Abstract
Fibroblast growth factor 21 (FGF21) is a metabolic regulator that is required for normal spermatogenesis and protects against diabetes-induced germ cell apoptosis. Here, we tried to define whether diabetes-induced germ cell apoptosis that is predominantly due to increased oxidative stress was associated with impaired glucose and fatty acid metabolism, by examining the effects of Fgf21 gene knockout (FGF21-KO) or FGF21 treatment on the glucose and fatty acid metabolic pathways in streptozotocin-induced diabetic mice. Western blottings revealed that protein kinase B (AKT)-mediated glucose signaling was down-regulated in diabetic testes and further decreased in FGF21-KO diabetic group both 10 days and 2 months after diabetes onset, reflected by reduced glycogen synthase (GS) kinase (GSK)-3β phosphorylation and increased GS phosphorylation. Deletion of the Fgf21 gene also inactivated fatty acid metabolism-related factors, AMP-activated protein kinase (AMPK), sirtuin 1 (Sirt1), and peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α), along with exacerbating diabetes-induced testicular oxidative stress and damage. Treatment with recombinant FGF21 partially prevented these diabetic effects. In FGF21-KO nondiabetic mice, testicular AMPK/Sirt1/PGC-1α signaling was down-regulated and AKT1 and murine double minute 2 were inactivated along with the increased p53 expression but not AKT2, GSK-3β, and GS. These results suggest that the role of FGF21 in maintaining spermatogenesis is associated with its activation of AKT1 and inhibition of p53. Deletion of the Fgf21gene significantly exacerbates diabetes-induced down-regulation of testicular AKT/GSK-3β/GS and AMPK/Sirt1/PGC-1α pathways and testicular oxidative stress and cell apoptosis.
Fibroblast growth factor 21 (FGF21) is a secretory protein that acts as a metabolic regulator of glucose homeostasis, insulin sensitivity and ketogenesis (1, 2). The liver is the main site of production of FGF21 (1), but nonhepatic tissues, such as adipose tissue, skeletal muscle, heart, and testes, also express FGF21 at mRNA and protein levels (3–5). Numerous studies have focused on the role of FGF21 in the metabolic regulation of liver, adipose tissue, and skeletal muscle (1, 3, 6); however, the effect of FGF21 in other organs has not been thoroughly studied. In a previous study, we showed that testis expresses FGF21 at mRNA level, and its expression did not respond fasting-induced expression that is a well-known stimulus for FGF21 expression in other organs but was significantly up-regulated in diabetes condition (7). Fgf21 gene knockout (FGF21-KO) enhanced the spontaneous and diabetes-induced testicular cell apoptosis, suggesting that FGF21 may be required for the maintenance of spermatogenesis and the prevention of germ cell apoptosis (7).
Increasing evidence indicates that testicular cell apoptosis in diabetes is associated with increased oxidative stress and damage (8). Metabolic abnormalities in diabetes cause elevated generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS). ROS and/or RNS cause cell damage either by its own or acting together, causing oxidative and/or nitrosative damage. For instance, nitric oxide, a RNS, interacts with superoxide, a ROS, to form the highly reactive peroxynitrite known to cause protein nitration. This leads to protein dysfunction and associated cell signaling abnormality. Oxidative and/or nitrosative stress and damage can occur when cellular ROS and/or RNS levels overwhelm endogenous antioxidant defenses (9). FGF21 was reported to have an antioxidative effect in cardiac cells through suppression of ROS production (5). Our previous study showed that deletion of the Fgf21 gene exacerbated diabetes-induced oxidative damage in testis (7). However, whether the antioxidative effect of FGF21 is due to its homeostasis of glucose and fatty acid metabolism has not been yet investigated.
By activating its receptor, insulin stimulates phosphatidylinositol 3 kinase and its downstream target, protein kinase B (AKT), which is essential for insulin-induced glucose and fatty acid metabolism. Activated AKT inhibits the activity of glycogen synthase (GS) kinase (GSK)-3 by phosphorylating its N-terminal serine residues, releasing its inhibitory effect on GSs (10). There are 2 isoforms of GSK-3: GSK-3α and GSK-3β, the predominant regulator of GS in the testis (11). In diabetes, glucose use in testis is reduced due to the inactivation of AKT and the activation of GSK-3β (12).
Impaired glucose metabolism is often paralleled by compromised fatty acid metabolism as we have observed in diabetic heart (13) and testes (12). This is reflected in elevated peroxisome proliferator-activated receptor-α expression and decreased AMP-activated protein kinase (AMPK) phosphorylation and PPAR-γ coactivator 1α (PGC-1α). AMPK is an important metabolic energy sensor and master regulator of metabolic homeostasis (14) and is predominantly activated by liver kinase 1 (LKB1) (15). Activation of LKB1 was found to stimulate fatty acid oxidation and mitochondrial biogenesis in a number of tissues, including the testis (16, 17). Recently, AMPK was reported to enhance nicotinamide adenine dinucleotide+-dependent type III deacetylase sirtuin 1 (Sirt1) activity (14). Sirt1 plays several physiological roles including regulation of glucose metabolism, cell survival, and mitochondrial respiration. Deletion of the Sirt1 gene markedly attenuated spermatogenesis, confirming the important role of Sirt1 protein in spermatogenesis and germ cell survival (18). Both AMPK and Sirt1 act in concert with the main regulator of mitochondrial biogenesis, PGC-1α, to regulate fatty acid oxidation, ATP synthesis and lipid homeostasis (14, 19).
In diabetes, impaired glucose and fatty acid metabolism lead to oxidative stress and testicular damage (20). Recent studies suggest that FGF21 lowered blood glucose levels (21) and enhanced insulin sensitivity and energy metabolism through an AMPK-Sirt1-PGC-1α-dependent mechanism in adipocytes (21, 22). FGF21 protects cardiac cells against ischemia-reperfusion injury by stimulating the AKT-GSK-3β pathway and reducing oxidative stress (23).
In addition to stimulating glucose metabolism, AKT signaling functions as a cell survival mediator. For instance, AKT protects germ cells against apoptotic cell death associated with radiation injury (24) and neonatal hypothyroidism (25). Spermatogenesis is disrupted and spontaneous apoptosis is increased in the testis of Akt1 gene knockout mice (26). AKT regulates cell survival by modulating the activity of several apoptotic proteins, including suppression of p53 activation (27), which may be related to enhanced function of the p53 negative regulator, murine double minute 2 (MDM2) (28).
Due to the stimulation of AKT signaling, insulin also plays an important role in maintaining normal spermatogenesis. It has been reported that insulin regulates the proliferation of Sertoli cells (29) and mediates testosterone generation in Leydig cells (30). Therefore, the present study used our established rodent model of streptozotocin (STZ)-induced diabetes (7), to investigate the effect of FGF21 on glucose and fatty acid metabolism-related signaling pathways along with AKT expression and function.
Materials and Methods
Animals and diabetes model
FGF21-KO mice with C57BL/6J background were a gift from Dr Steve Kliewer, University of Texas Southwestern Medical Center. Age-matched wild-type (WT) (C57BL/6J) controls were purchased from The Jackson Laboratory and housed in the University of Louisville Research Resources Center at 22°C with a 12-hour light, 12-hour dark cycle and free access to food (standard Laboratory Rodent Diet, 5001; LabDiet) and water. All procedures were approved by Institutional Animal Care and Use Committee, which is certified by the American Association for Accreditation of Laboratory Animal Care.
Twenty-four male 10-week-old FGF21-KO and 16 age- and gender-matched WT mice were randomly allocated into 5 groups (n = 8), including WT control (WT-CON), WT diabetes (WT-DM), FGF21-KO control (KO-CON), FGF21-KO diabetes (KO-DM), and KO-DM with treatment of exogenous FGF21 (KO-DM-FGF21). To produce type 1 diabetes, STZ (Sigma-Aldrich) was dissolved in 0.1M sodium citrate (pH 4.5) and given ip to WT-DM, KO-DM, and KO-DM-FGF21 mice at 200 mg/kg body weight. Corresponding control mice were given the same volume of sodium citrate buffer. Whole-blood glucose was measured in mouse tail vein blood at the third day after the STZ injection. Mice with blood glucose levels more than or equal to 250 mg/dL were considered diabetic and used for this study. The above 4 groups were further divided into either the acute study or the chronic study.
For the acute study, 4 groups were euthanized at 10 days after diabetes onset. In addition, some mice in the KO-DM-FGF21 group were injected ip with FGF21 at 100 μg/kg body weight daily during these 10 days, whereas the remaining mice were given the same volume of phosphate buffer. The FGF21 as a recombinant human FGF21 was gifted from Dr Huiyan Wang (31). Its purity exceeds 96% with less than 1.0-EU/mL endotoxin levels. These conditions are considered acceptable compared with commercially available proteins and also meet the requirements of pharmaceutical research in vivo (31). All mice were euthanized 6 hours after the last injection of FGF21 on the 10th day. For the chronic study, 4 groups of mice were euthanized 2 months after diabetes onset. For both studies the testes of these mice were harvested. One testis from each mouse was fixed in 10% buffered formalin for histopathological studies, whereas the other was stored at −80°C for biochemical studies.
Testicular immunofluorescent staining
Testis tissue was fixed in 10% formalin for 24 hours, embedded in paraffin, and sectioned at 5 μm. Tissue sections were deparaffinized, rehydrated, and then incubated with antibodies against 3-nitrotyrosine (3-NT) (1:400 dilution, AB5411; Millipore), 4-hydroxy-2-nonenal (4-HNE) (1:400 dilution, HNE11-S; Alpha Diagnostic International), Sirt1 (1:400 dilution, ab-12193; Abcam), and β-actin (1:500, sc-1616; Santa Cruz Biotechnology, Inc) overnight at 4°C. The secondary antibodies, cyanine3-conjugated IgG (at 1:200 dilution, ab-50503; Abcam) and fluorescein isothiocyanate-conjugated IgG (at 1:100 dilution, ab-6881; Abcam), were applied and counterstained with 4, 6-diamidino-2-phenylindole dihydrochloride (DAPI) (0.0002% solution, 32670; Sigma-Aldrich). Slides were analyzed by fluorescent microscopy (Nikon).
For 3-NT and 4-HNE staining, testicular cells, including germ cells and interstitial cells with red cytoplasmic staining, were considered positive. For Sirt1 staining, germ cells with blue nucleolus staining plus red Sirt1 staining were considered positive. To quantify the expression of Sirt1, both red stained and nonstained germ cells located in seminiferous tubules were counted manually in a blinded fashion, ie, the examiner was unaware of the grouping. From each testis section, we randomly selected 30 seminiferous tubules in cross-section without repetition, and 8 animals in each group were analyzed. The germ cells that were counted include spermatogonia, primary spermatocytes and secondary spermatocytes, but not spermatids and spermatozoa because those nuclei were not easily quantified. Results are presented as the percentage of positively staining nuclei in the experimental groups compared with that in the control group.
Western blotting
Testicular tissues were homogenized in radioimmunoprecipitation assay lysis buffer (Santa Cruz Biotechnology, Inc), and total proteins were extracted. Proteins were separated by 10% SDS-PAGE electrophoresis and transferred to a nitrocellulose membrane. Membranes were blocked with 5% nonfat milk for 1 hour and incubated overnight at 4°C with the primary antibodies against: apoptosis-induced factor (AIF), phospho-AKT (Ser473, 4058S), phospho-ERK1/2 (Tyr202/ Phe204, 4370S), phospho-GSK-3β (Ser9, 9336S), phospho-GS (Ser641, 3891S), phospho-AKT1 (Ser473, 9018S), phospho-AKT2 (Ser474, 8599S), phospho-LKB1 (Ser428, 3482S), phospho-AMPK (T172, 4188S), phospho-p53 (Ser15, 9284S), phospho-MDM2 (Ser166, 3521S), AKT (9272S), GSK-3β (9315S), ERK1/2 (4696S), GS (3893S), AKT1 (2938S), AKT2 (2964S), LKB1 (3047S), AMPK (2793S), and p53 (2524S), all of which were purchased from Cell Signaling. The primary antibodies also included those against Sirt1 (ab-12193) and PGC-1α (ab-54481) (both of which were purchased from Abcam), β-actin (1:2000 dilution, sc-1616; Santa Cruz Biotechnology, Inc), 4-HNE (1:4000 dilution, HNE11-S, Alpha Diagnostic International), and 3-NT (1:3000 dilution, ab-5411, Millipore). Antibodies from Cell Signaling and Abcam were used at 1:1000 dilutions, and the dilutions of the remaining antibodies are indicated above. After the unbound antibodies were removed, the membranes were incubated with the horseradish peroxidase-conjugated secondary antibody (1:2000 dilution, sc-2055, sc-2004, or sc-2020; Santa Cruz Biotechnology, Inc) for 1 hour at room temperature. Specific bands were visualized using an enhanced chemoluminescence detection kit (Thermo Scientific). For some polyclonal antibodies such as p-GS and Sirt1 antibodies, 2 very closed bands were shown on the gels due to the possible presence of multiple isoforms of these antigens (target proteins) in different tissues; therefore, both bands either for phospho-GS or Sirt1 were analyzed. Quantitative densitometry was performed on the identified bands by using Image Quant 5.2 software.
Statistical analysis
Data were presented as mean ± SD (n = 8). Comparisons were performed by One-way ANOVA for the different groups, followed by Tukey's test in pairwise repetitive comparisons with Origin 7.5 software. Statistical significance was considered as P < .05.
Results
Deletion of the Fgf21 gene increases diabetes-induced oxidative damage in testis
Deletion of the Fgf21 gene did not affect the fasting blood glucose levels and plasma triglyceride level at baseline but slightly increased both fasting blood glucose and plasma triglyceride levels under diabetic condition (Supplemental Figure 1, A and B). Treatment of FGF21-KO diabetic mice with FGF21 reduced blood glucose level but did not affect plasma triglyceride levels (Supplemental Figure 1, A and B). Our previous study demonstrated that deletion of the Fgf21 gene increased spontaneous and diabetes-induced testicular germ cell apoptosis (7). Consistent with those findings, Supplemental Figure 1C shows that diabetes increased terminal deoxynucleotidyl transferase dUTP nick end labeling-positive staining (mainly among spermatogonia and, to a lesser degree, primary spermatocytes) and AIF expression. Deletion of the Fgf21 gene increased spontaneous and diabetes-induced TUNEL-positive cells and AIF expression. Supplementation of FGF21 to FGF21-KO diabetic mice prevented diabetes-increased cell death (Supplemental Figure 1C).
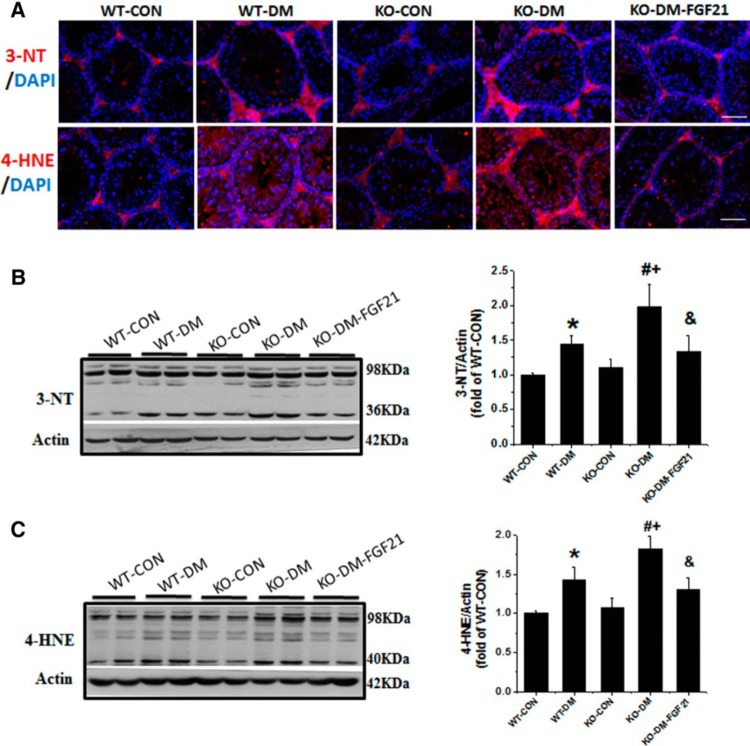
Germ cell apoptosis in diabetes is considered associated with increased oxidative stress and lipid peroxidation (7). To further confirm the oxidative and nitrosative stress in diabetic and FGF21-KO mice, testis tissue was used for immunofluorescent staining and Western blot analysis to examine 3-NT (a marker of protein nitration) and 4-HNE (a marker of lipid peroxidation), shown in Figure 1A. Positive staining for 3-NT and 4-HNE is seen in the cytoplasm of all testicular cells, including germ cells and interstitial cells. These markers were significantly increased in the WT-DM group and further increased in FGF21-KO diabetic mice (Figure 1A). Intraperitoneal injection of FGF21 to FGF21-KO diabetic mice prevented diabetes-induced testicular 3-NT and 4-HNE (Figure 1, B and C). It is noted that the expression of oxidative stress was predominantly in spermatogonia and spermatocytes that were consistent with the finding of apoptotic cells observed in our recent study (7) and the present findings (Supplemental Figure 1C). These results suggest that deletion of the Fgf21 gene promotes germ cell apoptosis via increasing oxidative stress induced by diabetes but not in nondiabetic animals.
Figure 1.
Deletion of the Fgf21 gene increases diabetes-induced oxidative damage in the testis. Type 1 diabetes was induced with STZ (200 mg/kg ip). Diabetic and age-matched control mice were administered daily ip injections of FGF21 (100 μg/kg) or PBS for 10 days. To measure the oxidative damage in testis, immunofluorescence staining (A) was done with anti-3-NT antibody and anti-4-HNE antibody, and the nuclear staining was done with DAPI (blue) on testis tissue sections (400×). Scale bar, 50 μm. Expression of 3-NT (B) and 4-HNE (C) was also detected by Western blot analysis, for which multiple bands were grouped as one for the quantitative densitometry analysis so that the results presented in the figures were the combined results. Data are presented as mean ± SD (n = 8). *, P < .05 vs WT-CON; #, P < .05 vs WT-DM; +, P < .05 vs KO-CON; &, P < .05 vs KO-DM.
Deletion of the Fgf21 gene down-regulates glucose metabolism in the testis of diabetic mice
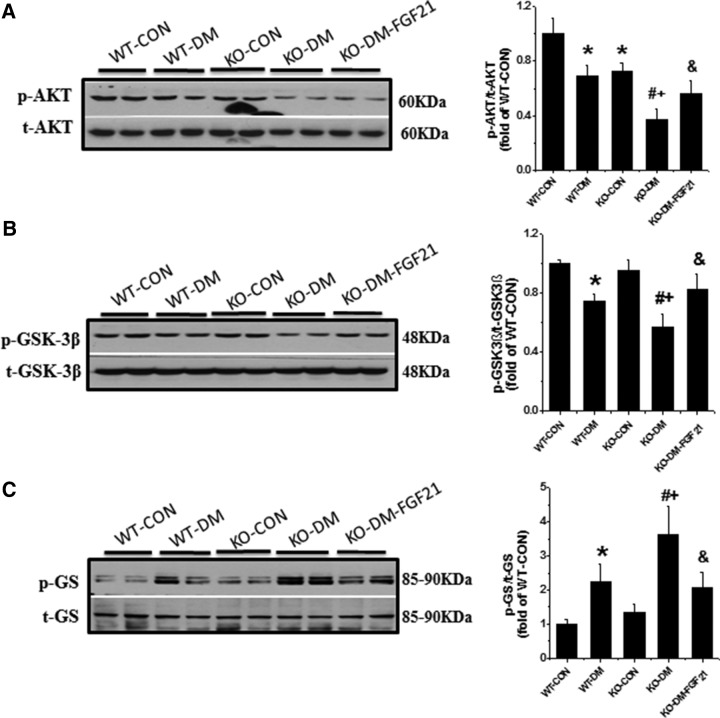
Diabetes-induced testicular oxidative stress and damage was thought to be associated with reduced glucose use due to decreased AKT and GSK-3β phosphorylation and increased GS phosphorylation (12). To explain how FGF21 inhibits oxidative stress in diabetes, the expression of the 3 glucose metabolic components was examined. Deletion of the Fgf21 gene decreased AKT phosphorylation (Figure 2A) but did not affect GSK-3β or GS phosphorylation, in control mice (Figure 2, B and C). AKT phosphorylation was decreased in the testis of diabetic mice 10 days after diabetes onset (Figure 2A). Diabetes significantly increased GSK-3β activity, shown by decreased GSK-3β phosphorylation level (Figure 2B). The increased GSK-3β activity inhibited GS activity, shown by increased phosphorylation of GS (Figure 2C). These results suggest that deletion of the Fgf21 gene aggravates the AKT/GSK-3β/GS signaling defect in diabetic testes, which is associated with increased oxidative stress and germ cell apoptosis (Supplemental Figure 1C) (7).
Figure 2.
Deletion of the Fgf21 gene increases the deficiency of glucose metabolism-associated pathway, AKT/GSK-3β/GS in testes of diabetic mice. Testicular tissue was used for Western blot analysis of phosphorylated and total AKT (A), GSK-3β (B), and GS (C). Data are presented as mean ± SD (n = 8). *, P < .05 vs WT-CON; #, P < .05 vs WT-DM; +, P < .05 vs KO-CON; &, P < .05 vs KO-DM.
Deletion of the Fgf21 gene inactivates AKT1 in nondiabetics and aggravates diabetes-induced inactivation of both AKT1 and AKT2
Some studies have concluded that both AKT1 and AKT2 play an important role in spermatogenesis and germ cell apoptosis, respectively (24, 32). Therefore, isoforms of AKT expression were examined in the testis. In WT mice, diabetes did not affect the level of AKT1 phosphorylation; however, deletion of the Fgf21 gene significantly inhibited AKT1 phosphorylation. Compared with WT diabetic mice, the phosphorylation of AKT1 was further decreased in FGF21-KO diabetic mice but restored by treatment with FGF21 (Figure 3A).
Figure 3.
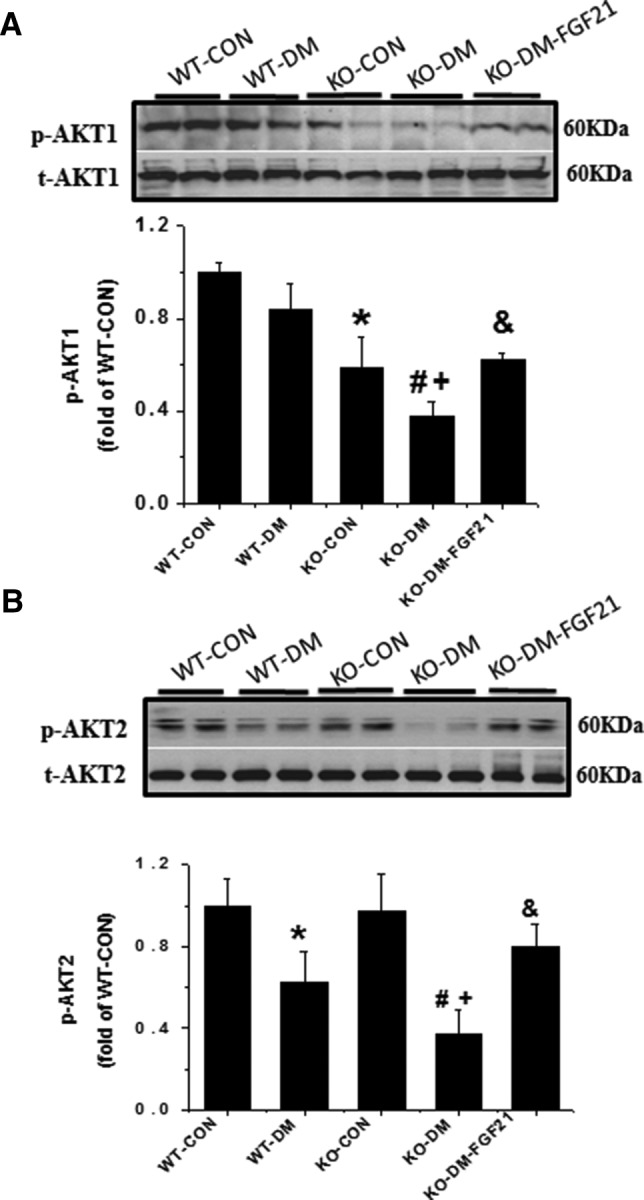
Effects of diabetes and the Fgf21 gene deletion on AKT1 and AKT2 in testis. Phosphorylated and total AKT1 (A) and AKT2 (B) were examined by Western blot analysis. Data are presented as mean ± SD (n = 8). *, P < .05 vs WT-CON; #, P < .05 vs WT-DM; +, P < .05 vs KO-CON; &, P < .05 vs KO-DM.
On the other hand, diabetes significantly decreased the phosphorylation of AKT2 in WT mice (Figure 3B), which is coincident with the changes of GSK-3β and GS phosphorylation (Figure 2, B and C). Deletion of the Fgf21 gene further degraded the diabetes-induced decrease in AKT2 phosphorylation, which, however, was also almost completely prevented by treatment with FGF21. There was no significant difference in AKT2 phosphorylation between FGF21-KO and WT-CON mice (Figure 3B).
Deletion of the Fgf21 gene exacerbates spontaneous and diabetes-induced inactivation of AMPK, Sirt1, and PGC-1α
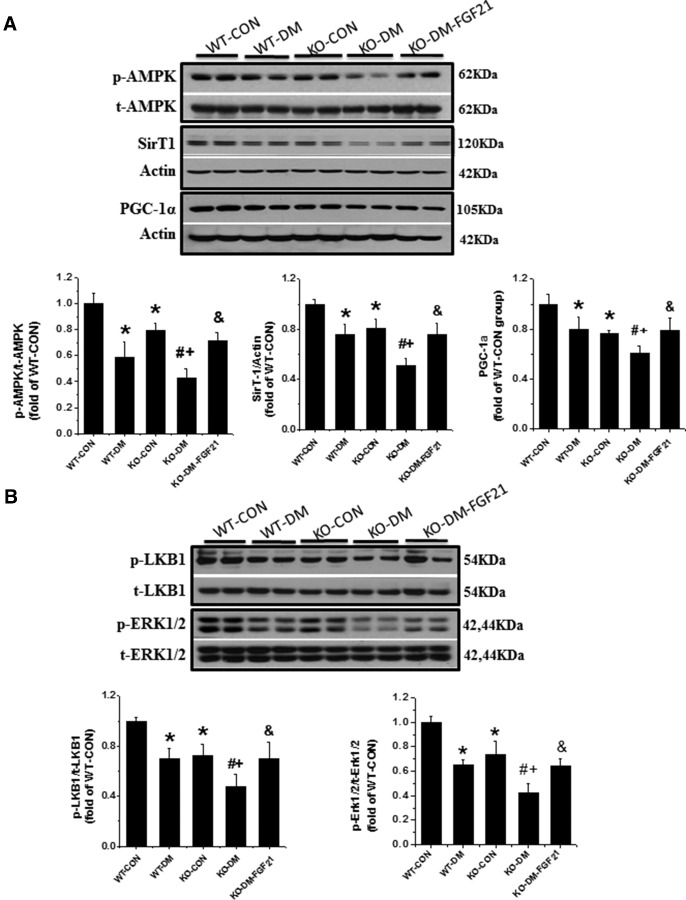
Our previous studies showed that the increased germ cell apoptosis in diabetes is associated with a disarrangement of fatty acid metabolism (12). Because FGF21 could regulate energy metabolism through an AMPK-Sirt1-PGC-1α-dependent mechanism in adipocytes (22), AMPK, an important mediator of fatty acid oxidation stimulation, was examined. Diabetes or deletion of the Fgf21 gene significantly decreased testicular AMPK phosphorylation (Figure 4A). Deletion of the Fgf21 gene further reduced testicular AMPK phosphorylation in diabetic mice, which could be restored by treatment with FGF21 (Figure 4A).
Figure 4.
Effects of diabetes and the Fgf21 gene deletion on AMPK/Sirt1/PGC-1α pathway associated with energy metabolism. Western blot analyses were used to detect the expression of phosphorylated and total AMPK, Sirt1 and PGC-1α (A) as well as LKB1 and ERK1/2 (B). Data are presented as mean ± SD (n = 8). *, P < .05 vs WT-CON; #, P < .05 vs WT-DM; +, P < .05 vs KO-CON; &, P < .05 vs KO-DM.
Sirt1 not only regulates glucose and lipid metabolism but also helps to maintain spermatogenesis and germ cell function (18). PGC-1α, a master regulator of mitochondrial biogenesis, has been identified as a target protein of Sirt1 and contributes to antioxidative effects (33). The expression of these 2 mediators was examined in the testes of FGF21-KO nondiabetic and diabetic mice. Coincident with the decline in AMPK phosphorylation, the expression level of Sirt1 and PGC-1α was also significantly decreased in the testes of FGF21-KO nondiabetic and diabetic mice (Figure 4A). Deletion of the Fgf21 gene also significantly exacerbated diabetes-induced reduction of Sirt1 and PGC-1α expression (Figure 4A), which was restored by FGF21 treatment (Figure 4A).
Sirt1 activation was further examined with immunofluorescence staining for the nuclear localization (Figure 5A), which appeared decreased in WT and FGF21-KO diabetic mice (Figure 5B). LKB1 regulates AMPK activity (15) and FGF21 activates FGF receptors through beta-klotho and ERK1/2 to phosphorylate LKB1 (34). Therefore, we examined LKB1 and ERK1/2 phosphorylation. As shown in Figure 4B, phosphorylation levels of LKB1 and ERK1/2 were significantly decreased in the testes of both WT diabetic and FGF21-KO nondiabetic mice. FGF21-KO diabetic mice showed a further decrease in the ratios of phosphorylated to total expression of LKB1 and ERK 1/2, compared with diabetic or FGF21-KO nondiabetic mice, which was relieved by FGF21 supplementation (Figure 4B).
Figure 5.
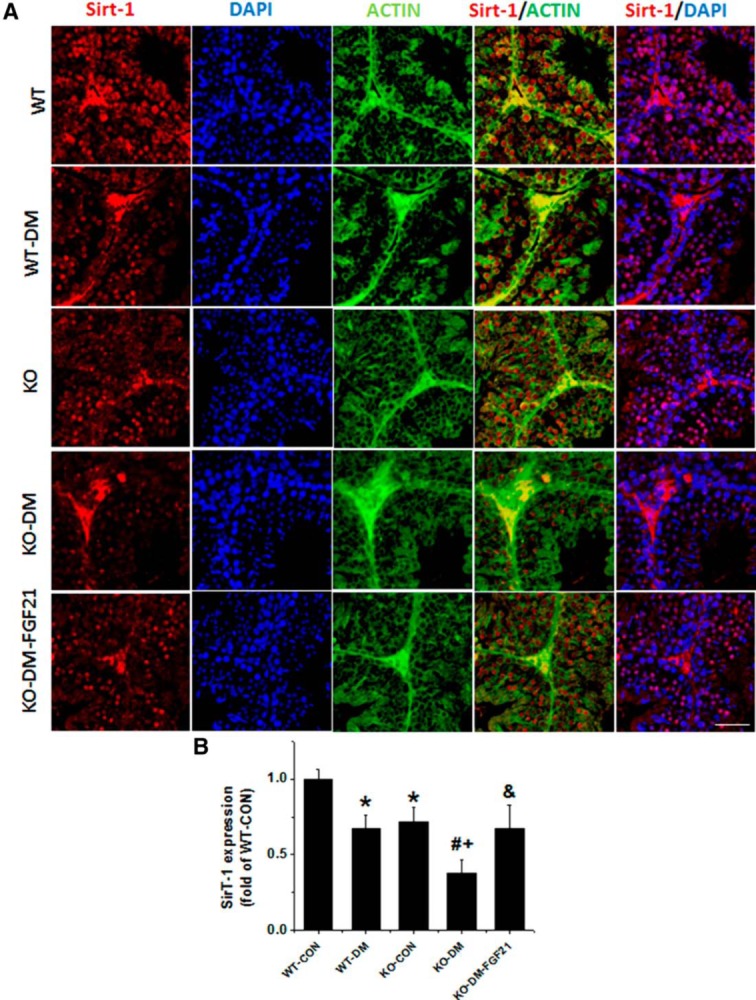
Immunofluorescence staining for Sirt1. A, Immunofluorescence staining using anti-Sirt1 antibody (5), anti-β-actin antibody (green), and DAPI for nuclei (blue) on testicular tissues (400×). Scale bar, 25 μm. B, Percentage of nuclei that was positively stained for Sirt1. Data are presented as mean ± SD (n = 8). *, P < .05 vs WT-CON; #, P < .05 vs WT-DM; +, P < .05 vs KO-CON; &, P < .05 vs KO-DM.
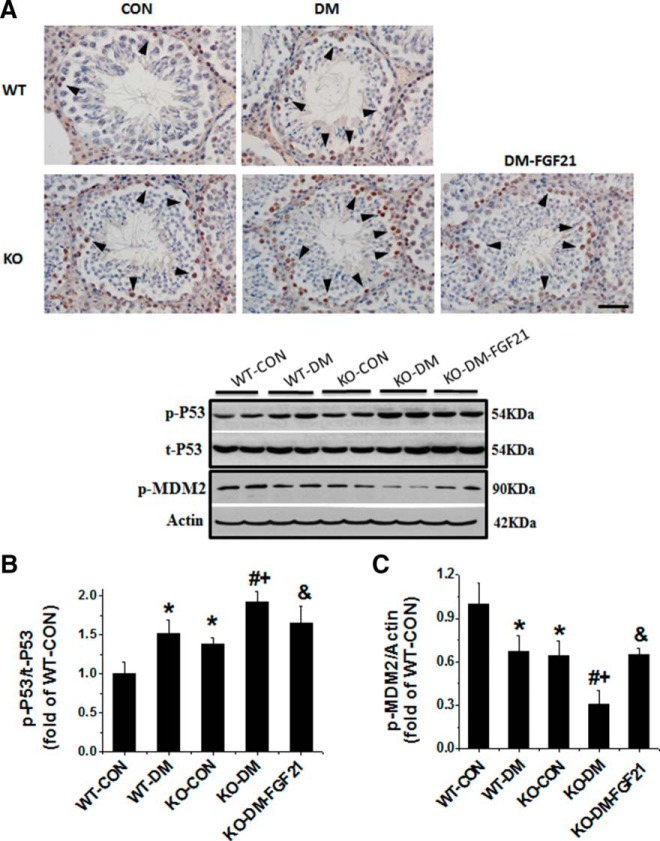
Deletion of the Fgf21 gene promotes spontaneous and diabetes-induced activation of p53
p53 plays an important role in testicular apoptosis induced by various oxidative stressors associated with the mitochondrial apoptotic pathway (35). Therefore, we examined the activation of testicular p53 and its ubiquitin ligase of MDM2 in both WT and FGF21-KO diabetic mice. p53 activation was first detected by immunohistochemistry. Positive nuclear staining was predominantly seen in spermatogonia, primary and secondary spermatocytes (Figure 6A), which is consistent with the distribution of apoptotic germ cells in our previous study (Figure 1A) (7). The incidence of positive staining germ cells was higher in the WT diabetic and KO-CON mice than WT-CON (Figure 6A). Western blot analysis revealed a significant increase in p53 phosphorylation in the testes of diabetic mice, KO-CON mice, and FGF21-KO diabetic mice (Figure 6B). Treatment of FGF21-KO diabetic mice with FGF21 completely prevented diabetes-increased p53 phosphorylation (Figure 6B).
Figure 6.
Effects of diabetes and the Fgf21 gene deletion on p53 and cell apoptosis associated protein. A, Immunohistochemistry staining using antiphosphorylated p53 antibody (400×). Scale bar, 50 μm. B, Western blot analysis was used to quantify p53 activation. C, Western blot analysis to quantify MDM2 activation. Data are presented as mean ± SD (n = 8). *, P < .05 vs WT-CON; #, P < .05 vs WT-DM; +, P < .05 vs KO-CON; &, P < .05 vs KO-DM.
In parallel with the increase of p53 phosphorylation in the testis, MDM2 phosphorylation at Ser166 was significantly decreased in the testis of FGF21-KO nondiabetic and WT diabetic mice. This was further decreased in the FGF21-KO diabetic mice and was attenuated by FGF21 treatment (Figure 6C).
Chronic effects of diabetes and the Fgf21 gene deletion on testicular apoptosis and associated cell signaling components
As shown in acute study above, deletion of the Fgf21 gene did not affect the fasting blood glucose levels (120.4 ± 21.6 mg/dL for WT-CON vs 112.6 ± 9.1 mg/dL for KO-CON, P > .05) and plasma triglyceride levels (82.9 ± 14.0 mg/dL for WT-CON vs 72.34 ± 9.8 mg/dL for KO-CON, P > .05) at baseline but slightly increased fasting blood glucose (476.4 ± 47.2 mg/dL for WT-DM vs 422.25 ± 62.9 mg/dL for KO-DM, P < .05) without effect on diabetic plasma triglyceride levels (141.8 ± 25.6 mg/dL for WT vs 139.0 ± 24.2 mg/dL for FGF21-KO, P > .05).
To examine the chronic effects of the Fgf21 gene deletion on testicular apoptosis and associated cell signaling components, apoptotic cell death was examined in the testis of mice with diabetes for 2 months. Testis weight of WT-DM for 2 months was slightly decreased compared with age-matched WT-CON mice (Supplemental Figure 2). The testis weight in FGF21-KO nondiabetic mice was also slightly lower than that of WT nondiabetic mice, and there was no difference for the testis weight between WT and FGF21-KO diabetic mice (Supplemental Figure 2). TUNEL staining showed the significant increase in the positive cells in both WT diabetic and FGF21-KO nondiabetic mice and deletion of the Fgf21 gene exacerbated diabetes-induced apoptotic cell death (Figure 7A), which was confirmed by Western blotting for AIF (Figure 7B).
Figure 7.
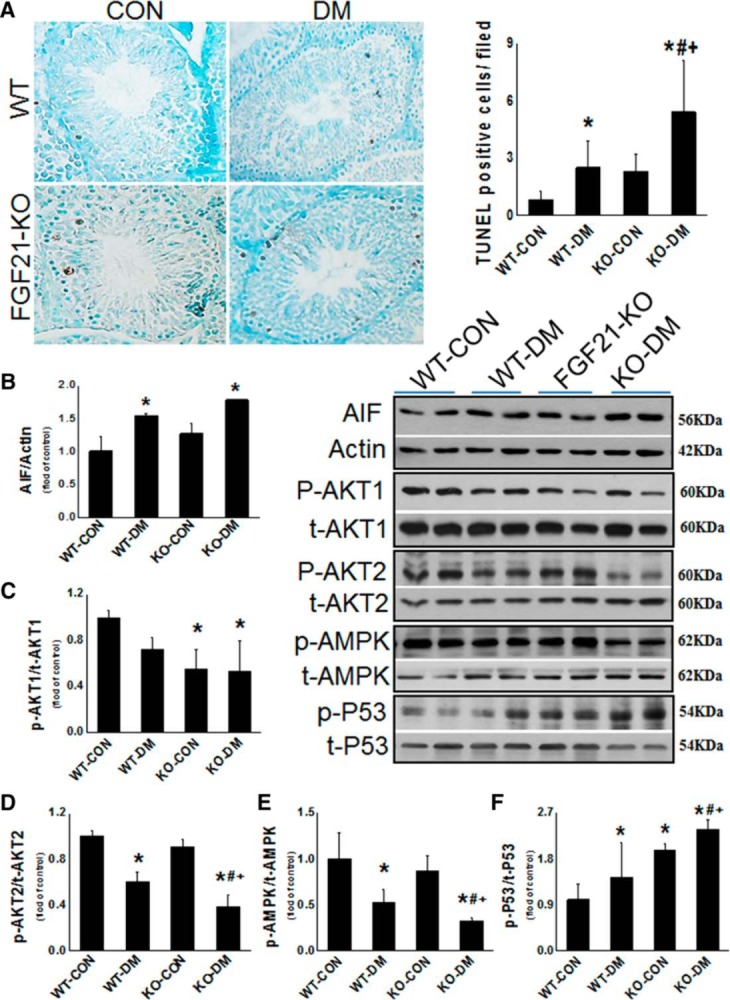
Chronic effects of diabetes and the Fgf21 gene deletion on testicular apoptosis and associated cell signaling components. Animals were killed at 2 months after diabetes onset. A and B, Apoptotic cell death was examined with TUNEL staining (A) and Western blot analysis for AIF (B). C–F, Western blots were also used to detect the phosphorylated and total expression of AKT1 (C), AKT2 (D), AMPK (E), and p53 (F). Data are presented as mean ± SD (n = 4–5). *, P < .05 vs WT-CON; #, P < .05 vs WT-DM; +, P < .05 vs KO-CON.
As discovered in the above acute study, we demonstrated that deletion of the Fgf21 gene significantly decreased the phosphorylation levels of testicular AKT1 (Figure 7C) but not AKT2 (Figure 7D). Two-month diabetes also slightly decreased AKT1 and significantly decreased AKT2 phosphorylation (Figure 7, C and D). Compared with WT-DM, deletion of the Fgf21 gene further decreased AKT2 phosphorylation but not AKT1 (Figure 7, C and D).
As observed in the above acute study (Figure 4A), testicular AMPK phosphorylation level was significantly decreased in WT diabetic group and further decreased in FGF21-KO diabetic groups at 2 months after diabetes (Figure 7E). We also found that both deletion of the Fgf21 gene and 2-month diabetes significantly increased p53 phosphorylation, which was synergistically increased in FGF21-KO diabetic group (Figure 7F).
Discussion
The testes of diabetic mice exhibit impaired AKT-mediated glucose metabolism, as reflected by decreased activation of AKT (12). The results of the present study are consistent with the previous finding in that diabetes significantly inhibited testicular AKT activation and up-regulated its downstream mediator, GSK-3β, to release the inhibitory effect on GS (Figure 2). As a consequence of impaired glucose metabolism, testicular oxidative stress was significantly increased, as shown by the accumulation of 3-NT and 4-HNE in the testes of diabetic mice (Figure 1). We demonstrated here for the first time that deletion of the Fgf21 gene exacerbated the diabetic effect on AKT-mediated signaling changes and testicular oxidative stress, which is attenuated by FGF21 treatment. These results are consistent with a recent study, showing that FGF21 significantly attenuated ischemia-reperfusion-induced damage to cardiac cells by preventing oxidative stress through the AKT-GSK-3β pathway (23). Therefore, FGF21 might compensate for impairment of the glucose metabolism pathway, AKT/GSK-3β/GS, to reduce oxidative stress and provide protection from diabetes-induced germ cell apoptosis (Figure 8).
Figure 8.
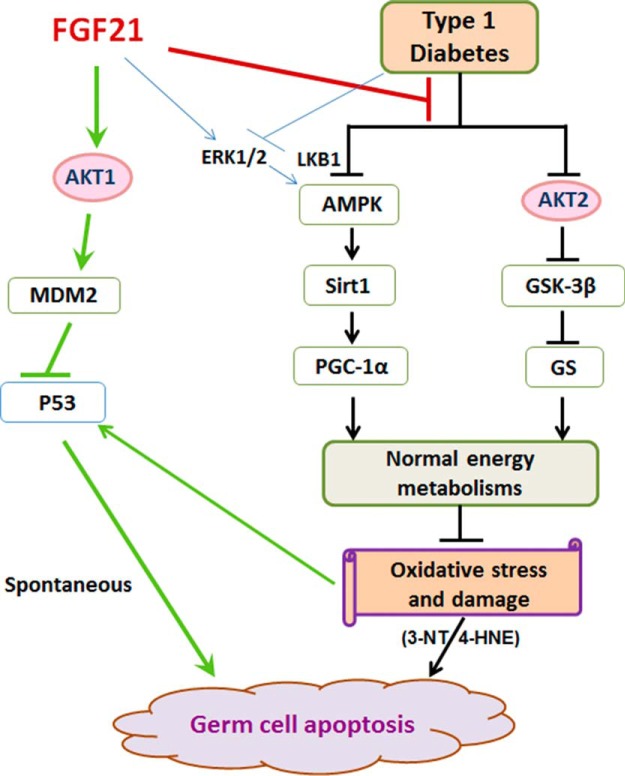
Illustration of the working mechanisms for FGF21 prevention of spontaneous and diabetes-induced germ cell apoptosis. Diabetes adversely affects glucose metabolism (AKT/GSK-3β/GS signaling) and fatty acid oxidation (AMPK/Sirt1/PGC-1α) in testis, leading to the accumulation of metabolic intermediates that cause testicular oxidative damage and germ cell apoptosis. FGF21 deficiency exacerbates these defects, leading to testicular cell apoptosis. In nondiabetics, FGF21 preserves almost normal spermatogenesis through the AKT-signaling pathway to inhibit p53 via AKT1/MDM2.
We found that AMPK, as a fatty acid oxidation stimulator, was significantly decreased in diabetic testes at 10 days (Figure 4A) and 2 months (Figure 7E) after diabetes. Sirt1, recently recognized as an AMPK downstream gene, was significantly reduced in diabetic testes (Figures 4A and 5). In addition, PGC-1α, an essential factor for the maintenance of optimal mitochondrial fatty acid oxidation, was significantly decreased in diabetic testes (Figure 4A). Prediabetes was recently reported to compromise testicular mitochondrial function by repressing PGC-1α/Sirt3, reducing respiratory capacity and increasing oxidative stress (36). Likewise, up-regulation of the AMPK-PGC1α-Sirt3 axis has been proposed to preserve peripheral nerve function in type 1 diabetic rats (37). Similarly, our results imply that diabetes-induced decreases in the phosphorylation of AMPK and the expression of Sirt1 and PGC-1α may be associated with testicular dysfunction. However, deletion of the Fgf21 gene augmented the diabetes-induced inhibition of the 3 factors, AMPK, Sirt1, and PGC-1α (Figures 4A and 5). These findings suggest that deletion of the Fgf21 gene exacerbates diabetes-induced compromised fatty acid metabolism, leading to the accumulation of fatty acid metabolic intermediates that cause testicular oxidative stress (Figure 1) and germ cell apoptosis (7), as illustrated by the working mechanism (Figure 8). Our results are consistent with a previous work, showing that activation of AMPK-Sirt1-PGC-1α by FGF21 in adipocytes enhanced mitochondrial oxidative capacity as demonstrated by increased oxygen consumption and citrate synthase activity to maintain energy homeostasis (22).
FGF21 function requires LKB1 to activate AMPK (22). FGF21 also activates FGF receptors to stimulate ERK1/2 that in turn regulates LKB1 activity (34, 38). In line with this, inactivation of ERK1/2 and LKB1 in the testis of FGF21-KO and WT diabetic mice parallels the inactivation of AMPK (Figures 4B and 7E). In addition, the preventive effect by FGF21 on diabetes-induced cytotoxicity in pancreatic β-cells (39) and kidney (40) has been reported to be mediated by activating the ERK1/2 pathway. Those results imply that the interaction of ERK1/2 and LKB1 is required for FGF21's action on testicular energy homeostasis through AMPK activation, as shown in Figure 8.
In nondiabetic mice, deletion of the Fgf21 gene also decreased fatty acid oxidation, reflected by the decreased activation of AMPK (Figures 4A and 7E) and reduced expression of Sirt1 and PGC-1α (Figure 4A). However, decreased fatty acid oxidation was not accompanied by increased oxidative stress (Figure 1). Meanwhile, AKT-mediated glucose metabolism was unchanged in FGF21-KO nondiabetic mice as shown by the normal level of GSK-3β and GS expression and phosphorylation (Figure 2, B and C). These results demonstrate that, unlike diabetes, deletion of the Fgf21 gene and associated impairment of fatty acid oxidation alone is insufficient to induce testicular oxidative stress and apoptosis. However, there may be other mechanisms or factors unrelated to oxidative stress that promote spontaneous germ cell apoptosis in the testis of FGF21-KO nondiabetic mice, which will be explored in future studies.
Interestingly, although GSK-3β and GS are expressed at normal levels in FGF21-KO mice (Figure 2, B and C), AKT activation is significantly decreased (Figure 2A). AKT is important for cell survival in many tissues, including testis (21). There are 3 AKT isoforms with different expression patterns and functions: AKT1 is the predominant isoform in many mammalian tissues and affects both organ development and cell survival, AKT2 is strongly expressed in insulin-responsive tissues and mainly regulates glucose metabolism, whereas AKT3 is expressed primarily in the brain (41). A testicular hypertrophic phenotype has also been described for AKT1-KO mice, which exhibited several morphological abnormalities in the testis (10, 26). In contrast, there was no significant decrease in the absolute testis weight or the ratio of testis weight to body weight in either AKT2-KO or AKT3-KO mice, although there was a significantly decreased ratio of brain weight to the body weight (10). These studies suggest that although AKT3 expression exists in both brain and testis, it plays a critical role in brain, but not in the testis, for the growth and development. However, our study was to investigate how the testis with normal development responds to testicular germ cell death induced by diabetes-derived metabolic abnormality. AKT1 has been implicated in inhibiting cell death induced by different stimuli, including growth factor withdrawal, cell cycle discordance, DNA damage, and loss of cell adhesion (24–26). Radiation exposure is a well-characterized germ cell injury model leading to cell cycle arrest or apoptosis and loss of Akt1 results in an earlier onset of germ cell apoptosis and enhanced sensitivity of mitotic spermatogonia to ionizing radiation. Furthermore, there was no change of either AKT2 or AKT3 expression in AKT1-KO mice exposed to radiation (24). Therefore, the AKT1-signaling pathway plays a major role in the protection of germ cells after injury.
Based on the above findings, we have focused on the expression and function of AKT1 and AKT2 isoforms in an effort to explain the different regulatory actions of FGF21 on normal and diabetes conditions. Deletion of the Fgf21gene significantly decreased AKT1 activation (Figures 3A and 7C) but not AKT2 (Figures 3B and 7D). However, diabetes in WT mice inhibited AKT2 activation (Figures 3B and 7D) but did not, or not significantly, affect AKT1 (Figures 3A and 7C). On the other hand, both AKT1 and AKT2 activation were reduced by deletion of the Fgf21 gene in diabetic mice (Figures 3, A and B, and 7, C and D). Therefore, we confirmed that under normal conditions, FGF21 affects germ cell spontaneous apoptosis predominantly through AKT1, whereas both AKT1 and AKT2 are involved in diabetes-mediated germ cell apoptosis when the Fgf21 gene is defect. This result is in line with other recent studies, in which AKT1-KO mice showed an increase in spontaneous testicular apoptosis and attenuation of spermatogenesis but not in the diabetic phenotype (26, 32). AKT1 protected from testicular apoptosis caused by radiation or neonatal hypothyroidism (24, 25).
AKT regulates cell survival by modulating the activity of several apoptotic and survival proteins, including p53 (27). p53 was reported to play a critical role in spermatogenesis and germ cell apoptosis in response to a variety of stimuli (42). In the present study, we observed p53 activation in the testes of diabetic mice (Figures 6, A and B, and 7F). Deletion of the Fgf21 gene enhanced the activation of testicular p53 in both diabetic and nondiabetic conditions (Figures 6, A and B, and 7F). Our previous study (7) and present results (Figures 1, A and B, and 7, A and B) showed that deletion of the Fgf21 gene caused an increase in spontaneous germ cell apoptosis, which was associated with the activation of mitochondria-dependent cell death pathway (7). p53 activation can rapidly interact with B-cell lymphoma 2/B-cell lymphoma 2-associated X proteins and activate the mitochondria-dependent cell death cascade (35). Therefore, FGF21 may inhibit spontaneous mitochondria-associated germ cell apoptosis by inactivating p53 via the AKT-signaling pathway. It is known that MDM2 is an ubiquitin ligase for p53 (43), and exogenous MDM2 promoted spermatogonia survival and inhibited apoptosis by reducing p53 protein (44). AKT-mediated phosphorylation of MDM2 at Ser166 and Ser186 increases the ubiquitination and degradation of p53 (43). Which isoform of AKT is the predominant player in MDM2 inhibition of p53 remains unclear, because it is reported that AKT1 could regulate MDM2 under normal conditions, but in AKT1-KO condition, AKT2 preserved the activation of MDM2 (45, 46). As shown in Figure 3, deletion of the Fgf21 gene reduced AKT1 (Figures 3A and 7C) as well as MDM2 phosphorylation in normal and diabetic mice (Figure 6C), but reduced AKT2 phosphorylation only in diabetic mice (Figures 3B and 7D). In the present study, therefore, AKT1 seems to be the major AKT isoform regulating MDM2 phosphorylation in testis.
Besides regulating fatty acid metabolism and mitochondrial respiration, Sirt1 functions as a down-stream signaling mediator of AKT to affect glucose metabolism and cell survival (47). Sirt1 deficiency markedly attenuates spermatogenesis, suggesting a direct effect of Sirt1 on germ cell survival and function (18). Therefore, it can be assumed that FGF21 deficiency increased spontaneous and diabetes-induced testicular apoptosis by exacerbating the down-regulation of AKT function (Figure 2A) and Sirt1 expression (Figures 4A and 5).
Besides the possible mechanisms discussed above, there may be a concern whether systemic and testicular levels of testosterone have any effect on testicular apoptotic cell death. We have previously shown the significant decrease in testosterone level in the blood of diabetic animals, which could be mildly attenuated by antioxidant N-acetylcysteine treatment that can significantly decrease diabetes-induced testicular apoptosis (48), suggesting that the reduction of oxidative stress in the testis is the major cause of testicular apoptosis under diabetic conditions. Therefore, it may be not a very critical mechanism here for the effect of FGF21 on diabetes-induced testicular cell death.
The present study may lack a direct link between the previously reported (7) and the presently demonstrated loss of cells due to apoptosis and the signaling described above. Our previous study (49) described that STZ-induced type 1 diabetes induced apoptotic cell death in the heart began at 3 days, peaked at 1–3 weeks, maintained an increased incidence for 1 to 2 months, and, at the late stage of diabetes, demonstrated a very low incidence of apoptotic cell death. It is clear that there is no way to study the cell death-signaling pathway directly in the apoptotic cells. However, because apoptotic cell death signaling was activated by the cell death stimuli that persists in different cells of the tissue at different stages, such cell death must occur gradually rather than suddenly. Accordingly, studies investigating cell death signaling under an in vivo model have to use the remaining surviving and/or preapoptotic cells (with cell death signaling activated) to elucidate any mechanisms. For this reason, we selected a short time point (2 wk after diabetes) for the mechanistic study, followed by a confirming study at 2 months after diabetes.
It should be mentioned that because the predominant apoptotic cells induced by diabetes in the testes were predominantly from spermatogonia and primary spermatocytes, the use of whole testis to extract total protein included the proteins derived from secondary spermatocytes, spermatids, sperm, and even somatic cells. This may dilute true signaling levels that would, ordinarily, be observed. Here, we have used immunochemical staining to show that the predominant indicators, such as oxidative damage, p53, and Sirt1, were localized among spermatogonia and spermatocytes, which are types of apoptotic cells we have observed in the recently published study and present findings (Supplemental Figure 1 and Figure 7A). Ideally, pure spermatogonia and spermatocytes isolated from testis would be used in future studies.
In summary, we showed previously that FGF21 deficiency causes and enhances spontaneous diabetes-induced germ cell apoptosis (7). We demonstrate for the first time that FGF21 deficiency exacerbates the impairment of AKT/GSK-3β/GS and AMPK/Sirt1/PGC-1α-signaling pathways in diabetic testes, which leads to accumulation of metabolic intermediates that cause testicular oxidative damage and germ cell apoptosis. In nondiabetics, however, FGF21 preserves normal spontaneous germ cell apoptosis and spermatogenesis homeostasis through AKT, especially AKT1, to suppress p53 activation via MDM2. This schema is illustrated in Figure 7. Based on the beneficial effects of FGF21 on energy homeostasis, a clinical trial was recently reported using LY2405319 (an analog of FGF21). It showed a significant improvement of dyslipidemia and reduction of body weight, fasting insulin, and adiponectin levels in patients with type 2 diabetes (50). These results suggest that FGF21-based therapies may represent a novel treatment for the metabolic syndrome and its complications.
Supplementary Material
Acknowledgments
We thank Dr Stephen J. Winters, Department of Medicine, University of Louisville, for his editorial assistance. We also thank Leroy R. Sachleben Jr for his help in editing this manuscript.
This study was supported in part by the National Science Foundation of China Young Scientist Award 81201218 (to Y.X.), Jilin Provincial Science and Technology Foundation Grants 20110465 (to Y.X.) and 20130522038JH and 20140414035GH (to X.J.), and the Jilin Department of Health Foundation Grant 3D511AK03426 (to Y.X.).
Disclosure Summary: The authors have nothing to disclose.
Abbreviations
- AIF
apoptosis-induced factor
- AKT
protein kinase B
- AMPK
AMP-activated protein kinase
- DAPI
4, 6-diamidino-2-phenylindole dihydrochloride
- FGF21
fibroblast growth factor 21
- GS
glycogen synthase
- GSK
GS kinase
- 4-HNE
4-hydroxy-2-nonenal
- KO-CON
FGF21-Knockout control
- KO-DM
FGF21-KO diabetes
- KO-DM-FGF21
KO-DM with treatment of exogenous FGF21
- LKB1
liver kinase 1
- MDM2
murine double minute 2
- 3-NT
3-nitrotyrosine
- PGC-1α
PPAR-γ coactivator 1α
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
- Sirt1
sirtuin 1
- STZ
streptozotocin
- WT
wild type
- WT-CON
WT control
- WT-DM
WT diabetes.
References
- 1. Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–437. [DOI] [PubMed] [Google Scholar]
- 2. Gälman C, Lundåsen T, Kharitonenkov A, et al. . The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARα activation in man. Cell Metab. 2008;8:169–174. [DOI] [PubMed] [Google Scholar]
- 3. Hondares E, Iglesias R, Giralt A, et al. . Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J Biol Chem. 2011;286:12983–12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fon Tacer K, Bookout AL, Ding X, et al. . Research resource: comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol Endocrinol. 2010;24:2050–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Planavila A, Redondo I, Hondares E, et al. . Fibroblast growth factor 21 protects against cardiac hypertrophy in mice. Nat Commun. 2013;4:2019. [DOI] [PubMed] [Google Scholar]
- 6. Cuevas-Ramos D, Aguilar-Salinas CA, Gómez-Pérez FJ. Metabolic actions of fibroblast growth factor 21. Curr Opin Pediatr. 2012;24:523–529. [DOI] [PubMed] [Google Scholar]
- 7. Jiang X, Zhang C, Xin Y, et al. . Protective effect of FGF21 on type 1 diabetes-induced testicular apoptotic cell death probably via both mitochondrial- and endoplasmic reticulum stress-dependent pathways in the mouse model. Toxicol Lett. 2013;219:65–76. [DOI] [PubMed] [Google Scholar]
- 8. Zhao Y, Tan Y, Dai J, et al. . Exacerbation of diabetes-induced testicular apoptosis by zinc deficiency is most likely associated with oxidative stress, p38 MAPK activation, and p53 activation in mice. Toxicol Lett. 2011;200:100–106. [DOI] [PubMed] [Google Scholar]
- 9. Cai L, Klein JB, Kang YJ. Metallothionein inhibits peroxynitrite-induced DNA and lipoprotein damage. J Biol Chem. 2000;275:38957–38960. [DOI] [PubMed] [Google Scholar]
- 10. Dummler B, Tschopp O, Hynx D, Yang ZZ, Dirnhofer S, Hemmings BA. Life with a single isoform of Akt: mice lacking Akt2 and Akt3 are viable but display impaired glucose homeostasis and growth deficiencies. Mol Cell Biol. 2006;26:8042–8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guo TB, Chan KC, Hakovirta H, et al. . Evidence for a role of glycogen synthase kinase-3 β in rodent spermatogenesis. J Androl. 2003;24:332–342. [DOI] [PubMed] [Google Scholar]
- 12. Zhao Y, Tan Y, Dai J, et al. . Zinc deficiency exacerbates diabetic down-regulation of Akt expression and function in the testis: essential roles of PTEN, PTP1B and TRB3. J Nutr Biochem. 2012;23:1018–1026. [DOI] [PubMed] [Google Scholar]
- 13. Wang Y, Feng W, Xue W, et al. . Inactivation of GSK-3β by metallothionein prevents diabetes-related changes in cardiac energy metabolism, inflammation, nitrosative damage, and remodeling. Diabetes. 2009;58:1391–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. [DOI] [PubMed] [Google Scholar]
- 15. Shaw RJ, Kosmatka M, Bardeesy N, et al. . The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA. 2004;101:3329–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Galardo MN, Riera MF, Pellizzari EH, Cigorraga SB, Meroni SB. The AMP-activated protein kinase activator, 5-aminoimidazole-4-carboxamide-1-b-D-ribonucleoside, regulates lactate production in rat Sertoli cells. J Mol Endocrinol. 2007;39:279–288. [DOI] [PubMed] [Google Scholar]
- 17. Riera MF, Galardo MN, Pellizzari EH, Meroni SB, Cigorraga SB. Molecular mechanisms involved in Sertoli cell adaptation to glucose deprivation. Am J Physiol Endocrinol Metab. 2009;297:E907–E914. [DOI] [PubMed] [Google Scholar]
- 18. Coussens M, Maresh JG, Yanagimachi R, Maeda G, Allsopp R. Sirt1 deficiency attenuates spermatogenesis and germ cell function. PLoS One. 2008;3:e1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reznick RM, Shulman GI. The role of AMP-activated protein kinase in mitochondrial biogenesis. J Physiol. 2006;574:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alves MG, Martins AD, Rato L, Moreira PI, Socorro S, Oliveira PF. Molecular mechanisms beyond glucose transport in diabetes-related male infertility. Biochim Biophys Acta. 2013;1832:626–635. [DOI] [PubMed] [Google Scholar]
- 21. Holland WL, Adams AC, Brozinick JT, et al. . An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 2013;17:790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chau MD, Gao J, Yang Q, Wu Z, Gromada J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1α pathway. Proc Natl Acad Sci USA. 2010;107:12553–12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cong WT, Ling J, Tian HS, et al. . Proteomic study on the protective mechanism of fibroblast growth factor 21 to ischemia-reperfusion injury. Can J Physiol Pharmacol. 2013;91:973–984. [DOI] [PubMed] [Google Scholar]
- 24. Rasoulpour T, DiPalma K, Kolvek B, Hixon M. Akt1 suppresses radiation-induced germ cell apoptosis in vivo. Endocrinology. 2006;147:4213–4221. [DOI] [PubMed] [Google Scholar]
- 25. Santos-Ahmed J, Brown C, Smith SD, et al. . Akt1 protects against germ cell apoptosis in the postnatal mouse testis following lactational exposure to 6-N-propylthiouracil. Reprod Toxicol. 2011;31:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen WS, Xu PZ, Gottlob K, et al. . Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15:2203–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fuchs SY, Adler V, Buschmann T, Wu X, Ronai Z. Mdm2 association with p53 targets its ubiquitination. Oncogene. 1998;17:2543–2547. [DOI] [PubMed] [Google Scholar]
- 29. Pitetti JL, Calvel P, Zimmermann C, et al. . An essential role for insulin and IGF1 receptors in regulating sertoli cell proliferation, testis size, and FSH action in mice. Mol Endocrinol. 2013;27:814–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spaliviero JA, Jimenez M, Allan CM, Handelsman DJ. Luteinizing hormone receptor-mediated effects on initiation of spermatogenesis in gonadotropin-deficient (hpg) mice are replicated by testosterone. Biol Reprod. 2004;70:32–38. [DOI] [PubMed] [Google Scholar]
- 31. Wang H, Xiao Y, Fu L, et al. . High-level expression and purification of soluble recombinant FGF21 protein by SUMO fusion in Escherichia coli. BMC Biotechnol. 2010;10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim ST, Omurtag K, Moley KH. Decreased spermatogenesis, fertility, and altered Slc2A expression in Akt1−/− and Akt2−/− testes and sperm. Reprod Sci. 2012;19:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mudò G, Mäkelä J, Di Liberto V, et al. . Transgenic expression and activation of PGC-1α protect dopaminergic neurons in the MPTP mouse model of Parkinson's disease. Cell Mol Life Sci. 2012;69:1153–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sapkota GP, Kieloch A, Lizcano JM, et al. . Phosphorylation of the protein kinase mutated in Peutz-Jeghers cancer syndrome, LKB1/STK11, at Ser431 by p90(RSK) and cAMP-dependent protein kinase, but not its farnesylation at Cys(433), is essential for LKB1 to suppress cell vrowth. J Biol Chem. 2001;276:19469–19482. [DOI] [PubMed] [Google Scholar]
- 35. Li GY, Xie P, Li HY, Hao L, Xiong Q, Qiu T. Involment of p53, Bax, and Bcl-2 pathway in microcystins-induced apoptosis in rat testis. Environ Toxicol. 2011;26:111–117. [DOI] [PubMed] [Google Scholar]
- 36. Rato L, Duarte AI, Tomás GD, et al. . Pre-diabetes alters testicular PGC1-α/SIRT3 axis modulating mitochondrial bioenergetics and oxidative stress. Biochim Biophys Acta. 2014;1837:335–344. [DOI] [PubMed] [Google Scholar]
- 37. Yu X, Zhang L, Yang X, et al. . Salvianolic acid A protects the peripheral nerve function in diabetic rats through regulation of the AMPK-PGC1α-Sirt3 axis. Molecules. 2012;17:11216–11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Esteve-Puig R, Canals F, Colome N, Merlino G, Recio JA. Uncoupling of the LKB1-AMPKα energy sensor pathway by growth factors and oncogenic BRAF. PLoS One. 2009;4:e4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wente W, Efanov AM, Brenner M, et al. . Fibroblast growth factor-21 improves pancreatic β-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes. 2006;55:2470–2478. [DOI] [PubMed] [Google Scholar]
- 40. Kim HW, Lee JE, Cha JJ, et al. . Fibroblast growth factor 21 improves insulin resistance and ameliorates renal injury in db/db mice. Endocrinology. 2013;154:3366–3376. [DOI] [PubMed] [Google Scholar]
- 41. Hay N. Akt isoforms and glucose homeostasis - the leptin connection. Trends Endocrinol Metab. 2011;22:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yin Y, Stahl BC, DeWolf WC, Morgentaler A. p53-mediated germ cell quality control in spermatogenesis. Dev Biol. 1998;204:165–171. [DOI] [PubMed] [Google Scholar]
- 43. Ogawara Y, Kishishita S, Obata T, et al. . Akt enhances Mdm2-mediated ubiquitination and degradation of p53. J Biol Chem. 2002;277:21843–21850. [DOI] [PubMed] [Google Scholar]
- 44. De Miguel MP, Donovan PJ. Determinants of retroviral-mediated gene delivery to mouse spermatogonia. Biol Reprod. 2003;68:860–866. [DOI] [PubMed] [Google Scholar]
- 45. Limesand KH, Schwertfeger KL, Anderson SM. MDM2 is required for suppression of apoptosis by activated Akt1 in salivary acinar cells. Mol Cell Biol. 2006;26:8840–8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang L, Sun S, Zhou J, et al. . Knockdown of Akt1 promotes Akt2 upregulation and resistance to oxidative-stress-induced apoptosis through control of multiple signaling pathways. Antioxid Redox Signal. 2011;15:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Samuel SM, Thirunavukkarasu M, Penumathsa SV, Paul D, Maulik N. Akt/FOXO3a/SIRT1-mediated cardioprotection by n-tyrosol against ischemic stress in rat in vivo model of myocardial infarction: switching gears toward survival and longevity. J Agric Food Chem. 2008;56:9692–9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhao H, Xu S, Wang Z, et al. . Repetitive exposures to low-dose x-rays attenuate testicular apoptotic cell death in streptozotocin-induced diabetes rats. Toxicol Lett. 2010;192:356–364. [DOI] [PubMed] [Google Scholar]
- 49. Cai L, Li W, Wang G, Guo L, Jiang Y, Kang YJ. Hyperglycemia-induced apoptosis in mouse myocardium: mitochondrial cytochrome C-mediated caspase-3 activation pathway. Diabetes. 2002;51:1938–1948. [DOI] [PubMed] [Google Scholar]
- 50. Gaich G, Chien JY, Fu H, et al. . The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 2013;18:333–340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.