Abstract
Enhancing the endogenous capacity of myelin repair is a major therapeutic challenge in demyelinating diseases such as multiple sclerosis. We found that progesterone and the synthetic 19-norprogesterone derivative 16-methylene-17α-acetoxy-19-norpregn-4-ene-3,20-dione (Nestorone) promote the remyelination of axons by oligodendrocytes after lysolecithin-induced demyelination in organotypic cultures of cerebellar slices taken from postnatal rats or mice. The intracellular progesterone receptors (PR) mediate the proremyelinating actions of Nestorone, because they are not observed in slices from PR knockout mice. Notably, Nestorone was less efficient in heterozygous mice, expressing reduced levels of PR, suggesting PR haploinsufficiency in myelin repair. Using mice expressing the enhanced green fluorescent protein (EGFP) under the control of the proteolipid gene promoter, we showed that both progesterone and Nestorone strongly increased the reappearance of cells of the oligodendroglial lineage in the demyelinated slices. In contrast to Nestorone, the pregnane derivative medroxyprogesterone acetate had no effect. The increase in oligodendroglial cells by Nestorone resulted from enhanced NG2+ and Olig2+ oligodendrocyte progenitor cell (OPC) recruitment. In cocultures of lysolecithin-demyelinated cerebellar slices from wild-type mice apposed to brain stem slices of proteolipid gene promoter-EGFP mice, Nestorone stimulated the migration of OPC towards demyelinated axons. In this coculture paradigm, Nestorone indeed markedly increased the number of EGFP+ cells migrating into the demyelinated cerebellar slices. Our results show that Nestorone stimulates the recruitment and maturation of OPC, two steps which are limiting for efficient myelin repair. They may thus open new perspectives for the use of progestins, which selectively target PR, to promote the endogenous regeneration of myelin.
Progesterone, in addition to its well-known role in reproduction, also exerts marked influences on the function and viability of neural cells throughout the nervous system (1–3). Because of its neuroprotective actions, documented by a series of experimental studies, progesterone is now considered as a promising therapeutic candidate for brain injuries (4).
A particular asset of progesterone is that it also promotes the formation of new myelin sheaths, necessary not only for the saltatory conduction of action potentials but also for axonal integrity. A role of progesterone in myelin formation was first demonstrated in peripheral nerves and dorsal root ganglia (5). Progesterone also accelerates axonal myelination by oligodendrocytes during brain development, as shown in organotypic cultures of cerebellar slices taken from postnatal rats, at a stage when the myelination of axons is very intense (6). The proremyelinating actions of progesterone involve the stimulation of oligodendrocyte progenitor cell (OPC) proliferation and maturation and require the presence of progesterone receptors (PR), because they are not observed in PR knockout (KO) mice (6, 7). Consistent with a role of progesterone in oligodendrocyte maturation are the observations that its addition to the medium of glial cell cultures prepared from neonatal rat brains increased the number of oligodendrocytes (8) and that it also increased the branching of oligodendrocytes in purified cultures (9).
After destruction of the myelin sheath, as a consequence of neuronal injury, axonal dysfunction or immune attacks of myelin, as occurs in demyelinating diseases such as multiple sclerosis, oligodendrocytes are primed to repair to some extend the lost myelin sheaths. This process, called remyelination or myelin repair, requires the recruitment of OPC present in the demyelinated lesions or recruited from undamaged areas, their proliferation, and differentiation into mature oligodendrocytes (10, 11). Unfortunately, the endogenous capacity of myelin repair has its limits, and remyelination efficiency progressively decreases during relapsing/remitting multiple sclerosis. Thus, boosting this process would be of great therapeutic benefit.
Studying the process of remyelination is facilitated by the availability of cellular markers allowing to identify OPC, e.g. NG2 proteoglycan, or the more mature stages of oligodendrocytes (12, 13). However, it is important to distinguish between developmental myelination and remyelination. Indeed, although some developmental events are recapitulated during myelin repair (14), some of the intracellular signaling mechanisms involved in both processes may differ (15, 16).
Progesterone has also been shown to have a beneficial influence on the remyelination of axons after toxin-induced demyelination of the cerebellar peduncle of 9-month-old male rats, an age when the endogenous capacity of myelin repair is already much reduced (17). In an experimental model of rat spinal cord injury, resulting in the death of oligodendrocytes and demyelination, the administration of progesterone increased the proliferation of OPC and their maturation (18).
Thus, besides its potential for neuroprotective interventions, progesterone may also be considered as a promising proremyelinating agent. In addition to the use of natural progesterone, synthetic progestins may offer an interesting option. Indeed, they show greater and prolonged activity when compared with progesterone and may be devoid of some of the side effects associated with the natural hormone. On the other hand, progestins have been designed to target the so called “classical” intracellular PR for contraceptive purposes or to protect the uterus during hormone replacement therapies, and they do not necessarily mimic all the actions of progesterone (1). Indeed, progesterone and its neuroactive metabolites have been demonstrated to bind to multiple target proteins, including neurotransmitter receptors and membrane receptors of progesterone, which are not necessarily targeted by the synthetic analogs (19, 20).
The aim of the present study was to determine whether 16-methylene-17α-acetoxy-19-norpregn-4-ene-3, 20-dione (Nestorone), a 19-norprogesterone derivative, efficiently stimulates remyelination. Nestorone has been designed to selectively target PR, does not possess any significant androgenic, estrogenic, or mineralocorticoid-like activity, and it is about 100 times more potent than natural progesterone in bioassays (21, 22). In addition, it binds to glucocorticoid receptor (GR) with low affinity but does not manifest glucocorticoid-like effects, possibly because of rapid metabolism in the liver. We examined the role of the PR in mediating the effects of Nestorone on remyelination, and we characterized its effects at the level of oligodendroglial cells. We also compared the actions of Nestorone with those of another synthetic progestin, medroxyprogesterone acetate (MPA). Although progesterone and Nestorone stimulate neuroprotective responses, this is not the case for MPA (23). For these studies, we used an experimental system of lysolecithin-induced demyelination in organotypic slice cultures of the rat and mouse cerebellum, a powerful experimental system to study the effects of proremyelinating factors and to explore the underlying mechanisms (24–26).
Materials and Methods
Slice cultures and cocultures
Newborn postnatal day (P)10 Sprague Dawley male rats (Janvier, Le Genest-Saint-Isle, France) and transgenic animals, male mice expressing the enhanced green fluorescent protein (EGFP) under the control of the proteolipid gene promoter (PLP) (PLP-EGFP mice) and PRKO mice were used. PRKO mice are here referred to as wild-type PR+/+, heterozygous PR+/−, and homozygous KO PR−/− mice, generated by inserting the lacZ reporter and neomycin resistance genes into exon 1 of the PR gene to effectively disrupt its transcription (27). Newborn pups of P0 (the day of birth) from PLP-EGFP mice were used in the coculture experiment. After decapitation, brains were dissected out into cold Gey's balanced salt solution containing 5 mg/ml glucose, and meninges were removed. Cerebellar parasagittal slices (350 μm thick) were cut on a MacIlwain tissue chopper and transferred onto membranes of 30-mm Millipore culture inserts with a 0.4-μm pore size (Millicell; Millipore, Bedford, MA). Slices were maintained in culture on top of the membranes in six-well plates containing 1 ml of medium at 35 C in an atmosphere of humidified 5% CO2. The medium was composed of 50% basal medium with Earle's salts (Invitrogen, Gaithersburg, MD), 25% Hanks ' balanced salt solution (Life Technologies, Grand Island, NY), 25% horse serum (Life Technologies), l-glutamine (1 mm), and 5 mg/ml glucose.
To induce demyelination, the medium was removed from the wells after 7 d in vitro (DIV), and fresh medium containing lysophosphatidylcholine (lysolecithin 0.5 mg/ml; Sigma, St. Louis, MO) was added to the cultures for approximately 17 h. The medium was then changed, and the slices incubated for four additional days (4 DIV) in the absence or presence of progesterone, Nestorone, or MPA. Steroids were dissolved in ethanol (0.01%), and control cultures were treated with vehicle alone. The medium with the respective steroids was replaced once after 2 d.
Cocultures were prepared as follows. After lysolecithin treatment, P10 cerebellar slices were transected with a glass knife through lobules III and VIII under a dissecting microscope to remove deep nuclei zone. The two parts were gently separated to ensure complete axotomy. The dorsal parts, containing the upper parts of lobules and the fiber tract area, were apposed with portions of P0 brain stem slices from PLP-EGFP+ mice. This coculture system allowed us to study the migration of PLP-EGFP+ oligodendroglial cells into lysolecithin-demyelinated slices. Cocultures were treated with Nestorone or vehicle alone for 4 d, the medium being replaced after 2 d, and after 10 d, the number of EGFP+ cells that had migrated into the demyelinated slice and the distance of their migration from the limit between the two slices were quantified. The migrating cells were also analyzed for the expression of the oligodendrocyte transcription factor Olig2.
Steroids
Steroids were diluted at a final concentration of 0.1% ethanol. Control media contained the same amount of vehicle. Dose-response curves were determined by treating cerebellar slices with different concentrations (1–100 μm) of each compound, and we show here only results for the dose with maximal efficiency (20 μm).
Steroids used were progesterone (Sigma) and synthetic progestins (gifts of the Population Council, New York, NY): the 19-norprogesterone derivative Nestorone and 17-OH progesterone derivative MPA.
Antibodies and staining procedures
The following primary antibodies were used: rabbit polyclonal antibody against Calbindin D-28K (1/10,000 dilution; Swant, Bellinzona, Switzerland) to visualize Purkinje cells, monoclonal antibodies against myelin basic protein (MBP) (1/1000 dilution; Millipore) to examine the extent of myelination, Olig2 (1/200, rabbit polyclonal; Millipore), and rabbit anti-NG2 chondroitin sulfate proteoglycan polyclonal antibody (1/1000 dilution; Millipore). Primary antibodies were detected, respectively, with secondary goat antirabbit cyanine 3-labeled antibody (1/250 dilution; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) and antimouse Alexa Fluor 488-labeled antibody (1/1000 dilution; Molecular Probes, Leiden, Netherlands). 4′,6-Diamidino-2-phenylindole (Sigma) was used to label cell nuclei. Slice cultures were fixed in 4% paraformaldehyde in phosphate buffer (0.1 m) (pH 7.4), for 1 h at room temperature. After washing in PBS, slices were taken off the Millicell and processed for immunohistochemistry. They were incubated for 1 h in a PBS blocking solution [0.12 m (pH 7.4)] containing 0.9% NaCl, 0.25% Triton X-100, 0.1% gelatin, and 0.1% sodium azide (PBSGTA) and lysine (0.1 m). Incubations with primary antibodies were performed in PBSGTA overnight at 4 C. Secondary antibodies were incubated for 2 h at room temperature in PBSGTA. Slices were washed in PBS and then mounted in Fluoromount-G mounting medium.
Quantification of myelination, OPC density, and OPC migration
To determine the number of PLP-EGFP+ cells in the slices, fluorescent cells were directly visualized and counted under a fluorescence microscope (Carl Zeiss, Inc., Oberkochen, Germany). Under these conditions, we counted the total number of surviving PLP-EGFP+ cells in three different cerebellar lobules and calculated the means. MBP staining was quantified in these areas with NIH image software [Ghoumari et al. (6)] to evaluate the extent of remyelination and oligodendrocyte density. Images of the immunostained oligodendrocytes and myelin fibers in organotypic slice cultures of rat or mice cerebella were acquired using the image analyzing system Axiovision 4 (Carl Zeiss, Inc.). Quantification of intact myelin segments (MBP+ fibers) per field was performed by numbering the myelin segments (without interrupted area) from three random fields per slice. Half of the apical fields (∼105 μm2) of the lobule of interest were used for this quantification, by calibrating ×20 objective images, under a fluorescence microscope (Carl Zeiss, Inc.). At least 18 slices were used per treatment. The thickness of axons and fibers (axon + myelin) and the g ratios (axon diameter/fiber diameter) were also evaluated by calibrating ×100 objective images, using a confocal Zeiss LSM 410 (Carl Zeiss, Inc.). The NG2 staining density was measured using NIH image software. This staining density was quantified on a continuous scale of 0–255 (darkest). To minimize differences between different measurements, we set as control an arbitrary level of staining at the value of 100. NG2 staining density was evaluated as percentage (light pixels/light + dark pixels).
In cocultures, only the EGFP-PLP+ cells visualized in the white matter of the demyelinated P10 cerebellar slices were counted within a surface of 2.105 μm2. The mean lengths of the longest distances of EGFP-PLP+ cell migration, from the limit between the two slices and invading the white matter of P10 cerebellar slices, were measured using a confocal Zeiss LSM 410 (Carl Zeiss, Inc.) image analyzing system. For each measurement, at least four cocultures, each prepared from a different wild-type and transgenic animal, were used.
Western blot analysis
Cerebellar slices treated with lysolecithin were cultured in the absence or presence of 20 μm Nestorone for 3 h. Slices were then washed with Gey's balanced salt solution containing 5 mg/ml glucose and dissolved in a Triton lysis buffer containing 50 mm Tris (pH 7.4), 150 mm NaCl, 1 mm EGTA, 1 mm Na3VO4, 100 mm NaF, 5 μm ZnCl2, 1% Triton X-100, 10% glycerol, and a cocktail of protease inhibitors (Sigma). After homogenization, extracts were clarified by centrifugation (14,000 × g for 10 min at 4 C). For baseline (time 0), cerebellum was directly homogenized in the lysis buffer. The concentrations of soluble proteins in the supernatants were quantified by the Bradford method (Bio-Rad, France). Extracts were resolved (20 μg) by SDS-PAGE (10% polyacrylamide gel) and electrophoretically transferred to polyvinylidene fluoride membranes (Millipore). Membranes were incubated with 5% nonfat dry milk in Tris-buffered saline containing 0.1% Tween 20 at room temperature for 1 h and probed overnight at 4 C with an anti-MBP (see above) or with antitotal p38 antibodies (total MAPK; Cell Signaling Technology, Inc., Danvers, MA). After washing with Tris-buffered saline containing 0.1% Tween 20, membranes were incubated for 1 h at room temperature with peroxidase-conjugated AffiniPure goat antirabbit (1/20,000 dilution; Jackson ImmunoResearch Laboratories, Inc.). After addition of chemiluminescence reagent (GE Healthcare, Buckinghamshire, UK), blots were exposed to G:box iChemi System (Syngene, Cambridge, UK) for developing, and images were captured using Genesnap software (Syngene). Results were quantified using Genetools software (Syngene).
Statistical analysis
Data were expressed as means ± sem of at least n = 18 cerebellar slices from four animals. The significance of differences between means was evaluated by Newman-Keuls tests after two- or one-way ANOVA for multiple group comparisons. The level of significance was set at P < 0.05.
Results
Progesterone and Nestorone promote remyelination in organotypic cerebellar slice cultures
To determine whether progesterone promotes the remyelination of axons, cerebellar slices taken from P10 rats were cultured for 7 DIV allowing axons to become myelinated (Fig. 1A). They were then treated with 0.5 mg/ml of lysolecithin for approximately 17 h, resulting in marked demyelination but sparing the axons of Purkinje neurons (24). Four days after lysolecithin-induced demyelination, immunostaining for the myelin marker MBP still appeared sparse and irregular when slices were cultured in medium without the addition of progestins (Fig. 1B). However, in slices cultured for 4 d in the presence of progesterone (20 μm), MBP immunoreactive processes were aligned, and there was a 1.5-fold increase in MBP staining (P < 0.05) (Fig. 1, C and E). Adding a micromolar concentration of progesterone to the culture medium is necessary to stimulate myelin formation, because the hormone does not easily penetrate the 350-μm-thick cerebellar slices, which are cultured on top of a microporous membrane and not submerged by the culture medium (6). When slices were exposed to the same concentration of the potent synthetic progestin Nestorone, dense networks of well-organized MBP-positive myelinated fibers were observed, and MBP immunostaining was increased 2-fold when compared with slices grown in the absence of exogenous progestins (P < 0.01) (Fig. 1, D and E). Nestorone treatment increased MBP staining, reaching the level of controls. No significant difference was seen between these two groups. The marked increase in MBP expression in response to Nestorone treatment was confirmed by Western blot analysis (Fig. 1F). Progesterone (20 μm) also enhanced remyelination, but its effect remained statistically lower than the one of Nestorone (56.8 ± 5.1 vs. 74.48 ± 4.3% staining intensity). In contrast to Nestorone, the 17-OH progesterone derivative MPA (20 μm) had no significant effect on MBP-immunoreactive myelin (Fig. 1E).
Fig. 1.
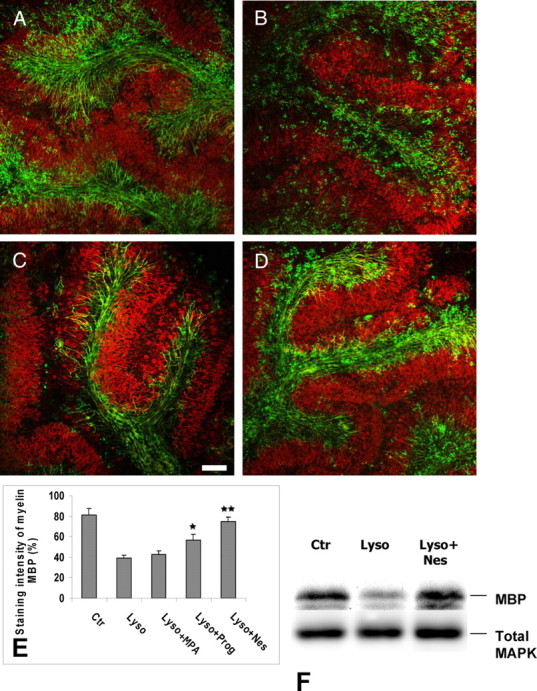
Progesterone and Nestorone promote remyelination after lysolecithin-induced demyelination. Confocal images of whole mount immunostaining for the myelin marker, MBP (green), and the Purkinje cell marker (Calbindin) (red) were performed in slice cultures of P10 rat cerebellum in absence (A, control) or presence of 0.5 mg/ml lysolecithin for approximately 17 h (B). Control cultures were exposed to vehicle (ethanol) alone. C and D, Slices treated with 20 μm progesterone or Nestorone, respectively, after removal of lysolecithin. Slice cultures treated by lysolecithin show broken myelin staining (B), suggestive of damaged myelin. Four-day (4 DIV) treatment with progestin enhances (MBP)-positive cell staining. In contrast to progesterone and Nestorone, the 17-OH progesterone derivative MPA (20 μm) had no significant effect on MBP-immunoreactive myelin (same as B). E, Quantification of MBP staining intensity under different treatment, measured using NIH image software to determine the degree of MBP+ remyelinated axons (calculated as staining intensity of myelin MBP). F, Analysis of MBP expression by Western blotting after slice treatment with lysolecithin alone or with lysolecithin and thereafter with Nestorone (mean ± sem; **, P < 0.01; *, P < 0.05, when compared with the lysolecithin-treated condition using Newman-Keuls tests after one-way ANOVA). Nes, Nestorone; Ctr, control; Lyso, lysolecithin; Prog, progesterone. Scale bar in A–D, 100 μm.
We examined the proremyelinating effect of Nestorone by confocal microscopy at high magnification. In control cerebellar cultures, thick Purkinje axons were observed, most of which were myelinated throughout (Fig. 2, A1–A3). In contrast, 4 d after lysolecithin-induced demyelination, some axons were still completely devoid of myelin, whereas myelinated axons were only surrounded by very thin myelin as shown by double-label immunostaining (Fig. 2, B1–B3). When slices were treated with Nestorone (20 μm), Purkinje axons were surrounded by thick myelin. Consistent with a proremyelinating effect of Nestorone, g ratios (axon diameter/fiber diameter) were significantly decreased by the progestin (controls, 0.71 ± 0.02; lysolecithin, 0.80 ± 0.01; and lysolecithin + Nestorone, 0.64 ± 0.01; P < 0.05). We also observed more MBP-positive oligodendrocytes close to the remyelinated axons in response to Nestorone treatment (Fig. 2, C1 and C3).
Fig. 2.
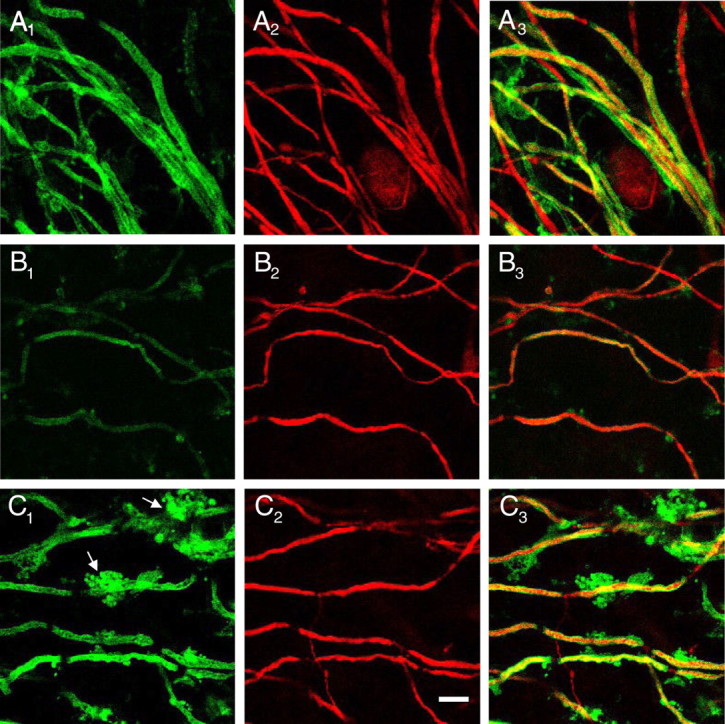
Action of progestin Nestorone on myelin sheath thickness. P10 rat cerebellar slices were not treated (control, A), treated with lysolecithin (B) alone or with lysolecithin, and 20 μm Nestorone (C) for 4 DIV. MBP and Calbindin were used for immunostaining of myelin (green) and axon (red), respectively. Fibers (axon + myelin) at high magnification show thick or thin myelin surrounding axons. Arrowheads indicate myelinating oligodendrocytes. Images were analyzed using confocal microscopy. Scale bar, 10 μm.
The proremyelinating action of Nestorone requires the intracellular PR
Nestorone is a 19-norprogesterone derivative, designed to efficiently target the intracellular PR. Although Nestorone does not exert androgenic, estrogenic, or glucocorticoid activity in different in vivo assays, it has been shown to bind to the GR and to cause thymus involution, although only at high doses (21, 22). To determine whether Nestorone promotes myelin repair via PR, we used adult male wild-type PR+/+, heterozygous PR+/−, and homozygous KO PR−/− mice. Cerebellar slices of PR+/+, PR+/−, and PR−/− mice were sampled at P10. As in the previous experiments, slices were cultured for 7 DIV, then demyelinated by an overnight treatment with lysolecithin and cultured for 4 d in the absence or presence of Nestorone (20 μm).
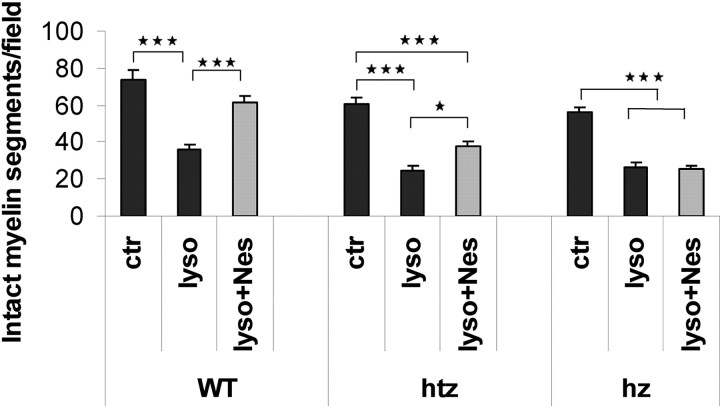
As for rats, mouse cerebellar slices were abundantly myelinated after 7 DIV, and transient treatment with lysolecithin produced a marked demyelination of Purkinje axons, independent of the PR genotype (Fig. 3). Two-way ANOVA revealed highly significant effects of PR genotype (P < 0.001) and treatment (P < 0.001) on the number of remyelinated MBP+ segments, and a significant interaction between both factors (P < 0.01). Treatment with Nestorone markedly stimulated remyelination in cerebellar slices of PR+/+ mice, and slightly in PR+/− slices, and it was without effect in PR−/− animals (Fig. 3). Nestorone more efficiently stimulated myelination in PR+/+ slices when compared with PR+/− slices (P < 0.001). Also, no statistically significant difference was observed between control and Nestorone-treated slices (P > 0.05). Together, these results strongly suggest that PR expression levels are a limiting factor for efficient remyelination in response to progesterone and Nestorone.
Fig. 3.
The intracellular progesterone receptor (PR) is necessary for Nestorone effect on remyelination. Cerebellar slices from P10 wild-type (WT) or PRKO mice (Htz, Heterozygous; Hz, homozygous) were cultured for 7 d and treated with lysolecithin (0.5 mg/ml) for approximately 17 h. Then, Nestorone (20 μm) was added to cultures for 4 DIV. The apical fields (∼105 μm2) of the lobule of interest were used for this quantification. Results expressed as means ± sem; ***, P < 0.001; **, P < 0.01; *, P < 0.05 when compared by Newman-Keuls test after two-way ANOVA (genotype × treatment). Nes, Nestorone; ctr, control; lyso, lysolecithin; WT, wild-type; htz, heterozygous; hz, homozygous.
Progesterone and Nestorone, but not MPA, increase the number of oligodendroglial cells
We next investigated whether the stimulation of axonal remyelination by progestins after lysolecithin-induced demyelination is accompanied by a replenishment of lost oligodendrocytes. We prepared cerebellar slices from P10 transgenic mice expressing EGFP driven by the mouse myelin PLP gene promoter (28). In these mice, EGFP expression is high in all cells of the oligodendrocyte lineage and can be easily detected in the postnatal brain. After 7 DIV, PLP-EGFP+ oligodendroglial cells are largely distributed in the cerebellar white matter tracts, and both cell bodies and myelinated processes were intensely stained (Fig. 4A). Four days after overnight exposure to lysolecithin, the number of oligodendroglial cells was reduced by 70% when cerebellar slices were treated with vehicle (Fig. 4, B and F). However, in the presence of progesterone or Nestorone (both at 20 μm), oligodendroglial cells were significantly replenished (Fig. 4, C, E, and F). In contrast, MPA (20 μm) failed to increase the number of PLP-EGFP+ cells (Fig. 4, D and F).
Fig. 4.
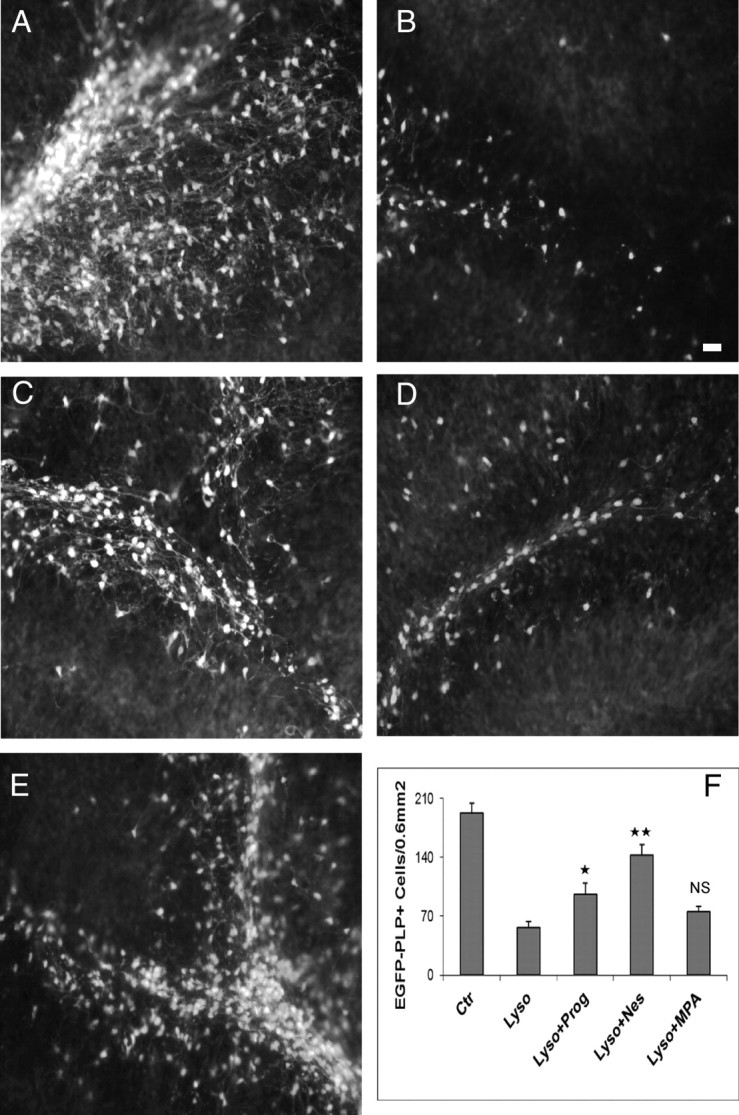
Progestins restore the number of oligodendrocyte PLP-GFP+ cells after lysolecithin treatment. PLP-EGFP mice (GFP under the control of the PLP) were used to label oligodendrocytes in the white matter tracts of the cerebellum. A, Nontreated slices (controls). Slices showing lower expression of PLP-EGFP when treated with lysolecithin (B) and high expression when incubated with progesterone or Nestorone (C and E). However, no increase in PLP-EGFP was seen when slices were treated with 20 μm MPA (D). F, The number of PLP-EGFP+ cells was markedly increased after progesterone and Nestorone treatment in comparison with slices treated with MPA. Areas of 0.6 mm2 in cerebellar lobule were used for quantification, and images were acquired with an image analyzing system, Axiovision 4 (Carl Zeiss, Inc.) (mean ± sem; **, P < 0.01; *, P < 0.05. ns, Nonsignificant, when compared with the lysolecithin-treated groups by Newman-Keuls tests after one-way ANOVA). Nes, Nestorone; Ctr, control; Lyso, lysolecithin. Scale bar, 100 μm.
Effects of Nestorone on oligodendrocyte progenitors
The increase in oligodendroglial cells by Nestorone after the exposure of cerebellar slices to lysolecithin suggests a stimulation of OPC recruitment to demyelinated areas. In EGFP-PLP mice, the fluorescent protein is expressed at all stages of the oligodendrocyte lineage. Thus, an additional marker is required to specifically label OPC. The most often used one is the proteoglycan NG2 (11). We have previously shown that progesterone treatment stimulates the proliferation of NG2+ oligodendrocyte precursors in P7 cerebellar slices (7). Here, we show that Nestorone treatment rapidly increases the density of NG2+ cells after lysolecithin-induced demyelination. In cerebellar slices of PLP-EGFP+ mice, cultured for 7 DIV, we observed a more than 60% reduction in the number of PLP-EGFP+ oligodendroglial cells 24 h after lysolecithin exposure. However, adding 20 μm Nestorone for 24 h to the culture medium after the removal of lysolecithin caused an approximately 2- and 3-fold increase in the number EGFP+ and NG2+ cells, respectively (Fig. 5, A, B, and E).
Fig. 5.
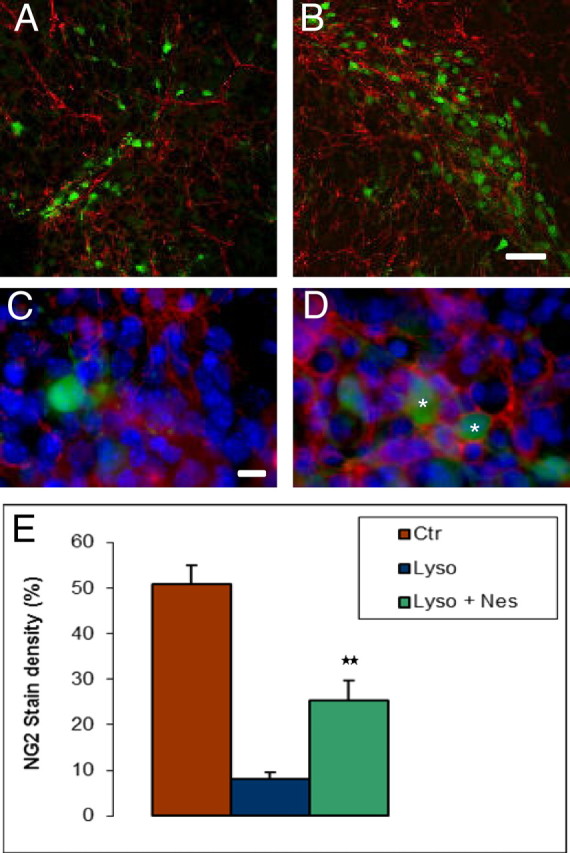
Oligodendrocyte progenitor marker NG2 and EGFP-PLP+ cells increase after lysolecithin exposure followed by Nestorone treatment for 24 h. A and B, Representative fluorescent Axioskop-FS microscope images of NG2+ cells and EGFP-PLP+ cells in P10 cerebellar slices treated by lysolecithin or by lysolecithin followed by 20 μm Nestorone, respectively. C and D, Asterisks indicate triple-positive cells, 4′,6-diamidino-2-phenylindole (DAPI) (blue), EGFP-PLP (green), and NG2 (red), indicating an increase in the density of OPC. E, Quantification of NG2 staining in control, lysolecithin and lysolecithin + Nestorone-treated slices (error bars represent sem; **, P < 0.01, when compared with the lysolecithin-treated condition using Newman-Keuls tests after one-way ANOVA). Nes, Nestorone; Ctr, control; Lyso, lysolecithin. Scale bar, 50 μm (A and B) and 10 μm (C and D).
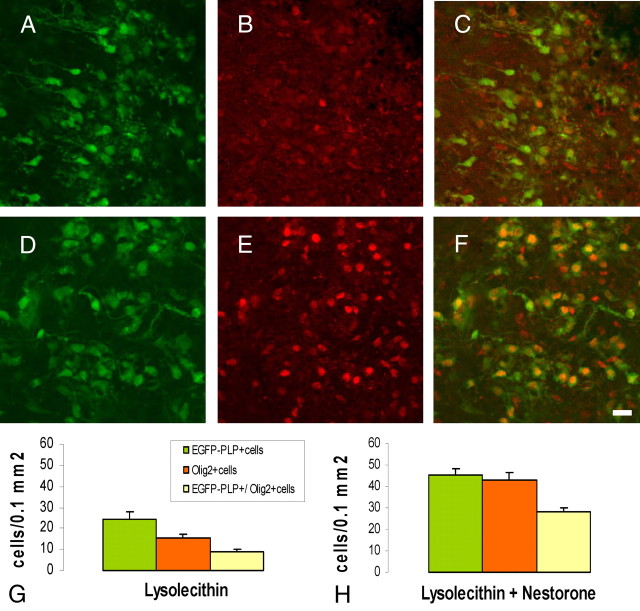
Importantly, Nestorone also markedly increased the density of NG2+ processes surrounding EGFP+ cells, indicating an increase in OPC density (Fig. 5, A–E). The EGFP+ cells were indeed OPC responding to demyelination, because they also expressed Olig2, a basic helix-loop-helix transcription factor required for the specification of the oligodendrocyte lineage (29, 30). Nestorone treatment significantly increased the density of Olig2+ and Olig2+/EGFP-PLP+ cells (Fig. 6). Thus, the progestin promotes the remyelination of axons by stimulating the recruitment of OPC and their differentiation into mature oligodendroglial cells.
Fig. 6.
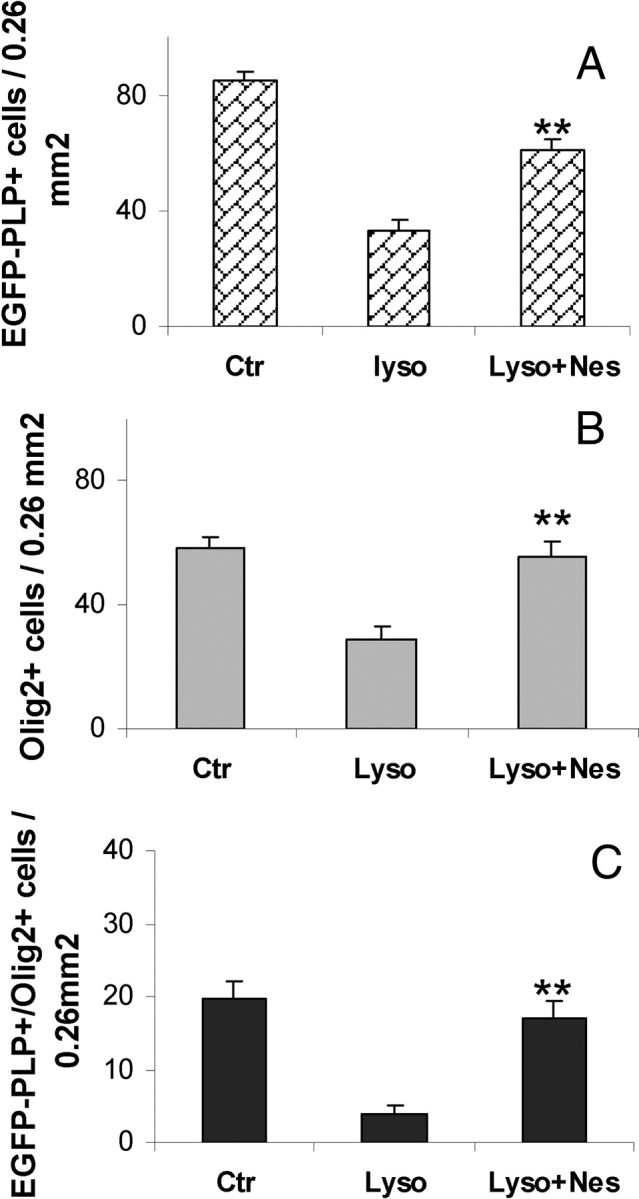
Progestins promote oligodendrocyte differentiation. Olig2, a basic helix-loop-helix transcription factor, increases after lysolecithin exposure and Nestorone treatment for 24 h. A–C, Quantifications of EGFP-PLP+, Olig2+, and EGFP-PLP+/Olig2+ cells in control, lysolecithin, and lysolecithin + (20 μm) Nestorone-treated slices, respectively. Error bars represent sem; **, P < 0.01, when compared with the lysolecithin-treated condition using Newman-Keuls tests after one-way ANOVA. Nes, Nestorone; Ctr, control; Lyso, lysolecithin.
Migration of oligodendrocytes and remyelination in the presence of Nestorone
The recruitment of OPC after a demyelinating lesion can result not only from their proliferation and specification but also their migration toward the demyelinated axons. Indeed, the vicinity of demyelinated axons becomes depleted in oligodendrocytes and their progenitors, eventually resulting in the formation of chronically demyelinated lesions (31). We thus investigated whether Nestorone also promotes the migration of OPC toward a demyelinated area. As an experimental system, we set up cocultures to observe the migration of OPC from P0 brain stem slices of PLP-EGFP mice into P10 cerebellar slices of wild-type mice, demyelinated by exposure to lysolecithin as described above. The demyelinated P10 cerebellar slices were transected with a glass knife through lobules III and VIII under a dissecting microscope to remove the deep nuclei zone. The dorsal part of the slices, containing Purkinje neuron soma with their proximal portions of demyelinated axons, were then apposed to the P0 brain stem slices containing EGFP+ OPC, and slices were then cocultured for 10 d (Fig. 7A, inset).
Fig. 7.
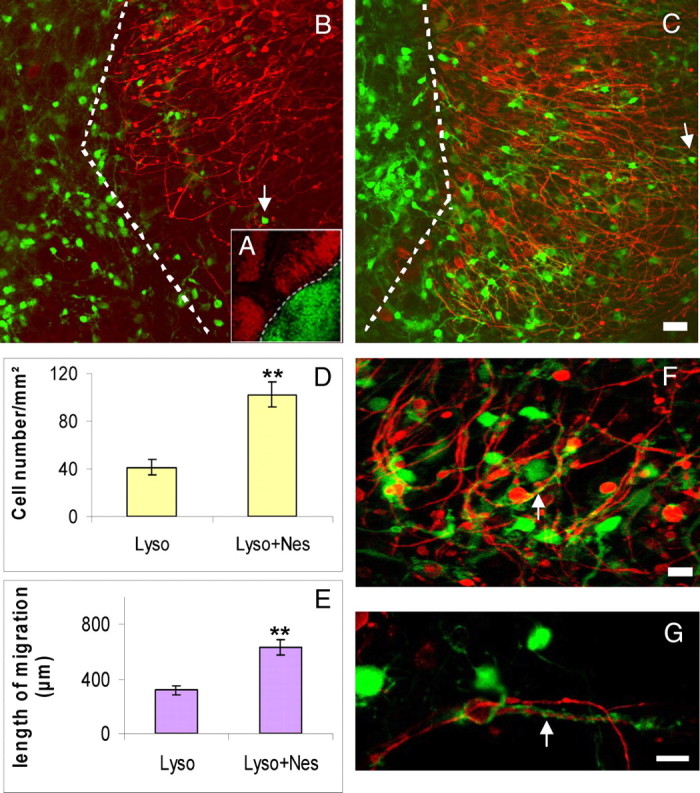
Migration of oligodendrocyte progenitors and remyelination in presence of Nestorone. A, Model of axotomy and coculture of cerebellar and brain stem slices. Some cultures were transected with a glass knife through lobules III and VIII under a dissecting microscope to remove deep nuclei zone. The two parts were gently separated to ensure a complete axotomy. The dorsal parts were apposed with portions of brain stem from P0-PLP-EGFP mice, which allowed us, in coculture slices, a more precise analysis of the fate of the migrating PLP-EGFP+ oligodendrocytes. Dashed lines indicate the limit between the two cultures. B, After lysolecithin treatment, axotomized P10 cerebellar slices (Purkinje cells in red) were cocultured with portions of P0 brain stem slices from PLP-EGFP+ oligodendrocytes (green), showing low number of migrating oligodendrocytes, which invade P10 cerebellar slices. However, treatment of the same cocultures with Nestorone (20 μm) highly increases the number of oligodendrocytes and their migration onto transected and lysolecithin-treated P10 cerebellar slices (C and D). Analysis of migrating oligodendrocytes was performed at 10 DIV after lysolecithin removal, and Nestorone allows immature oligodendrocytes to migrate far into P10 cerebellar slices, represented by the mean lengths of the longest distances of EGFP-PLP+ cell migration (E). F, Under Nestorone treatment, cocultures show increased process extension and complexity, normally a feature of oligodendrocyte maturation. PLP-EGFP+ extensions wind up Purkinje axons, showed at high magnification (G). Arrows indicate migrating EGFP-PLP+ oligodendrocytes in B and C and 10 μm (F and G).and surrounding Purkinje axons in F and G. For each group, 16 cocultures from four different mice were used. Mean ± sem; **, P < 0.01. Nes, Nestorone; Ctr, control; Lyso, lysolecithin. Scale bar, 100 μm (B and C).
Under these conditions, only a few EGFP+ cells migrated into the lysolecithin-demyelinated cerebellar slices (Fig. 7B). However, when slices were cocultured in the presence of Nestorone (20 μm), there was a nearly 3-fold increase in the number of EGFP+ cells migrating into the cerebellar slices (Fig. 7, C and D). Moreover, treatment with Nestorone doubled the OPC migration distance (Fig. 7, C and E). Importantly, the EGFP+ cells that migrated toward demyelinated Purkinje axons extended long processes, and some of the EGFP+ extensions tightly interacted with Purkinje cell axons (Fig. 7. F and G). These are clearly features of oligodendrocyte maturation.
We confirmed that coculturing brain stem slices of EGFP-PLP mice with demyelinated cerebellar slices of wild-type mice for 10 d in the presence of 20 μm Nestorone markedly increased the number of migrating EGFP+ cells (Fig. 8). Moreover, in the presence of Nestorone, a significantly greater proportion of migrating EGFP+ cells coexpressed the oligodendrocyte transcription factor Olig2 (vehicle, 40.2 ± 5.7%; Nestorone, 63.2 ± 2.9%; **, P < 0.01). Thus, Nestorone promotes both the recruitment of OPC toward demyelinated axons and their maturation into oligodendrocytes.
Fig. 8.
Increased number and differentiation of oligodendrocytes in presence of Nestorone. Coculture of cerebellar slices from P10 wild-type mice and brain stem slices from P0 transgenic PLP-EGFP mice were treated by lysolecithin for approximately 17 h and thereafter by vehicle (A–C) or Nestorone (20 μm) (D–F) for 3 d. Ten days later, cocultures were immunolabeled with Olig2 transcription factor (red). A and D, EGFP-PLP+ cells; B and E, Olig2+ cells; C and F, EGFP-PLP+/Olig2+ cells. G and H, Quantification of these different markers in untreated and Nestorone-treated cocultures. Treatment of cocultures with Nestorone highly increases the number and differentiation of immature migrating oligodendrocytes. Mean + sem. Scale bar, 10 μm.
Discussion
Our results show that progesterone and Nestorone, but not MPA, increase remyelination in cerebellar slice cultures after a demyelinating insult with lysolecithin. The proremyelinating effect of Nestorone requires the presence of PR, because it is not observed in cerebellar slices from PRKO mice. This observation is consistent with the high affinity and selectivity of Nestorone for PR and points to a potential role for PR in myelin repair. Importantly, Nestorone stimulates the recruitment and maturation of OPC, which are both needed for efficient axonal remyelination (11).
Progesterone has previously been shown to exert a beneficial influence on myelin repair in different in vivo paradigms of demyelination/remyelination (17, 18, 32). Moreover, progesterone treatment attenuated the severity of disease symptoms in mice with experimental autoimmune encephalomyelitis, an experimental model of multiple sclerosis. It improved spinal axon myelination and had protective effects on axons (33, 34). However, the signaling mechanisms involved in the promyelinating actions of progesterone are not well defined. This is an important question, because progesterone acts on multiple targets within the nervous system (1).
Our present findings identify PR as key mediators of the proremyelinating actions of progesterone, because they were not observed in PR−/− mice. Importantly, progesterone was also much less efficient in stimulating the remyelination of axons in PR+/− mice, in which PR binding has been shown to be reduced by half in progesterone target tissues, including brain and uterus (35, 36). Together, these results point to an influence of PR deficiency and haploinsufficiency on remyelination, and they also suggest that PR may be a limiting factor in progesterone-driven myelin repair. It is interesting to note that decreased PR expression in hypothalamus or uterus of PR+/− mice does not result in a particular reproductive phenotype (35).
This raises the important question of the cellular targets of progesterone and progestins, which has been previously addressed. Thus, immunohistochemical analyses showed that PR within the developing cerebellum is expressed in postnatal Purkinje neurons (37). This suggests that the proremyelinating effects of progesterone may mainly involve neuronal PR, consistent with the key role played by neuron-derived factors in the maturation of oligodendrocytes and the formation of myelin sheaths (38). However, PR expression has also been demonstrated in brain glial cells (39, 40). In particular, PR signaling in astrocytes may be involved in myelination by stimulating growth factor production (41, 42). Whether oligodendrocytes also express PR and are a direct target for the actions of progesterone remains to be clarified (9, 43). On the contrary, microglial cells have been reported to be devoid of PR (44).
PR may thus be promising targets for promoting myelin repair in demyelinating diseases such as multiple sclerosis. Consequently, our results open the way to a new therapeutic indication for some synthetic progestins, designed to highly target PR and already used in contraception, menopausal hormone therapy, and the treatment of gynecological diseases (22). However, it is important to keep in mind that not all progestins are the same in terms of their actions on various targets. They belong to different classes, and even small changes in their structure may result in considerable differences in their pharmacological actions (22, 45). It is thus important to determine which synthetic progestin(s) efficiently stimulate remyelination and whether their proremyelinating effects involve PR.
We report here that Nestorone is a potent proremyelinating agent. This progestin belongs to the class of synthetic 19-norprogesterone derivatives, which show great selectivity for PR and may provide therapeutic benefits for relapsing/remitting multiple sclerosis by promoting endogenous myelin repair. This result gains significance in light of an ongoing phase II clinical trial testing another 19-norprogesterone derivative, nomegestrol acetate, in combination with estradiol, for the prevention of postpartum relapses in multiple sclerosis (46).
By contrast, the progestin MPA failed to promote the replenishment of oligodendrocytes and the formation of new myelin after lysolecithin-induced demyelination of axons. Thus, not all progestins may be useful for the treatment of demyelinating diseases. MPA was the progestin used in the Women's Health Initiative study, a large prospective clinical trial aimed to test the efficacy of hormone replacement therapy in postmenopausal women. MPA probably contributed to the negative outcomes of the Women's Health Initiative trial (1, 47). Recent experimental studies have shown that MPA lacks neuroprotective activity, antagonizes the neuroprotective and promnesic effects of estrogen and exacerbates excitotoxic damage to neurons (23, 48, 49).
Would the presence of the GR in cerebellar slices (50) have a mediating Nestorone effect on remyelination? Several points may argue for the nonimplication or at the least for a very small role of the GR in this effect:
1) MPA, which exerts a strong glucocorticoid effect, did not induce the same positive response as Nestorone did; 2) dexamethasone, known to bind with very high affinity to GR, also did not influence remyelination in cerebellar slices (data not shown); and 3) a previous study by Chari et al. (51) showed that glucocorticoid delay oligodendrocyte-mediated remyelination in toxin-induced demyelinating in vivo model. Therefore, it is very unlikely that the remyelinating action seen relates to the GR binding. However, further studies with a selective blocker of the GR without blocking the PR (because mifepristone does both) would be needed to fully exclude this possibility.
Progestins are particularly interesting for treating degenerative diseases of the nervous system, not only because they easily cross blood-brain barrier and rapidly diffuse throughout nervous tissues but also exert multiple beneficial effects. Thus, progesterone and Nestorone are efficient neuroprotective agents to promote the formation of new myelin sheaths and to modulate neuroinflammatory responses (23, 52). Likewise, Nestorone stimulates the formation of new myelin sheaths by acting on multiple key steps of the remyelination process: the recruitment and maturation of OPC. These are encouraging findings, because impaired recruitment of OPC and failure of their differentiation may be a major cause of remyelination failure, and agents stimulating both processes may be of great interest for multiple sclerosis patients (11, 53).
Nestorone is a fourth-generation synthetic progestin, and it is currently undergoing testing as a contraceptive in both women and men (54, 55). It may thus become useful as a proremyelinating agent in both female and male multiple sclerosis patients. Its strong progestational activity, combined with the lack of androgenic, estrogenic, and glucocorticoid-like activities, confers special advantages to the therapeutic use of Nestorone.
Acknowledgments
We thank John P. Lydon and Bert W. O'Malley (Department of Molecular and Cellular Biology, Baylor College of Medicine, Houston, TX) for generous gifts of PR-KO mice and Philippe Leclerc for image analysis.
R.S.-W. is supported by a grant from BARUS for this research.
Disclosure Summary: M.E.-E., N.K., R.S.-W., M.S., and A.M.G. are inventor on United States Patent WO/2011/040048. R.H., O.G., R.J., and W.B.M. have nothing to disclose.
Abbreviation:
- DIV
Day in vitro
- EGFP
enhanced green fluorescent protein
- GR
glucocorticoid receptor
- KO
knockout
- MBP
myelin basic protein
- MPA
medroxyprogesterone acetate
- Nestorone
16-methylene-17α-acetoxy-19-norpregn-4-ene-3,20-dione
- OPC
oligodendrocyte progenitor cell
- P
postnatal day
- PBSGTA
PBS blocking solution [0.12 m (pH 7.4)] containing 0.9% NaCl, 0.25% Triton X-100, 0.1% gelatin, and 0.1% sodium azide
- PLP
proteolipid gene promoter
- PR
progesterone receptor.
References
- 1. Schumacher M, Guennoun R, Ghoumari A, Massaad C, Robert F, El-Etr M, Akwa Y, Rajkowski K, Baulieu EE. 2007. Novel perspectives for progesterone in hormone replacement therapy, with special reference to the nervous system. Endocr Rev 28:387–439 [DOI] [PubMed] [Google Scholar]
- 2. Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J. 2008. Progesterone receptors: form and function in brain. Front Neuroendocrinol 29:313–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stein DG. 2008. Progesterone exerts neuroprotective effects after brain injury. Brain Res Rev 57:386–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stein DG, Wright DW. 2010. Progesterone in the clinical treatment of acute traumatic brain injury. Expert Opin Investig Drugs 19:847–857 [DOI] [PubMed] [Google Scholar]
- 5. Koenig HL, Schumacher M, Ferzaz B, Thi AN, Ressouches A, Guennoun R, Jung-Testas I, Robel P, Akwa Y, Baulieu EE. 1995. Progesterone synthesis and myelin formation by Schwann cells. Science 268:1500–1503 [DOI] [PubMed] [Google Scholar]
- 6. Ghoumari AM, Ibanez C, El-Etr M, Leclerc P, Eychenne B, O'Malley BW, Baulieu EE, Schumacher M. 2003. Progesterone and its metabolites increase myelin basic protein expression in organotypic slice cultures of rat cerebellum. J Neurochem 86:848–859 [DOI] [PubMed] [Google Scholar]
- 7. Ghoumari AM, Baulieu EE, Schumacher M. 2005. Progesterone increases oligodendroglial cell proliferation in rat cerebellar slice cultures. Neuroscience 135:47–58 [DOI] [PubMed] [Google Scholar]
- 8. Jung-Testas I, Hu ZY, Baulieu EE, Robel P. 1989. Neurosteroids: biosynthesis of pregnenolone and progesterone in primary cultures of rat glial cells. Endocrinology 125:2083–2091 [DOI] [PubMed] [Google Scholar]
- 9. Marin-Husstege M, Muggironi M, Raban D, Skoff RP, Casaccia-Bonnefil P. 2004. Oligodendrocyte progenitor proliferation and maturation is differentially regulated by male and female sex steroid hormones. Dev Neurosci 26:245–254 [DOI] [PubMed] [Google Scholar]
- 10. Miller RH, Mi S. 2007. Dissecting demyelination. Nat Neurosci 10:1351–1354 [DOI] [PubMed] [Google Scholar]
- 11. Franklin RJ, Ffrench-Constant C. 2008. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci 9:839–855 [DOI] [PubMed] [Google Scholar]
- 12. Baumann N, Pham-Dinh D. 2001. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev 81:871–927 [DOI] [PubMed] [Google Scholar]
- 13. Miller RH. 2002. Regulation of oligodendrocyte development in the vertebrate CNS. Prog Neurobiol 67:451–467 [DOI] [PubMed] [Google Scholar]
- 14. John GR, Shankar SL, Shafit-Zagardo B, Massimi A, Lee SC, Raine CS, Brosnan CF. 2002. Multiple sclerosis: re-expression of a developmental pathway that restricts oligodendrocyte maturation. Nat Med 8:1115–1121 [DOI] [PubMed] [Google Scholar]
- 15. Arnett HA, Fancy SP, Alberta JA, Zhao C, Plant SR, Kaing S, Raine CS, Rowitch DH, Franklin RJ, Stiles CD. 2004. bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science 306:2111–2115 [DOI] [PubMed] [Google Scholar]
- 16. Stidworthy MF, Genoud S, Li WW, Leone DP, Mantei N, Suter U, Franklin RJ. 2004. Notch1 and Jagged1 are expressed after CNS demyelination, but are not a major rate-determining factor during remyelination. Brain 127:1928–1941 [DOI] [PubMed] [Google Scholar]
- 17. Ibanez C, Shields SA, El-Etr M, Baulieu EE, Schumacher M, Franklin RJ. 2004. Systemic progesterone administration results in a partial reversal of the age-associated decline in CNS remyelination following toxin-induced demyelination in male rats. Neuropathol Appl Neurobiol 30:80–89 [DOI] [PubMed] [Google Scholar]
- 18. Labombarda F, González SL, Lima A, Roig P, Guennoun R, Schumacher M, de Nicola AF. 2009. Effects of progesterone on oligodendrocyte progenitors, oligodendrocyte transcription factors, and myelin proteins following spinal cord injury. Glia 57:884–897 [DOI] [PubMed] [Google Scholar]
- 19. Belelli D, Lambert JJ. 2005. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci 6:565–575 [DOI] [PubMed] [Google Scholar]
- 20. Thomas P, Pang Y, Dong J, Groenen P, Kelder J, de Vlieg J, Zhu Y, Tubbs C. 2007. Steroid and G protein binding characteristics of the seatrout and human progestin membrane receptor α subtypes and their evolutionary origins. Endocrinology 148:705–718 [DOI] [PubMed] [Google Scholar]
- 21. Kumar N, Koide SS, Tsong Y, Sundaram K. 2000. Nestorone: a progestin with a unique pharmacological profile. Steroids 65:629–636 [DOI] [PubMed] [Google Scholar]
- 22. Sitruk-Ware R. 2008. Pharmacological profile of progestins. Maturitas 61:151–157 [DOI] [PubMed] [Google Scholar]
- 23. Liu L, Zhao L, She H, Chen S, Wang JM, Wong C, McClure K, Sitruk-Ware R, Brinton RD. 2010. Clinically relevant progestins regulate neurogenic and neuroprotective responses in vitro and in vivo. Endocrinology 151:5782–5794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Birgbauer E, Rao TS, Webb M. 2004. Lysolecithin induces demyelination in vitro in a cerebellar slice culture system. J Neurosci Res 78:157–166 [DOI] [PubMed] [Google Scholar]
- 25. Mi S, Miller RH, Tang W, Lee X, Hu B, Wu W, Zhang Y, Shields CB, Zhang Y, Miklasz S, Shea D, Mason J, Franklin RJ, Ji B, Shao Z, Chédotal A, Bernard F, Roulois A, Xu J, Jung V, Pepinsky B. 2009. Promotion of central nervous system remyelination by induced differentiation of oligodendrocyte precursor cells. Ann Neurol 65:304–315 [DOI] [PubMed] [Google Scholar]
- 26. Miron VE, Ludwin SK, Darlington PJ, Jarjour AA, Soliven B, Kennedy TE, Antel JP. 2010. Fingolimod (FTY720) enhances remyelination following demyelination of organotypic cerebellar slices. Am J Pathol 176:2682–2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ismail PM, Li J, DeMayo FJ, O'Malley BW, Lydon JP. 2002. A novel LacZ reporter mouse reveals complex regulation of the progesterone receptor promoter during mammary gland development. Mol Endocrinol 16:2475–2489 [DOI] [PubMed] [Google Scholar]
- 28. Mallon BS, Shick HE, Kidd GJ, Macklin WB. 2002. Proteolipid promoter activity distinguishes two populations of NG2-positive cells throughout neonatal cortical development. J Neurosci 22:876–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ligon KL, Kesari S, Kitada M, Sun T, Arnett HA, Alberta JA, Anderson DJ, Stiles CD, Rowitch DH. 2006. Development of NG2 neural progenitor cells requires Olig gene function. Proc Natl Acad Sci USA 103:7853–7858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fancy SP, Zhao C, Franklin RJ. 2004. Increased expression of Nkx2.2 and Olig2 identifies reactive oligodendrocyte progenitor cells responding to demyelination in the adult CNS. Mol Cell Neurosci 27:247–254 [DOI] [PubMed] [Google Scholar]
- 31. Mason JL, Toews A, Hostettler JD, Morell P, Suzuki K, Goldman JE, Matsushima GK. 2004. Oligodendrocytes and progenitors become progressively depleted within chronically demyelinated lesions. Am J Pathol 164:1673–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Acs P, Kipp M, Norkute A, Johann S, Clarner T, Braun A, Berente Z, Komoly S, Beyer C. 2009. 17β-estradiol and progesterone prevent cuprizone provoked demyelination of corpus callosum in male mice. Glia 57:807–814 [DOI] [PubMed] [Google Scholar]
- 33. Garay L, Deniselle MC, Lima A, Roig P, de Nicola AF. 2007. Effects of progesterone in the spinal cord of a mouse model of multiple sclerosis. J Steroid Biochem Mol Biol 107:228–237 [DOI] [PubMed] [Google Scholar]
- 34. Garay L, Deniselle MC, Meyer M, Costa JJ, Lima A, Roig P, de Nicola AF. 2009. Protective effects of progesterone administration on axonal pathology in mice with experimental autoimmune encephalomyelitis. Brain Res 1283:177–185 [DOI] [PubMed] [Google Scholar]
- 35. Mani SK, Blaustein JD, O'Malley BW. 1997. Progesterone receptor function from a behavioral perspective. Horm Behav 31:244–255 [DOI] [PubMed] [Google Scholar]
- 36. Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Shyamala G, Conneely OM, O'Malley BW. 1995. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9:2266–2278 [DOI] [PubMed] [Google Scholar]
- 37. Sakamoto H, Ukena K, Tsutsui K. 2001. Effects of progesterone synthesized de novo in the developing Purkinje cell on its dendritic growth and synaptogenesis. J Neurosci 21:6221–6232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Simons M, Trajkovic K. 2006. Neuron-glia communication in the control of oligodendrocyte function and myelin biogenesis. J Cell Sci 119:4381–4389 [DOI] [PubMed] [Google Scholar]
- 39. Jung-Testas I, Renoir JM, Gasc JM, Baulieu EE. 1991. Estrogen-inducible progesterone receptor in primary cultures of rat glial cells. Exp Cell Res 193:12–19 [DOI] [PubMed] [Google Scholar]
- 40. Labombarda F, Guennoun R, Gonzalez S, Roig P, Lima A, Schumacher M, De Nicola AF. 2000. Immunocytochemical evidence for a progesterone receptor in neurons and glial cells of the rat spinal cord. Neurosci Lett 288:29–32 [DOI] [PubMed] [Google Scholar]
- 41. Lacroix-Fralish ML, Tawfik VL, Nutile-McMenemy N, Harris BT, Deleo JA. 2006. Differential regulation of neuregulin 1 expression by progesterone in astrocytes and neurons. Neuron Glia Biol 2:227–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chesik D, De Keyser J. 2010. Progesterone and dexamethasone differentially regulate the IGF-system in glial cells. Neurosci Lett 468:178–182 [DOI] [PubMed] [Google Scholar]
- 43. Chan JR, Phillips LJ, Glaser M. 1998. Glucocorticoids and progestins signal the initiation and enhance the rate of myelin formation. Proc Natl Acad Sci USA 95:10459–10464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sierra A, Gottfried-Blackmore A, Milner TA, McEwen BS, Bulloch K. 2008. Steroid hormone receptor expression and function in microglia. Glia 56:659–674 [DOI] [PubMed] [Google Scholar]
- 45. Hapgood JP, Koubovec D, Louw A, Africander D. 2004. Not all progestins are the same: implications for usage. Trends Pharmacol Sci 25:554–557 [DOI] [PubMed] [Google Scholar]
- 46. Vukusic S, Ionescu I, El-Etr M, Schumacher M, Baulieu EE, Cornu C, Confavreux C. 2009. The prevention of post-partum relapses with progestin and estradiol in multiple sclerosis (POPART'MUS) trial: rationale, objectives and state of advancement. J Neurol Sci 286:114–118 [DOI] [PubMed] [Google Scholar]
- 47. Taylor HS, Manson JE. 2011. Update in hormone therapy use in menopause. J Clin Endocrinol Metab 96:255–264 [DOI] [PubMed] [Google Scholar]
- 48. Nilsen J, Brinton RD. 2002. Impact of progestins on estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology 143:205–212 [DOI] [PubMed] [Google Scholar]
- 49. Nilsen J, Morales A, Brinton RD. 2006. Medroxyprogesterone acetate exacerbates glutamate excitotoxicity. Gynecol Endocrinol 22:355–361 [DOI] [PubMed] [Google Scholar]
- 50. Lawson A, Ahima RS, Krozowski Z, Harlan RE. 1992. Postnatal development of corticosteroid receptor immunoreactivity in the rat cerebellum and brain stem. Neuroendocrinology 55:695–707 [DOI] [PubMed] [Google Scholar]
- 51. Chari DM, Zhao C, Kotter MR, Blakemore WF, Franklin RJ. 2006. Corticosteroids delay remyelination of experimental demyelination in the rodent central nervous system. J Neurosci Res 83:594–605 [DOI] [PubMed] [Google Scholar]
- 52. El-Etr M, Ghoumari A, Sitruk-Ware R, Schumacher M. 2011. Hormonal influences in multiple sclerosis: new therapeutic benefits for steroids. Maturitas 68:47–51 [DOI] [PubMed] [Google Scholar]
- 53. Kuhlmann T, Miron V, Cui Q, Cuo Q, Wegner C, Antel J, Brück W. 2008. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain 131:1749–1758 [DOI] [PubMed] [Google Scholar]
- 54. Sitruk-Ware R, Small M, Kumar N, Tsong YY, Sundaram K, Jackanicz T. 2003. Nestorone: clinical applications for contraception and HRT. Steroids 68:907–913 [DOI] [PubMed] [Google Scholar]
- 55. Mahabadi V, Amory JK, Swerdloff RS, Bremner WJ, Page ST, Sitruk-Ware R, Christensen PD, Kumar N, Tsong YY, Blithe D, Wang C. 2009. Combined transdermal testosterone gel and the progestin nestorone suppresses serum gonadotropins in men. J Clin Endocrinol Metab 94:2313–2320 [DOI] [PMC free article] [PubMed] [Google Scholar]