The prevalence of obesity has reached alarming proportions globally, and continues to rise in both developed and developing countries. At least 2.8 million people each year die as a result of being overweight or obese. According to the estimates by the World Health Organization in 2008 more than 1.4 billion adults were overweight in 2008, and more than half a billion were obese, and the number of overweight and obese adults is projected to increase to 2.2 billion and 1.1 billion, respectively, by 2030. As the numbers of obese women increases the associated comorbidities like type 2 diabetes, coronary heart disease, cancers and reproductive disorders will also increase.
Women with obesity have a number of reproductive disorders including problems with ovulation, decreased rates of conception, infertility, early pregnancy loss, birth defects, and reduced assisted reproductive technology success (1, 2). The increased incidence of miscarriages and assisted reproductive technology failure in obese women (3, 4) is attributed in large part to poor oocyte quality manifest as impaired oocyte maturation and disrupted spindle morphology. A number of investigators provide evidence of metabolic perturbations in oocytes from obese mothers including high levels of intracellular lipid (5), elevated endoplasmic reticulum (6), and oxidative stress (7, 8), increased accumulation of oocyte mRNA (9), and mitochondrial dysfunction (1, 7). These obesity-induced alterations in ovarian follicle development and oocyte quality are associated with activation of systemic or local inflammatory pathways (10–12).
In this issue of Endocrinology, Xie et al (13) find that obesity-dependent increases in proinflammatory signaling regulates the abundance of oocyte specific mRNAs (Figure 1). This journey began when this group found that a subset of maternal RNAs, including maternal effect genes, are elevated in oocytes from mature obese mice (9) and women with metabolic dysfunction (14). These observations are important because the increased abundance of maternal effect genes is known to interfere with normal embryonic development (15, 16). In the present study, to induce the obese phenotype the investigators fed young adult female mice normal rodent chow or a high-fat diet containing 45% or 60% of its calories from fat. The high-fat diet induced increases in adipose tissue and whole body weight but did not alter the weight or gross morphology of the ovary. This study revealed increases in the abundance of maternal effect gene transcripts (developmental pluripotency-associated protein 3 [Dppa3], pluripotency factors POU class 5 homeobox 1 [Pou5f1], and basonuclin 1 [Bnc1]) in ovaries collected from the obese females. The authors also report diet-induced obesity was associated with the induction of an ovarian inflammatory response characterized by increased expression of the inflammatory cytokine tumor necrosis factor, Tnf, and activation of intracellular signals associated with the inflammatory response (ie, signal transducer and activator of transcription 3 [STAT3] and nuclear factor kappa B [NFκB]). Surprisingly, the inflammatory signaling molecules were found in different portions of the ovary; STAT3 localized to the oocyte, whereas NFκB localized to the somatic cells of the ovary. This team then directly linked phosphorylated STAT3 to the promotor region of Pou5f1 in oocytes from obese females, a critical finding linking obesity, inflammatory pathways and oocyte dysfunction. Additional studies are required to identify how inflammatory signals cause obesity-dependent increases in oocyte mRNAs and determine how this negatively impacts embryo viability. It will also be important to determine whether adiposity associated NFκB signaling in somatic cells contributes to reported alterations in inflammation, mitochondrial dysfunction and oxidative stress.
Figure 1.
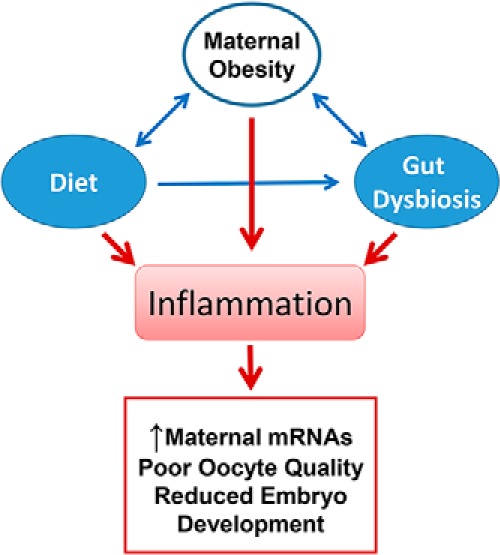
Consumption of a high-fat diet results in obesity and inflammatory responses that are linked to increased expression of maternal effects genes in oocytes, which contribute to poor quality oocytes and impaired embryo development. High-fat diet also shifts the gut microbiome resulting in dysbiosis and inflammatory responses that are correlated with increased expression of maternal effects genes, which contribute to infertility in women.
Another characteristic of obesity that is correlated with chronic inflammatory responses is gut dysbiosis (17). Dysbiosis refers to disturbances in the interdependent relationship between intestinal lumen cells and gut microbes (17, 18). Changes in the relative abundance of specific microbial populations in the gut are correlated with increased intestinal and systemic inflammation (19–22). In this issue of Endocrinology, Xie et al used their mouse model of obesity to determine whether gut microbes contribute to ovarian inflammation and increased expression of ovarian mRNAs. Analysis of cecum samples revealed the presence of a number of microbe families, and the microbe Lachnospiraceae was significantly elevated in animals fed the high-fat diet. Multivariate linear regression analysis revealed positive correlations between the ovarian abundance of Dppa3, Pou5f1, and Bnc1 transcripts. Additionally, a positive correlation was identified with abundance of Lachnospiraceae and levels of ovarian Tnf. These observations are the first report demonstrating a positive correlation between abundance gut microbiota species and ovarian transcription. Collectively the findings in this report draw correlations between obesity induced changes in gut microbial composition, makers of ovarian inflammatory responses and oocyte gene expression (Figure 1). Diet-induced increases in systemic inflammation mediated in part by changes in the composition of the gut microbiome may contribute to infertility and other reproductive disorders.
With the developing epidemics of overweight/obesity and physical inactivity strategies are need for women (and men) to combat physiological disturbances linked to inflammation. Weight loss alone does not appear to be an effective way to improve oocyte quality and reproductive outcomes in the obese patient. Programs designed to lose weight by lowering of caloric intake and increasing physical activity have relatively low compliance and low success rates, and modest, short-term weight loss does not improve in vitro fertilization success in obese and overweight patients (23). Studies in mice show that dietary interventions fail to reverse obesity-induced problems in oocytes (24). However, recent studies using a mouse model of exercise (25) report that exercise improves certain aspects of lipid metabolism in oocytes. Fatty acid β-oxidation is required for oocyte maturation and embryo development (26) and defects in β-oxidation can result in fertility defects in mice and humans (5). Moderate exercise in this mouse model of obesity had a beneficial effect on lipid metabolism, but exercise was not able to reverse other damage to oocytes on a high-fat diet. Exercise is also postulated to reduce levels of proinflammatory cytokines and systemic inflammation (27), which may account for the beneficial effects on oocyte quality. A recent study suggests that treatment of obese mice with inhibitors of endoplasmic reticulum stress restored oocyte quality and embryo development (6). It is also intriguing to consider how modification of gut microbial communities could provide a therapy for female infertility; however, a deeper understanding of the relationship between relative gut microbial populations and the function of the reproductive tract in mammalian systems is needed. In support of such an idea is a recent report demonstrating that feeding mice a novel probiotic mixture shifted the abundance of certain gut microbes, which subsequently altered systemic levels of inflammatory immune cell populations and reduced development of experimental hepatocellular carcinoma (28). Although much additional research is required to define mechanisms, the present report opens new avenues for approaching the complex issue of obesity and reproductive disorders in women.
Acknowledgments
This work was supported by the Olson Center for Women's Health and the Department of Veterans Affairs, Office of Research and Development Biomedical Laboratory Research and Development Program.
Disclosure Summary: The author has nothing to disclose.
Abbreviations
- NFκB
nuclear factor-kappa B
- Pou5f1
POU class 5 homeobox 1
- STAT3
signal transducer and activator of transcription 3.
Footnotes
See article in Endocrinology 2016;157:1630–1643
References
- 1. Grindler NM, Moley KH. Maternal obesity, infertility and mitochondrial dysfunction: potential mechanisms emerging from mouse model systems. Mol Hum Reprod. 2013;19:486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Catalano PM. Obesity, insulin resistance, and pregnancy outcome. Reproduction. 2010;140:365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brewer CJ, Balen AH. The adverse effects of obesity on conception and implantation. Reproduction. 2010;140:347–364. [DOI] [PubMed] [Google Scholar]
- 4. Linné Y. Effects of obesity on women's reproduction and complications during pregnancy. Obes Rev. 2004;5:137–143. [DOI] [PubMed] [Google Scholar]
- 5. Dunning KR, Russell DL, Robker RL. Lipids and oocyte developmental competence: the role of fatty acids and β-oxidation. Reproduction. 2014;148:R15–R27. [DOI] [PubMed] [Google Scholar]
- 6. Wu LL, Russell DL, Wong SL, et al. . Mitochondrial dysfunction in oocytes of obese mothers: transmission to offspring and reversal by pharmacological endoplasmic reticulum stress inhibitors. Development. 2015;142:681–691. [DOI] [PubMed] [Google Scholar]
- 7. Hou YJ, Zhu CC, Duan X, Liu HL, Wang Q, Sun SC. Both diet and gene mutation induced obesity affect oocyte quality in mice. Sci Rep. 2016;6:18858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Igosheva N, Abramov AY, Poston L, et al. . Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS One. 2010;5:e10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pohlmeier WE, Xie F, Kurz SG, Lu N, Wood JR. Progressive obesity alters the steroidogenic response to ovulatory stimulation and increases the abundance of mRNAs stored in the ovulated oocyte. Mol Reprod Dev. 2014;81:735–747. [DOI] [PubMed] [Google Scholar]
- 10. Nteeba J, Ortinau LC, Perfield JW 2nd, Keating AF. Diet-induced obesity alters immune cell infiltration and expression of inflammatory cytokine genes in mouse ovarian and peri-ovarian adipose depot tissues. Mol Reprod Dev. 2013;80:948–958. [DOI] [PubMed] [Google Scholar]
- 11. Nteeba J, Ganesan S, Keating AF. Progressive obesity alters ovarian folliculogenesis with impacts on pro-inflammatory and steroidogenic signaling in female mice. Biol Reprod. 2014;91:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boots CE, Jungheim ES. Inflammation and human ovarian follicular dynamics. Semin Reprod Med. 2015;33:270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xie F, Anderson CL, Timme KR, Kurz SG, Fernando SC, Wood JR. Obesity-dependent increases in oocyte mRNAs are associated with increases in pro-inflammatory signaling and gut microbial abundance of Lachnospiraceae in female mice. Endocrinology. 2016:en20151851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wood JR, Dumesic DA, Abbott DH, Strauss JF 3rd. Molecular abnormalities in oocytes from women with polycystic ovary syndrome revealed by microarray analysis. J Clin Endocrinol Metab. 2007;92:705–713. [DOI] [PubMed] [Google Scholar]
- 15. Park MW, Kim KH, Kim EY, Lee SY, Ko JJ, Lee KA. Associations among Sebox and other MEGs and its effects on early embryogenesis. PLoS One. 2015;10:e0115050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Giraldez AJ, Mishima Y, Rihel J, et al. . Zebrafish MiR-430 promotes deadenylation and clearance of maternal mRNAs. Science. 2006;312:75–79. [DOI] [PubMed] [Google Scholar]
- 17. Turnbaugh PJ, Hamady M, Yatsunenko T, et al. . A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bäckhed F, Ding H, Wang T, et al. . The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hamilton MK, Boudry G, Lemay DG, Raybould HE. Changes in intestinal barrier function and gut microbiota in high-fat diet-fed rats are dynamic and region dependent. Am J Physiol Gastrointest Liver Physiol. 2015;308:G840–G851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ding S, Chi MM, Scull BP, et al. . High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS One. 2010;5:e12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cani PD, Amar J, Iglesias MA, et al. . Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. [DOI] [PubMed] [Google Scholar]
- 22. Kim KA, Gu W, Lee IA, Joh EH, Kim DH. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One. 2012;7:e47713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chavarro JE, Ehrlich S, Colaci DS, et al. . Body mass index and short-term weight change in relation to treatment outcomes in women undergoing assisted reproduction. Fertil Steril. 2012;98:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reynolds KA, Boudoures AL, Chi MM, Wang Q, Moley KH. Adverse effects of obesity and/or high-fat diet on oocyte quality and metabolism are not reversible with resumption of regular diet in mice. Reprod Fertil Dev. 2015;27:716–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boudoures AL, Chi M, Thompson A, Zhang W, Moley KH. The effects of voluntary exercise on oocyte quality in a diet-induced obese murine model. Reproduction. 2016;151:261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dunning KR, Cashman K, Russell DL, Thompson JG, Norman RJ, Robker RL. β-Oxidation is essential for mouse oocyte developmental competence and early embryo development. Biol Reprod. 2010;83:909–918. [DOI] [PubMed] [Google Scholar]
- 27. Woods JA, Vieira VJ, Keylock KT. Exercise, inflammation, and innate immunity. Immunol Allergy Clin North Am. 2009;29:381–393. [DOI] [PubMed] [Google Scholar]
- 28. Li J, Sung CY, Lee N, et al. . Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc Natl Acad Sci USA. 2016;113:E1306–E1315. [DOI] [PMC free article] [PubMed] [Google Scholar]


