Abstract
Impacts on brain and behavior have been reported in laboratory rodents after developmental exposure to bisphenol A (BPA), raising concerns about possible human effects. Epidemiological data suggest links between prenatal BPA exposure and altered affective behaviors in children, but potential mechanisms are unclear. Disruption of mesolimbic oxytocin (OT)/vasopressin (AVP) pathways have been proposed, but supporting evidence is minimal. To address these data gaps, we employed a novel animal model for neuroendocrine toxicology: the prairie vole (Microtus ochrogaster), which are more prosocial than lab rats or mice. Male and female prairie vole pups were orally exposed to 5-μg/kg body weight (bw)/d, 50-μg/kg bw/d, or 50-mg/kg bw/d BPA or vehicle over postnatal days 8–14. Subjects were tested as juveniles in open field and novel social tests and for partner preference as adults. Brains were then collected and assessed for immunoreactive (ir) tyrosine hydroxylase (TH) (a dopamine marker) neurons in the principal bed nucleus of the stria terminalis (pBNST) and TH-ir, OT-ir, and AVP-ir neurons in the paraventricular nucleus of the hypothalamus (PVN). Female open field activity indicated hyperactivity at the lowest dose and anxiety at the highest dose. Effects on social interactions were also observed, and partner preference formation was mildly inhibited at all dose levels. BPA masculinized principal bed nucleus of the stria terminalis TH-ir neuron numbers in females. Additionally, 50-mg/kg bw BPA-exposed females had more AVP-ir neurons in the anterior PVN and fewer OT-ir neurons in the posterior PVN. At the 2 lowest doses, BPA eliminated sex differences in PVN TH-ir neuron numbers and reversed this sex difference at the highest dose. Minimal behavioral effects were observed in BPA-exposed males. These data support the hypothesis that BPA alters affective behaviors, potentially via disruption of OT/AVP pathways.
Bisphenol A (BPA) is a high-volume production chemical routinely detectable in humans, because it is ubiquitous in the built and wild environment (1, 2). Concerns have been raised regarding the potential long-term human health effects of environmentally relevant BPA exposure (2, 3), particularly on neural development and behavior (4). Addressing these concerns and maximizing confidence in the translational value of experimental data obtained for this purpose requires employing the most appropriate animal model. Here, we demonstrate the utility of the prairie vole (Microtus ochrogaster), a more prosocial animal model than laboratory rats or mice, for assessing BPA impacts on neuroendocrine development and behaviors related to sociality and social investigation.
Elevated gestational urinary concentrations of BPA have been correlated with adverse behavioral outcomes in children, including hyperactivity, anxiety, and executive function deficits (5–7), suggesting the possibility that early life exposure to BPA impacts neural development. Effects on sociality remain unknown but plausible, because successful social performance, including affiliation, attachment, social reciprocity, and mate seeking, are heavily influenced by affect and cognition (8). Although BPA-related effects in experimental animal studies are reasonably concordant with the available human data (9–11), information regarding impacts on social behaviors are sparse and inconsistent. Moreover, the mechanisms by which BPA-related behavioral changes manifest remain poorly understood (12). One barrier to filling these data gaps is that traditional rodent animal model systems are not highly social. Novel to toxicology, but commonly used for basic and biomedical studies of sociality, prairie voles are advantageous because they display affiliative behaviors more typical of humans, including social monogamy and alloparental care. They also form family units and display relatively low levels of aggression (13). Transformative work, spanning decades and involving several vole species with varying degrees of sociality, has linked prosocial traits to the oxytocin (OT)/vasopressin (AVP) system and its interactions with estrogen and mesolimbic dopamine pathways (13). This comprehensive knowledge makes the prairie vole an ideal model for interrogating the mechanisms by which BPA and other endocrine disrupting compounds (EDCs) alter sociality. Importantly, the translational value of the vole model has now been demonstrated in humans (reviewed in Ref. 14). For example, intranasal OT administration is presently being explored as a potential therapy for autism, and manipulation of OT and AVP is being considered therapeutically for depression. Only 1 study to date has used voles to explore the impact of environmental EDC exposure on brain and behavior. Female pine voles (Microtus pinetorum), which are socially monogamous like prairie voles, born to dams orally exposed to 2 mg/kg · d of the estrogenic pesticide methoxychlor (15, 16) during gestation and lactation displayed reduced physical contact during the partner preference test, indicating impaired sociality (17). OT receptor binding in the cingulate cortex was also reduced. These data support our rational that the vole is a potentially invaluable, yet underused, animal model for testing the hypothesis that early life exposure to BPA (and other EDCs) alters social behavior and related, coordinating, pathways.
Available data (although limited) support the hypothesis that developmental exposure to BPA alters the organization and function of OT and AVP pathways (18, 19) and suggest that effects may persist across generations (10, 20). Early life exposure to BPA has also been shown to affect the dopamine system in varying manners (reviewed in Ref. 21). For example, gestational exposure to human-relevant exposure levels of BPA decreased midbrain dopamine neuron numbers in monkeys (22). Additionally, 3 rodent studies have linked BPA-related disruption in the dopamine system with hyperactivity (11, 23, 24), further supporting the overarching hypothesis that BPA exposure may have an organizational effect on the mesolimbic dopaminergic system and related behaviors.
BPA is primarily thought to act by perturbing estrogen action, although other modes of action, including epigenetic changes and other mechanisms, are plausible (11, 12). Because OT/AVP and dopaminergic pathways are heavily influenced by sex steroids across the lifespan (25–29), the ontogeny of OT/AVP and dopaminergic pathways may be particularly vulnerable to BPA. In prairie voles, neonatal manipulation of sex steroids alters affiliative behaviors later in life, and estradiol administration during adulthood affects estrus and activity (26, 30–32). Manipulation of OT/AVP levels as a result of direct exposures to exogenous hormones, agonists, or by altering the social environment can modify the number of OT, AVP, and dopaminergic neurons in the paraventricular nucleus of the hypothalamus (PVN), thereby resulting in behavioral outcomes, such as anxiety-like behavior and alterations of prototypical male and female sociosexual behavior (33–37). Thus, these endpoints were a primary focus of the present studies.
We also quantified the density of dopaminergic neurons in the principal bed nucleus of the stria terminalis (pBNST); an interconnection site for brain areas integral for social behaviors related to reproduction and defense, including locomotor activity and motivation (38–40) as well as social stress (41, 42). Dopaminergic pathways in this area are thought to regulate corticotropic affective states (43). Male prairie voles have significantly more dopaminergic neurons (identified by tyrosine hydroxylase [TH]-immunoreactivity [ir]) in the pBNST than females and less prosocial rodents (13). This sex- and species-specific difference in pBNST TH-ir neuronal density is important for sex-specific prosocial behavior. Because pBNST neuron numbers are sensitive to sex steroid hormones (39, 40, 44, 45), we predicted that this population may be particularly vulnerable to endocrine disruption. For the present studies, we tested the hypothesis that alteration in OT-ir, AVP-ir, and TH-ir neuron numbers in the PVN and sex-specific TH-ir in the pBNST may contribute to the expression of social behavior, defensive behavior, and locomotor activity effects associated with BPA exposure.
Materials and Methods
Subjects
Husbandry
The animals used in this study were laboratory-reared prairie voles that originated from wild stock from Urbana. The prairie vole is a well-established rodent model for examining prosocial behaviors in the field and laboratory (reviewed in Ref. 13) but has housing, diet, and husbandry requirements that differ from conventional laboratory rodents (for details on basic ethology and laboratory housing needs, please refer to Refs. 46–50). Animals were maintained on a 14-hour light, 10-hour dark cycle in thoroughly washed polysulfone cages and provided with Purina high fiber rabbit chow (Purina) and water ad libitum at the Northeast Ohio Medical University in an Association for Assessment and Accreditation of Laboratory Animal Care approved facility affiliated with the Cushing lab at University of Akron. Rabbit chow is the established diet for laboratory prairie voles, because it is more similar to their natural diet than rat chow. This diet contains phytoestrogens from alfalfa and other ingredients vital for vole health and reproductive success in captive environments (48). Thus, although use of a phytoestrogen-free diet is typically preferable for studies evaluating the impacts of EDCs like BPA in animals (51, 52), this is not feasible for voles. On the day of birth, animals were sexed and marked for identification via toe clip. Litters were weaned at 21 days of age and housed in same-sex sibling pairs in 12 × 18 × 28-cm cages.
Exposure
Male and female prairie vole pups were orally exposed across postnatal day (PND)8–PND14, which has been identified as the crucial sociosexual developmental window in this species and akin to the neonatal period in rats/mice (30). On PND8, litters were removed from their home cage and placed in a clean cage on cotton bedding. All individuals were randomly assigned to exposure groups and weighed. Animals received an oral dose of one of 3 doses of BPA 5-μg/kg body weight (bw), 50-μg/kg bw (established reference dose), and 50-mg/kg bw (lowest observed adverse effect level) (53). Doses were based upon average weight of pups on PND8 and dissolved in 2.7 g of hydroxypropyl β cyclodextrin (Pharm Grade) in 10-mL 0.9% NaCl. A fourth group received vehicle only. For all groups, delivery was 25 μL orally to the pups by micropipette (as described previously for mice, see Ref. 54). This oral route was chosen because it is less stressful than orogastric gavage, a procedure that has recently been found to affect hypothalamic gene expression (55). All litters contained at least 1 control and no more than 1 exposed animal per exposure per sex per litter. Pups were returned to the parents as a group after exposure. Dosing was repeated daily on PND9–PND14. Specific pharmacokinetics regarding BPA uptake and metabolism are not available for the vole but presumed to be similar to rats, mice, and rhesus monkeys (56). A detailed pharmacological assessment was beyond the scope of the present study. An estrogen-exposed group was not included, because previous work in our lab has shown that BPA effects on nonreproductive behavior are not consistent with those induced by estrogen (18), Thus, estrogen was not assumed to be an appropriate positive control for effect. Additionally, it is unclear what the appropriate dose would be in a prairie vole, because information about how early life estrogen exposure influences neuroendocrine development and behavior in this species remains limited. Housing and all procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were preapproved by the NEOMED Institutional Animal Care and Use Committee. Although testing materials were polycarbonate, they were not considered a significant source of BPA contamination in the laboratory (52).
Behavior
Open field (OF) test
One week after weaning (PND28), subjects participated in a 10-minute OF test (40-cm2 Plexiglas test area), developed to ascertain anxiety-related and locomotor activities (57). Tests were video recorded from above on a digital video recorder, and then TopScan (Clever Sys, Inc) software was used to analyze time spent in the center, perimeter, and corners; frequency of transition between sections; distance traveled by section; and total distance traveled. Time spent in a freezing position was hand scored by an observer blind to exposure groups. Final animal numbers were as follows. Females: control, n = 23; 5 μg, n = 25; 50 μg, n = 15; and 50 mg, n = 16. Males: control, n = 12; 5 μg, n = 16; 50 μg, n = 12; and 50 mg, n = 16.
Novel social test
On PND30 (2 d after OF testing), subjects were tested for 1 hour in the novel social arena as described previously (58, 59), using a gently tethered, unrelated stimulus animal (same sex, size, and age matched and from an unexposed litter). Latency to enter the stimulus animal cage, frequency to movement between cages, number of contact bouts, duration in the stimulus animal cage, duration of exploratory behavior, and time spent in side-by-side physical contact were analyzed using TopScan software (Clever Sys, Inc). Endpoints were then validated/scored by an exposure-blinded observer to better determine the type of contact. All investigatory data were collected by hand. Prairie voles display very low levels of aggression; thus, injury to the stimulus animal is low or absent. As a precautionary measure, however, an observer remained in the room throughout the test period to monitor signs of aggression (not observed in any tests). Final animal numbers were as follows. Females: control, n = 24; 5 μg, n = 24; 50 μg, n = 15; and 50 mg, n = 17. Males: control, n = 16; 5 μg, n = 13; 50 μg, n = 11; and 50 mg, n = 14.
Partner preference test
Adult (PND60–PND75) animals were tested with an opposite sex conspecific for the effects of exposure on social interaction and formation of partner preference, using a standard, well-established paradigm detailed previously (13, 59). Briefly, test subjects were cohabitated for 3 hours with an unrelated, sexually naïve “partner” a length of time sufficient to induce a pair bond even though mating typically does not occur (60). No mating was observed, and females were in anestrus, because, unlike rats and mice, female prairie voles do not undergo spontaneous estrus, and estrogen levels remain low unless a female is exposed to a male for an extended period of time (up to 24 h). All tests were videotaped and scored with TopScan software (Clever Sys, Inc). Time spent in side-by-side contact (huddling) with each animal was scored and analyzed. By definition, a partner preference is formed if a test animal spends significantly more time in physical contact with the partner than the stranger (13). Final animal numbers were as follows. Females: control, n = 21; 5 μg, n = 17; 50 μg, n = 13; 50 mg, n = 17. Males: control, n = 15; 5 μg, n = 16; 50 μg, n = 10; and 50 mg, n = 16.
Immunohistochemistry
Animals were euthanized over PND60–PND90. Subjects were given 0.05-mL buprenorphine ip and then deeply anesthetized 15 minutes later with 0.05 mL of a ketamine-xylazine (at a concentration of 67.7 and 13.33 mg/kg, respectively) mixture administered sc. Brains were removed and immersion fixed in 4% paraformaldehyde for 24 hours at 4°C and transferred to fresh solution at 2 and 4 hours. The brains were then cryoprotected in 30% buffered sucrose with 0.1% sodium azide and shipped to the Patisaul lab, where they were stored in fresh cryoprotectant overnight at 4°C and then flash frozen and stored at −80°C. Brains were coronally sectioned at 35 μm on a frozen sliding microtome. Then sections for each individual corresponding to the regions of interest (ROIs) were collected and processed for immunohistochemistry.
PVN OT and AVP
For each individual, 8 sequential sections of the PVN were collected and processed for immunohistochemical staining of OT and AVP using routine procedures described previously (19). Sections were washed in cold potassium phosphate buffer solution (KBPS) and preincubated for 24 hours in 0.02M KPBS, 0.3% Triton X-100, and 2% normal goat serum at 4°C. Sections were then incubated in a primary antibody cocktail consisting of 1:12 000 monoclonal mouse anti-OT (catalog number MAB5296; Millipore) and polyclonal rabbit anti-AVP (catalog number 20069; Immunostar) for 72 hours on a shaker at 4°C. Sections were then washed and incubated in a cocktail of Alexa Fluor 568 goat antimouse and Alexa Fluor 488 goat antirabbit secondary antibodies (both at 1:200) for 120 minutes. After a final wash in cold KPBS, sections were mounted on Fisher super frost plus glass slides, coverslipped with a glycerol mountant, and stored at −20°C (for list of antibodies, please see Table 1).
Table 1.
Antibodies Used
| Peptide/Protein Target | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised in: Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|
| OT | Anti-OT clone 4G11 | MAB5296; Millipore | Mouse monoclonal | 1:12 000 |
| AVP | AVP antibody | 20069; Immunostar | Rabbit polyclonal | 1:12 000 |
| TH | Anti-TH hydroxylase antibody | AB152; Millipore | Rabbit polyclonal | 1:4000 |
PVN and pBNST TH
For each individual, 3 consecutive caudal PVN and pBNST sections were collected and processed for the immunohistochemical staining of TH using routine lab procedures (19) and counterstained with Hoescht. After a 24-hour preincubation, in cold 0.02M KPBS with 0.3% Triton X-100, and 2% normal donkey serum, the sections were then incubated with the polyclonal rabbit anti-TH (catalog number AB152; Millipore) primary antibody at 1:4000 for 72 hours on a shaker at 4°C. Sections were then washed in cold KPBS and incubated with the Alexa Fluor 488 donkey antirabbit secondary antibody at 1:200 for 120 minutes, washed, and counterstained with Hoechst (catalog number H3569; Invitrogen Life Technologies) via a 45-second incubation. After a final wash in cold KPBS, sections were mounted on Fisher super frost plus glass slides, coverslipped with a glycerol mountant, and stored at −20°C.
Quantification and analysis
A prairie vole brain atlas is not available; thus, the Paxinos and Watson rat brain atlas (61) was used to identify the PVN and its surrounding landmarks (as described in Ref. 62) along with available studies identifying anatomical subregions of the vole PVN (63, 64). Although relatively little is known about the functional distribution of specific neuronal phenotypes in the vole PVN, most significantly for the present study, most anterior and medial OT/AVP neurons in the prairie vole PVN have been putatively characterized as magnocellular neurohypophysial neurons, whereas the posterior PVN is known to contain a population projecting to the hindbrain and spinal cord; features indicative of parvocellular neurons (64).
With these subdivisions in mind, we subdivided the PVN into anterior, medial, and posterior regions based on results and figures published by Ross et al (64). Anterior sections correspond with Paxinos and Watson plates 38–41, medial sections correspond with plates 42–47, and posterior sections correspond with plates 48–51. From these 14 consecutive sections, 2 anterior, 4 medial, and 2 posterior sections from the middle of each subregion (anterior, medial, and posterior) were selected. All OT-ir and AVP-ir neurons were then bilaterally counted and averaged for each individual using a fluorescent Leica DM5000 scope. Two consecutive sections from the most caudal (posterior) PVN region were assessed for TH-ir, where we found bilateral populations, round, located lateral and dorsal to the third ventricle, corresponding to the PVN, posterior part and A13 dopamine cells in the depicted in plates 51–52 in Paxinos and Watson. The TH-ir cells confined to this region were manually counted and averaged for each animal. The pBNST of each animal was imaged at ×200 using a Leica DM5500 confocal microscope. We then used ImageJ to quantify the TH-ir cells found in a dense cluster within 3 consecutive sections corresponding to plates 21–23 in the Swanson atlas (65), as described in Ref. 39, but, most specifically, plate 21, where the most densely populated area was found.
Statistical analysis
Behavior
All OF and novel social behavior results were analyzed by a two-way ANOVA with exposure and sex as factors. A one-way ANOVA was then performed within sex, because the behaviors analyzed are known to be sexually dimorphic (13). If overall exposure effects were significant (P ≤ .05), then a protected Fisher's least significant differences post hoc test was performed to evaluate pair-wise differences (P ≤ .05). All analyses were performed using Prism 6, and potential statistical outliers were identified using the Robust Regression and Outlier Removal Method (ROUT) method (as noted). All results were considered significant if P ≤ .05.
Time spent with the partner vs the stranger was analyzed using an unpaired t test with the Holm-Sidak method to correct for multiple comparisons (with the α set at 5%) for each exposure and sex. A partner preference was considered to have occurred if time in side-by-side contact with the partner was greater than the stranger with P ≤ .05.
Neuroanatomy
To detect sex and exposure group differences in numbers of OT-ir, AVP-ir, and TH-ir cells, average cell numbers per region (anterior, medial, and posterior PVN, pBNST) were analyzed by a two-way ANOVA and followed up with a one-way ANOVA and protected Fisher's least significant difference post-hoc test as described for behavior analysis.
Results
All results (behavioral and neuroanatomical) are summarized in Table 2. Significant sex and exposure effects are shown.
Table 2.
Summary of Results on Behavioral and Neuroanatomical Endpoints
| Endpoint | Female Control | Female 5-μg BPA | Female 50-μg BPA | Female 50-mg BPA | Male Control | Male 5-μg BPA | Male 50-μg BPA | Male 50-mg BPA |
|---|---|---|---|---|---|---|---|---|
| OF | M = F | ↑Activity | NS | ↓Activity | M = F | NS | NS | NS |
| Novel social | M > F | NS | NS | ↑Sniff time (M < F) | M > F | NS | ↓Sniff time | ↓Sniff time (M < F) |
| Partner preference | Significant preference | No preference | No preference | No preference | No preference | No preference | No preference | No preference |
| Posterior PVN OT-ir | M = F | NS | NS | ↓OT | M = F | NS | NS | NS |
| Anterior PVN AVP-ir | M = F | NS | NS | ↑AVP | M = F | NS | NS | NS |
| Posterior PVN TH-ir | M > F | ↑TH (M = F) | ↑TH (M = F) | ↑TH (M = F) | M > F | ↓TH (M = F) | ↓TH (M = F) | ↓TH (M < F) |
| pBNST TH-ir | M > F | ↑TH (M = F) | NS | NS | M > F | NS | NS | NS |
Abbreviations: NS, non-significant; M, male; F, female.
Behavior
Open field
A significant exposure by sex interaction was detected by a two-way ANOVA for total overall bouts (all entries made into any area of the arena) made in the OF arena (F(3,114) = 2.866, P ≤ .05) (Figure 1A) as well as total distance traveled (F(3,115) = 3.883, P ≤ .01) (Figure 1B). Within females, main effects of BPA exposure were identified for total overall bouts (F(3,69) = 6.496, P ≤ .001) (Figure 1A), center bouts (F(3,70) = 3.120, P ≤ .03) (Figure 1C), corner bouts (F(3,70) = 4.441, P ≤ .01) (Figure 1E), and perimeter bouts (F(3,70) = 5.564, P ≤ .002) (Figure 1G). The 5-μg BPA group made significantly more bouts than controls overall (P ≤ .005) and into the center (P ≤ .05) and perimeter (P ≤ .01), whereas the 50-mg BPA female group made significantly fewer bouts into the corners than the controls (P ≤ .05). No significant differences in duration of time spent in any portion of the OF arena were found (Figure 1, D, F, and H). No significant effects of BPA were identified in the males.
Figure 1.
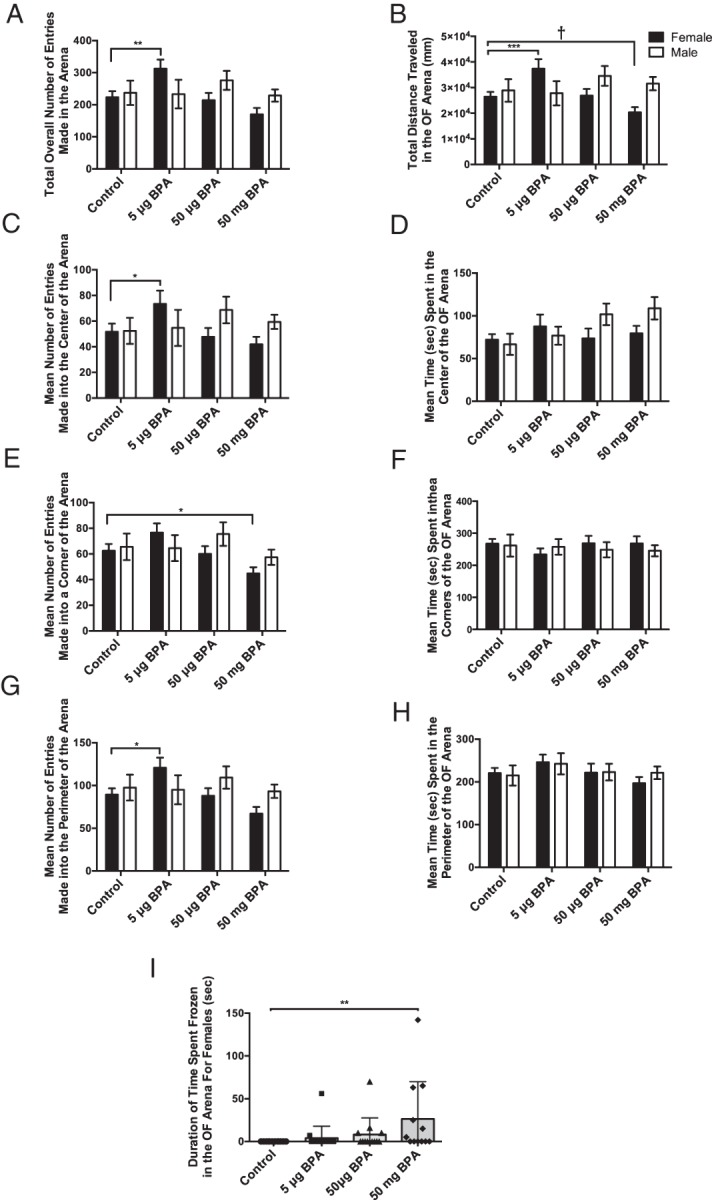
Early life exposure to BPA caused a dose-specific effect on activity levels in female prairie voles, with the low dose increasing and high dose decreasing activity. A, Females exposed to 5-μg BPA made significantly more entries into the OF zones overall, than control females. B, Females exposed to 5-μg BPA traveled significantly more, whereas females exposed to 50-mg BPA traveled less than control females. C, Females exposed to 5-μg BPA made significantly more entries into the center of the OF arena than control females but (D) duration of time spent in the center was unchanged. E, Females exposed to 50-mg BPA made fewer entries into the corners compared with control conspecifics. F, However, duration of time spent in the corners was unchanged. G, Females exposed to 5-μg BPA made significantly more entries into the perimeter of the OF compared with controls. H, However, duration of time spent in the perimeter was unchanged. I, Females exposed to 50-mg BPA froze significantly more than controls in the OF arena. Data are shown as the mean ± SE. Differences from same sex controls indicated by *, P ≤ .05; **, P ≤ .01; ***, P ≤ .001; †, P ≤ .1.
One-way ANOVA revealed that freezing behavior was significantly elevated in the female 50-mg BPA group compared with controls (F(3,49) = 2.854, P ≤ .05) (Figure 1I). This group had high variation so ROUT was employed to identify outliers and 6 were identified. If these 6 outliers were removed from the dataset, the significant difference was lost. Thus, we considered this statistical phenomenon to be indicative of biologically meaningful, phenotypic variation for this trait and did not consider any animals to be true “outliers.” No significant exposure effects on freezing behavior were found for males.
Novel social
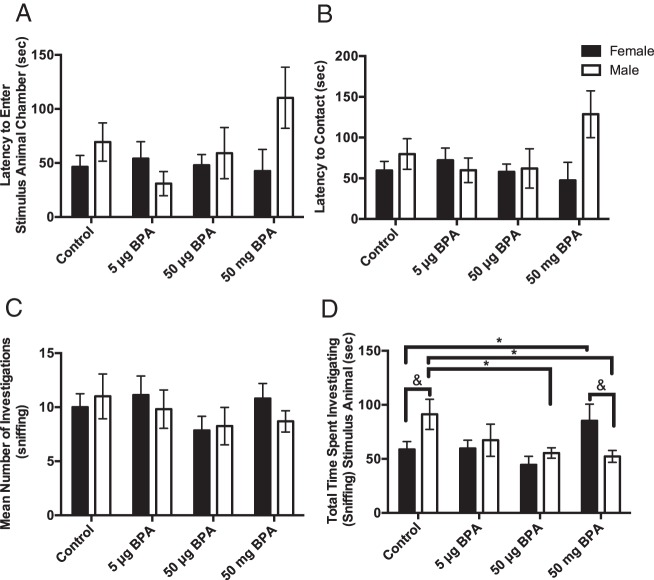
No significant effects or interactions were found for latency to enter the stimulus animal chamber (Figure 2A), latency to contact the stimulus animal (Figure 2B), or number of investigations (sniffing) of the stimulus animal (Figure 2C). Two-way ANOVA revealed a significant interaction between sex and exposure for time spent investigating the stimulus animal (F(3,95) = 3.894, P ≤ .01) (Figure 2D). Control males spent significantly more time investigating the stimulus animal than did the control females (P ≤ .02), 50-μg BPA-exposed males (P ≤ .03) males, and 50-mg BPA-exposed males (P ≤ .01). Females exposed to 50-mg BPA spent significantly more time investigating than did the control females (P ≤ .05) and males exposed to 50-mg BPA (P ≤ .02). Thus, the sex difference observed in the control animals was reversed in the 50-mg BPA exposure group (Figure 2D).
Figure 2.
Postnatal BPA caused a sex- and dose-specific response in duration of time spent sniffing a novel stimulus animal. No significant differences were found in the latency to enter the stimulus animal's chamber (A), latency to contact the stimulus animal (B), or number of investigations of a stimulus animal (C). D, Control males spent significantly more time investigating the stimulus animal than control females. Exposure to 50-μg and 50-mg BPA significantly decreased the time males spent investigating, and exposure to 50-mg BPA increased the amount of time females spent investigating the stimulus animal. Sex differences in investigation were lost in the 50-μg BPA and 50-mg BPA dose groups. Data are shown as the mean ± SE. Significant difference from control indicated by *, P ≤ .05.
Partner preference
The control females formed a partner preference, spending significantly more time in side-by-side contact with the partner vs the stranger (t20 = 2.53, P ≤ .02) (Figure 3A). In contrast, females treated with any dose of BPA failed to form a partner preference, because there was no significant difference in the duration of side-by-side contact between the partner and the stranger (5 μg [t16 = 1.39, P = .17], 50 μg [t12 = 1.78, P = .09], and 50 mg [t16 = 1.77, P = .09]). None of the male exposure groups, including controls, spent significantly more time in side-by-side contact with either the partner or the stranger (Figure 3B).
Figure 3.
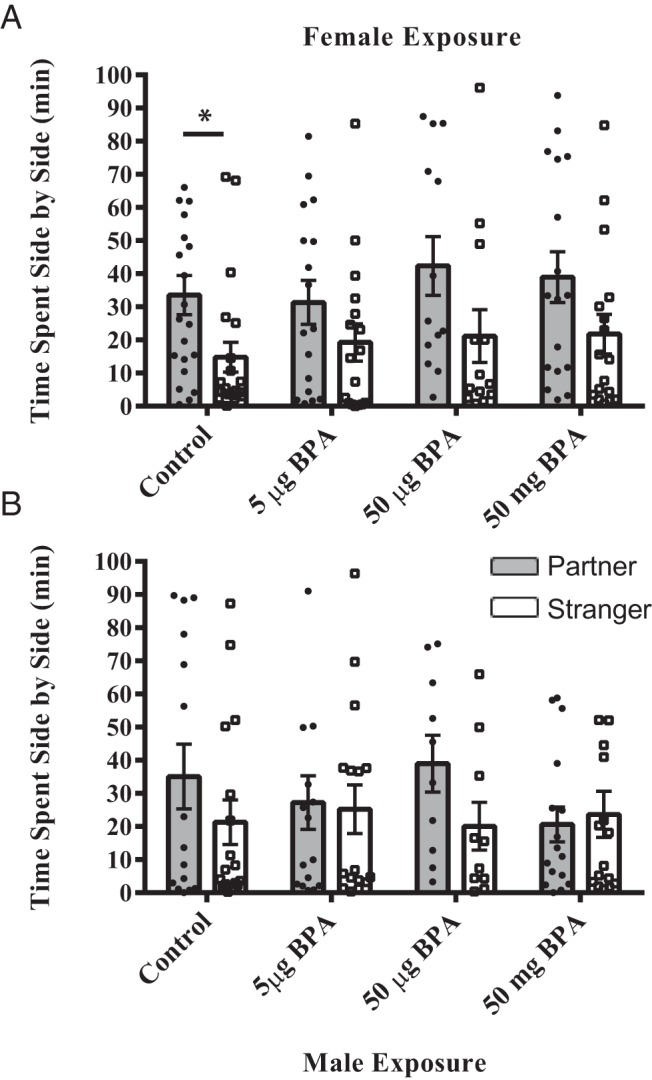
Postnatal BPA exposure resulted in a loss of statistical significance in the partner preference test. A, Control females spent significantly more time huddling with the stranger vs the partner. In the BPA-exposed groups, females still spent more time beside their partner, but the statistical significance in time spent with the partner vs a stranger was lost. B, None of the male groups showed a significant partner preference. For each group, the individual data points are depicted as well as the mean ± SE. Significant difference from control indicated by *, P ≤ .05.
Number of OT-ir, AVP-ir, and TH-ir neurons in the PVN
Oxytocin
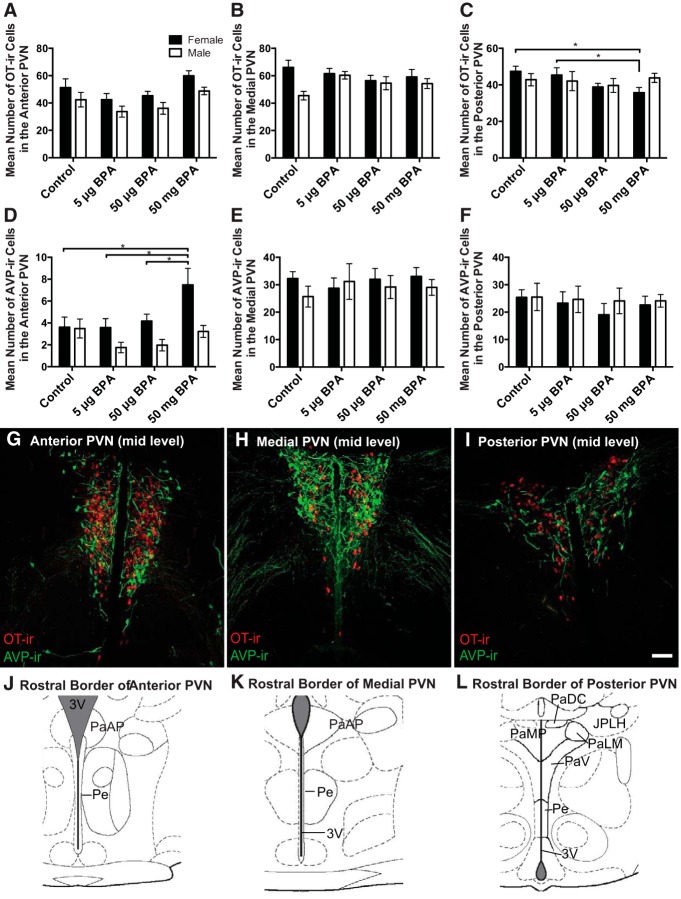
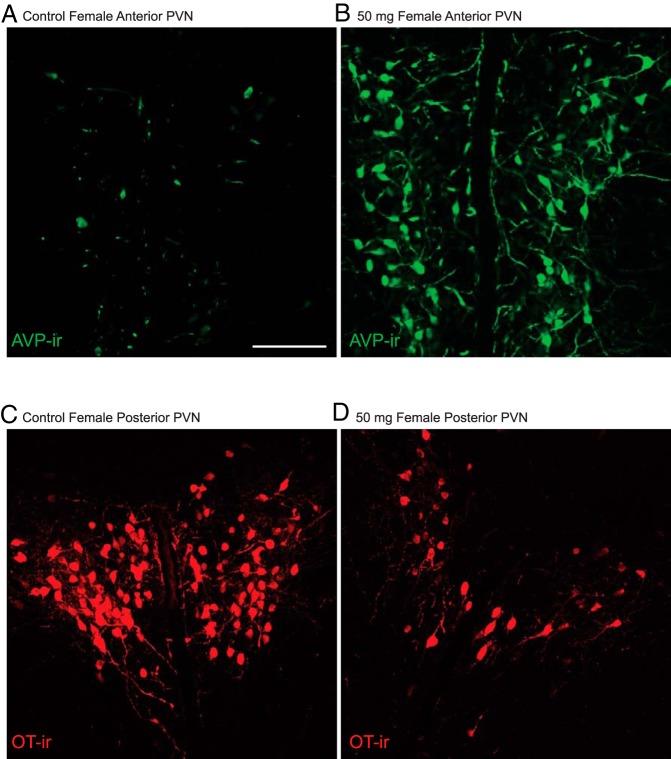
No significant interaction between sex and exposure was found by two-way ANOVA but a one-way ANOVA within sex indicated a significant main effect of exposure on female OT-ir (F(3,43) = 3.010, P ≤ .04) neuron numbers in the anterior PVN. Females exposed to 50-mg BPA had significantly more OT-ir neurons than the 5-μg (P ≤ .01) and 50-μg (P ≤ .05) groups (Figure 4A) but did not statistically differ from controls. Differences in medial PVN OT-ir neuron numbers did not reach statistical significance (Figure 4B). There was a significant main effect of exposure on OT-ir (F(3,42) = 2.754, P ≤ .054) (Figure 4C) neuron numbers in the posterior PVN. The 50-mg BPA group had significantly fewer OT-ir neurons compared with controls (P ≤ .02) (Figures 4C and 5, E and F) and 5-μg BPA-exposed females (P ≤ .05).
Figure 4.
Early life exposure to 50-mg/kg BPA resulted in a significant decrease in OT-ir cells number and a significant increase in the AVP-ir cell numbers in the female prairie vole PVN. A and B, BPA exposure did not significantly alter OT-ir cell numbers in the anterior or medial PVN of either sex, but females exposed to 50-mg BPA had significantly fewer OT-ir cells in the posterior PVN (C) and significantly more AVP-ir cells in the anterior PVN (D). E and F, Exposure to BPA had no significant effect on AVP-ir cell numbers in the medial and posterior PVN of either sex. G–I, Representative images representing a midlevel immuolabeled section for each of the ROIs in the PVN (anterior, medial, and posterior). J–L, Corresponding illustrations adapted from the Paxinos and Watson rat brain atlas depicting the landmarks used to identify the rostral border of each subregion: anterior (plates 38–41), medial (plates 42–47), or posterior (plates 48–51). Scale bars, 100 μm. PaAP, paraventricular nucleus of the hypothalamus anterior parvicellular; PaDC, paraventricular nucleus of the hypothalamus dorsal cap; PalM, paraventricular nucleus of the hypothalamus lateral magnocellular; PaMP, paraventricular nucleus of the hypothalamus medial parvicellular; PaPo, paraventricular nucleus of the hypothalamus, posterior; PaV, paraventricular nucleus ventral portion; Pe, periventricular hypothalamic nucleus; ZI, zona incerta. Data are shown as the mean ± SE. Significant difference from control indicated by *, P ≤ .05.
Figure 5.
Representative images depicting increased AVP-ir cell numbers in the female anterior PVN (A and B) and decreased OT-ir cell numbers in the posterior PVN of 50-mg/kg BPA-exposed females compared with control conspecifics (C and D). Scale bars, 100 μm.
A significant main effect of exposure was found for male OT-ir (F(3,42) = 3.341, P ≤ .03) (Figure 4A) neuron numbers in the anterior PVN. Males exposed to 50-mg BPA had significantly more OT-ir cells than 5-μg BPA (P ≤ .01) and 50-μg BPA (P ≤ .05). No significant effect of exposure was found for OT-ir neuron number in the medial or posterior PVN (Figure 4, C and B).
Vasopressin
No significant interaction was found by a two-way ANOVA, but one-way ANOVA within sex indicated a significant main effect of exposure on female AVP-ir (F(3,42) = 3.406, P ≤ .03) neuron numbers in the anterior PVN. Females exposed to 50-mg BPA had significantly more AVP-ir neurons than all other groups (controls, P ≤ .01 [Figures 4D and 5, A and B]; 5 μg, P ≤ .01, and 50 μg, P ≤ .05). No significant effects were found for female medial (Figure 4B) or posterior PVN AVP-ir neuron numbers (Figure 4C).
In males, no effects of exposure were found for male AVP-ir neuron number in the anterior PVN (Figure 4D), the medial PVN (Figure 4E), or posterior PVN (Figure 4F).
Tyrosine hydroxylase
A two-way ANOVA revealed a significant exposure by sex interaction (F(3,39) = 19.61, P ≤ .0001). Male controls had significantly more TH-ir cells than the female controls (P ≤ .0001) (Figure 6, A, D, and E). Exposure to 5-μg, 50-μg, or 50-mg BPA caused a significant decrease in TH-ir cell number in males (P ≤ .01, P ≤ .001, P ≤ .0001 respectively) and a significant increase in cell number in females (P ≤ .0001, P ≤ .001, P ≤ .0001, respectively) (Figure 6, A and D–G). The sex difference in TH-ir cells was lost in the 5-μg BPA group and reversed in the 50-μg and 50-mg BPA exposure groups compared with unexposed controls.
Figure 6.
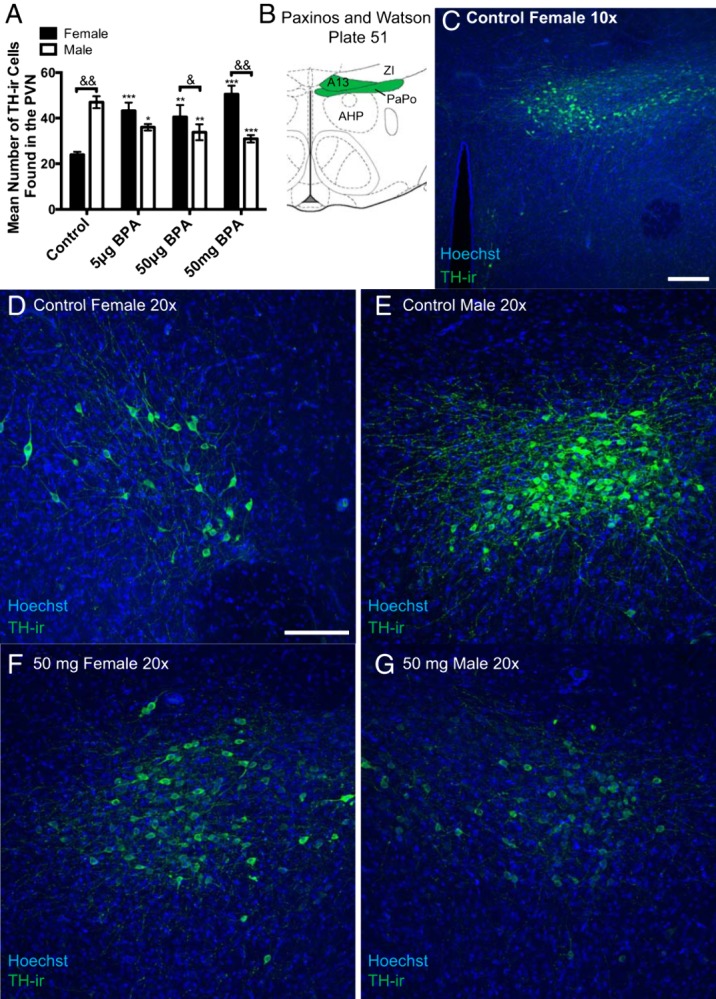
In the posterior PVN, postnatal BPA exposure significantly increased the number of TH-ir cells in females and significantly decreased their numbers in males, thus reversing the sex difference in density. A, Control males had significantly more TH-ir cells than control females in the posterior PVN area, but this was reversed by BPA exposure due to a significant loss of TH-ir cells in males and a significant increase in females. B, The shaded portion of the diagram adapted from Paxinos and Watson plates 51 depicting the ROI. C, Representative image depicting the rounded, dense cluster of TH-ir cells found in the A13 region and quantified. D and E, Representative images depicting that TH-ir density is sexually dimorphic with females (D) having significantly fewer than males (E). F and G, Representative images revealing that 50-mg/kg BPA reverses the sexual dimorphism in posterior PVN TH-ir density. Scale bars, 100 μm. Data are shown as the mean ± SE. A significant difference from control indicated by *, P ≤ .01; **, P ≤ .001; ***, P ≤ .0001 within sex. Significant difference between sexes within exposure indicated by &, P ≤ .01; &&, P ≤ .0001. A13, A13 dopamine cells; AHP, anterior hypothalamic area, posterior; DA, dorsal hypothalamic area; PaPo, paraventricular nucleus, posterior; Pe, periventricular hypothalamic nucleus; ZI, zona incerta.
Number of TH-ir neurons in the pBNST
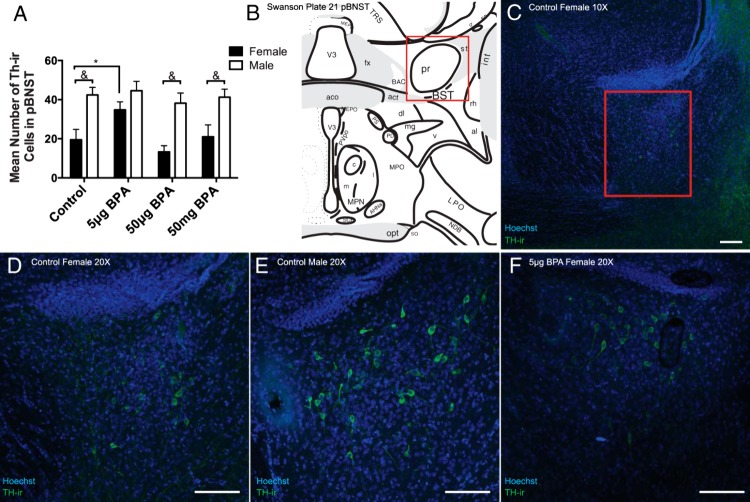
A two-way ANOVA indicated main effects of exposure (F(3,51) = 2.708, P ≤ .05) and sex (F(1,51) = 30.67, P ≤ .0001) but no significant interaction. Males had significantly more TH-ir cells than females in the control (P ≤ .005), 50-μg BPA (P ≤ .005), and 50-mg BPA (P ≤ .01) exposure groups (Figure 7, A and D–F). Postnatal exposure to 5-μg BPA caused a significant increase (P ≤ .05) in TH-ir cell number in the female pBNST; thus, attenuating the sex difference.
Figure 7.
Early life exposure to 5-μg/kg BPA caused a significant increase in female TH-ir cell number in the pBNST. A, pBNST TH-ir was significantly elevated in the 5-μg/kg BPA females compared with unexposed conspecifics but unaltered in the other exposure groups. B, An illustration adapted from the Swanson rat brain atlas plate 21 depicting the ROI (boxed). C, Low magnification image of the population of TH-ir cells quantified in this region. D–F, Representative images showing the pronounced sex difference in pBNST TH-ir and the significant increase in TH-ir cell numbers in the female 5-μg/kg bw BPA exposure group. Scale bars, 100 μm. Data are shown as the mean ± SE. A significant difference from control indicated by *, P ≤ .05. Significant difference between sexes within the same exposure group indicated by &, P ≤ .01. pr, pBNST.
Discussion
Consistent with what has been reported in other species (9, 12, 66, 67), here, we show that BPA had sex-specific effects on the expression of behaviors associated with anxiety, activity, and sociality in prairie voles (summarized in Table 2). In females, BPA exposure affected exploratory activity and behavior but only minimally inhibited partner preference formation. Behavioral outcomes were accompanied by dose- and sex-dependent changes in TH-ir in the pBNST, and TH-ir, OT-ir, and AVP-ir neuron numbers in the PVN resulting in the loss of region-specific sex differences at some doses. Collectively, these studies provide further evidence that developmental exposure to BPA can significantly impact the brain and behavior and highlight the utility of the prairie vole model when seeking to explore the effects of exposure to BPA or other EDCs on prosocial behaviors and the neuroendocrine systems that coordinate them.
Female prairie voles exposed to the lowest BPA dose (5-μg/kg bw) demonstrated heightened exploratory activity in the OF, suggestive of hyperactivity; an observation consistent with what has been reported in mice (9–11, 68), rats (23, 24, 69, 70), zebrafish (71), and young children (5–7). Similarly, the novel social test and partner preference test results indicate that BPA altered the time course/development of prosocial behavior in a sex-specific manner. Control males investigated novel individuals significantly more than females, and BPA exposure either eliminated or reversed this effect, with females exposed to the highest dose of BPA investigating novel individuals more than exposed males. In the 2 lower dose groups, the sex difference in investigation of novel conspecifics was lost. These findings suggest that BPA, although not altering total time spent with novel conspecifics, alters how individuals interact socially.
The formation of a partner preference is the initial and critical event in the establishment of long-term pair bonds, and an essential aspect of social monogamy (13). In females, BPA precluded the emergence of a statistically significant partner preference. The fact that there still appears to be a strong tendency (Figure 3A) to spend more time in contact with the partner suggests that BPA, instead of disrupting the ultimate formation of a preference, altered aspects of this process. One possibility is that BPA increases the total time needed to form a preference and bond. This hypothesis is supported by the heightened activity and social investigation observed in the other tasks. In contrast to females, BPA had no effect on partner preference in males. This is not surprising given that control males did not form a preference, an outcome consistent with previous studies indicating that mating is required for males to form a bond unless OT or AVP is centrally administered (13). Neonatally castrated males fail to form a bond even after infusion of OT or AVP (32); thus, a logical follow up would be to determine whether early exposure to BPA also makes males refractory. Additionally, because studies in mice have shown that social behavior and preference may be further impacted if both individuals (the test subject and the stimulus animal) have been exposed to EDCs (72, 73), examining partner preference outcomes when both sexes have been exposed is also of interest.
Enhanced freezing behavior provides further evidence that BPA may induce a high anxiety phenotype (10, 12). The proportion of females engaging in this behavior was highest in the 50-mg BPA group, and variability in the expression of this behavior increased with dose; a phenomenon suggesting that some individuals are more predisposed to respond to an environmental stressor with this type of activity than others. Essentially, within the 50-mg BPA group, there were “responders” and “nonresponders,” and the statistically significant difference in freezing behavior was lost if the 6 most robust data points were removed as “statistical outliers” (identified by a ROUT test). Our interpretation of this behavioral variability is that the responders may represent individuals within a population that are more sensitive to a change in the environment. Limited information is available regarding genetic and other differences within a population contributing to variation in coping styles (74, 75) but may result from intrauterine position and other environmental factors (76). Further work in this area is critically needed, because the concept of “adaptation” (or “resilience”) is emerging as a pivotal but controversial concept in endocrine disruption toxicology (77). Voles are an ideal model for addressing this, because laboratory colonies are derived from wild caught individuals, thus preserving naturally occurring genomic and phenotypic variance (78, 79). Additionally, voles have a very different life history than rats and mice, which may account for why no impacts of BPA were observed on other anxiety-assessment aspects of OF behavior. For example, avoidance of the center is typically considered a hallmark measure of anxiety, but this may not be an appropriate measure of vole “anxiety,” because they typically move about in grass runways where they are openly exposed to predators.
Although available data are limited, the potential for BPA to disruption of the OT/AVP system has previously been shown by us and others. We have shown, for example, that developmental exposure to BPA via drinking water (1 mg/L), resulting in serum levels approximately equivalent to humans (4), elevated anxiety-related behaviors in juvenile rats and decreased Esr2 (estrogen receptor [ER]β) and melanocortin 4 receptor (Mc4r) expression levels in the amygdala (18). These genes play crucial roles in regulating the production and release of OT and AVP in the PVN. Specifically, agonism of melanocortin 4 receptor (Mc4R) in magnocellular neurons induces dendritic secretion of OT (80), an effect which is anxiolytic (81, 82). In female rats, neonatal BPA exposure (50-mg/kg bw or 50-μg/kg bw by sc injection) significantly increased OT-ir neuron numbers in the anterior PVN of female rats (19), a result interpreted as potentially indicative of OT sequestration. Mice reared on a diet delivering approximately 170-μg/kg bw BPA (to the dams) during gestation displayed transgenerational changes in sociality coinciding with decreased AVP and OT mRNA expression levels in whole embryonic brains. A subsequent study revealed hyperactivity in the F3 generation accompanied by increased investigative sniffing of a novel conspecific (10, 20). Collectively, these findings support the hypothesis that BPA exposure may disrupt the organization of OT/AVP pathways arising in the PVN, thereby impacting related behaviors.
The present data enhance available information about how BPA might be altering PVN organization by revealing that only specific subpopulations of PVN OT, AVP, and TH neurons may be vulnerable to endocrine disruption. Interpreting the functional significance of these region-specific changes is complicated by the limited information regarding the subarchitecture of the vole PVN, because the only study to date attempting to identify-specific OT/AVP neuronal populations, and their projections, in the vole PVN found that magnocellular and parvocellular cells are intermixed and that their projections may not entirely match those of the rat (64). Although we hypothesize that the BPA-impacted population of OT-ir and AVP-ir neurons is parvocellular, the possibility that BPA alters the density of magnocellular neurons cannot be ruled out. This would suggest a potential mechanism for homeostatic disruptions associated with BPA exposure, including cardiovascular effects and hypertension (83, 84). Similarly, in the rat, parvocellular OT and AVP neurons in the affected areas of the PVN, along with coordinating input from the BNST, are involved in the stress response (42), supporting the possibility that BPA may influence the hypothalamic-pituitary-adrenal (HPA) axis.
Actions on ERs are likely a primary mechanism by which BPA induces effects on dopaminergic and OT/AVP pathways. We have shown that developmental BPA exposure can perturb ERα and ERβ expression throughout the rat mesolimbic dopamine system, including the PVN and BNST (55, 85–88), across the lifespan. Altered distribution of ERs in limbic nuclei central to sociality may underpin the observed behavioral and structural changes reported here. Critically, OT pathways coordinating social recognition are well known to be regulated by estrogen, with both ERα and ERβ knockout mice showing social deficits (89, 90), and ERβ knockout females failing to generate OT or AVP mRNA expression in response to exogenous estrogen administration (91, 92). The distribution of ERα differs across social and nonsocial species and plays a significant role in regulating species-specific sociality (93). Increased ERα in the pBNST, for example, reduces male prosocial behaviors (13, 94). The male prairie vole pBNST is also richly populated with TH-ir cells, some of which are colocalized with ERα. Few of these colocalized cells have been detected in the female vole pBNST or the BNST of polygamous rodent species (in either sex), and this unique neuronal population is more responsive to estrogen administration in maintaining TH (13), suggesting that ERα mediates BNST dopamine production. These data suggest that future work should focus on EDC-related impacts on ER levels within vole limbic nuclei, and male social interactions mediated by them, including aggression. Although the specific functional role of limbic ERβ remains poorly understood, ERβ in the PVN, BNST, and associated structures plays a fundamental role in mediating motivational and anxiety-related behaviors (95–97). Moreover, in female prairie voles, estradiol administration induces estrous but also modulates locomotor activities (98, 99), suggesting a role for ERs in heightened exploration.
Conclusions
Exposure to BPA altered the expression of social behavior and the associated limbic system in prairie voles. The effects were sexually dimorphic and dose dependent, with most effects occurring in females. The increase in activity in females treated with the lowest level of BPA, indicative of hyperactivity, is consistent with available human and mouse data. In contrast, the subtle, but significant changes in social interaction seen in the novel social test and formation of partner preferences represent new findings that may not be observable in less social laboratory rodents. Behavioral changes were accompanied by altered OT-ir and AVP-ir cell numbers in subregions of the PVN, and TH-ir cell numbers in the pBNST. Although the specific functional significance of these changes needs to be further elucidated, impacts are likely wide ranging, because these brain regions and mechanisms are known to be involved in the regulation of prosocial behaviors as well as responses to stress. Collectively, the data indicate that using a more socially relevant species may be critical for understanding the neurobehavioral effects of BPA, as well as other EDCs, in humans.
Acknowledgments
We thank Jacob Kahn for tissue preparation and Lisa McGraw for her critical reading of the manuscript. A.W.S. acknowledges Joseph and Ayla Grappe' for their requisite support.
This work was supported by pilot funds granted to H.B.P. through North Carolina State University.
Disclosure Summary: H.B.P. is serving as a Guest Associate Editor for Endocrinology and serves on the Scientific Advisory Board of the Glass Packing Institute. All other authors have nothing to disclose.
Abbreviations
- AVP
vasopressin
- BPA
bisphenol A
- bw
body weight
- EDC
endocrine disrupting compound
- ER
estrogen receptor
- KBPS
potassium phosphate buffer solution
- ir
immunoreactivity
- OF
open field
- OT
oxytocin
- pBNST
principal bed nucleus of the stria terminalis
- PND
postnatal day
- PVN
paraventricular nucleus of the hypothalamus
- ROI
region of interest
- ROUT
Robust Regression and Outlier Removal Method
- TH
tyrosine hydroxylase.
References
- 1. Vogel SA. The politics of plastics: the making and unmaking of bisphenol a “safety.” Am J Public Health. 2009;99(suppl 3):S559–S566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Cien Saude Colet. 2012;17(2):407–434. [DOI] [PubMed] [Google Scholar]
- 3. Richter CA, Birnbaum LS, Farabollini F, et al. . In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007;24(2):199–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. FAO/WHO. Toxicological and Health Aspects of Bisphenol A: Report of Joint FAO/WHO Expert Meeting and Report of Stakeholder Meeting on Bisphenol A. Ottawa, Canada: World Health Organization; 2011. [Google Scholar]
- 5. Braun JM, Kalkbrenner AE, Calafat AM, et al. . Impact of early-life bisphenol a exposure on behavior and executive function in children. Pediatrics. 2011;128(5):873–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Braun JM, Yolton K, Dietrich KN, et al. . Prenatal bisphenol A exposure and early childhood behavior. Environ Health Perspect. 2009;117(12):1945–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harley KG, Gunier RB, Kogut K, et al. . Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children. Environ Res. 2013;126:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Adkins-Regan E. Neuroendocrinology of social behavior. ILAR J. 2009;50(1):5–14. [DOI] [PubMed] [Google Scholar]
- 9. Williams SA, Jasarevic E, Vandas GM, et al. . Effects of developmental bisphenol A exposure on reproductive-related behaviors in California mice (Peromyscus californicus): a monogamous animal model. PLoS One. 2013;8(2):e55698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wolstenholme JT, Goldsby JA, Rissman EF. Transgenerational effects of prenatal bisphenol A on social recognition. Horm Behav. 2013;64(5):833–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kundakovic M, Gudsnuk K, Franks B, et al. . Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc Natl Acad Sci USA. 2013;110(24):9956–9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wolstenholme JT, Rissman EF, Connelly JJ. The role of bisphenol A in shaping the brain, epigenome and behavior. Horm Behav. 2011;59(3):296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Young KA, Gobrogge KL, Liu Y, Wang Z. The neurobiology of pair bonding: insights from a socially monogamous rodent. Front Neuroendocrinol. 2011;32(1):53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Modi ME, Young LJ. The oxytocin system in drug discovery for autism: animal models and novel therapeutic strategies. Horm Behav. 2012;61(3):340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gray LE Jr, Ostby J. Effects of pesticides and toxic substances on behavioral and morphological reproductive development: endocrine versus nonendocrine mechanisms. Toxicol Ind Health. 1998;14(1–2):159–184. [DOI] [PubMed] [Google Scholar]
- 16. Gray LE Jr, Ostby J, Cooper RL, Kelce WR. The estrogenic and antiandrogenic pesticide methoxychlor alters the reproductive tract and behavior without affecting pituitary size or LH and prolactin secretion in male rats. Toxicol Ind Health. 1999;15(1–2):37–47. [DOI] [PubMed] [Google Scholar]
- 17. Engell MD, Godwin J, Young LJ, Vandenbergh JG. Perinatal exposure to endocrine disrupting compounds alters behavior and brain in the female pine vole. Neurotoxicol Teratol. 2006;28(1):103–110. [DOI] [PubMed] [Google Scholar]
- 18. Patisaul HB, Sullivan AW, Radford ME, et al. . Anxiogenic effects of developmental bisphenol a exposure are associated with gene expression changes in the juvenile rat amygdala and mitigated by soy. PLoS One. 2012;7(9):e43890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adewale HB, Todd KL, Mickens JA, Patisaul HB. The impact of neonatal bisphenol-a exposure on sexually dimorphic hypothalamic nuclei in the female rat. Neurotoxicology. 2011;32(1):38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wolstenholme JT, Edwards M, Shetty SR, et al. . Gestational exposure to bisphenol A produces transgenerational changes in behaviors and gene expression. Endocrinology. 2012;153(8):3828–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Masuo Y, Ishido M. Neurotoxicity of endocrine disruptors: possible involvement in brain development and neurodegeneration. J Toxicol Environ Health B Crit Rev. 2011;14(5–7):346–369. [DOI] [PubMed] [Google Scholar]
- 22. Elsworth JD, Jentsch JD, Vandevoort CA, Roth RH Jr, DE, Leranth C. Prenatal exposure to bisphenol A impacts midbrain dopamine neurons and hippocampal spine synapses in non-human primates. Neurotoxicology. 2013;35:113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ishido M, Masuo Y, Kunimoto M, Oka S, Morita M. Bisphenol A causes hyperactivity in the rat concomitantly with impairment of tyrosine hydroxylase immunoreactivity. J Neurosci Res. 2004;76(3):423–433. [DOI] [PubMed] [Google Scholar]
- 24. Masuo Y, Morita M, Oka S, Ishido M. Motor hyperactivity caused by a deficit in dopaminergic neurons and the effects of endocrine disruptors: a study inspired by the physiological roles of PACAP in the brain. Regul Pept. 2004;123(1–3):225–234. [DOI] [PubMed] [Google Scholar]
- 25. Cushing BS, Kramer KM. Mechanisms underlying epigenetic effects of early social experience: the role of neuropeptides and steroids. Neurosci Biobehav Rev. 2005;29(7):1089–1105. [DOI] [PubMed] [Google Scholar]
- 26. Lonstein JS, Rood BD, De Vries GJ. Unexpected effects of perinatal gonadal hormone manipulations on sexual differentiation of the extrahypothalamic arginine-vasopressin system in prairie voles. Endocrinology. 2005;146(3):1559–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cavanaugh BL, Lonstein JS. Androgenic and oestrogenic influences on tyrosine hydroxylase-immunoreactive cells of the prairie vole medial amygdala and bed nucleus of the stria terminalis. J Neuroendocrinol. 2010;22(4):217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kabelik D, Schrock SE, Ayres LC, Goodson JL. Estrogenic regulation of dopaminergic neurons in the opportunistically breeding zebra finch. Gen Comp Endocrinol. 2011;173(1):96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Simerly RB, Swanson LW, Handa RJ, Gorski RA. Influence of perinatal androgen on the sexually dimorphic distribution of tyrosine hydroxylase-immunoreactive cells and fibers in the anteroventral periventricular nucleus of the rat. Neuroendocrinology. 1985;40(6):501–510. [DOI] [PubMed] [Google Scholar]
- 30. Kramer KM, Perry AN, Golbin D, Cushing BS. Sex steroids are necessary in the second postnatal week for the expression of male alloparental behavior in prairie voles (Microtus ochragaster). Behav Neurosci. 2009;123(5):958–963. [DOI] [PubMed] [Google Scholar]
- 31. Roberts RL, Zullo AS, Carter CS. Sexual differentiation in prairie voles: the effects of corticosterone and testosterone. Physiol Behav. 1997;62(6):1379–1383. [DOI] [PubMed] [Google Scholar]
- 32. Cushing BS, Okorie U, Young LJ. The effects of neonatal castration on the subsequent behavioural response to centrally administered arginine vasopressin and the expression of V1a receptors in adult male prairie voles. J Neuroendocrinol. 2003;15(11):1021–1026. [DOI] [PubMed] [Google Scholar]
- 33. Yamamoto Y, Cushing BS, Kramer KM, Epperson PD, Hoffman GE, Carter CS. Neonatal manipulations of oxytocin alter expression of oxytocin and vasopressin immunoreactive cells in the paraventricular nucleus of the hypothalamus in a gender-specific manner. Neuroscience. 2004;125(4):947–955. [DOI] [PubMed] [Google Scholar]
- 34. Martin MM, Liu Y, Wang Z. Developmental exposure to a serotonin agonist produces subsequent behavioral and neurochemical changes in the adult male prairie vole. Physiol Behav. 2012;105(2):529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ahern TH, Young LJ. The impact of early life family structure on adult social attachment, alloparental behavior, and the neuropeptide systems regulating affiliative behaviors in the monogamous prairie vole (Microtus ochrogaster). Front Behav Neurosci. 2009;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Curtis JT, Stowe JR, Wang Z. Differential effects of intraspecific interactions on the striatal dopamine system in social and non-social voles. Neuroscience. 2003;118(4):1165–1173. [DOI] [PubMed] [Google Scholar]
- 37. Lieberwirth C, Liu Y, Jia X, Wang Z. Social isolation impairs adult neurogenesis in the limbic system and alters behaviors in female prairie voles. Horm Behav. 2012;62(4):357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, posterior division: implications for cerebral hemisphere regulation of defensive and reproductive behaviors. J Comp Neurol. 2004;471(4):396–433. [DOI] [PubMed] [Google Scholar]
- 39. Northcutt KV, Wang Z, Lonstein JS. Sex and species differences in tyrosine hydroxylase-synthesizing cells of the rodent olfactory extended amygdala. J Comp Neurol. 2007;500(1):103–115. [DOI] [PubMed] [Google Scholar]
- 40. Northcutt KV, Lonstein JS. Neuroanatomical projections of the species-specific tyrosine hydroxylase-immunoreactive cells of the male prairie vole bed nucleus of the stria terminalis and medial amygdala. Brain Behav Evol. 2011;77(3):176–192. [DOI] [PubMed] [Google Scholar]
- 41. Been LE, Petrulis A. Chemosensory and hormone information are relayed directly between the medial amygdala, posterior bed nucleus of the stria terminalis, and medial preoptic area in male Syrian hamsters. Horm Behav. 2011;59(4):536–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Herman JP, Cullinan WE, Watson SJ. Involvement of the bed nucleus of the stria terminalis in tonic regulation of paraventricular hypothalamic CRH and AVP mRNA expression. J Neuroendocrinol. 1994;6(4):433–442. [DOI] [PubMed] [Google Scholar]
- 43. Meloni EG, Gerety LP, Knoll AT, Cohen BM, Carlezon WA. Behavioral and anatomical interactions between dopamine and corticotropin-releasing factor in the rat. J Neurosci. 2006;26(14):3855–3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Northcutt KV, Lonstein JS. Sex differences and effects of neonatal aromatase inhibition on masculine and feminine copulatory potentials in prairie voles. Horm Behav. 2008;54(1):160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Northcutt KV, Lonstein JS. Social contact elicits immediate-early gene expression in dopaminergic cells of the male prairie vole extended olfactory amygdala. Neuroscience. 2009;163(1):9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Carter CS, Getz LL, Gavish L, McDermott JL, Arnold P. Male-related pheromones and the activation of female reproduction in the prairie vole (Microtus ochrogaster). Biol Reprod. 1980;23(5):1038–1045. [DOI] [PubMed] [Google Scholar]
- 47. Carter CS, Witt DM, Thompson EG, Carlstead K. Effects of hormonal, sexual, and social history on mating and pair bonding in prairie voles. Physiol Behav. 1988;44(6):691–697. [DOI] [PubMed] [Google Scholar]
- 48. Cushing BS, Martin JO, Young LJ, Carter CS. The effects of peptides on partner preference formation are predicted by habitat in prairie voles. Horm Behav. 2001;39(1):48–58. [DOI] [PubMed] [Google Scholar]
- 49. Solomon NG, Crist TO. Estimates of reproductive success for group-living prairie voles, Microtus ochrogaster, in high-density populations. Anim Behav. 2008;76(3):881–892. [Google Scholar]
- 50. Solomon NG, Richmond AR, Harding PA, et al. . Polymorphism at the avpr1a locus in male prairie voles correlated with genetic but not social monogamy in field populations. Mol Ecol. 2009;18(22):4680–4695. [DOI] [PubMed] [Google Scholar]
- 51. Thigpen JE, Setchell KD, Ahlmark KB, et al. . Phytoestrogen content of purified, open- and closed-formula laboratory animal diets. Lab Anim Sci. 1999;49(5):530–536. [PubMed] [Google Scholar]
- 52. Thigpen JE, Setchell KD, Kissling GE, et al. . The estrogenic content of rodent diets, bedding, cages, and water bottles and its effect on bisphenol A studies. J Am Assoc Lab Anim Sci. 2013;52(2):130–141. [PMC free article] [PubMed] [Google Scholar]
- 53. Geens T, Aerts D, Berthot C, et al. . A review of dietary and non-dietary exposure to bisphenol-A. Food Chem Toxicol. 2012;50(10):3725–3740. [DOI] [PubMed] [Google Scholar]
- 54. Palanza PL, Howdeshell KL, Parmigiani S, vom Saal FS. Exposure to a low dose of bisphenol A during fetal life or in adulthood alters maternal behavior in mice. Environ Health Perspect. 2002;110(suppl 3):415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cao J, Rebuli ME, Rogers J, et al. . Prenatal bisphenol A exposure alters sex-specific estrogen receptor expression in the neonatal rat hypothalamus and amygdala. Toxicol Sci. 2013;133(1):157–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yang X, Doerge DR, Fisher JW. Prediction and evaluation of route dependent dosimetry of BPA in rats at different life stages using a physiologically based pharmacokinetic model. Toxicol Appl Pharmacol. 2013;270(1):45–59. [DOI] [PubMed] [Google Scholar]
- 57. Palanza P, Morellini F, Parmigiani S, vom Saal FS. Ethological methods to study the effects of maternal exposure to estrogenic endocrine disrupters: a study with methoxychlor. Neurotoxicol Teratol. 2002;24(1):55–69. [DOI] [PubMed] [Google Scholar]
- 58. Insel TR, Preston S, Winslow JT. Mating in the monogamous male: behavioral consequences. Physiol Behav. 1995;57(4):615–627. [DOI] [PubMed] [Google Scholar]
- 59. Cushing BS, Carter CS. Peripheral pulses of oxytocin increase partner preferences in female, but not male, prairie voles. Horm Behav. 2000;37(1):49–56. [DOI] [PubMed] [Google Scholar]
- 60. Carter CS, Witt DM, Schneider J, Harris ZL, Volkening D. Male stimuli are necessary for female sexual behavior and uterine growth in prairie voles (Microtus ochrogaster). Horm Behav. 1987;21(1):74–82. [DOI] [PubMed] [Google Scholar]
- 61. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th ed London, UK: Academic Press; 2007. [Google Scholar]
- 62. Gobrogge KL, Liu Y, Jia X, Wang Z. Anterior hypothalamic neural activation and neurochemical associations with aggression in pair-bonded male prairie voles. J Comp Neurol. 2007;502(6):1109–1122. [DOI] [PubMed] [Google Scholar]
- 63. Wang Z, Zhou L, Hulihan TJ, Insel TR. Immunoreactivity of central vasopressin and oxytocin pathways in microtine rodents: a quantitative comparative study. J Comp Neurol. 1996;366(4):726–737. [DOI] [PubMed] [Google Scholar]
- 64. Ross HE, Cole CD, Smith Y, et al. . Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009;162(4):892–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Swanson LW. Brain Maps: Structure of the Rat Brain. 2nd ed Amsterdam, The Netherlands: Elsevier; 1998. [Google Scholar]
- 66. Gioiosa L, Fissore E, Ghirardelli G, Parmigiani S, Palanza P. Developmental exposure to low-dose estrogenic endocrine disruptors alters sex differences in exploration and emotional responses in mice. Horm Behav. 2007;52(3):307–316. [DOI] [PubMed] [Google Scholar]
- 67. Cox KH, Gatewood JD, Howeth C, Rissman EF. Gestational exposure to bisphenol A and cross-fostering affect behaviors in juvenile mice. Horm Behav. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Anderson OS, Peterson KE, Sanchez BN, Zhang Z, Mancuso P, Dolinoy DC. Perinatal bisphenol A exposure promotes hyperactivity, lean body composition, and hormonal responses across the murine life course. FASEB J. 2013;27(4):1784–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ishido M, Masuo Y, Terasaki M, Morita M. Rat hyperactivity by bisphenol A, but not by its derivatives, 3-hydroxybisphenol A or bisphenol A 3,4-quinone. Toxicol Lett. 2011;206(3):300–305. [DOI] [PubMed] [Google Scholar]
- 70. Ishido M, Yonemoto J, Morita M. Mesencephalic neurodegeneration in the orally administered bisphenol A-caused hyperactive rats. Toxicol Lett. 2007;173(1):66–72. [DOI] [PubMed] [Google Scholar]
- 71. Saili KS, Corvi MM, Weber DN, et al. . Neurodevelopmental low-dose bisphenol A exposure leads to early life-stage hyperactivity and learning deficits in adult zebrafish. Toxicology. 2012;291(1–3):83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Crews D, Gore AC, Hsu TS, et al. . Transgenerational epigenetic imprints on mate preference. Proc Natl Acad Sci USA. 2007;104(14):5942–5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Crews D. Epigenetics, brain, behavior, and the environment. Hormones (Athens). 2010;9(1):41–50. [DOI] [PubMed] [Google Scholar]
- 74. Koolhaas JM, de Boer SF, Coppens CM, Buwalda B. Neuroendocrinology of coping styles: towards understanding the biology of individual variation. Front Neuroendocrinol. 2010;31(3):307–321. [DOI] [PubMed] [Google Scholar]
- 75. Koolhaas JM, Bartolomucci A, Buwalda B, et al. . Stress revisited: a critical evaluation of the stress concept. Neurosci Biobehav Rev. 2011;35(5):1291–1301. [DOI] [PubMed] [Google Scholar]
- 76. Ryan BC, Vandenbergh JG. Intrauterine position effects. Neurosci Biobehav Rev. 2002;26(6):665–678. [DOI] [PubMed] [Google Scholar]
- 77. Andersen ME, Dennison JE, Thomas RS, Conolly RB. New directions in incidence-dose modeling. Trends Biotechnol. 2005;23(3):122–127. [DOI] [PubMed] [Google Scholar]
- 78. McGraw LA, Davis JK, Lowman JJ, et al. . Development of genomic resources for the prairie vole (Microtus ochrogaster): construction of a BAC library and vole-mouse comparative cytogenetic map. BMC Genomics. 2010;11:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. McGraw LA, Young LJ. The prairie vole: an emerging model organism for understanding the social brain. Trends Neurosci. 2010;33(2):103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sabatier N, Rowe I, Leng G. Central release of oxytocin and the ventromedial hypothalamus. Biochem Soc Trans. 2007;35(pt 5):1247–1251. [DOI] [PubMed] [Google Scholar]
- 81. Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65(6):768–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. McCarthy MM, Altemus M. Central nervous system actions of oxytocin and modulation of behavior in humans. Mol Med Today. 1997;3(6):269–275. [DOI] [PubMed] [Google Scholar]
- 83. Shankar A, Teppala S. Urinary bisphenol A and hypertension in a multiethnic sample of US adults. J Environ Public Health. 2012;2012:481641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Melzer D, Rice NE, Lewis C, Henley WE, Galloway TS. Association of urinary bisphenol a concentration with heart disease: evidence from NHANES 2003/06. PLoS One. 2010;5(1):e8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cao J, Joyner L, Mickens JA, Leyrer SM, Patisaul HB. Sex-specific Esr2 mRNA expression in the rat hypothalamus and amygdala is altered by neonatal bisphenol A exposure. Reproduction. 2014;147(4):537–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cao J, Mickens JA, McCaffrey KA, Leyrer SM, Patisaul HB. Neonatal Bisphenol A exposure alters sexually dimorphic gene expression in the postnatal rat hypothalamus. Neurotoxicology. 2012;33(1):23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Adewale HB, Todd KL, Mickens JA, Patisaul HB. The impact of neonatal Bisphenol-A (BPA) exposure on sexually dimorphic hypothalamic nuclei in the female rat. Neurotoxicology. 2011; 32:38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Rebuli ME, Cao J, Sluzas E, et al. . Investigation of the effects of subchronic low dose oral exposure to bisphenol A (BPA) and ethinyl estradiol (EE) on estrogen receptor expression in the juvenile and adult female rat hypothalamus. Toxicol Sci. 2014;140(1):190–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Choleris E, Devidze N, Kavaliers M, Pfaff DW. Steroidal/neuropeptide interactions in hypothalamus and amygdala related to social anxiety. Prog Brain Res. 2008;170:291–303. [DOI] [PubMed] [Google Scholar]
- 90. Choleris E, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW, Ogawa S. An estrogen-dependent four-gene micronet regulating social recognition: a study with oxytocin and estrogen receptor-α and -β knockout mice. Proc Natl Acad Sci USA. 2003;100(10):6192–6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Patisaul HB, Scordalakes EM, Young LJ, Rissman EF. Oxytocin, but not oxytocin receptor, is regulated by oestrogen receptor β in the female mouse hypothalamus. J Neuroendocrinol. 2003;15(8):787–793. [DOI] [PubMed] [Google Scholar]
- 92. Nomura M, McKenna E, Korach KS, Pfaff DW, Ogawa S. Estrogen receptor-β regulates transcript levels for oxytocin and arginine vasopressin in the hypothalamic paraventricular nucleus of male mice. Brain Res Mol Brain Res. 2002;109:84–94. [DOI] [PubMed] [Google Scholar]
- 93. Cushing BS, Wynne-Edwards KE. Estrogen receptor-α distribution in male rodents is associated with social organization. J Comp Neurol. 2006;494(4):595–605. [DOI] [PubMed] [Google Scholar]
- 94. Lei K, Cushing BS, Musatov S, Ogawa S, Kramer KM. Estrogen receptor-α in the bed nucleus of the stria terminalis regulates social affiliation in male prairie voles (Microtus ochrogaster). PLoS One. 2010;5(1):e8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-β on anxiety-related behaviors. Endocrinology. 2005;146(2):797–807. [DOI] [PubMed] [Google Scholar]
- 96. Kudwa AE, McGivern RF, Handa RJ. Estrogen receptor β and oxytocin interact to modulate anxiety-like behavior and neuroendocrine stress reactivity in adult male and female rats. Physiol Behav. 2014;129:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sullivan AW, Hamilton P, Patisaul HB. Neonatal agonism of ERβ impairs male reproductive behavior and attractiveness. Horm Behav. 2011;60(2):185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Cushing BS, Hite R. Effects of estradiol on sexual receptivity, wheel-running behavior, and vaginal estrus in virgin prairie voles. Physiol Behav. 1996;60(3):829–832. [DOI] [PubMed] [Google Scholar]
- 99. Cushing BS, Marhenke S, McClure PA. Estradiol concentration and the regulation of locomotor activity. Physiol Behav. 1995;58(5):953–957. [DOI] [PubMed] [Google Scholar]