Abstract
Antimicrobial multidrug-resistant microorganisms (MDRO) can be transmitted between companion animals and their human owners. Aim of this study was to determine the prevalence of extended spectrum beta-lactamase-producing Enterobacteriaceae (ESBL-E) and Staphylococcus aureus including methicillin-resistant S. aureus (MRSA) in different companion animal species. Dogs (n = 192), cats (n = 74), and rabbits (n = 17), treated in a veterinary practice and hospital or living in an animal shelter and private households, were sampled. All facilities were located in a region characterized by a high density of pig production. Nasal, buccal and perianal swabs were enriched and cultured on solid chromogenic selective media. A subgroup of 20 animals (13 dogs, 3 cats, 4 rabbits) was analyzed for the presence of staphylococci other than S. aureus. Amongst all animals (n = 283), twenty dogs (10.4%) and six cats (8.1%) carried S. aureus. MRSA was found in five dogs (2.6%) and two cats (2.7%). Isolates were of spa types t011, t034, t108 (all mecA-positive, ST398), and t843 (mecC-positive, ST130), typical for livestock-associated (LA)-MRSA. Except for one dog, MRSA-positive animals did not have direct contact to husbandry. ESBL-Escherichia coli (blaCTX-M/blaTEM/blaSHV genes) were present in seven dogs (3.6%), one cat (1.4%) possessed a cefotaxime-resistant Citrobacter freundii isolate (blaTEM/blaCMY-2 genes). MDRO carriage was associated with animals from veterinary medical settings (p<0.05). One dog and one rabbit carried methicillin-resistant coagulase-negative staphylococci. The exclusive occurrence of MRSA lineages typically described for livestock stresses the impact of MDRO strain dissemination across species barriers in regional settings. Presence of ESBL-E and LA-MRSA among pets and probable dissemination in clinical settings support the necessity of a “One Health” approach to address the potential threats due to MDRO-carrying companion animals.
Introduction
Today, companion animals such as cats and dogs are often considered family members and close proximity or direct animal contact are given on a daily basis in many households. This causes the potential risk of transmission of a multitude of pathogenic microorganisms, including multidrug-resistant bacteria, between pets and their human owners [1–3]. Coagulase-positive staphylococci, such as methicillin-resistant (MR) Staphylococcus aureus (MRSA), Staphylococcus pseudintermedius (MRSP) and Staphylococcus intermedius (MRSI), as well as MR coagulase-negative staphylococci (MR-CoNS) and extended-spectrum beta-lactamase (ESBL) producing Enterobacteriaceae (ESBL-E) have been shown to colonize companion animals and cause infections in both pets and humans [4–9].
The ability of bacteria to inhabit different hosts poses the additional threat of resistance gene transfer, resulting in decreased susceptibility against antimicrobials in increasing numbers of bacteria [4,10]. Resistance genes of particular interest comprise the mec genes (mecA, mecB, mecC, and mecD) causing methicillin resistance in members of the genera Staphylococcus and Macrococcus, and the bla genes (blaCMY-2, blaSHV, blaTEM, blaCTX-M) encoding beta-lactamases (especially ESBL) in Enterobacterales comprising Enterobacteriaceae and related families [11–16].
The aim of this study was to assess carriage and antimicrobial resistance of opportunistic animal and human pathogens in companion animals in North-West Germany by means of culture-based phenotypic and molecular methods.
Materials and methods
Sample collection
Bacterial isolates were obtained from dogs (n = 192), cats (n = 74), and rabbits (n = 17) either healthy or undergoing veterinary examination between May 2015 and March 2016. Animals treated in a private veterinary practice and a veterinary hospital or living in an animal shelter and private households were included. Swab samples (Transwab Amies MW 172P, Medical Wire & Equipment, Corsham Wiltshire, England) were collected from each animal’s nasal vestibules (both sides using one swab), oral mucosa and perianal area. Samples from animals in the hospital were taken prior to treatment. In the veterinary practice, animals were treated as outpatients. In order to participate in the study, pet owners had to answer a full consent form and a questionnaire. Samples were stored for a maximum of three days at room temperature before further processing.
Cultivation
For isolation of S. aureus, nasal and buccal swabs were streaked onto selective chromogenic medium (chromID S. aureus, bioMérieux, Marcy l´Étoile, France), then suspended in 5 ml of tryptic soy broth (TSB, Becton Dickinson, Franklin Lakes, NJ, USA) supplemented with 6.5% NaCl. Solid and liquid cultures were incubated at 37°C for 24 h. Subsequently, chromID S. aureus agar was inoculated with 10 μl of the enriched liquid culture. Furthermore, 1 ml of the culture was used on chromogenic medium selective for MRSA (chromID MRSA, bioMérieux) and 1 ml was suspended in 9 ml phenolred mannitol broth supplemented with ceftizoxim/aztreonam (PHMB+C/AZ, Mediaproducts BV, Groningen, Netherlands). Cultures were grown at 37°C for either 24 h (chromID S. aureus, bioMérieux and PHMB+C/AZ, Mediaproducts BV) or 48 h (chromID MRSA, bioMérieux). From the PHMB+C/AZ (Mediaproducts BV) culture, 1 ml was used for further incubation on chromID MRSA (bioMérieux) at 37°C for 48 h.
For a subset of samples from 20 animals (13 dogs, 3 cats and 4 rabbits), which were the first animals included in the study, swabs were additionally streaked onto 5% sheep blood agar supplemented with aztreonam and colistin (CAP) (Oxoid, Wesel, Germany) and incubated at 37°C for 24 h for the detection of staphylococcal species apart from S. aureus.
In order to detect ESBL-E, swab samples from the perianal area were suspended in TSB and incubated at 37°C for 24 h. Chromogenic selective medium (chromID ESBL, bioMérieux) was inoculated with 10 μl of the liquid culture and further incubated at 37°C for 24 h.
Identification of isolates
Colonies grown on chromID MRSA (bioMérieux), chromID S. aureus (bioMérieux), and CAP agar (Oxoid) were isolated based on conventional phenotypic characteristics, such as colony morphology, pigmentation, Gram staining and production of clumping factor (Pastorex Staph Plus; bioMérieux). Colonies grown on chromID ESBL medium (bioMérieux) were selected according to their colony morphology and pigmentation. Single colonies were inoculated onto Columbia blood agar (Becton Dickinson) and further cultivated at 37°C for 24 h.
Identification of pure cultures down to the species level was accomplished via matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF MS, Bruker Daltonics, Billerica, MA) as described elsewhere [17,18]. In brief, cells from freshly grown single colonies were smeared on a ground steel target plate (Bruker Daltonics) and covered with 1 μl of alpha-cyano-4-hydroxycinnamic acid (HCCA) dissolved in 50.0% acetonitrile and 2.5% trifluoroacetic acid. Analysis of co-crystallized samples was performed with flexControl 3.3 (Bruker Daltonics). Spectra were evaluated with the MALDI Biotyper 4.0 (Bruker Daltonics) considering an m/z range of 4,000–10,000 Da. The score threshold for explicit determination on species level was set to ≥ 2.0.
Bacterial isolates yielding scores ≤ 2.0 in MALDI-TOF MS analysis were subjected to biochemical identification in the VITEK 2 automated system (bioMérieux) using VITEK 2 GP ID cards for nasal and buccal samples or VITEK 2 GN ID cards (bioMérieux) for perianal samples, respectively.
If identification results via MALDI-TOF MS and VITEK 2 were ambiguous, the 16S rRNA gene was sequenced. For this, total genomic DNA was extracted using the QIAamp DNA MiniKit following the manufacturer’s instructions (Qiagen, Venlo, Netherlands). Primers 27f and 907r(m) were used to target the V1-V4 hypervariable regions of the 16S rRNA gene [19,20]. Purification of PCR products was achieved using the MinElute Kit (Qiagen) according to manufacturer’s instructions. Sequencing was performed on an ABI 3730XL sequencing machine using the cycle sequencing technology (Eurofins Genomics, Ebersberg, Germany). For sequence analysis, comparison against sequences obtained from validly described type strains and isolates was carried out using the Seqmatch function of the RDP-II database [21]. Sequences yielding a similarity score of ≥ 98% were assigned at species level.
Characterization of isolates
Antimicrobial susceptibility testing (AST) of isolates identified as staphylococci or Enterobacteriaceae was carried out with the VITEK 2 system (bioMérieux) according to the manufacturer’s instructions using the test cards AST-P632 for staphylococci and AST-N214 for Enterobacteriaceae. AST results were interpreted according to The European Committee on Antimicrobial Susceptibility Testing (EUCAST) clinical breakpoints [22].
S. pseudintermedius and S. intermedius isolates were further characterized regarding their phenotypic resistance by disk diffusion using oxacillin (1 μg) as recommended by EUCAST [22]. The presence of the methicillin resistance determinants mecA, mecB and mecC in staphylococci was tested by PCR as previously described [11,23]. S. aureus isolates were genotyped by means of their spa gene and multilocus sequence typing (MLST) was performed on MRSA isolates as described elsewhere [24,25].
In Enterobacteriaceae, ESBL-production was confirmed using the MASTDISCS ID Extended-Spektrum-β-Laktamasen (ESβL)-Set (CPD10) D67C (MAST Diagnostica, Reinfeld, Germany) according to the manufacturer’s instruction. Resistance was further classified by detection of blaSHV, blaTEM and blaCTX-M genes by multiplex PCR as introduced by Monstein et al. [26]. The gene blaCMY-2 was tested as described by Souna et al. [27]. Additionally, the eazyplex SuperBug assay (AmplexDiagnostics GmbH, Gars am Inn, Germany) to detect carbapenemase-producing Enterobacteriaceae (CRE) was applied following the manufacturer’s instructions.
Association of ESBL-E/cefotaxime-resistant Enterobacteriaceae and MRSA carriage with potential risk factors was assessed separately for cats, dogs and rabbits applying Fisher’s exact test in GraphPad Prism v.5.00 (GraphPad Software, La Jolla, CA, USA); p < 0.05 was considered significant.
Results
Sample group
A total of 283 animals was sampled including 192 dogs, 74 cats and 17 rabbits. The mean age of the dogs was 5.6 years (range: 0.1–16 years) with 85 animals being male and 107 being female. Among the male dogs, 37.6% (n = 32) were castrated, 39.2% (n = 42) of female dogs were neutered. The group of cats had a mean age of 4.2 years (range: 0.1–17 years), the group of rabbits a mean age of 2.4 years (range 0.3–10 years). Among cats, 63.8% (23/36) of male cats were castrated and 55.3% (21/38) female cats were neutered. The gender distribution in the group of rabbits was nine male and eight female animals. Among these, 33.3% (3/9) of male and 25% (2/8) of female rabbits were neutered.
Across the complete group of animals, 31.4% (89/283) were healthy whereas 68.6% (194/283) were sampled before undergoing veterinary examinations. Altogether, antibiotics had been administered to 27.9% of animals (79/283) within six months prior to sampling. More details about the sample groups are provided in Table 1.
Table 1. Metadata of animals sampled.
| Characteristics | Dogs | Cats | Rabbits | Total | |
|---|---|---|---|---|---|
| Age (y) | Mean | 5.6 | 4.2 | 2.4 | 5.1 |
| Range | 0.1–16 | 0.1–17 | 0.3–10 | 0.1–17 | |
| Gendera | Male | 85 (32) | 36 (23) | 9 (3) | 130 (58) |
| Female | 107 (42) | 38 (21) | 8 (2) | 153 (65) | |
| Origin of sample | Animal shelter | 4 | 17 | 0 | 21 |
| Private household | 45 | 19 | 0 | 64 | |
| Private veterinary practice | 91 | 31 | 17 | 139 | |
| Veterinary hospital | 52 | 7 | 0 | 59 | |
| Veterinary examination | Yes | 128 | 50 | 16 | 194 |
| No | 64 | 22 | 1 | 89 | |
| Reason for veterinary examinationb |
Standard examination | 47 | 18 | 14 | 79 |
| Internal diseases | 20 | 19 | 0 | 39 | |
| Surgical intervention | 41 | 12 | 2 | 55 | |
| Orthopedics | 20 | 1 | 0 | 21 | |
| Antibiotic treatmentc | Topical | 9 | 5 | 0 | 14 |
| Systemic | 41 | 19 | 0 | 60 | |
| Both | 2 | 2 | 1 | 5 | |
| No antibiotics | 140 | 48 | 16 | 204 | |
| Contact with other animals |
Livestock | 35 | 14 | 4 | 53 |
| Horses | 79 | 19 | 0 | 98 | |
| Dogs | 78 | 23 | 2 | 103 | |
| Cats | 40 | 53 | 0 | 93 | |
| Rodents | 13 | 1 | 12 | 26 | |
| Birds | 1 | 1 | 0 | 2 | |
| Owner stayed abroadc | 72 | 17 | 4 | 93 | |
| Total | 192 | 74 | 17 | 283 | |
aNumbers in brackets give total numbers of neutered animals
bStandard examination: general examination and consultation, vaccination, parasite prophylaxis, or dental cleaning. Internal diseases: diseases of digestive tract, urogenital tract, circulatory system, nervous system, skin, eyes, ears, or metabolism. Surgical intervention: Sterilization, wound management, orthopedic surgery, or tumor resection. Orthopedics: diagnosis of lameness, radiography, or bandage management.
c within six months prior to sampling.
Colonization with beta-lactam-resistant Enterobacteriaceae
Among all animals (n = 283), seven (2.5%) were found to carry ESBL-Escherichia coli; one sample was positive for cefotaxime-resistant Citrobacter freundii (Table 2). Whereas all E. coli isolates originated from the perianal area of dogs (7/192, 3.6%), the C. freundii isolate was detected in the perianal sample of a cat (1/74, 1.4%). All ESBL-E/cefotaxime-resistant Enterobacteriaceae isolates were susceptible to ertapenem, imipenem, meropenem and tigecycline. The assay for the detection of CRE remained negative. Four E. coli isolates harbored single bla genes (blaTEM: n = 1; blaCTX-M: n = 3), while four isolates carried combinations of bla genes (C. freundii: blaTEM and blaCMY-2: n = 1; E. coli: blaTEM and blaSHV: n = 1, blaTEM and blaCTX-M: n = 2) (Table 2).
Table 2. Resistance profiles of cefotaxime-resistant Enterobacteriaceae found in 283 companion animals.
| Species | Animal hosta | Sampling site | Resistance genes | Phenotypic antimicrobial susceptibility test profile | |
|---|---|---|---|---|---|
| ESBL-Screeningb | Other resistancesc | ||||
| Citrobacter freundii | Cat (#2) | Perianal | blaTEM, blaCMY-2 | NEG | AMP, SAM, TZP, CXM, CPD, CTX, CAZ |
| Escherichia coli | Dog (#121) | Perianal | blaCTX-M | POS | AMP, SAM, CXM, CPD, CTX, SXT |
| Dog (#130) | Perianal | blaTEM | POS | AMP, SAM, CXM, CPD, CTX | |
| Dog (#146) | Perianal | blaTEM, blaSHV | POS | AMP, SAM, CPD, CAZ, MXF, SXT | |
| Dog (#147) | Perianal | blaCTX-M | POS | AMP, SAM, CXM, CPD, CTX, CAZ, SXT | |
| Dog (#160) | Perianal | blaTEM, blaCTX-M | POS | AMP, SAM, CXM, CPD, CTX, CIP, MXF | |
| Dog (#163) | Perianal | blaCTX-M | POS | AMP, SAM, CXM, CPD, CTX, CIP, MXF, SXT | |
| Dog (#182) | Perianal | blaTEM, blaCTX-M | POS | AMP, SAM, CXM, CPD, CTX | |
a Individual running numbers of animals are given in brackets.
b as determined by MASTDISC ID ESβL-Set (CPD10) D67C (MAST Diagnostica)
c MICs were detected with VITEK 2 (bioMérieux) and evaluated using breakpoints provided by EUCAST [22].
POS, positive; AMP, ampicillin; CAZ, ceftazidime; CIP, ciprofloxacin; CPD, cefpodoxime; CTX, cefotaxime; CXM, cefuroxime; MXF, moxifloxacin; SAM, ampicillin-sulbactam; SXT, trimethoprim-sulfamethoxazol; TZP, piperacillin-tazobactam.
The dogs colonized with ESBL-producing E. coli were 2–12 years old (mean: 7.7 years). Five dogs were male, the other two were female, all of which were neutered except for one male dog. All seven animals were undergoing veterinary examination either in a private veterinary practice (2/7) or a veterinary clinic (5/7). Reasons for the examination were internal diseases (4/7), orthopedics (2/7), or surgical interventions (1/7). Antibiotics had been administered to 4/7 dogs within six months before sampling. One dog had regular contact with livestock and other companion animals, three had contact with horses and companion animals, two had contact with other companion animals and one was not kept together with any other animals. Positive ESBL-E. coli samples from dogs admitted to the veterinary clinic (5/7) were detected within a short time interval between 01/2016 and 03/2016.
The cat carrying cefotaxime-resistant C. freundii was 0.5 years old, male and not neutered. It was brought into the private veterinary practice for surgical intervention and had not received any antibiotics prior to sampling. It was reported to live together with another cat and a dog.
Amongst the samples obtained from rabbits, no ESBL-E were detected.
Colonization with S. aureus
Twenty of 192 dogs (10.4%) carried S. aureus. Colonizing strains (n = 26) belonged to spa types t008, t034 (each n = 4), t091 (n = 3), t605, t786, t3750 (each n = 2), t002, t011, t019, t108, t275, t620, t630, t16020, and t16021 (each n = 1). In 11/20 animals, S. aureus was exclusively detected in the nose (55%). five animals (25%) were tested positive only in their mouth. The remaining four animals carried S. aureus in both habitats. In these four animals, isolates from nasal and buccal samples always belonged to the same spa type (t034, t605, t786 and t3750). In the nose of one dog, isolates associated with three different S. aureus spa types (t008, t091 and t620) were found. Methicillin resistance conferred by the gene mecA was found in six S. aureus isolates colonizing 5/192 dogs (2.6%). These isolates belonged to spa types t034 (n = 4), t011 and t108 (each n = 1), all associated with sequence type (ST) 398. All canine MRSA isolates (6/6) showed resistance against tetracycline (Table 3). MRSA positive animals were of variable age (0.2–7 years, mean 3.6 years) and gender (3 female, 2 male) and were either admitted to the private practice (n = 4) or the clinic (n = 1). One of the dogs was a healthy animal, accompanying another animal treated by the veterinarian. The other dogs were admitted to the veterinarian for standard examination (2/5) or surgical intervention (2/5). Among the the MRSA positive dogs, 1/5 were reported to have contact with livestock, 1/5 had contact with other companion animals, such as other dogs, cats, or rabbits, and 3/5 had contact with both horses and companion animals. Systemic antibiotics had been administered to 2/5 dogs.
Table 3. Resistance profiles of (i) methicillin-resistant Staphylococcus aureus (MRSA) from 283 companion animals and (ii) methicillin-resistant coagulase-negative staphylococci from 20 companion animals.
| Species | Animal hosta | Sampling site | spa type | Resistance genes | Phenotypic antimicrobial susceptibility test profile | |
|---|---|---|---|---|---|---|
| FOX screeningb | Other resistancesc | |||||
| Staphylococcus aureus | Dog (#12) | Nasal | t108 | mecA | POS | PEN, OXA, TET |
| Dog (#16) | Nasal | t034 | mecA | POS | PEN, OXA, CLI, ERY, TET | |
| Dog (#89) | Buccal | t011 | mecA | POS | PEN, OXA, LVX, TET | |
| Dog (#103) | Nasal | t034 | mecA | POS | PEN, CLI, TET | |
| Dog (#171) | Buccal | t034 | mecA | POS | PEN, OXA, LVX, TET, SXT | |
| Dog (#171) | Nasal | t034 | mecA | POS | PEN, OXA, LVX, TET, SXT | |
| Cat (#44) | Buccal | t843 | mecC | POS | PEN, OXA | |
| Cat (#44) | Nasal | t843 | mecC | POS | PEN | |
| Cat (#67) | Buccal | t011 | mecA | POS | PEN, OXA, LVX, TET | |
| Staphylococcus cohnii subsp. cohnii | Dog (#2) | Buccal | mecA | POS | OXA, FOF | |
| Staphylococcus cohnii subsp. urealyticus | Rabbit (#3) | Nasal | - | POS | OXA, FOF, FA | |
| Rabbit (#3) | Buccal | - | POS | OXA, FOF, FA | ||
| Staphylococcus pettenkoferi | Rabbit (#1) | Buccal | - | POS | OXA, FOF | |
| Staphylococcus saprophyticus subsp. saprophyticus | Rabbit (#3) | Nasal | mecA | POS | OXA, FOF, FA | |
a Individual running numbers of animals are given in brackets.
b as determined by VITEK 2 automated system (bioMérieux).
c MICs were detected with VITEK 2 (bioMérieux) and evaluated using breakpoints provided by EUCAST [22].
POS, positive; CLI, clindamycin; ERY, erythromycin; FOF, fosfomycin; FA, fusidic acid; LVX, levofloxacin; OXA, oxacillin; PEN, penicillin; TET, tetracycline; SXT, trimethoprim-sulfamethoxazol.
In the group of 74 cats, six (8.1%) were characterized as S. aureus carriers. Isolates (n = 9) were assigned to spa types t843 (n = 3), t011 and t11232 (each n = 2), and t015 and t1736 (each n = 1). In two of these cats, S. aureus was found only in the nose, in another two only in the mouth and in the remaining two in both habitats. Isolates colonizing different habitats in the same animal were shown to be of the same spa types (t843 and t11232). Three S. aureus isolates colonizing 2/74 animals (2.7%) were MR. Of these, one isolate (spa type t011, ST398) carried mecA, whereas the other two isolates, both spa type t843 and ST130 obtained from mouth and nose of the same animal, carried mecC. The mecA-carrying isolate showed further resistances against clindamycin, levofloxacin and tetracycline (Table 3). The two cats carrying MRSA were eight and ten years old, both female and neutered and both brought to the veterinarian practice because of internal diseases. Neither of them was reported having contact to livestock or horses, but both were kept together with other pets, such as dogs and other cats. The cat carrying the mecA-positive MRSA had received systemic and local antibiosis within six months prior to sampling. The animal carrying the mecC-positive t843 isolates in mouth and nose also carried another nasal t843 S. aureus strain which was methicillin-susceptible according to phenotypic and molecular tests.
Amongst the rabbits, no S. aureus/MRSA isolates were found.
Association of risk factors with MDRO carriage
In the group of dogs, a linkage between neutered animals and ESBL-E. coli carriage was shown (6/68 vs. 1/117; p = 0.0138). None of the other assessed risk factors was associated with ESBL-E/cefotaxime-resistant Enterobacteriaceae or MRSA carriage in the three groups of animals (p > 0.05 for all variables). However, when analyzing ESBL-E/cefotaxime-resistant Enterobacteriaceae and MRSA (i.e. MDRO) together and carriage among all tested animals (not separately for different species), we found that animals sampled in practices and hospitals carried MDRO more frequently than animals sampled in shelters and households (17/181 vs. 0/85; p = 0.0023). Moreover, veterinary treatment (16/178 vs. 1/88; p = 0.0161), castration (12/111 vs. 5/155; p = 0.0238) and contact to other pets (15/163 vs. 2/103; p = 0.0355), were associated with MDRO carriage.
Colonization with staphylococci other than S. aureus
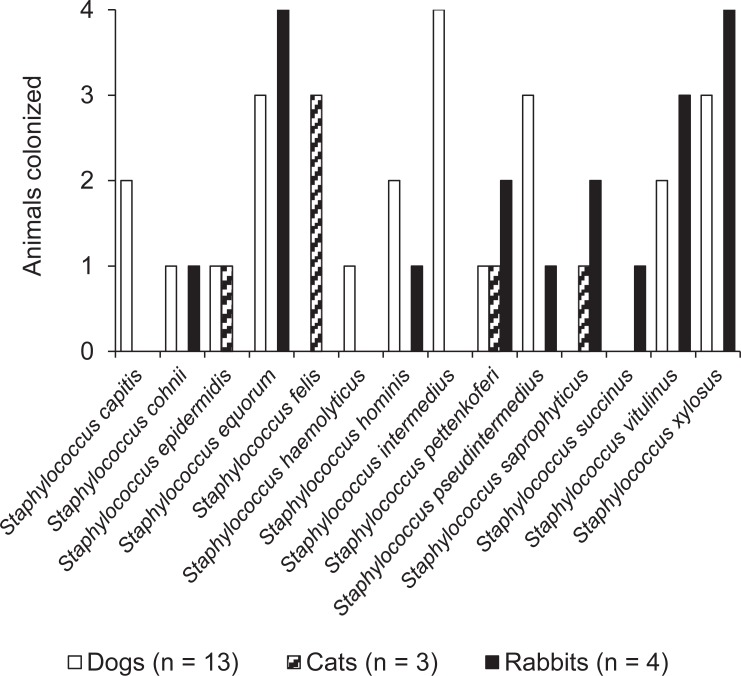
A subgroup of animals (n = 20), consisting of 13 dogs, three cats and four rabbits was further analyzed for staphylococcal species other than S. aureus. Altogether, 14 different staphylococcal species were detected across this subgroup (Fig 1).
Fig 1. Absolute numbers of companion animals (dogs, cats, rabbits) colonized with different staphylococcal species other than S. aureus in the subgroup of 20 animals.
In the group of dogs, eleven different species were detected with the coagulase-positive species S. intermedius (in 4/13 dogs; 30.8%) and S. pseudintermedius (in 3/13 dogs; 23.1%) and the CoNS Staphylococcus equorum and Staphylococcus xylosus (both in 3/13 dogs; 23.1%) being most prevalent. Amongst the cats, four different staphylococcal species were detected. In this group, Staphylococcus felis was found in 3/3 of cats whereas the remaining three species were found in 1/3 cats each. Throughout the group of rabbits, nine different staphylococcal species were detected, with S. equorum and S. xylosus both being detected in 4/4 of the rabbits. Other prevalent species were Staphylococcus vitulinus (in 3/4 rabbits), Staphylococcus pettenkoferi and Staphylococcus saprophyticus (each in 2/4 rabbits) (Fig 1).
Screenings for methicillin resistance among staphylococcal isolates revealed one Staphylococcus cohnii subsp. cohnii, two Staphylococcus cohnii subsp. urealyticus, one S. pettenkoferi, and one Staphylococcus saprophyticus subsp. saprophyticus isolate showing phenotypic resistance according to VITEK 2 testing. Presence of the gene mecA was verified for one MR-S. cohnii subsp. cohnii isolate in a dog and one MR-S. saprophyticus subsp. saprophyticus in a rabbit (Table 3). The animals carrying these two strains had not been in direct contact with livestock or horses but were both in contact with other companion animals. Moreover, the dog carrying MR-S. cohnii subsp. cohnii had received topical antibiotics before sampling and was at the veterinary practice for a surgical intervention. The rabbit carrying MR-S. saprophyticus subsp. saprophyticus had been brought to the veterinarian practice for a standard examination.
Discussion
The risk of zoonotic transmission of bacteria between animals and humans is a global problem challenging human and veterinary medicine and beyond, such as food industry and wildlife, which necessitates inter- and transdisciplinary linking within a One Health concept [1,5,28,29]. However, most studies focus on the analysis of zoonotic agents in livestock whereas comprehensive data on the colonization of companion animals are limited. In contrast to livestock, companion animals are often considered as family members living in very close contact with their owners. Thus, transmission between humans and animals in both directions is very likely [3]. This study gives an overview of the prevalence of ESBL-E and MRSA in companion animals. Moreover, it provides insights into the occurrence of other staphylococcal species constituting potential reservoirs for methicillin resistance determinants.
In this point-prevalence assessment, we found that the proportion of animals colonized with ESBL-E. coli (2.5% of all animals or 3.6% of the dogs) was comparable to other studies investigating the perianal/rectal carriage of companion animals [30–32]. On the other hand, consecutive screenings of feline and canine fecal samples revealed considerably higher abundances of ESBL-E [33,34], showing the different outputs generated by the two approaches. Naturally, perianal swab samples only yield small amounts of fecal material, resulting in the recovery of only a fraction of bacterial isolates when compared to the corresponding fecal sample. Nevertheless, we deliberately chose this approach in order to efficiently collect and maximize the number of samples. Moreover, a swab sample of the perianal area provides valuable information about the risk of ESBL-E transmission from pet to owner, as the animals tested positive in this habitat present a permanent risk factor for the people in close contact.
The prevalence of MRSA among cats and dogs was generally lower than described for livestock [35,36]. However, it corresponded with carriage rates reported for humans in the general German population [37–39]. Previous studies found similar data in companion animals (0.1–5.7%) [40–43]. Interestingly, all MRSA isolates found were linked to spa types belonging to the livestock-associated ST398 (t011, t034, t108) and ST130 (t843), respectively. This is particularly interesting, as the majority of these animals (6/7) did not live in direct contact with livestock. However, the samples of this study were obtained from animals living in rural districts in North-West Germany, an area well known for its high density of husbandry, in particular pig farming. In this area, a significant increase of LA-MRSA, particularly CC398, into human healthcare facilities has been observed over the last decades [44,45]. Accordingly, the high colonization rate of this clonal lineage in companion animals could reflect the occurrence of LA-MRSA in the general and hospitalized human population of this area [37,45,46]. Moreover, Bierowiec et al. (2016) demonstrated that the prevalence of S. aureus is significantly higher in domestic cats than in feral cats [47]. This underpins previous assumptions of a bacterial ‘spill over’ from owners to their pets, thus creating a reservoir for (re-)colonization and infection [5,48–50]. The findings of this study are also in line with data about epidemic extended-host-spectrum MRSA lineages, which instead of showing host specificity can rather be assigned to a specific geographic origin [10,51]. Moreover, it has been shown, that especially MRSA show a high potential to spread via dust and air into the closer farm environment [52, 53]. Apart from this, the fact that all MDROs were either obtained from one veterinary practice or one veterinary clinic, strongly suggest a nosocomial spread of these strains. A significant association of these factors could be demonstrated (Fisher’s exact test; p < 0.05). This finding is further substantiated by the close relation of LA-MRSA spa types found and the very short time interval where ESBL-E. coli were detected particularly in animals treated ambulatory in the veterinary clinic. A general rise in veterinary nosocomial outbreaks for different MDROs has been well-documented in recent years [5,54,55]. Again, the ‘spill over’ from veterinary personnel, other animal patients and their human owners, and the distinct geographic origin would be key factors for such an outbreak.
Other coagulase-positive staphylococcal species, also often described as the S. aureus counterparts in veterinary medicine, are S. intermedius and S. pseudintermedius. Both species are well-known for causing different types of pyogenic and skin infections, such as pyoderma, dermatitis and otitis in small companion animals, particularly in dogs [56–61]. Moreover, the prevalence of MR strains has been increasing rapidly, leading to significant health problems in animals, particularly in veterinary hospital settings [62–65]. In the subgroup of 20 animals analyzed for the colonization of different staphylococcal species, S. pseudintermedius was found in dogs (3/13) and rabbits (1/4), but not in cats. S. intermedius was found only in dogs (4/13). However, none of these strains showed resistance against methicillin.
Apart from the coagulase-positive species S. intermedius and S. pseudintermedius, another twelve different coagulase-negative staphylococcal species were shown to be present in the subset of 20 animals. Amongst these, one canine S. cohnii subsp. cohnii isolate and one S. saprophyticus subsp. saprophyticus isolate in a rabbit were shown to be phenotypically MR and to harbor the mecA gene. In humans, S. saprophyticus subsp. saprophyticus is well known as a frequent cause for urinary tract infections in young women [66–71]. In animals, S. saprophyticus has been described as a colonizer of the gastrointestinal tract of cattle and pigs, a causative agent for subclinical mastitis in cows, and as a contaminant of animal food products [72–74]. Less data are available on S. cohnii subsp. cohnii, which is described as a colonizer of dogs, goats and poultry, but has also been detected in the hospital environment and in clinical samples of humans suffering from a variety of infectious diseases [66,75–77]. In the past decades, the prevalence of MR-CoNS is rapidly increasing, reaching levels of up to over 80% within clinical samples of human patients [66,78–80]. In the animal cohort investigated here, at least 10.0% of animals (2/20) were colonized with MR-CoNS. Another two animals were colonized with other phenotypically resistant staphylococcal strains. These strains might either serve as a reservoir of methicillin resistance genes for other zoonotic pathogens or could be transferred directly to humans, such as pet owners or veterinary personnel.
Conclusion
Data of this study prove that resistances against beta-lactams and other antibiotic classes are ubiquitously present in medically significant pathogens colonizing companion animals. This situation constitutes a high risk of transmission of MDROs or resistance-encoding mobile elements to humans being in close contact to those animals and their microbiota. The exclusive occurrence of only those MRSA belonging to livestock-associated clonal lineages in companion animals without direct contact to livestock emphasizes the adverse effects of MDRO dissemination across species barriers in regions with high density of livestock husbandry. The findings underline the need for the consequent implementation of the One Health concept considering all interconnections between human and animal populations where pathogens and their resistance mechanisms can be transmitted.
Acknowledgments
We thank Melanie Bach, Damayanti Kaiser and Martina Schulte for excellent technical assistance.
Data Availability
All relevant data are within the manuscript.
Funding Statement
This work was supported in part by the German Federal Ministry for Education and Research (BMBF) within the research consortia MedVet-Staph (grant no. 01KI1301A to KB and RK), RESET (grant no. 01Kl1013C to UR) and the Research Network Zoonotic Infectious Diseases (project #1Health-PREVENT, grant no. 01KI1727A to KB and RK) and in part by the Bundesinstitut für Risikobewertung (BfR) (grant no. 1329-557 to KB). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wieler LH, Ewers C, Guenther S, Walther B, Lübke-Becker A. Methicillin-resistant staphylococci (MRS) and extended-spectrum beta-lactamases (ESBL)-producing Enterobacteriaceae in companion animals: Nosocomial infections as one reason for the rising prevalence of these potential zoonotic pathogens in clinica. Int J Med Microbiol. Elsevier GmbH.; 2011;301: 635–641. 10.1016/j.ijmm.2011.09.009 [DOI] [PubMed] [Google Scholar]
- 2.Weese JS, Fulford MB. Companion Animal Zoonoses. 1st ed Oxford, UK: Wiley-Blackwell; 2011. 10.1002/9780470958957 [Google Scholar]
- 3.Walther B, Hermes J, Cuny C, Wieler LH, Vincze S, Elnaga YA, et al. Sharing more than friendship—nasal colonization with coagulase-positive staphylococci (CPS) and co-habitation aspects of dogs and their owners. PLoS One. 2012;7: e35197 10.1371/journal.pone.0035197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pomba C, Rantala M, Greko C, Baptiste KE, Catry B, van Duijkeren E, et al. Public health risk of antimicrobial resistance transfer from companion animals. J Antimicrob Chemother. Oxford University Press; 2017;72: 957–968. 10.1093/jac/dkw481 [DOI] [PubMed] [Google Scholar]
- 5.Walther B, Tedin K, Lübke-Becker A. Multidrug-resistant opportunistic pathogens challenging veterinary infection control. Vet Microbiol. Elsevier; 2017;200: 71–78. 10.1016/J.VETMIC.2016.05.017 [DOI] [PubMed] [Google Scholar]
- 6.Vincze S, Stamm I, Kopp PA, Hermes J, Adlhoch C, Semmler T, et al. Alarming proportions of methicillin-resistant Staphylococcus aureus (MRSA) in wound samples from companion animals, Germany 2010–2012. PLoS One. 2014;9: e85656 10.1371/journal.pone.0085656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Misic AM, Davis MF, Tyldsley AS, Hodkinson BP, Tolomeo P, Hu B, et al. The shared microbiota of humans and companion animals as evaluated from Staphylococcus carriage sites. Microbiome. 2015;3: 1–19. 10.1186/s40168-014-0066-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mani I, Maguire JH. Small Animal Zoonoses and Immuncompromised Pet Owners. Top Companion Anim Med. 2009;24: 164–174. 10.1053/j.tcam.2009.07.002 [DOI] [PubMed] [Google Scholar]
- 9.Schmiedel J, Falgenhauer L, Domann E, Bauerfeind R, Prenger-Berninghoff E, Imirzalioglu C, et al. Multiresistant extended-spectrum β-lactamase-producing Enterobacteriaceae from humans, companion animals and horses in central Hesse, Germany. BMC Microbiol. 2014;14: 1–13. 10.1186/1471-2180-14-187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vincze S, Stamm I, Monecke S, Kopp PA, Semmler T, Wieler LH, et al. Molecular analysis of human and canine Staphylococcus aureus strains reveals distinct extended-host-spectrum genotypes independent of their methicillin resistance. Appl Environ Microbiol. 2013;79: 655–662. 10.1128/AEM.02704-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Becker K, van Alen S, Idelevich EA, Schleimer N, Seggewiß J, Mellmann A, et al. Plasmid-Encoded Transferable mecB-Mediated Methicillin Resistance in Staphylococcus aureus. Emerg Infect Dis. 2018;24: 242–248. 10.3201/eid2402.171074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Becker K, Ballhausen B, Köck R, Kriegeskorte A. Methicillin resistance in Staphylococcus isolates: The “mec alphabet” with specific consideration of mecC, a mec homolog associated with zoonotic S. aureus lineages. Int J Med Microbiol. 2014;304: 794–804. 10.1016/j.ijmm.2014.06.007 [DOI] [PubMed] [Google Scholar]
- 13.Schwendener S, Cotting K, Perreten V. Novel methicillin resistance gene mecD in clinical Macrococcus caseolyticus strains from bovine and canine sources. Sci Rep. 2017;7: 1–11. 10.1038/s41598-016-0028-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ur Rahman S, Ali T, Ali I, Khan NA, Han B, Gao J. The Growing Genetic and Functional Diversity of Extended Spectrum Beta-Lactamases. Biomed Res Int. 2018;2018: 9519718 10.1155/2018/9519718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bush K, Jacoby GA. Updated functional classification of beta-lactamases. Antimicrob Agents Chemother. 2010;54: 969–76. 10.1128/AAC.01009-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cantón R, González-Alba JM, Galán JC. CTX-M enzymes: Origin and diffusion. Front Microbiol. 2012;3 10.3389/fmicb.2012.00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Idelevich EA, Schüle I, Grünastel B, Wüllenweber J, Peters G, Becker K. Rapid identification of microorganisms from positive blood cultures by MALDI-TOF mass spectrometry subsequent to very short-term incubation on solid medium. Clin Microbiol Infect. 2014;20: 1001–1006. 10.1111/1469-0691.12640 [DOI] [PubMed] [Google Scholar]
- 18.Kaspar U, Kriegeskorte A, Schubert T, Peters G, Rudack C, Pieper DH, et al. The culturome of the human nose habitats reveals individual bacterial fingerprint patterns. Environ Microbiol. 2016;18: 2130–2142. 10.1111/1462-2920.12891 [DOI] [PubMed] [Google Scholar]
- 19.Breitkopf C, Hammel D, Scheld HH, Peters G, Becker K. Impact of a molecular approach to improve the microbiological diagnosis of infective heart valve endocarditis. Circulation. 2005;111: 1415–1421. 10.1161/01.CIR.0000158481.07569.8D [DOI] [PubMed] [Google Scholar]
- 20.Lane DJ. 16S/23S rRNA Sequencing In: Stackebrandt E, Goodfellow M, editors. Nucleic acid techniques in bacterial systematics. Chichester, United Kingdom: John Wiley & Sons; 1991. pp. 115–175. 10.1007/s00227-012-2133-0 [Google Scholar]
- 21.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73: 5261–7. 10.1128/AEM.00062-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 8.0; 2018 [Internet]. Available from: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_8.0_Breakpoint_Tables.pdf
- 23.Kriegeskorte A, Ballhausen B, Idelevich EA, Köck R, Friedrich AW, Karch H, et al. Human MRSA isolates with novel genetic homolog, Germany. Emerg Infect Dis. 2012;18: 1016–1018. 10.3201/eid1806.110910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mellmann A, Friedrich AW, Rosenkötter N, Rothgänger J, Karch H, Reintjes R, et al. Automated DNA Sequence-Based Early Warning System for the Detection of Methicillin-Resistant Staphylococcus aureus Outbreaks. PLoS Med. 2006;3: e33 10.1371/journal.pmed.0030033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Enright MC, Day NPJ, Davies CE, Peacock SJ, Spratt BG. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol. 2000;38: 1008–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monstein HJ, Östholm-Balkhed Å, Nilsson M V., Nilsson M, Dornbusch K, Nilsson LE. Multiplex PCR amplification assay for the detection of blaSHV, blaTEM and blaCTX-M genes in Enterobacteriaceae. Apmis. 2007;115: 1400–1408. 10.1111/j.1600-0463.2007.00722.x [DOI] [PubMed] [Google Scholar]
- 27.Souna D, Amir AS, Bekhoucha SN, Berrazeg M, Drissi M. Molecular typing and characterization of TEM, SHV, CTX-M, and CMY-2 β-lactamases in Enterobacter cloacae strains isolated in patients and their hospital environment in the west of Algeria. Médecine Mal Infect. 2014;44: 146–152. 10.1016/J.MEDMAL.2014.01.008 [DOI] [PubMed] [Google Scholar]
- 28.Zinsstag J, Schelling E, Waltner-Toews D, Tanner M. From “one medicine”; to “one health” and systemic approaches to health and well-being. Prev Vet Med. 2011;101: 148–56. 10.1016/j.prevetmed.2010.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaumburg F, Mugisha L, Peck B, Becker K, Gillespie TR, Peters G, et al. Drug-Resistant Human Staphylococcus aureus in Sanctuary Apes Pose a Threat to Endangered Wild Ape Populations. Am J Primatol. 2012;74: 1071–1075. 10.1002/ajp.22067 [DOI] [PubMed] [Google Scholar]
- 30.Harada K, Morimoto E, Kataoka Y, Takahashi T. Clonal spread of antimicrobial-resistant Escherichia coli isolates among pups in two kennels. Acta Vet Scand. 2011;53: 11 10.1186/1751-0147-53-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gandolfi-Decristophoris P, Petrini O, Ruggeri-Bernardi N, Schelling E. Extended-spectrum β-lactamase-producing Enterobacteriaceae in healthy companion animals living in nursing homes and in the community. Am J Infect Control. 2013;41: 831–835. 10.1016/j.ajic.2012.11.013 [DOI] [PubMed] [Google Scholar]
- 32.Murphy C, Reid-Smith RJ, Prescott JF, Bonnett BN, Poppe C, Boerlin P, et al. Occurrence of antimicrobial resistant bacteria in healthy dogs and cats presented to private veterinary hospitals in southern Ontario: A preliminary study. Can Vet J = La Rev Vet Can. 2009;50: 1047–53 [PMC free article] [PubMed] [Google Scholar]
- 33.Baede VO, Wagenaar JA, Broens EM, Duim B, Dohmen W, Nijsse R, et al. Longitudinal study of extended-spectrum-β-lactamase- and AmpC-producing Enterobacteriaceae in household dogs. Antimicrob Agents Chemother. 2015;59: 3117–24. 10.1128/AAC.04576-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hordijk J, Schoormans A, Kwakernaak M, Duim B, Broens E, Dierikx C, et al. High prevalence of fecal carriage of extended spectrum β-lactamase/AmpC-producing Enterobacteriaceae in cats and dogs. Front Microbiol. Frontiers; 2013;4: 242 10.3389/fmicb.2013.00242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graveland H, Duim B, van Duijkeren E, Heederik D, Wagenaar JA. Livestock-associated methicillin-resistant Staphylococcus aureus in animals and humans. Int J Med Microbiol. 2011;301: 630–634. 10.1016/j.ijmm.2011.09.004 [DOI] [PubMed] [Google Scholar]
- 36.Becker K, Ballhausen B, Kahl BC, Köck R. The clinical impact of livestock-associated methicillin-resistant Staphylococcus aureus of the clonal complex 398 for humans. Vet Microbiol. 2017;200: 33–38. 10.1016/j.vetmic.2015.11.013 [DOI] [PubMed] [Google Scholar]
- 37.Becker K, Schaumburg F, Fegeler C, Friedrich AW, Köck R, Prevalence of Multiresistant Microorganisms PMM Study. Staphylococcus aureus from the German general population is highly diverse. Int J Med Microbiol. 2017;307: 21–27. 10.1016/j.ijmm.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 38.Köck R, Mellmann A, Schaumburg F, Friedrich AW, Kipp F, Becker K. The epidemiology of methicillin-resistant Staphylococcus aureus (MRSA) in Germany. Dtsch Arztebl Int. 2011;108: 761–7. 10.3238/arztebl.2011.0761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Köck R, Werner P, Friedrich AW, Fegeler C, Becker K, Bindewald O, et al. Persistence of nasal colonization with human pathogenic bacteria and associated antimicrobial resistance in the German general population. New Microbes New Infect. 2016;9: 24–34. 10.1016/j.nmni.2015.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoet AE, van Balen J, Nava-Hoet RC, Bateman S, Hillier A, Dyce J, et al. Epidemiological Profiling of Methicillin-Resistant Staphylococcus aureus -Positive Dogs Arriving at a Veterinary Teaching Hospital. Vector-Borne Zoonotic Dis. 2013;13: 385–393. 10.1089/vbz.2012.1089 [DOI] [PubMed] [Google Scholar]
- 41.Wedley AL, Dawson S, Maddox TW, Coyne KP, Pinchbeck GL, Clegg P, et al. Carriage of Staphylococcus species in the veterinary visiting dog population in mainland UK: Molecular characterisation of resistance and virulence. Vet Microbiol. 2014;170: 81–88. 10.1016/j.vetmic.2014.01.015 [DOI] [PubMed] [Google Scholar]
- 42.Ruzauskas M, Couto N, Kerziene S, Siugzdiniene R, Klimiene I, Virgailis M, et al. Prevalence, species distribution and antimicrobial resistance patterns of methicillin-resistant staphylococci in Lithuanian pet animals. Acta Vet Scand. 2015;57: 27 10.1186/s13028-015-0117-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davis JA, Jackson CR, Fedorka-Cray PJ, Barrett JB, Brousse JH, Gustafson J, et al. Carriage of methicillin-resistant staphylococci by healthy companion animals in the US. Lett Appl Microbiol. 2014;59: 1–8. 10.1111/lam.12254 [DOI] [PubMed] [Google Scholar]
- 44.van Alen S, Ballhausen B, Peters G, Friedrich AW, Mellmann A, Köck R, et al. In the centre of an epidemic: Fifteen years of LA-MRSA CC398 at the University Hospital Münster. Vet Microbiol. 2017;200: 19–24. 10.1016/j.vetmic.2016.01.021 [DOI] [PubMed] [Google Scholar]
- 45.Köck R, Harlizius J, Bressan N, Laerberg R, Wieler LH, Witte W, et al. Prevalence and molecular characteristics of methicillin-resistant Staphylococcus aureus (MRSA) among pigs on German farms and import of livestock-related MRSA into hospitals. Eur J Clin Microbiol Infect Dis. 2009;28: 1375–1382. 10.1007/s10096-009-0795-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Köck R, Siam K, Al-Malat S, Christmann J, Schaumburg F, Becker K, et al. Characteristics of hospital patients colonized with livestock-associated meticillin-resistant Staphylococcus aureus (MRSA) CC398 versus other MRSA clones. J Hosp Infect. Elsevier; 2011;79: 292–296. 10.1016/j.jhin.2011.08.011 [DOI] [PubMed] [Google Scholar]
- 47.Bierowiec K, Płoneczka-Janeczko K, Rypuła K. Prevalence and Risk Factors of Colonization with Staphylococcus aureus in Healthy Pet Cats Kept in the City Households. Biomed Res Int. 2016;2016: 3070524 10.1155/2016/3070524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morgan M. Methicillin-resistant Staphylococcus aureus and animals: zoonosis or humanosis? J Antimicrob Chemother. Oxford University Press; 2008;62: 1181–1187. 10.1093/jac/dkn405 [DOI] [PubMed] [Google Scholar]
- 49.Guardabassi L, Schwarz S, Lloyd DH. Pet animals as reservoirs of antimicrobial-resistant bacteria. J Antimicrob Chemother. 2004;54: 321–332. 10.1093/jac/dkh332 [DOI] [PubMed] [Google Scholar]
- 50.Strommenger B, Kehrenberg C, Kettlitz C, Cuny C, Verspohl J, Witte W, et al. Molecular characterization of methicillin-resistant Staphylococcus aureus strains from pet animals and their relationship to human isolates. J Antimicrob Chemother. 2006;57: 461–465. 10.1093/jac/dki471 [DOI] [PubMed] [Google Scholar]
- 51.Harrison EM, Weinert LA, Holden MTG, Welch JJ, Wilson K, Morgan FJE, et al. A shared population of epidemic methicillin-resistant Staphylococcus aureus 15 circulates in humans and companion animals. MBio. 2014;5: e00985–13. 10.1128/mBio.00985-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feld L, Bay H, Angen Ø, Larsen AR, Madsen AM. Survival of LA-MRSA in dust from swine farms. Ann Work Expo Heal. 2018;62: 147–156. 10.1093/annweh/wxx108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bos MEH, Verstappen KM, Van Cleef BAGL, Dohmen W, Dorado-García A, Graveland H, et al. Transmission through air as a possible route of exposure for MRSA. J Expo Sci Environ Epidemiol. 2016;26: 263–269. 10.1038/jes.2014.85 [DOI] [PubMed] [Google Scholar]
- 54.van Duijkeren E, Moleman M, Sloet van Oldruitenborgh-Oosterbaan MM, Multem J, Troelstra A, Fluit AC, et al. Methicillin-resistant Staphylococcus aureus in horses and horse personnel: An investigation of several outbreaks. Vet Microbiol. 2010;141: 96–102. 10.1016/j.vetmic.2009.08.009 [DOI] [PubMed] [Google Scholar]
- 55.Cuny C, Witte W. MRSA in equine hospitals and its significance for infections in humans. Vet Microbiol. 2017;200: 59–64. 10.1016/j.vetmic.2016.01.013 [DOI] [PubMed] [Google Scholar]
- 56.Ganiere J-P, Medaille C, Mangion C. Antimicrobial Drug Susceptibility of Staphylococcus intermedius Clinical Isolates from Canine Pyoderma. J Vet Med Ser B. 2005;52: 25–31. 10.1111/j.1439-0450.2004.00816.x [DOI] [PubMed] [Google Scholar]
- 57.Fazakerley J, Williams N, Carter S, McEwan N, Nuttall T. Heterogeneity of Staphylococcus pseudintermedius isolates from atopic and healthy dogs. Vet Dermatol. 2010;21: 578–585. 10.1111/j.1365-3164.2010.00894.x [DOI] [PubMed] [Google Scholar]
- 58.Hájek V. Staphylococcus intermedius, a New Species Isolated from Animals. Int J Syst Bacteriol. 1976;26: 401–408. 10.1099/00207713-26-4-401 [Google Scholar]
- 59.Biberstein EL, Jang SS, Hirsh DC. Species distribution of coagulase-positive staphylococci in animals. J Clin Microbiol. 1984;19: 610–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoekstra KA, Paulton RJL. Clinical prevalence and antimicrobial susceptibility of Staphylococcus aureus and Staph. intermedius in dogs. J Appl Microbiol. 2002;93: 406–413. 10.1046/j.1365-2672.2002.01708.x [DOI] [PubMed] [Google Scholar]
- 61.Shin JH, Kim SH, Jeong HS, Oh SH, Kim HR, Lee JN, et al. Identification of coagulase-negative staphylococci isolated from continuous ambulatory peritoneal dialysis fluid using 16S ribosomal RNA, tuf, and SodA gene sequencing. Perit Dial Int. 2011;31: 340–6. 10.3747/pdi.2010.00073 [DOI] [PubMed] [Google Scholar]
- 62.Weese JS, van Duijkeren E. Methicillin-resistant Staphylococcus aureus and Staphylococcus pseudintermedius in veterinary medicine. Vet Microbiol. Elsevier; 2010;140: 418–429. 10.1016/j.vetmic.2009.01.039 [DOI] [PubMed] [Google Scholar]
- 63.van Duijkeren E, Catry B, Greko C, Moreno MA, Pomba MC, Pyorala S, et al. Review on methicillin-resistant Staphylococcus pseudintermedius. J Antimicrob Chemother. Oxford University Press; 2011;66: 2705–2714. 10.1093/jac/dkr367 [DOI] [PubMed] [Google Scholar]
- 64.Perreten V, Kadlec K, Schwarz S, Andersson UG, Finn M, Greko C, et al. Clonal spread of methicillin-resistant Staphylococcus pseudintermedius in Europe and North America: An international multicentre study. J Antimicrob Chemother. 2010;65: 1145–1154. 10.1093/jac/dkq078 [DOI] [PubMed] [Google Scholar]
- 65.De Lucia M, Moodley A, Latronico F, Giordano A, Caldin M, Fondati A, et al. Prevalence of canine methicillin resistant Staphylococcus pseudintermedius in a veterinary diagnostic laboratory in Italy. Res Vet Sci. 2011;91: 346–348. 10.1016/j.rvsc.2010.09.014 [DOI] [PubMed] [Google Scholar]
- 66.Becker K, Heilmann C, Peters G. Coagulase-negative staphylococci. Clin Microbiol Rev. 2014;27: 870–926. 10.1128/CMR.00109-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wallmark G, Arremark I, Telander B. Staphylococcus saprophyticus: a frequent cause of acute urinary tract infection among female outpatients. J Infect Dis. 1978;138: 791–7. [DOI] [PubMed] [Google Scholar]
- 68.Schneider PF, Riley T V. Staphylococcus saprophyticus urinary tract infections: epidemiological data from Western Australia. Eur J Epidemiol. 1996;12: 51–54. 10.1007/BF00144428 [DOI] [PubMed] [Google Scholar]
- 69.Raz R, Colodner R, Kunin CM. Who Are You—Staphylococcus saprophyticus? Clin Infect Dis. 2005;40: 896–898. 10.1086/428353 [DOI] [PubMed] [Google Scholar]
- 70.Nys S, van Merode T, Bartelds AIM, Stobberingh EE. Urinary tract infections in general practice patients: diagnostic tests versus bacteriological culture. J Antimicrob Chemother. 2006;57: 955–958. 10.1093/jac/dkl082 [DOI] [PubMed] [Google Scholar]
- 71.Eriksson A, Giske CG, Ternhag A. The relative importance of Staphylococcus saprophyticus as a urinary tract pathogen: distribution of bacteria among urinary samples analysed during 1 year at a major Swedish laboratory. APMIS. 2013;121: 72–78. 10.1111/j.1600-0463.2012.02937.x [DOI] [PubMed] [Google Scholar]
- 72.Hedman P, Ringertz O, Eriksson B, Kvarnfors P, Andersson M, Bengtsson L, et al. Staphylococcus saprophyticus found to be a common contaminant of food. J Infect. 1990;21: 11–19. 10.1016/0163-4453(90)90554-L [DOI] [PubMed] [Google Scholar]
- 73.Persson Waller K, Aspán A, Nyman A, Persson Y, Grönlund Andersson U. CNS species and antimicrobial resistance in clinical and subclinical bovine mastitis. Vet Microbiol. 2011;152: 112–116. 10.1016/j.vetmic.2011.04.006 [DOI] [PubMed] [Google Scholar]
- 74.Hedman P, Ringertz O, Lindström M, Olsson K. The Origin of Staphylococcus saprophyticus from Cattle and Pigs. Scand J Infect Dis. 1993;25: 57–60. 10.1080/00365549309169670 [PubMed] [Google Scholar]
- 75.Piette A, Verschraegen G. Role of coagulase-negative staphylococci in human disease. Vet Microbiol. 2009;134: 45–54. 10.1016/j.vetmic.2008.09.009 [DOI] [PubMed] [Google Scholar]
- 76.Szewczyk EM, Nowak T, Cieślikowski T, Różalska M. Potential Role of Staphylococcus cohnii in a Hospital Environment. Microb Ecol Health Dis. 2003;15: 51–56. 10.1080/08910600310014908 [Google Scholar]
- 77.Koksal F, Yasar H, Samasti M. Antibiotic resistance patterns of coagulase-negative Staphylococcus strains isolated from blood cultures of septicemic patients in Turkey. Microbiol Res. 2009;164: 404–10. 10.1016/j.micres.2007.03.004 [DOI] [PubMed] [Google Scholar]
- 78.Hellmark B, Unemo M, Nilsdotter-Augustinsson Å, Söderquist B. Antibiotic susceptibility among Staphylococcus epidermidis isolated from prosthetic joint infections with special focus on rifampicin and variability of the rpoB gene. Clin Microbiol Infect. 2009;15: 238–244. 10.1111/j.1469-0691.2008.02663.x [DOI] [PubMed] [Google Scholar]
- 79.Jones ME, Karlowsky JA, Draghi DC, Thornsberry C, Sahm DF, Nathwani D. Epidemiology and antibiotic susceptibility of bacteria causing skin and soft tissue infections in the USA and Europe: a guide to appropriate antimicrobial therapy. Int J Antimicrob Agents. 2003;22: 406–419. 10.1016/S0924-8579(03)00154-7 [DOI] [PubMed] [Google Scholar]
- 80.Guggenheim M, Zbinden R, Handschin AE, Gohritz A, Altintas MA, Giovanoli P. Changes in bacterial isolates from burn wounds and their antibiograms: A 20-year study (1986–2005). Burns. 2009;35: 553–560. 10.1016/j.burns.2008.09.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.