-invasive and invasive squamous lesions of the skin.
Material and methods
We studied 89 cases of which 28 cases of actinic keratosis (KIN) and 61 cases of squamous carcinomas, belonging to patients admitted in the Dermatology and Surgery Clinics of the Emergency County Hospital Craiova. Surgical fragments were fixed in 10% buffered formaldehyde, processed by classic histopathological technique and hematoxylin-eosin (HE) stained. The classification of lesions has been made according to the literature recommendations [12,13]. The 28 cases diagnosed with KIN corresponded to KIN I in 22cases, KIN II in three cases and KIN III in three cases. The investigated squamous carcinomas corresponded in 18 cases to well-differentiated forms (16 in Stage I and two in Stage II), in 37 cases to moderately differentiated types (32 in Stage I, four in Stage II and one in Stage III) and in 6 cases to poorly differentiated tumors (three in Stage I, two in Stage II, and one in Stage III).
Seriated sections were subsequently processed for immunohistochemistry using a polymer amplification based detection system (Histofine Horseradish Peroxidase conjugated polymer, Nichirei, Japan, ready to use, code 414151F). For visualization of the reactions, DAB (3,3'-Diaminobenzidine) chromogen (code 3467, Dako) was used, with positive (spleen tissue) and negative external controls (by omitting the primary antibody) being used to validate the reactions. We used the mouse antihuman monoclonal CD44 antibody (clone DF 1485/Dako), diluted 1:80, with pH 6 citrate buffer as retrieval solution.
To evaluate the semi-quantitative expression of CD44, a scoring system was adopted, which was independently assigned by two specialists (CS and AS), based on the intensity of staining and the percentage of positive cells [14].
The intensity score was considered as: 1 for low intensity, 2 for moderate intensity and 3 for strong intensity. Reactions positivity cutoff was set at 5%. The labelled cell score was scored as 1 for 6-25% positive cells, 2 for 26-50% positive cells, 3 for 51-75% positive cells and 4 for >75% positive cells. The final staining score (FSS) was calculated by multiplying the intensity and labeled cells scores. For the statistical analysis, FSS were considered low for values between 1-4 and high for values between 6-12. In this study we used comparison tests (ANOVA, χ2 test) within Statistical Package for the Social Sciences (SPSS) 10 software. The study was approved by the local ethical committee (no.171/11.09.2017), and written informed consent was obtained from all the patients.
Results
CD44 expression analysis revealed a positive immunoreaction in 84 cases (92.3%), of which 28 cases of actinic keratosis and 56 cases of squamous carcinoma (Table 11).
Table 1.
Distribution of CD44 positive cases
| Lesion type | Actinic keratosis | Squamous cell carcinoma | ||||
| KIN I | KIN II | KIN III | WD | MD | PD | |
| Nr of cases | 22 | 3 | 3 | 16 | 34 | 6 |
KIN- keratinocyte intraepithelial neoplasia; WD-well differentiated; MD-moderate differentiated; PD-poorly differentiated
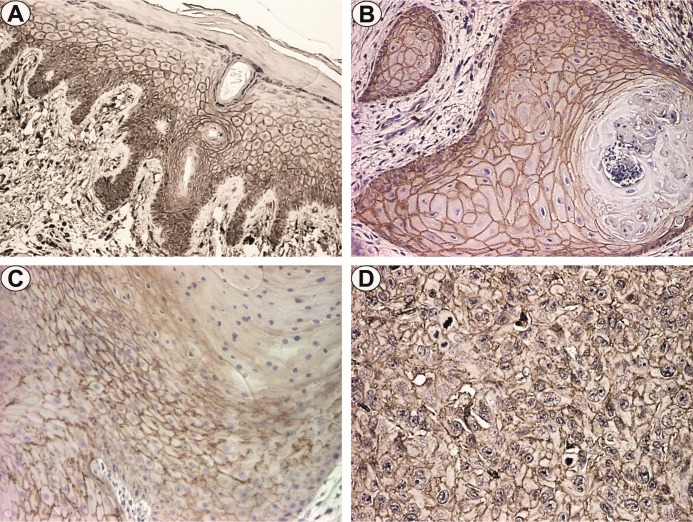
Analysis of the percentage of positive CD44 cells and intensity revealed the presence of the marker for all investigated actinic keratosis (100%). The distribution of the CD44 marker was observed membranous throughout the thickness of the epidermis (with the exception of the granular and corneous layer) with moderate or increased intensity (Fig.1A). The FSS score of CD44 for these cases ranged from 3 to 12, with the average values of 7.6 for KIN I, 7 for KIN II and 4.3 for KIN III (Table 2)
Figure 1.
CD44 immunostaining: a. KIN I, x100; b. Well differentiated squamous cell carcinoma, x100; c. Moderately differentiated squamous carcinoma, x100; d. Poorly differentiated squamous carcinoma, x100
Table 2.
Distribution of actinic keratosis cases depending of CD44 FSS
| KIN degree | KIN I | KIN II | KIN III |
| FSS / no. of positive cases | 7,6/22 | 7/3 | 4,3/3 |
* FSS-final staining scores; KIN- keratinocyte intraepithelial neoplasia
For the cases of squamous carcinomas, we noticed the positivity of reaction in 56 cases, representing 91.8% of investigated lesions.
We noticed variations in the CD44 FSS values according to tumor grade and stage (Table 3).
Table 3.
Distribution of cases of squamous cell carcinoma depending of CD44 FSS
| Degree / Stage | WD | MD | PD |
| Stage I: FSS / no. of positive cases | 7.5/14 | 5.6/30 | 6.3/3 |
| Stage II: FSS / no. of positive cases | 6/2 | 6,3/3 | 5/2 |
| Stage III: FSS / no. of positive cases | 0/0 | 4/1 | 2/1 |
* WD-well differentiated; MD-moderate differentiated; PD-poorly differentiated; FSS-final staining scores; KIN-keratinocyte intraepithelial neoplasia
We have found positivity for 16 (88.8%) of well-differentiated tumors and 34 (91.8%) and 6 (100%) for moderately respectively for the poorly differentiated tumors, irrespective of tumor stage. Depending on the tumor stage, we noticed positivity for all stage III tumors (100%), but also for Stage II (87.5%) and Stage I (92.1%) tumors
In the case of well-differentiated tumors, membranous immunoexpression of CD44 was observed with diffuse pattern and medium or increased intensity in tumoral islets (Fig.1B). CD44 immunostaining was also present in inflammatory cells of tumoral stroma. For moderate and poorly differentiated carcinoma, the CD44 reactions intensity was moderate or high (Fig.1C-D).
For squamous carcinomas FSS values varied between 2 and 12. For the stage I tumors, the FSS values were 7.5, 5.6 and 6.3 in well, moderately and respectively poorly differentiated forms. For the stage II tumors the FSS values were 6, 6.3 and 5 in the well, moderately and poorly differentiated lesions. For the stage III lesions, the FSS values were 0, 4 and 2 tumors in well, moderately and poorly differentiated tumors.
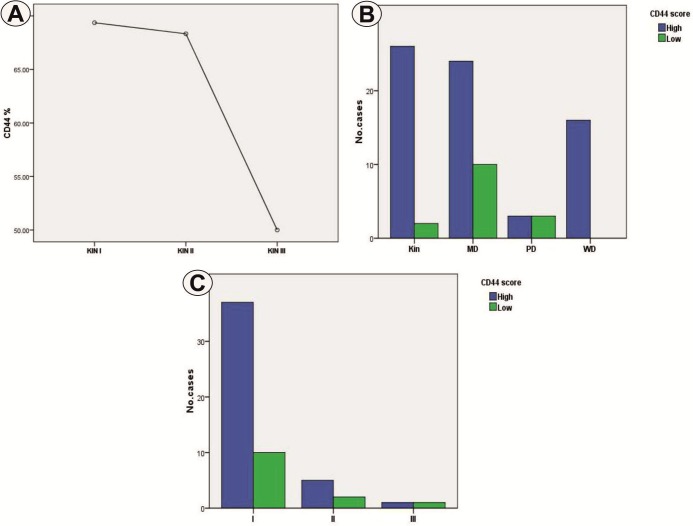
The statistical analysis indicated significantly higher values for KIN I and II, compared to KIN III (p=0.001, ANOVA test) (Fig.2A). There were also significantly higher differences in KIN lesions compared to carcinomas, as well as in well-differentiated carcinomas compared to moderate and poorly differentiated (p=0.005, χ 2 test) (Fig.2B). We did not find statistical associations of CD44 expression with the tumor stage (p=0.601, χ 2 test) (Fig.2C)
Figure 2.
a. Distribution of CD44 values depending on KIN grade; b. Distribution of CD44 positive cases in relation to lesion type and differentiation degree of carcinomas; c. Distribution of CD44 positive carcinomas depending on tumor stage
Discussion
CD44 antigen is a surface glycosylated polypeptide involved in various cellular functions including cell adhesion. Moreover, it has been demonstrated that CD44 expression appears to confer metastatic potential for cell lines originating from certain carcinomas.
In the normal skin, CD44 is expressed in keratinocytes along the epidermis, with the exception of granular and corneous layers, in the hair follicles, eccrine sweat glands and dermal dendritic cells [15,16]. Other studies have reported for the normal epidermis positivity in upper layers of cells, and their progressive decreasing to suprabasal layers [17]. In addition, the immunoexpression of CD44 was identified in various skin tumors, such as melanoma, Merkel carcinoma and squamous cell carcinoma [18,19,20].
In our study, we found CD44 immunoexpression in all cases of actinic keratosis. The distribution of the CD44 staining was membranous, throughout the thickness of the epidermis with moderate or increased intensity. Similar studies in the literature reported reduced expression of CD44 for actinic keratosis, focal or diffuse expression for Bowen disease [21] and intense expression for all invasive and metastatic squamous carcinomas [15,16,21]. Another recent study indicates differential expression of CD44 depending of the KIN grade, in KIN I and KIN II being located in basal and suprabasal layers, while in KIN III the expression was diffused across the thickness of the epidermis [17]. The authors suggest that differences of expression in normal skin and KIN, as well as in KIN of varying degrees, may be associated with alteration of stratification and differentiation of keratinocytes during the transformation of keratosis into squamous cell carcinoma [17].
The investigated squamous carcinomas were positive in 91.8% of cases. In the case of well-differentiated squamous carcinomas, the CD44 marker was present diffuse membranous, in the tumoral islets, with medium or increased intensity. For moderate and poorly differentiated carcinoma forms, the CD44 immunostaining was membranous and diffuse in the neoplastic cell islets with medium or high intensity. One study reported variable expression in squamous carcinomas depending on the degree of differentiation [22]. Prieto et al. find intense expression for CD44 in all invasive and metastatic squamous carcinomas [16]. Literature studies reach contradictory conclusions, some consider that CD44 expression in cutaneous squamous cell carcinomas is not related to malignant transformation, but may be related to tumor progression and metastasis [16], while others argue that CD44 expression is not correlated with the invasive and metastatic potential, and is rather related to the degree of tumor differentiation [22].
Conclusions
CD44 reactions were significantly higher in preinvasive and invasive lesions with low degree, with significantly higher differences of KIN lesions compared to squamous carcinomas and depending on the degree of differentiation. These observations suggest that CD44 expression plays a role in the progression of cutaneous squamous neoplasia.
References
- 1.Grimme HU, Termeer CC, Bennett KL, Weiss JM, Schöpf E, Aruffo A, Simon JC. Colocalization of basic fibroblast growth factor and CD44 isoforms containing the variably spliced exon v3 (CD44v3) in normal skin and in epidermal skin cancers. Br J Dermatol. 1999;141(5):824–832. doi: 10.1046/j.1365-2133.1999.03154.x. [DOI] [PubMed] [Google Scholar]
- 2.Dietrich A, Tanczos E, Vanscheidt W, Schöpf E, Simon JC. Detection of CD44 splice variants in formalin-fixed, paraffin-embedded specimens of human skin cancer. J Cutan Pathol. 1997;24(1):37–42. doi: 10.1111/j.1600-0560.1997.tb00783.x. [DOI] [PubMed] [Google Scholar]
- 3.Bourguignon LY, Singleton PA, Diedrich F. Hyaluronan-CD44 interaction with Rac1-dependent protein kinase N-gamma promotes phospholipase Cgamma1 activation, Ca(2+) signaling, and cortactin-cytoskeleton function leading to keratinocyte adhesion and differentiation. J Biol Chem. 2004;279(28):29654–29669. doi: 10.1074/jbc.M403608200. [DOI] [PubMed] [Google Scholar]
- 4.Bourguignon LY, Ramez M, Gilad E, Gilad E, Singleton PA, Man MQ, Crumrine DA, Elias PM, Feingold KR. Hyaluronan-CD44 interaction stimulates keratinocyte differentiation, lamellar body formation/secretion, and permeability barrier homeostasis. J Invest Dermatol. 2006;126(6):1356–1365. doi: 10.1038/sj.jid.5700260. [DOI] [PubMed] [Google Scholar]
- 5.Siiskonen H, Törrönen K, Kumlin T, Kumlin T, Rilla K, Tammi MI, Tammi RH. Chronic UVR causes increased immunostaining of CD44 and accumulation of hyaluronan in mouse epidermis. J Histochem Cytochem. 2011;59(10):908–917. doi: 10.1369/0022155411417874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orian-Rousseau V, Ponta H. Perspectives of CD44 targeting therapies. Arch Toxicol. 2015;89(1):3–14. doi: 10.1007/s00204-014-1424-2. [DOI] [PubMed] [Google Scholar]
- 7.Bourguignon LYW, Bikle D. Selective hyaluronan–CD44 signaling promotes miRNA-21 expression and interacts with vitamin D function during cutaneous squamous cell carcinomas progression following UV irradiation. Front Immunol. 2015;6:224–224. doi: 10.3389/fimmu.2015.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signaling regulators. Nat Rev Mol Cell Biol. 2003;4(1):33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 9.Pasonen-Seppänen S, Hyttinen JM, Rilla K, Jokela T, Noble PW, Tammi M, Tammi R. Role of CD44 in the organization of keratinocyte pericellular hyaluronan. Histochem Cell Biol. 2012;137(1):107–120. doi: 10.1007/s00418-011-0883-2. [DOI] [PubMed] [Google Scholar]
- 10.Orian-Rousseau V, Sleeman J. CD44 is a multidomain signaling platform that integrates extracellular matrix cues with growth factor and cytokine signals. Adv Cancer Res. 2014;123:231–254. doi: 10.1016/B978-0-12-800092-2.00009-5. [DOI] [PubMed] [Google Scholar]
- 11.Erfani E, Roudi R, Rakhshan A, Sabet MN, Shariftabrizi A, Madjd Z. Comparative expression analysis of putative cancer stem cell markers CD44 and ALDH1A1 in various skin cancer subtypes. Int J Biol Markers. 2016;31(1):53–61. doi: 10.5301/jbm.5000165. [DOI] [PubMed] [Google Scholar]
- 12.Weedon D, Morgan MB, Gross C, Nagore E. Squamous cell carcinoma. In: Yu LL, LeBoit PE, Burg G, Weedon D, Sarasin A, editors. Pathology and Genetics of Skin Tumours. Lyon: IARC Pres; 2006. pp. 30–32. [Google Scholar]
- 13.Cockerell CJ. Histopathology of incipient intraepidermal squamous cell carcinoma ("actinic keratosis"). Overexpression of HIF-1α, metallothionein and SLUG is associated with high TNM stage and lymph node metastasis. J Am Acad Dermatol. 2000;42(1):11–17. doi: 10.1067/mjd.2000.103344. [DOI] [PubMed] [Google Scholar]
- 14.Wang N, Dong CR, Jiang R, Tang C, Yang L, Jiang QF, Chen GG, Liu ZM. Overexpression of HIF-1α, metallothionein and SLUG is associated with high TNM stage and lymph node metastasis in papillary thyroid carcinoma. Int J Clin Exp Pathol. 2013;7(1):322–330. [PMC free article] [PubMed] [Google Scholar]
- 15.Prieto VG, Reed JA, McNutt NS, Bogdany JK, Lugo J, Shea CR. Differential expression of CD44 in malignant cutaneous epithelial neoplasms. Am J Dermatopathol. 1995;17(5):447–451. doi: 10.1097/00000372-199510000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Yasaka N, Furue M, Tamaki K. CD44 expression in normal human skin and skin tumors. J Dermatol. 1995;22(2):88–94. doi: 10.1111/j.1346-8138.1995.tb03349.x. [DOI] [PubMed] [Google Scholar]
- 17.Arciniegas E, Carrillo LM, Rojas H, Ramírez R, Reyes O, Suárez A, Ortega F. Mucin expression in focal epidermal dysplasia of actinic keratosis. Ann Transl Med. 2015;3(17):245–245. doi: 10.3978/j.issn.2305-5839.2015.10.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dabbs DJ. Immunohistochemistry of Skin Tumors. Philadelphia: Churchill Livingstone; 2009. Diagnostic Immunohistochemistry; pp. 313–406. [Google Scholar]
- 19.Penneys NS, Shapiro S. CD44 expression in Merkel cell carcinoma may correlate with risk ofmetastasis. J Cutan Pathol. 1994;21(1):22–26. doi: 10.1111/j.1600-0560.1994.tb00686.x. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Figueras MT. CD44 and melanocytic tumors: a possible role for standard CD44 in the epidermotropic spread melanoma. J Cutan Pathol. 1996;23(2):133–139. doi: 10.1111/j.1600-0560.1996.tb01286.x. [DOI] [PubMed] [Google Scholar]
- 21.Ko JW, Suh KS, Kim ST. CD44 Standard and Variants Expression in Keratinocytic Tumor. Korean J Dermatol. 2000;38(7):880–887. [Google Scholar]
- 22.Seelentag WK, Günthert U, Saremaslani P, Futo E, Pfaltz M, Heitz PU, Roth J. CD44 standard and variant isoform expression in human epidermal skin tumors is not correlated with tumor aggressiveness but down-regulated during proliferation and tumor de-differentiation. Int J Cancer. 1996;69(3):218–224. doi: 10.1002/(SICI)1097-0215(19960621)69:3<218::AID-IJC12>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]