Abstract
Despite the fact that acute gastroenteritis can be prevented, the disease still affects children, especially under the age of two. The increased levels of pediatric mortality in most developing regions make diarrheal diseases one of the most common causes of death in the children under the age of 5. The purpose of the study was to describe the cases of acute gastroenteritis reported as healthcare-associated infections in a pediatric hospital deserving the north-eastern urban and rural regions of Romania. Material and methods. A descriptive study was conducted on a group of 615 cases with acute bacterial gastroenteritis as healthcare-associated infections (HAIs), reported in “Sf. Maria” Emergency Hospital for Children, Iași, between 2012 and 2016. Results. Most cases of acute bacterial gastroenteritis were registered in 2015 (154 cases-25.04%), and the lowest in 2012 (12.84%). Male gender prevailed in almost all years of study, with no statistical significance (p≥0.05). Gastroenteritis with Campylobacter was most commonly reported in pediatric wards, especially in infants of 0-1 years old and children aged of 2-6 years. Cases of HAIs with Salmonella spp were also frequent. Conclusions. A competent management of HAIs especially as acute gastroenteritis in an emergency hospital for children from a region that includes developing rural areas, should be the most important issue for professionals involved in surveillance and control strategies, as well as clinicians, epidemiologist and microbiologist, in order to prevent HAIs burden occurrence and avoid antimicrobial resistance.
Keywords: acute gastroenteritis, bacterial, healthcare-associated infections, pediatrics
Introduction
Acute gastroenteritis affects 3 to 5 billion of children every year worldwide. Mortality caused by acute gastroenteritis has high levels of 1.5-2.5 million deaths per year or 12% of all deaths in children less than 5 years of age. In developed countries, acute gastroenteritis rarely causes deaths, but more than 300 deaths were reported in the United States yearly, lead to a heavy burden on the health system, with increased hospitalization costs and decreased quality of life in pediatric patients. In the United States also, acute gastroenteritis diagnosis leads to 1.5 million visits to primary care providers and 220,000 admissions to pediatric hospitals for children under the age of 5, representing 10% of all pediatric hospitalizations in this country (9-10 of 1000 yearly). In developing countries, the rate of hospitalizations for acute gastroenteritis is much higher than in developed countries. In the United Kingdom and Australia, hospitalization rates are around 12-15 per 1000 yearly. In pediatric hospitals from China, the hospitalization rate is 26 to 1,000 yearly. In Hong Kong, the rate of hospitalization is even higher than many developing countries. The difference can be explained by the level of primary health care and nutritional status, but also by the circulation of various pathogens involved in the occurrence of acute gastroenteritis in children and the decision to hospitalize the child by parents or the family as well as other social and economic factors [1,2,3].
Acute bacterial gastroenteritis accounts for 10% to 20% of all acute gastroenteritis. The most common bacterial etiologies are due to Salmonella, Campylobacter, Shigella, and Yersinia. Vibrio cholerae remains a major cause of diarrhea, especially after a social or natural disaster when sanitation is compromised [2,3].
Most cases of acute gastroenteritis tend to self-limitation and require only supportive therapy. Antibiotic treatment is suggested in patients with suspected invasive processes and severe diarrhea, systemic symptoms, fever or abdominal pain as well as in patients with toxic signs. Some studies have reported the association between the consumption of shrimp or crab and acute gastroenteritis with Escherichia coli, Vibrio spp, Aeromonas spp, Listeria monocytogenes, especially in developing countries with lower sanitation conditions [4].
Despite the fact that acute gastroenteritis can be prevented, the disease still affects children, especially under the age of two. The increased levels of pediatric mortality in most developing regions make diarrheal diseases the most common cause of death in the children under the age of 5 years after pneumonia. Although the burden of diarrheal disease in children under the age of 5 is difficult to count, prevention is a way to improve. Personal hygiene and food safety, including the use of clean water sources, are key measures to prevent the transmission of these diseases. Breastfeeding, especially under the age of 6 months, protects babies effectively [5].
The purpose of the study was to describe the cases of acute gastroenteritis reported as associated with health care in a pediatric hospital deserving the north-eastern urban and rural regions of Romania.
Material and methods
We conducted a descriptive study on a group of 615 cases with bacterial gastroenteritis as healthcare-associated infections (HAIs), admitted in “Sf. Maria” Emergency Hospital for Children, Iași, between 2012 and 2016.
Inclusion criteria were diagnosis of gastroenteritis with bacterial etiology detected as healthcare-associated infection (HAI). Exclusion criteria were diagnosis of viral gastroenteritis as well as non-infectious gastroenteritis. Age group, gender, and residence area were no selection criteria. =
The data were collected retrospectively from patients’ records and HAIs reporting files, subsequently statistically processed using the SPSS 20.0 software.
Results
Most cases of bacterial gastroenteritis diagnosed as HAIs were reported in 2015 (154 cases-25.04% of the total), and the lowest in 2012 (12.84%). There is an upward trend in their incidence (Fig.1).
Figure 1.
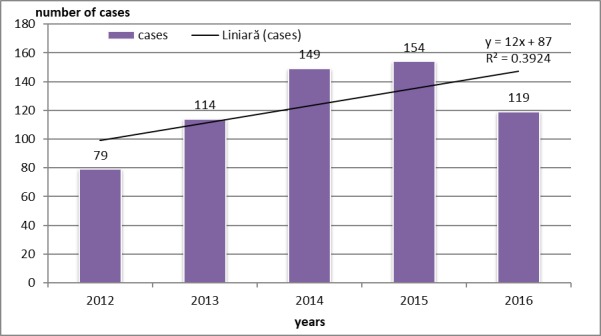
Patients distribution according to DLBCL treatment regimen
Gender distribution showed a slight predominance of boys (333 cases-54.14%). Male gender prevailed in almost all years of study, with no statistical significance (p≥0.05), except 2016 when M/F=1.53 with statistically significance of p<0.01 (Table 1).
Table 1.
Distribution of cases by year of study, gender and pathogens
| Pathogens | 2012 | 2013 | 2014 | 2015 | 2016 | ||||||||||
| M | F | Total | M | F | Total | M | F | Total | M | F | Total | M | F | Total | |
| Salmonella | 6 | 6 | 12 | 12 | 15 | 27 | 25 | 18 | 43 | 11 | 15 | 26 | 12 | 7 | 19 |
| Shigella | 0 | 1 | 1 | 1 | 3 | 4 | 2 | 2 | 4 | 0 | 1 | 1 | 2 | 0 | 2 |
| E.coli | 4 | 2 | 6 | 0 | 0 | 0 | 4 | 1 | 5 | 5 | 4 | 9 | 2 | 7 | 9 |
| Campylobacter | 24 | 30 | 54 | 46 | 34 | 80 | 46 | 49 | 95 | 69 | 46 | 115 | 53 | 31 | 84 |
| Yersinia enterocolitica | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Others | 4 | 2 | 6 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 2 | 3 | 3 | 2 | 5 |
| Total | 38 | 41 | 79 | 59 | 53 | 114 | 78 | 73 | 149 | 86 | 68 | 154 | 72 | 47 | 119 |
Regarding distribution by age, children of 0-1 years old were the most affected (478 cases-77.72%) (Table 2).
Table 2.
Distribution of bacterial infections cases by age groups
| Year of studyAge group | 0-1years | 2-5years | 6-11years | 12-18years | Total |
| 2012 | 61 | 10 | 6 | 2 | 79 |
| 2013 | 88 | 18 | 6 | 2 | 114 |
| 2014 | 108 | 20 | 10 | 11 | 149 |
| 2015 | 133 | 13 | 5 | 3 | 154 |
| 2016 | 88 | 18 | 4 | 9 | 119 |
| Total | 478 | 79 | 31 | 27 | 615 |
In order to describe the distribution of pathogens by year of study, the most important upward trend was of Campylobacter cases, followed by those due by Salmonella. Shigella spp and E. coli were detected in few cases as HAIs in children from the study group, and there were years with no cases with Yersinia enterocolitica (Fig.2).
Figure 2.
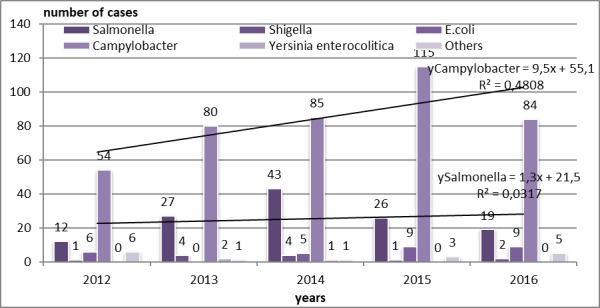
Distribution of pathogens by year of study
Pathogens total distribution by year indicated that Campylobacter was the “leader” of HAIs cases etiology, with 428 positive specimens (69.59% of total), followed by Salmonella specimens (127 cases-20.65% of total) and E.coli (29 cases-4.71%). Others etiologies summarized a total of 16 cases (2.60%), and Shigella with 12 cases (1.95%) and Yersinia enterocolitica etiologies (3 cases-0.48%) took last places (Fig.3).
Figure 3.
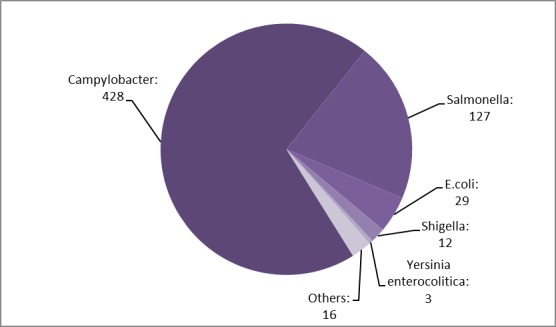
Distribution of pathogens by years-total cases
Campylobacter was the most detected pathogen in patients of that age group admitted with gastroenteritis (384 cases-80.81%), followed by cases with Salmonella gastroenteritis (67 cases-10.89%). Patients aged of 2-6 years suffered from gastroenteritis with same etiology ranked first Campylobacter (41 cases-6.67%), then Salmonella (38 cases-6.17%). Patients of 6-11 years old suffered from gastroenteritis with Salmonella (12 cases-1.95%) and Campylobacter (6 patients-0.97%). Teenagers of 12-18 years old were diagnosed with gastroenteritis with Salmonella as HAI (7 cases-1.13%), then E. coli (12 cases-1.95%). Shigella spp was isolated in children as HAI gastroenteritis in our study group in 11 cases (1.78%). Yersinia enterocolitica was also involved in gastroenteritis etiology, but in few cases (3 cases of our study group-0.55%) (Fig.4).
Figure 4.
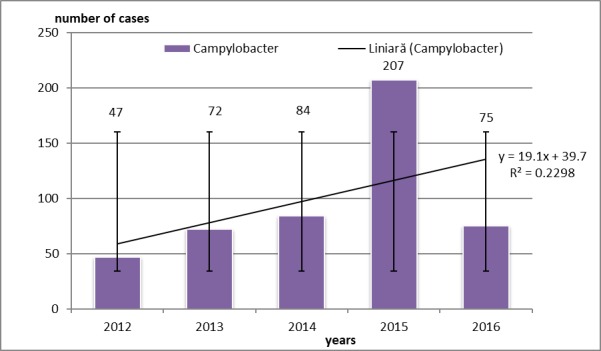
Campylobacter etiology in 0-1 aged children with gastroenteritis. Error bars with standard deviation
Discussion
Worldwide, one of the major etiologies of acute bacterial gastroenteritis is due to Campylobacter spp In the United States, the FoodNet Surveillance and Reporting System estimates that 1.3 million people are affected yearly by Campylobacter, but real incidence rates may be 35 times higher due to undiagnosed or undeclared cases. Geographical variations of campylobacteriosis incidence have been observed even in the United States between 1996 and 2006, with the average confirmed campylobacteriosis rate being 5 times higher in California (34 cases%ooo) compared to other states. This fact could be associated with more frequent visits to a physician, ordering laboratory tests, or exposure to risk factors in the California population compared to other regions [6].
Campylobacter habits the intestinal tracts of domestic animals such as poultry, cattle, pigs, and sheep, including cats and dogs. Disease rarely occurs in animals which eliminating the pathogen by faeces and contaminating environmental transmission routes such as meat and meat products or dairy products. In addition, they can contaminate water or ice. Another route of transmission may be through contact with carriers, especially cats and chickens. The incubation period for Campylobacter is 2 to 5 days, sometimes 10 days. Most cases are sporadic, but the incidence increases in March and during the summer months. Outbreaks of campylobacteriosis have been reported due to the consumption of raw milk or water from shafts contaminated with litter from farms and poultry. Children under the age of 4 are more likely to be involved, but also in people aged 15-44. Acute gastroenteritis with Campylobacter was also found in tourists from developed countries who visit developing regions [6,7].
The most common species associated with acute gastroenteritis are Campylobacter jejuni subsp. jejuni and Campylobacter coli. C. jejuni is responsible for over 90% of cases. Campylobacter upsaliensis was first isolated in dogs with diarrhea, but it can also cause disease in humans. The incidence of C. upsaliensis in patients with diarrhea can be underestimated as the pathogen cannot grow in selective media commonly used in clinical laboratories for Campylobacter evidence. Clinical microbiology studies have shown that Campylobacter fetus subsp. fetus, C. lari, C. concisus, C. jejuni subsp. doyle and C. hyointestinalis have been associated with acute gastroenteritis, especially in children. Before diarrhea occurred, more than 50% of symptomatic cases have been reported with fever, abdominal pain, and myalgia. Abdominal cramps can mimic the pain associated with acute appendicitis. In most cases, diarrhea had self-limitation, requiring only symptomatic treatment. Numerous studies have shown that in 5-10% of patients who received no treatment with antimicrobials, disease relapse occurs. Campylobacter may also cause extra-intestinal infections, including cases of bacteremia, urinary tract infections, cholecystitis, hepatitis, pancreatitis, nephritis, meningitis, abortion. and neonatal sepsis. Bacteremia with Campylobacter is rare in immunocompetent patients, but can occur frequently in patients with HIV, malignancies, or hepatitis. In neonates and elderly, bacteremia and extra-intestinal infections were more commonly reported compared to other patients [8,9,10].
Some studies have reported cases of autoimmune complications such as reactive arthritis and Guillain-Barré syndrome in patients suffered from acute gastroenteritis with Campylobacter. Reactive arthritis was found in 2-4% of cases and characterized by joint pain and swelling, lasting a few weeks to one year, but can be chronical or recurrent in 5% of cases. Symptoms usually begin in 3 to 40 days after acute gastroenteritis, most commonly affecting the knees. Guillain-Barré syndrome is an acute paralyzing disease of the peripheral nervous system and is in patients with campylobacteriosis with an onset of 2 to 21 days after diarrhea occurred in 25%-40% of cases. Various serological and microbiological studies have shown the presence of C. jejuni infection in patients with Guillain-Barré syndrome. Studies have also highlighted that only 1 in 1,000 patients exposed to Campylobacter infection developed Guillain-Barré syndrome. The isolation rate of C. jejuni from the culture of the Guillain-Barré syndrome patients ranges from 8% to 50%, and seropositivity ranges from 24% to 76%. The explanation could be related to the fact that C. jejuni lipopoligosaccharides act as a human ganglioside-like, with the formation of autoantibodies that react with peripheral receptors [11,12,13].
The main STEC reservoir is considered to be bovine and ruminant. Contamination of the environment with animal litter and animal products, inadequate control measures in the food and farm industry are factors that favor STEC detection in almost any environment. STEC gastroenteritis occurs as a result of the consumption of contaminated animal products or contaminated water, reported mainly during the summer months, but observed throughout the year. In the United States, the incidence of gastroenteritis with STEC is monitored by FoodNet. In 2012, the incidence of O157 STEC was 1.12%ooo, and the incidence of non-O157 STEC was 1.16%ooo. Of the STEC non-O157 strains, the most frequently isolated serotypes were O26, O103, O111, O121, O45, and O145. In other countries, the incidence of STEC varies from 0.4%ooo in Australia to 5.33%ooo in Ireland, and it is much higher in developing countries such as Argentina and India, but official data reported by surveillance systems are not available for these countries [2,14,15].
Acute gastroenteritis caused by Yersinia enterocolitica can have varied clinical forms such as diarrheal self-limiting enteric disease, low fever and abdominal pain or severe terminal ileitis and mesenteric lymphadenitis that can be confused with acute appendicitis. The incubation period is usually 24-48 hours after ingestion of water or contaminated food, and the disease lasts between 7 and 14 days, but the symptoms may persist for up to 2 to 12 months.
Immunocompromised patients are often affected by severe and prolonged illness. Severe cases may require hospitalization due to dehydration. Sepsis is uncommon and is often associated with cardiovascular, dermal, pulmonary and abscess conditions. Often, the disease could be associated with acute pharyngitis, sore throat and fever as predominant symptoms, sometimes with fulminant forms, difficulty in swallowing and breathing requiring urgent medical assistance [16,17,18].
The four Shigella species (Shigella dysenteriae, S. flexneri, S. boydii, and S. sonnei) are pathogens often encountered in the etiology of human acute gastroenteritis, but have been documented in rare cases in dogs and primate monkeys. Transmission routes are mainly food or contaminated water, but cases have also been reported to workers in microbiology laboratories. Due to the low infectious dose (10-100 microorganisms), direct transmission through person-to-person contact is common for Shigella spp [11,19].
Laboratories should provide routine microbiological examinations to the physician. Hospital laboratories that perform coprocultures can provide useful information about Salmonella, Shigella, STEC, and Campylobacter, in developed countries. In the future, the role of bacterial coprocultures would be diminished by non-culture test methods for the diagnosis of pathogens involved in acute gastroenteritis. Both traditional and contemporary molecular methods can only examine four or five microorganisms. The new technologies could have various issues related to test performance and the impossibility of being used as routine test in public health laboratories due to financial limitations. However, new technologies should be considered by hospitals and public health units, due to the possibility of identifying numerous pathogens, so that antimicrobial treatment can be applied with accuracy [20,21].
Medical and socio-economic implications of HAIs are complex, with high values of length of stay, worsening of the main disease, complications, increasing costs of laboratory examinations and therapies, leading to economic and psycho-emotional loss and decreasing patients’ quality of life. Gastroenteritis as HAIs in pediatric units represents a major burden and risk for immunosuppressed patients, infants, premature infants or patients with other conditions. There are many factors contributing to the epidemiological process, which we encounter in our hospital, also, related to the possibility of environmental contamination and rapid enteral transmission of microorganisms. Deficiencies in continued hygiene and crowding of medical or social activities (visitors, patient attendants) are also factors contributing to the spread of gastroenteritis as HAIs in pediatrics units.
Conclusions
Gastroenteritis with Campylobacter was most commonly reported in pediatric wards, especially in infants of 0-1 years old and children aged of 2-6 years. Cases of HAIs with Salmonella spp are also frequent.
A competent management of HAIs especially as gastroenteritis in a children emergency hospital from a region that includes developing rural areas, should be the most important issue for professionals involved in surveillance and control strategies, as well as clinicians, epidemiologist and microbiologist in order to prevent HAIs burden occurrence and avoid antimicrobial resistance.
References
- 1.Chow CM, Leung AK, Hon KL. Acute gastroenteritis: from guidelines to real life. Clin experim gastroenterol. 2010;3:97–112. doi: 10.2147/ceg.s6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elliott EJ. Acute gastroenteritis in children. BMJ. 2007;334(7583):35–40. doi: 10.1136/bmj.39036.406169.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guarino A, Albano F, Ashkenazi S, Vecchio AL, Shamir R, Szajewska H. European Society for Paediatric Gastroenterology, Hepatology, and Nutrition/European Society for Paediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe. J Pediatr Gastroenterol Nutr. 2008;46(Suppl 2):S81–S122. doi: 10.1097/MPG.0b013e31816f7b16. [DOI] [PubMed] [Google Scholar]
- 4.Lai CC, Ji DD, Wu FT, Mu JJ, Yang JR, Jiang DD, Lin WY, Chen WT, Yen MY, Wu HS, Chen TH. Etiology and risk factors of acute gastroenteritis in a Taipei Emergency Department: clinical features for bacterial gastroenteritis. J Epidemiol. 2016;26(4):216–223. doi: 10.2188/jea.JE20150061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howidi M, Al Kaabi N, El Khoury AC, Brandtmüller A, Nagy L, Richer E, Haddadin W, Miqdady MS. Burden of acute gastroenteritis among children younger than 5 years of age-a survey among parents in the United Arab Emirates. BMC Pediatrics. 2012;12:74–74. doi: 10.1186/1471-2431-12-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ailes E, Scallan E, Berkelman RL, Kleinbaum DG, Tauxe RV, Moe CL. Do differences in risk factors, medical care seeking, or medical practices explain the geographic variation in campylobacteriosis in Foodborne Diseases Active Surveillance Network (FoodNet) sites. Clin Infect Dis. 2012;54(Suppl 5):S464–S471. doi: 10.1093/cid/cis050. [DOI] [PubMed] [Google Scholar]
- 7.CDC. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food-10 states. MMWR Morb Mortal Wkly Rep. 2009;59(14):418–422. [PubMed] [Google Scholar]
- 8.Jay-Russell MT, Mandrell RE, Yuan J, Bates A, Manalac R, Mohle-Boetani J, Kimura A, Lidgard J, Miller WG. Using major outer membrane protein typing as an epidemiological tool to investigate outbreaks caused by milk-borne Campylobacter jejuni isolates in California. J Clin Microbiol. 2013;51(1):195–201. doi: 10.1128/JCM.01845-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez-Cruz A, Munoz P, Mohedano R, Valerio M, Marin M, Alcala L, Rodriguez-Creixems M, Cercenado E, Bouza E. Campylobacter bacteremia: clinical characteristics, incidence, and outcome over 23 years. Medicine (Baltimore) 2010;89(5):319–330. doi: 10.1097/MD.0b013e3181f2638d. [DOI] [PubMed] [Google Scholar]
- 10.Schonberg-Norio D, Mattila L, Lauhio A, Katila ML, Kaukoranta SS, Koskela M, Pajarre S, Uksila J, Eerola E, Sarna S, Rautelin H. Patient-reported complications associated with Campylobacter jejuni infection. Epidemiol Infect. 2010;138(7):1004–1011. doi: 10.1017/S0950268809991099. [DOI] [PubMed] [Google Scholar]
- 11.Humphries RM, Linscott AJ. Laboratory diagnosis of bacterial gastroenteritis. Clin Microbiol Rev. 2015;28(1):3–31. doi: 10.1128/CMR.00073-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pope JE, Krizova A, Garg AX, Thiessen-Philbrook H, Ouimet JM. Campylobacter reactive arthritis: a systematic review. Semin Arthritis Rheum. 2007;37(1):48–55. doi: 10.1016/j.semarthrit.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nyati KK, Nyati R. Role of Campylobacter jejuni infection in the pathogenesis of Guillain-Barré syndrome: an update. BioMed Res Internat. 2013;2013:852195–852195. doi: 10.1155/2013/852195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vally H, Hall G, Dyda A, Raupach J, Knope K, Combs B, Desmarchelier P. Epidemiology of Shiga toxin producing Escherichia coli in Australia, 2000-2010. BMC Public Health. 2012;12:63–63. doi: 10.1186/1471-2458-12-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gould LH, Bopp C, Strockbine N, Atkinson R, Baselski V, Body B, Carey R, Crandall C, Hurd S, Kaplan R, Neill M, Shea S, Somsel P, Tobin-D'Angelo M, Griffin PM, Gerner-Smidt P. Recommendations for diagnosis of Shiga toxin-producing Escherichia coli infections by clinical laboratories. MMWR Recomm Rep. 2009;58((RR-12)):1–14. [PubMed] [Google Scholar]
- 16.Rosner BM, Werber D, Hohle M, Stark K. Clinical aspects and self-reported symptoms of sequelae of Yersinia enterocolitica infections in a population-based study, Germany 2009-2010. BMC Infect Dis. 2013;13:236–236. doi: 10.1186/1471-2334-13-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fredriksson-Ahomaa M, Cernela N, Hächler H, Stephan R. Yersinia enterocolitica strains associated with human infections in Switzerland 2001-2010. Eur J Clin Microbiol Infect Dis. 2012;31(7):1543–1550. doi: 10.1007/s10096-011-1476-7. [DOI] [PubMed] [Google Scholar]
- 18.Tusevljak N, Rajic A, Waddell L, Dutil L, Cernicchiaro N, Greig J, Wilhelm BJ, Wilkins W, Totton S, Uhland FC, Avery B, McEwen SA. Prevalence of zoonotic bacteria in wild and farmed aquatic species and seafood: a scoping study, systematic review, and meta-analysis of published research. Foodborne Pathog Dis. 2012;9(6):487–497. doi: 10.1089/fpd.2011.1063. [DOI] [PubMed] [Google Scholar]
- 19.Khan WA, Griffiths JK, Bennish ML. Gastrointestinal and extra-intestinal manifestations of childhood shigellosis in a region where all four species of Shigella are endemic. PLoS One. 2013;8(5):e64097–e64097. doi: 10.1371/journal.pone.0064097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchan BW, Olson WJ, Pezewski M, Marcon MJ, Novicki T, Uphoff TS, Chandramohan L, Revell P, Ledeboer NA. Clinical evaluation of a real-time PCR assay for identification of Salmonella, Shigella, Campylobacter (Campylobacter jejuni and C. coli), and Shiga toxin-producing Escherichia coli isolates in stool specimens. J Clin Microbiol. 2013;51:4001–4007. doi: 10.1128/JCM.02056-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones TF, Gerner-Smidt P. Nonculture diagnostic tests for enteric diseases. Emerg Infect Dis. 2012;18(3):513–514. doi: 10.3201/eid1803.111914. [DOI] [PMC free article] [PubMed] [Google Scholar]


